Abstract
INTRODUCTION:
Mitral regurgitation (MR) in chronic heart failure (CHF) patients frequently worsens with exercise. Cardiac resynchronization therapy (CRT) reduces MR at rest, but its effects on exercise-induced worsening of MR are incompletely explored. The present study examined the influence of CRT on MR during submaximal exercise in CHF patients.
METHODS:
Eleven patients with CHF who were treated with CRT underwent echocardiography while performing steady-state exercise during four conduction modes (intrinsic rhythm, right ventricular [RV], biventricular [BiV] and left ventricular [LV] pacing). Measurements of MR were jet area planimetry, effective regurgitant orifice area, peak MR flow rate and regurgitant volume.
RESULTS:
At rest and during exercise, there were no differences in dyssynchrony between intrinsic rhythm and RV pacing. BiV and LV pacing reduced dyssynchrony at rest and during exercise compared with intrinsic conduction and RV pacing, and there were no differences in the magnitude of these effects between these two pacing modes. At rest, RV pacing increased MR compared with intrinsic conduction (MR regurgitant volume; P<0.05), whereas BiV and LV pacing reduced MR (reductions in effective regurgitant orifice area and jet area; P<0.02, and MR flow rate; P<0.05 with BiV pacing from intrinsic conduction). MR significantly increased on exercise with intrinsic rhythm and RV pacing, whereas with LV and BiV pacing, there were no significant exercise-induced increases in any MR variable. There were relationships between changes in measures of dyssynchrony and reductions in MR at rest and during exercise.
CONCLUSIONS:
CRT reduces MR at rest and during exercise, and prevents exercise-induced MR. Reductions in MR during exercise correlate with improvements in dyssynchrony.
Keywords: Cardiac resynchronization therapy, Heart failure, Mitral regurgitation
Abstract
INTRODUCTION :
Chez les patients atteints d’insuffisance cardiaque chronique (ICC), la régurgitation mitrale (RM) s’aggrave souvent à l’effort. Le traitement par resynchronisation cardiaque (TRC) réduit la RM au repos, mais ses effets sur l’aggravation de la RM à l’effort restent à élucider. La présente étude s’est penchée sur l’influence du TRC sur la RM durant un exercice modéré chez des patients atteints d’ICC.
MÉTHODES :
Onze patients atteints d’ICC qui étaient traités par TRC ont subi une échocardiographie d’effort à l’équilibre sous quatre modes de conduction (rythme intrinsèque, stimulation ventriculaire droite [VD], biventriculaire [BiV] et ventriculaire gauche [VG]). Les mesures de la RM ont porté sur la planimétrie du jet passant, l’ouverture effective de régurgitation mitrale, le flux de RM de pointe et le volume de régurgitation.
RÉSULTATS :
Au repos et à l’effort, on n’a noté aucune différence de dyssynchronie entre le rythme intrinsèque et la stimulation VD. La stimulation BiV et VG a réduit la dyssynchronie au repos et à l’effort comparativement à la conduction intrinsèque et la stimulation VD et on n’a observé aucune différence quant à l’ampleur de ces effets entre les deux modes de stimulation. Au repos, la stimulation VD a augmenté la RM comparativement à la conduction intrinsèque (volume régurgitant, P<0,05), tandis que la stimulation BiV et VG a réduit la RM (réduction de l’ouverture effective de régurgitation et du jet passant, P<0,02 et du flux de RM, P<0,05 avec stimulation BiV par rapport à la conduction intrinsèque). La RM a significativement augmenté à l’effort avec le rythme intrinsèque et la stimulation VD, tandis qu’avec la stimulation VG et BiV, on n’a noté aucune augmentation significative des variables de la RM à l’effort. On a noté des liens entre les changements des mesures de dyssynchronie et les réductions de la RM au repos et à l’effort.
CONCLUSIONS :
Le TRC réduit la RM au repos et à l’effort et empêche la RM induite par l’exercice. Les réductions de la RM durant l’exercice sont en corrélation avec les améliorations de la dyssynchronie.
Significant secondary, or ‘functional’, mitral regurgitation (MR) is seen in 40% to 60% of patients with chronic heart failure (CHF) due to left ventricular (LV) systolic dysfunction (LVSD) (1,2). Significant MR decreases effective stroke volume, limits stroke volume adaptation during exercise and predicts a worse outcome in patients with heart failure (2,3). MR increases during exercise in patients with systolic heart failure (4–6), and although the degree of MR at rest is unrelated to that during exercise (6), the presence of even mild MR at rest is associated with a lower functional capacity (7,8). The degree of regurgitation at rest relates directly to the impairment in exercise capacity (9). A significant increase in MR with exercise can lead to acute pulmonary edema (10) and confers an adverse prognosis in CHF patients (11,12).
The severity of MR in CHF patients relates to the degree of dyssynchrony both at rest and during exercise (13), and early reports of biventricular (BiV) pacing have documented that cardiac resynchronization therapy (CRT) was associated with reductions in MR (14). Since then, further data have confirmed that univentricular left-sided (15) and BiV pacing can reduce the degree of MR at rest acutely (16,17) and chronically (18–20). In ‘responders’ to CRT, the MR reduces in line with reverse LV remodelling (21) and correlates with the degree of improved synchronicity when examined by tissue Doppler imaging (20,21) and papillary muscle strain mapping (22). There are two reports examining the effects of CRT on MR induced by peak exercise (23,24), and one demonstrating reductions in MR and dyssynchrony during heart rate-matched workloads in patients following CRT (25). We aimed to add to the current literature on MR in patients with resynchronization therapy. First, we contrasted the impact of right ventricular (RV), LV and BiV pacing compared with intrinsic conduction on the measures of MR at rest. Second, we examined the impact of submaximal exercise on the severity of MR during the different pacing and conduction modes. The choice of a submaximal target allowed an equivalent workload to be maintained during all measurement periods. Finally, we explored the impact of different conduction and pacing modes, as well as exercise on measures of ventricular dyssynchrony, and how changes in these variables relate to changes in measures of the severity of mitral insufficiency.
PATIENTS AND METHODS
Patients
Patients who had recently undergone implantation of a CRT device were eligible to participate in the study. All patients had a history of advanced CHF secondary to systolic LV dysfunction due to ischemic heart disease and an ejection fraction lower than 35%. All had been in New York Heart Association class III at the time of implantation. Patients were in normal sinus rhythm with preserved atrioventricular conduction, and all had a left bundle branch block intraventricular conduction pattern with a QRS duration of greater than 130 ms. In addition, all patients had significant dyssynchrony on at least two of the variables described below at the time of implantation, and objective evidence of impairment of exercise capacity on exercise testing with metabolic gas analysis. During the recruitment phase, consecutive patients were approached at their follow-up appointment. Patients with musculoskeletal disorders limiting exercise capacity (n=1), atrial fibrillation, those with poor echocardiographic windows (n=1) or those residing in another province (n=1) were not enrolled. Each patient underwent a cycle-based peak exercise test to establish peak oxygen consumption and maximal workload approximately one month following implantation of their CRT device. At this time, two-dimensional tissue Doppler and transmitral echocardiography were used to optimize intra-, inter- and atrioventricular timing at rest, aiming for simultaneous radial and longitudinal contraction and a normalized mitral inflow pattern. Patients were not selected based on response to CRT. Written, informed consent was obtained from all subjects. The study was approved by the human subjects ethics review board of Mount Sinai Hospital, Toronto, Ontario.
Exercise echocardiographic procedures
Image aquisition
Within two months of implantation, patients were brought to the echocardiography laboratory. Their devices were randomly set to one of four modes: RV, BiV, LV pacing and no pacing (intrinsic conduction [single-chamber ventricular pacemaking 30 beats/min]). Atrioventricular conduction was set to the same level for each pacing mode (150 ms). The patient, echocardiographer and supervising physician were blinded to the pacing mode. Resting echocardiography was performed as described below (Vivid 7; Vingmed, Norway), and the images were stored digitally on commercially available equipment (Echopac PC; Vingmed, Norway). The patient was then asked to perform exercise in the supine position at 30% of the peak workload achieved during the postimplantation assessment of peak oxygen consumption using a commercial supine ergometry system. This level of exercise was chosen to be lower than the anaerobic threshold of all subjects, allowing it to be maintained for the time required to achieve steady state (26) and record the images. A percentage of peak workload was chosen to ensure that the same proportion of peak work was being performed by all individuals during the test. During exercise, but after at least 3 min (27), repeat echocardiography was performed. After acquisition, the patient was allowed to rest for a minimum of 20 min, until basic hemodynamic variables had returned to resting levels, and the pacemaker was reprogrammed to the next mode. This procedure was repeated until images had been acquired during rest and steady-state exercise for each of the four modes of pacing. The order of each of the subsequent pacing modes was also randomized. At the time of the study, images were coded to ensure blinding of the reporter to both pacing mode and rest or exercise. They were then analyzed offline. Blinding was maintained until the end of the recruitment period.
Assessment of MR
In assessing the degree of MR, the recommendations of the American Society of Echocardiography (28) were used:
Jet area by planimetry in the four-chamber view.
- The proximal isovelocity surface area (PISA) or flow convergence. This calculation uses the velocity of the regurgitant jet and the radius of the hemisphere of increasing colour flow signal seen proximal to the regurgitant orifice (29). Maximal effective regurgitant orifice area (EROA) can then be derived using the equation:
where Va is the aliasing velocity at the orifice and PkVreg is the peak velocity of the regurgitant jet on continuous wave Doppler (30). This allowed recording of the MR flow rate from the PISA calculation. The velocity-time integrals for aortic outflow, mitral inflow, and the areas of the mitral and aortic valves to calculate LV inflow and outflow volumes were measured; the difference between the volumes was used to estimate MR volume (MRV) (31).
Assessment of dyssynchrony
The assessment of dyssynchrony remains an expanding field. Electrocardiographic variables are not specific enough to predict either the presence of dyssynchrony or the response to therapy (32–34). In addition to a left bundle branch block on electrocardiogram, several methods were used to confirm intraventricular and interventricular dyssynchrony in patients referred for CRT.
M-mode echocardiography across the parasternal long-axis or short-axis views identified intraventricular delays. A septal to posterior wall motion delay of longer than 130 ms is generally the accepted value for significant radial dyssynchrony (35).
Tissue Doppler echocardiography of the mitral annulus examines the longitudinal motion of the base of the heart. Although the exact degree of lateral annular delay identifying potential responders varies slightly among published articles, longitudinal delays of time to peak velocity of systolic motion that are longer than approximately 65 ms between the septal and lateral portions of the mitral annulus are thought to represent significant intraventricular dyssynchrony (intraventricular delay) (36,37).
Interventricular dyssynchrony can be measured by comparing the timing of aortic outflow with that of pulmonary outflow. This remains the simplest and most reproducible measure of dyssynchrony, and a delay of longer than 40 ms was a criterion for recruitment to a major randomized trial in CRT (38).
In addition to the variables discussed above, tissue Doppler imaging was used to examine longitudinal interventricular dyssynchrony by looking at the time to peak velocity of the systolic motion of the lateral portion of the mitral annulus, and comparing this with the timing of systolic motion of the tricuspid annulus in the four-chamber view (interventricular delay).
Other echocardiographic variables
RV systolic pressure at rest and during exercise was estimated from the systolic transtricuspid pressure gradient with the use of the modified Bernoulli equation. LV ejection fraction was assessed using biplane-modified Simpson’s rule.
Statistical analysis
Results are reported as mean ± SE. Using a commercially available statistics program (Statview; SAS Institute, USA), the different pacing modalities between subjects and the effects of exercise were compared. ANOVA was used to examine the changes in MR and dyssynchrony variables, comparing the three pacing modes against intrinsic conduction and each other. Multiple comparisons were compensated for using Fisher’s protected least significant difference correction. Paired t tests were used to examine changes within a pacing modality at rest and during exercise. Simple linear regression was used to explore whether there were systematic relationships between continuous variables. P<0.05 was considered to be significant.
RESULTS
Table 1 displays the characteristics of patients at baseline. The mean PR interval was 184±34 ms. There was a small but nonsignificant increase in peak oxygen consumption at the one-month exercise test above that performed preimplantation (10.7±1.6 versus 13.1±1.2; P not significant). Two patients had no subjective improvement in symptoms.
TABLE 1.
Subject characteristics (n=11)
| Characteristic | Baseline value |
|---|---|
| Age (years) | 59±2 |
| Days after implantation | 45±1 |
| Height (cm) | 170±1 |
| Weight (kg) | 90±2 |
| Systolic blood pressure (mmHg) | 103±2 |
| QRS duration (ms) | 172±3 |
| Baseline creatinine (μmol/L) | 135±4 |
| Ischemic heart disease (n) | 6 |
| Left ventricular ejection fraction (%) | 25±7 |
| Peak oxygen consumption (mL/kg/min) | 12.1±0.5 |
| VE/VCO2 slope | 38.2±1.1 |
| Drugs | |
| Furosemide*, mg | 83±8 |
| Angiotensin-converting enzyme inhibitor/angiotensin II inhibitor (n) | 11 |
| Spironolactone (n) | 6 |
| Beta-blocker (n) | 11 |
Values are presented as mean ± SE unless indicated otherwise.
The mean daily dose at implantation in furosemide equivalent. VE/VCO2 Ratio of ventilation to carbon dioxide production
The average heart rates at rest and during exercise were 67±1 beats/min and 90±2 beats/min, respectively. Systolic blood pressure was 103±2 mmHg at rest and 123±2 mmHg during exercise. There were no differences between pacing modes at rest or during exercise for either of these variables.
Devices and implantation
All patients had LV leads placed transvenously using conventional percutaneous methods. All but two patients received true bipolar leads. In those with unipolar leads, the pacing circuit was programmed to integrated bipolar (LV tip to RV coil).
Reproducibility of echocardiographic variables
Continuous and colour Doppler have previously been used as reproducible methods for assessing MR during cycle exercise (39,40).
Two scans on each individual (one at rest and one during exercise) were chosen at random to calculate a coefficient of variance for each important MR variable. The coefficient of variance for jet area was 14% and 20% for the PISA-calculated EROA. MR flow rate and MRV measurements yielded coefficients of variance of 21% and 26%, respectively.
Dyssynchrony
Table 2 shows the resting and exercise dyssynchrony and the MR variables for intrinsic conduction. Tables 3 and 4 show the changes in dyssynchrony variables from intrinsic conduction with the three pacing modes at rest and on exercise. At rest, there were no significant differences in measures of dyssynchrony between intrinsic rhythm and RV pacing. However, BiV and LV pacing reduced dyssynchrony compared with both intrinsic conduction and RV pacing (Table 3). There were no differences between LV pacing and BiV pacing.
TABLE 2.
Echocardiographic characteristics at rest and during exercise for intrinsic conduction
| At rest | During exercise | P | |
|---|---|---|---|
| SPWMD (ms) | 217±67 | 194±78 | NS |
| APD (ms) | 58±28 | 38±24 | NS |
| Longitudinal IV delay (ms) | 66±36 | 44±37 | NS |
| Longitudinal VV delay (ms) | 81±51 | 85±46 | NS |
| RVP (mmHg) | 21±7 | 41±14 | <0.005 |
| MR EROA (cm2) | 0.17±0.15 | 0.29±0.17 | <0.005 |
| MR flow rate (mL/s) | 88±78 | 149±83 | <0.02 |
| MR regurgitant volume (mL) | 24±27 | 45±30 | <0.02 |
| MR jet area (cm2) | 5.0±4.0 | 8.6±4.8 | <0.05 |
Values are presented as mean ± SE. APD Aortopulmonary outflow delay; EROA Effective regurgitant orifice area; IV Intraventricular; MR Mitral regurgitation; NS Not significant; RVP Right ventricular pressure; SPWMD Septal to posterior wall motion delay; VV Interventricular
TABLE 3.
Changes in dyssynchrony and mitral regurgitation (MR) variables from intrinsic conduction at rest for each pacing mode
| RV pacing | LV pacing | BiV pacing | |
|---|---|---|---|
| SPWMD (ms) | −38±23 | −145±56*** | −185±27*** |
| APD (ms) | −10.9±9.4 | −49.7±13.9*** | −35.8±10.5* |
| Longitudinal IV delay (ms) | −18±12 | −50±15 | −75±29*** |
| Longitudinal VV delay (ms) | −5±27 | −48±25 | −74±32 |
| RVP (mmHg) | 3.1±1.9 | 0.7±5.2 | 0.7±3.6 |
| MR EROA (cm2) | 0.003±0.01 | −0.05±0.02 | −0.09±0.03*** |
| MR flow rate (mL/s) | −0.5±14.5 | −27.4±8.9 | −45.7±16.7*** |
| MR regurgitant volume (mL) | 5.7±5.2* | −5.3±3.7 | −13.4±6.2** |
| MR jet area (cm2) | 0.4±0.2 | −1.0±0.4 | −2.1±1.0*** |
Values are presented as mean ± SE. APD Aortopulmonary outflow delay; BiV Biventricular; EROA Effective regurgitant orifice area; IV Intraventricular; LV Left ventricular; RV Right ventricular; RVP Right ventricular pressure; SPWMD Septal to posterior wall motion delay; VV Interventricular.
P<0.05 from intrinsic conduction;
P<0.05 from RV pacing
TABLE 4.
Changes in dyssynchrony and mitral regurgitation (MR) variables from intrinsic conduction during exercise for each pacing mode
| RV pacing | LV pacing | BiV pacing | |
|---|---|---|---|
| SPWMD (ms) | −63±38 | −220±45*** | −162±45* |
| Aortiopulmonary outflow delay (ms) | −3.4±7.0 | −46.0±13.9*** | −43.8±9.5*** |
| Longitudinal IV delay (ms) | 31±20 | −90±23*** | −50±32 |
| Longitudinal VV delay (ms) | −22±20 | −81±21 | −52±24 |
| RVP (mmHg) | 2.7±1.9 | −7.9±4.0** | −7.8±4.5** |
| MR EROA (cm2) | −0.03±0.03 | −0.14±0.03*** | −0.13±0.03*** |
| MR flow rate (mL/s) | −9.7±18.6 | −64.5±21.3*** | −63.4±14.5*** |
| MR regurgitant volume (mL) | −4.4±5.2 | −21.4±5.2*** | −22.0±5.4*** |
| MR jet area (cm2) | −0.3±0.9 | −3.8±1.3*** | −3.7±1.1*** |
Values are presented as mean ± SE. BiV Biventricular; EROA Effective regurgitant orifice area; IV Intraventricular; LV Left ventricular; RV Right ventricular; RVP Right ventricular pressure; SPWMD Septal to posterior wall motion delay; VV Interventricular.
P<0.05 from intrinsic conduction;
P<0.05 from RV pacing
During exercise, there were no differences in measures of dyssynchrony between RV pacing and intrinsic rhythm or between LV and BiV pacing (Table 4), and both LV and BiV pacing were associated with significantly less dyssynchrony than intrinsic conduction and RV pacing.
MR
At rest, RV pacing tended to be associated with greater MR (MRV, EROA and jet area) compared with intrinsic conduction. This difference was significant for MRV (Table 3). MR variables tended to be lower than intrinsic conduction with both BiV and LV pacing, but the greatest changes were seen with BiV pacing when all measures except MRV were significantly lower.
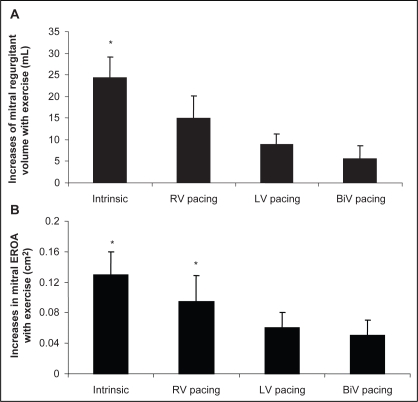
Changes in MR induced by exercise were examined (by comparing exercise with rest) for each of the conduction phases. Intrinsic rhythm and RV pacing were associated with increases in MR, whereas there were no significant changes in any of the MR variables during exercise with LV and BiV pacing (Figure 1 and Table 5).
Figure 1).
A and B Absolute increases from resting values of mitral valve regurgitant volume and effective regurgitant orifice area (EROA) during exercise between pacing modes. BiV Biventricular; LV Left ventricular; RV Right ventricular. *P<0.05
TABLE 5.
Changes in mitral regurgitation (MR) variables during exercise from rest for each pacing phase
| Intrinsic conduction | RV pacing | LV pacing | BiV pacing | |
|---|---|---|---|---|
| MR EROA (cm2) | 0.13±0.03* | 0.08±0.03* | 0.06±0.02 | 0.05±0.02 |
| MR flow rate (mL/s) | 69.7±17.2* | 45.3±20.5* | 35.1±14.0 | 28.8±9.3 |
| MR regurgitant volume (mL) | 24.4±4.8* | 15.1±5.0 | 8.9±2.5 | 5.6±3.0 |
| MR jet area (cm2) | 4.4±0.6* | 3.9±1.1* | 1.2±1.0 | 1.6±0.8 |
Values are presented as mean ± SE. BiV Biventricular; EROA Effective regurgitant orifice area; LV Left ventricular; RV Right ventricular.
P<0.05 from rest
Measures of changes were not different in the two patients with no subjective response compared with those who felt improved.
Correlations
Table 6 shows the correlations of changes in dyssynchrony with changes in MR variables during rest and exercise. At rest, there were modest but significant relationships between changes in aortopulomonary outflow delay and changes in MR. The change in septal to posterior wall motion delay also frequently correlated with changes in MR variables. During exercise, changes in each dyssynchrony variable (except longitudinal intraventricular delay) were significantly correlated with changes in each of the MR variables.
TABLE 6.
Correlations between mitral regurgitation (MR) and dyssynchrony variables at rest and during exercise
| MR variable | Δ SPWMD (ms) | ΔAorto- pulmonary outflow delay (ms) | ΔLongitudinal IV delay (ms) | ΔLongitudinal VV delay (ms) |
|---|---|---|---|---|
| Rest | ||||
| ΔMR EROA (cm2) | 0.40* | 0.52* | 0.27 | 0.23 |
| ΔMR flow rate (mL/s) | 0.33** | 0.38* | 0.27 | 0.23 |
| ΔMR regurgitant volume (mL) | 0.32 | 0.59* | 0.52* | 0.34* |
| ΔMR jet area (cm2) | 0.42* | 0.51* | 0.16 | 0.01 |
| Exercise | ||||
| ΔMR EROA (cm2) | 0.38* | 0.45* | 0.17 | 0.31* |
| ΔMR flow rate (mL/s) | 0.36* | 0.38* | 0.35* | 0.31* |
| ΔMR regurgitant volume (mL) | 0.39* | 0.40* | 0.20 | 0.49* |
| ΔMR jet area (cm2) | 0.50* | 0.41* | 0.35* | 0.40* |
Data are presented as r values. EROA Effective regurgitant orifice area; IV Intraventricular; SPWMD Septal to posterior wall motion delay; VV Interventricular.
P<0.05;
P<0.02
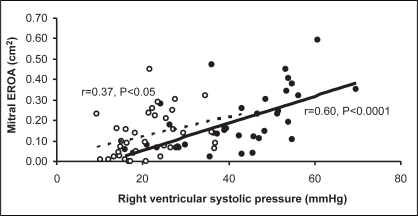
RV systolic pressure increased significantly during exercise for all conduction patterns, the greatest change being with RV pacing (P<0.0001), and correlated directly during exercise with EROA (r=0.60; P<0.0001) (Figure 2), MR regurgitant volume (r=0.56, P=0.0004) and jet area (r=0.76; P<0.0001). RV systolic pressure also correlated directly with changes in dyssynchrony variables during exercise (r=0.65; P<0.0001, for change in aortopulmonary outflow delay, and r=0.32; P=0.05 for change in septal to posterior wall motion delay).
Figure 2).
Relationship between mitral valve effective regurgitant orifice area (EROA) and right ventricular systolic pressure at rest (unfilled circles and dashed trendline) (r=0.60; P<0.0001) and exercise (filled circles and solid trendline) (r=0.37; P<0.05)
DISCUSSION
Our data demonstrate that LV and BiV pacing reduced MR at rest and during exercise, and that CRT prevents the increase in MR on exercise seen in patients with LV dysfunction. The present study also demonstrated that the reductions in MR are systematically related to the improvements in dyssynchrony indexes induced by CRT both at rest and during exercise.
MR in CHF
Reducing the degree of MR is seen as an important goal in medical and surgical therapies for heart failure (41). The increased volume by the LV seen in patients with MR and LV dysfunction is thought to contribute to adverse remodelling, leading to further dilation and worsening of the MR. Patients with significant MR have a higher symptom class and mortality, and more frequent admissions to hospital than those with lesser degrees of mitral incompetence (42–44). Patients who have a large increase in MR during exercise, likely a consequence of ventricular dilation due to increases in both preload and afterload, have a higher mortality and morbidity than those with more limited increases in MR (10,12).
Medical therapies have achieved some success in reducing MR and preventing the adverse remodelling associated with it. In acute heart failure with significant MR, dobutamine reduces the regurgitant fraction and improves forward stroke volume (45). Nitroglycerine has also been shown to reduce MRV and the regurgitant orifice area (46). Long-term therapy with angiotensin-converting enzyme inhibitors (47,48) and beta-blockers can reduce the degree of MR at rest and during moderate exercise (49,50). Nevertheless, there are significant numbers of patients with heart failure in whom significant MR persists at rest and during exercise despite maximal medical therapy.
Functional MR in patients with heart failure is thought to be due to poor mitral valve leaflet coaptation as a consequence of ventricular dilation. However, in patients with LV dysfunction and left bundle branch block, ventricular dilation is not the only mechanism involved. Intra-and interventricular dyssynchrony are associated with MR (13) presumably by interfering with the correct timing of coaptation of the mitral valve leaflets (51). Although there are data suggesting that severe MR is a predictor of poor response to CRT (52), there are now several studies demonstrating that CRT acutely (16,17) and over prolonged periods (18–20,38) improves MR at rest in patients with mechanical dyssynchrony. Strain imaging has recently allowed confirmation that this is, at least in part, due to improved timing of papillary muscle activation (22).
MR and CRT
The present study is the first blinded study to systematically examine changes in MR at rest and during steady-state submaximal exercise, with randomization of the four different conduction patterns described (intrinsic conduction, RV pacing, LV pacing and BiV pacing). Previous studies have shown reductions with CRT at rest: two have examined the effects of CRT on MR after peak exercise (23,24) and one at matched heart rates during exercise, which demonstrated that although patients exercised more when their CRT devices were active, they had less MR at peak (25). The present study adds to these previous data in demonstrating that even at moderate workloads, representative of daily activities, CRT almost completely eliminates the increase in MR observed during both intrinsic conduction and RV pacing. We have confirmed previous observations made at rest (22) –that reductions in dyssynchrony relate to reductions in MR. We have documented that reductions in measures of dyssynchrony are also correlated with the reduction in MR observed during exercise and confirmed previous observations that even after several months of therapy, discontinuing biventricular stimulation leads to a worsening of echocardiographic variables (53). Our data also confirmed previous suggestions that CRT leads to a reduction in pulmonary artery pressure during exercise (24), but we were able to suggest that this is related to changes in MR.
Our study controlled the workload carefully to 30% of a previously achieved peak to ensure that each patient performed the same work during each phase. The order of the phases was randomized to reduce carryover effects, and the analysis of the echocardiographs was blinded. There were reductions in MR variables at rest and during exercise with LV and BiV pacing compared with intrinsic conduction (left bundle branch block) and RV pacing, although the impact of BiV pacing was greatest. Both LV and BiV pacing prevented the exercise-induced increases in MR that occurred during intrinsic conduction and RV pacing.
From our data, it was apparent that the degree of MR related to the degree of mechanical dyssynchrony, and while improvements of inter-and intraventricular dyssynchrony with LV or BiV pacing correlated with reductions in MR during rest, the relationship was most striking during exercise. The present data also showed that although MR was related to changes in intraventricular dyssynchrony as assessed by M-mode echocardiography-measured posterior wall motion delay at rest and during exercise, the most consistent relationship was between changes in aortopulmonary delay and MR variables.
Limitations
The present study, although carried out in a double-blinded, randomized fashion, and with careful and reproducible data collection, was performed on a small number of unselected patients. This limits the applicability of the results obtained.
CONCLUSIONS
Our study demonstrated that in addition to the established benefits of CRT on LV dyssynchrony, CRT also reduces MR at rest and during exercise, and eliminates the increase in MR that occurs during exercise. CRT also reduces the rise in pulmonary artery pressure during exercise. Both of these effects may contribute to the improvements of symptoms in CRT-treated CHF patients. The degree of MR and its improvement by LV and BiV pacing is related to the improvement in intra- and interventricular resynchronization.
Footnotes
FINANCIAL SUPPORT: Dr Witte has received educational funding from Guidant (Canada) and Medtronic (UK). Dr Parker holds a Career Investigator Award from the Heart and Stroke Foundation of Ontario.
REFERENCES
- 1.Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–91. doi: 10.1016/j.cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–43. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 3.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 4.Keren G, Katz S, Gage J, Strom J, Sonnenblick EH, LeJemtel TH. Effect of isometric exercise on cardiac performance and mitral regurgitation in patients with severe congestive heart failure. Am Heart J. 1989;118:973–9. doi: 10.1016/0002-8703(89)90232-9. [DOI] [PubMed] [Google Scholar]
- 5.Spinarova L, Toman J, Stejfa M, Soucek M, Richter M, Kara T. Systolic and diastolic function in patients with chronic heart failure at rest and during exercise. Int J Cardiol. 1997;59:251–6. doi: 10.1016/s0167-5273(97)02924-0. [DOI] [PubMed] [Google Scholar]
- 6.Lancellotti P, Lebrun F, Piérard LA. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2003;42:1921–8. doi: 10.1016/j.jacc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Tada H, Tamai J, Takaki H, Ohnishi E, Okano Y, Yoshioka T. Mild mitral regurgitation reduces exercise capacity in patients with idiopathic dilated cardiomyopathy. Int J Cardiol. 1997;58:41–5. doi: 10.1016/s0167-5273(96)02839-2. [DOI] [PubMed] [Google Scholar]
- 8.Lapu-Bula R, Robert A, Van Craeynest D, et al. Contribution of exercise-induced mitral regurgitation to exercise stroke volume and exercise capacity in patients with left ventricular systolic dysfunction. Circulation. 2002;106:1342–8. doi: 10.1161/01.cir.0000028812.98083.d9. [DOI] [PubMed] [Google Scholar]
- 9.Lapu-Bula R, Robert A, De Kock M, et al. Relation of exercise capacity to left ventricular systolic function and diastolic filling in idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 1999;83:728–34. doi: 10.1016/s0002-9149(98)00979-5. [DOI] [PubMed] [Google Scholar]
- 10.Piérard LA, Lancellotti P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med. 2004;351:1627–34. doi: 10.1056/NEJMoa040532. [DOI] [PubMed] [Google Scholar]
- 11.Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation. 2003;108:1713–7. doi: 10.1161/01.CIR.0000087599.49332.05. [DOI] [PubMed] [Google Scholar]
- 12.Lancellotti P, Gérard PL, Piérard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J. 2005;26:1528–32. doi: 10.1093/eurheartj/ehi189. [DOI] [PubMed] [Google Scholar]
- 13.Ennezat PV, Maréchaux S, Le Tourneau T, et al. Myocardial asynchronism is a determinant of changes in functional mitral regurgitation severity during dynamic exercise in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Eur Heart J. 2006;27:679–83. doi: 10.1093/eurheartj/ehi682. [DOI] [PubMed] [Google Scholar]
- 14.Bakker PF, Meijburg HW, de Vries JW, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol. 2000;4:395–404. doi: 10.1023/a:1009854417694. [DOI] [PubMed] [Google Scholar]
- 15.Blanc JJ, Bertault-Valls V, Fatemi M, Gilard M, Pennec PY, Etienne Y. Midterm benefits of left univentricular pacing in patients with congestive heart failure. Circulation. 2004;109:1741–4. doi: 10.1161/01.CIR.0000124479.89015.64. [DOI] [PubMed] [Google Scholar]
- 16.Yu CM, Lin H, Fung WH, Zhang Q, Kong SL, Sanderson JE. Comparison of acute changes in left ventricular volume, systolic and diastolic functions, and intraventricular synchronicity after biventricular and right ventricular pacing for heart failure. Am Heart J. 2003;145:E18. doi: 10.1016/S0002-8703(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 17.Breithardt OA, Sinha AM, Schwammenthal E, et al. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure J Am Coll Cardiol 200341765–70.(Erratum in 2003;41:1852) [DOI] [PubMed] [Google Scholar]
- 18.Chan KL, Tang AS, Achilli A, et al. Functional and echocardiographic improvement following multisite biventricular pacing for congestive heart failure. Can J Cardiol. 2003;19:387–90. [PubMed] [Google Scholar]
- 19.St John Sutton MG, Plappert T, Abraham WT, et al. Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–90. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 20.Porciani MC, Macioce R, Demarchi G, et al. Effects of cardiac resynchronization therapy on the mechanisms underlying functional mitral regurgitation in congestive heart failure. Eur J Echocardiogr. 2006;7:31–9. doi: 10.1016/j.euje.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Yu CM, Fung WH, Lin H, Zhang Q, Sanderson JE, Lau CP. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684–8. doi: 10.1016/s0002-9149(02)03404-5. [DOI] [PubMed] [Google Scholar]
- 22.Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J., III A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: Insights from mechanical activation strain mapping. J Am Coll Cardiol. 2004;44:1619–25. doi: 10.1016/j.jacc.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Lancellotti P, Mélon P, Sakalihasan N, et al. Effect of cardiac resynchronization therapy on functional mitral regurgitation in heart failure. Am J Cardiol. 2004;94:1462–5. doi: 10.1016/j.amjcard.2004.07.154. [DOI] [PubMed] [Google Scholar]
- 24.Ennezat PV, Gal B, Kouakam C, et al. Cardiac resynchronisation therapy reduces functional mitral regurgitation during dynamic exercise in patients with chronic heart failure: An acute echocardiographic study. Heart. 2006;92:1091–5. doi: 10.1136/hrt.2005.071654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bordachar P, Lafitte S, Reuter S, et al. Echocardiographic assessment during exercise of heart failure patients with cardiac resynchronization therapy. Am J Cardiol. 2006;97:1622–5. doi: 10.1016/j.amjcard.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 26.Witte KK, Clark AL. Is the elevated slope relating ventilation to carbon dioxide production in chronic heart failure a consequence of slow metabolic gas kinetics? Eur J Heart Fail. 2002;4:469–72. doi: 10.1016/s1388-9842(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 27.Witte KK, Thackray SD, Lindsay KA, Cleland JG, Clark AL. Metabolic gas kinetics depend upon the level of exercise performed. Eur J Heart Fail. 2005;7:991–6. doi: 10.1016/j.ejheart.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. American Society of Echocardiography Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 29.Bargiggia GS, Tronconi L, Sahn DJ, et al. A new method for quantitation of mitral regurgitation based on color flow Doppler imaging of flow convergence proximal to regurgitant orifice. Circulation. 1991;84:1481–9. doi: 10.1161/01.cir.84.4.1481. [DOI] [PubMed] [Google Scholar]
- 30.Schwammenthal E, Chen C, Benning F, Block M, Breithardt G, Levine RA. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: Clinical data and experimental testing. Circulation. 1994;90:307–22. doi: 10.1161/01.cir.90.1.307. [DOI] [PubMed] [Google Scholar]
- 31.Enriquez-Sarano M, Bailey KR, Seward JB, Tajik AJ, Krohn MJ, Mays JM. Quantitative Doppler assessment of valvular regurgitation. Circulation. 1993;87:841–8. doi: 10.1161/01.cir.87.3.841. [DOI] [PubMed] [Google Scholar]
- 32.Kass DA. Predicting cardiac resynchronization response by QRS duration: The long and short of it. J Am Coll Cardiol. 2003;42:2125–7. doi: 10.1016/j.jacc.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–3. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 34.Yu CM, Yang H, Lau CP, et al. Regional left ventricle mechanical asynchrony in patients with heart disease and normal QRS duration: Implication for biventricular pacing therapy. Pacing Clin Electrophysiol. 2003;26:562–70. doi: 10.1046/j.1460-9592.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- 35.Pitzalis MV, Iacoviello M, Romito R, et al. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol. 2002;40:1615–22. doi: 10.1016/s0735-1097(02)02337-9. [DOI] [PubMed] [Google Scholar]
- 36.Bax JJ, Marwick TH, Molhoek SG, et al. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol. 2003;92:1238–40. doi: 10.1016/j.amjcard.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Bax JJ, Molhoek SG, van Erven L, et al. Usefulness of myocardial tissue Doppler echocardiography to evaluate left ventricular dyssynchrony before and after biventricular pacing in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;91:94–7. doi: 10.1016/s0002-9149(02)03009-6. [DOI] [PubMed] [Google Scholar]
- 38.Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 39.Lebrun F, Lancellotti P, Piérard LA. Quantitation of functional mitral regurgitation during bicycle exercise in patients with heart failure. J Am Coll Cardiol. 2001;38:1685–92. doi: 10.1016/s0735-1097(01)01605-9. [DOI] [PubMed] [Google Scholar]
- 40.Irvine T, Li XK, Sahn DJ, Kenny A. Assessment of mitral regurgitation. Heart. 2002;88(Suppl 4):iv11–9. doi: 10.1136/heart.88.suppl_4.iv11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madani MM. Mitral valve repair in the treatment of heart failure. Curr Treat Options Cardiovasc Med. 2004;6:305–11. doi: 10.1007/s11936-004-0032-5. [DOI] [PubMed] [Google Scholar]
- 42.Venturi F, Gianfaldoni ML, Melina G, et al. Mitral effective regurgitant orifice area versus left ventricular ejection fraction as prognostic indicators in patients with dilated cardiomyopathy and heart failure. Ital Heart J. 2004;5:755–61. [PubMed] [Google Scholar]
- 43.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144:524–9. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 44.Grayburn PA, Appleton CP, DeMaria AN, et al. BEST Trial Echocardiographic Substudy Investigators Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: The Beta-blocker Evaluation of Survival Trial (BEST) J Am Coll Cardiol. 2005;45:1064–71. doi: 10.1016/j.jacc.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 45.Keren G, Katz S, Strom J, Sonnenblick EH, LeJemtel TH. Dynamic mitral regurgitation. An important determinant of the hemodynamic response to load alterations and inotropic therapy in severe heart failure. Circulation. 1989;80:306–13. doi: 10.1161/01.cir.80.2.306. [DOI] [PubMed] [Google Scholar]
- 46.Keren G, Bier A, Strom JA, Laniado S, Sonnenblick EH, LeJemtel TH. Dynamics of mitral regurgitation during nitroglycerin therapy: A Doppler echocardiographic study. Am Heart J. 1986;112:517–25. doi: 10.1016/0002-8703(86)90516-8. [DOI] [PubMed] [Google Scholar]
- 47.Evangelista-Masip A, Bruguera-Cortada J, Serrat-Serradell R, et al. Influence of mitral regurgitation on the response to captopril therapy for congestive heart failure caused by idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:373–6. doi: 10.1016/0002-9149(92)90236-r. [DOI] [PubMed] [Google Scholar]
- 48.Levine AB, Muller C, Levine TB. Effects of high-dose lisinopril-isosorbide dinitrate on severe mitral regurgitation and heart failure remodeling. Am J Cardiol 1998;82:1299–301,A10. [DOI] [PubMed]
- 49.Stevenson LW, Brunken RC, Belil D, et al. Afterload reduction with vasodilators and diuretics decreases mitral regurgitation during upright exercise in advanced heart failure. J Am Coll Cardiol. 1990;15:174–80. doi: 10.1016/0735-1097(90)90196-v. [DOI] [PubMed] [Google Scholar]
- 50.Waagstein F, Strömblad O, Andersson B, et al. Increased exercise ejection fraction and reversed remodeling after long-term treatment with metoprolol in congestive heart failure: A randomized, stratified, double-blind, placebo-controlled trial in mild to moderate heart failure due to ischemic or idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2003;5:679–91. doi: 10.1016/s1388-9842(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 51.Littmann L, Symanski JD. Hemodynamic implications of left bundle branch block. J Electrocardiol. 2000;33(Suppl):115–21. doi: 10.1054/jelc.2000.20330. [DOI] [PubMed] [Google Scholar]
- 52.Díaz-Infante E, Mont L, Leal J, et al. SCARS Investigators Predictors of lack of response to resynchronization therapy. Am J Cardiol. 2005;95:1436–40. doi: 10.1016/j.amjcard.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Brandt RR, Reiner C, Arnold R, Sperzel J, Pitschner HF, Hamm CW. Contractile response and mitral regurgitation after temporary interruption of long-term cardiac resynchronization therapy. Eur Heart J. 2006;27:187–92. doi: 10.1093/eurheartj/ehi558. [DOI] [PubMed] [Google Scholar]