Abstract
OBJECTIVES:
Adiponectin, an adipocyte-specific protein, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) play a crucial role in arteriosclerosis and plaque disruption. The present study was designed to elucidate the relationship of adiponectin and the ratio of MMP-9/TIMP-1 and their effects on the stability of plaque in acute coronary syndrome (ACS).
METHODS:
The concentrations of adiponectin, MMP-9, TIMP-1 and interleukin-10 were analyzed using ELISA in 56 consecutive unselected patients divided into two groups, stable angina (n=13) and ACS (n=43), and were compared with 19 healthy control subjects. The 56 patients were also angiographically studied and divided into two groups, simple lesion (n=22) and complex lesion (n=34), based on coronary plaque morphology.
RESULTS:
The ratio of MMP-9/TIMP-1 showed significantly higher values in the ACS group compared with the control group (0.22±0.10 versus 0.11±0.03; P<0.001). Adiponectin was negatively correlated with the ratio of MMP-9/TIMP-1 (r=–0.332; P=0.008) and positively correlated with interleukin-10 (r=0.651; P=0.001). Multivariate logistic regression analysis showed that adiponectin (P=0.046) and MMP-9/TIMP-1 (P=0.044) are independent predictors for ACS, and MMP-9/TIMP-1 (P=0.013) is an independent predictor for complex lesion morphology plaques.
CONCLUSION:
In the present study, it was found that adiponectin has a negative relationship with the ratio of MMP-9/TIMP-1 in patients with ACS, and that the ratio of MMP-9/TIMP-1 is an independent predictor of the stability of atherosclerotic plaque and the severity of coronary atherosclerosis.
Keywords: Acute coronary syndrome, Adiponectin, Atherosclerosis, Complex lesion, MMP-9, TIMP-1
Abstract
OBJECTIFS :
L’adiponectine, une protéine sécrétée par les adipocytes, les métalloprotéinases matricielles (MMP) et les inhibiteurs tissulaires des métalloprotéinases (ITMP) jouent un rôle crucial dans l’artériosclérose et la rupture de plaque. La présente étude était conçue pour déterminer la relation de l’adiponectine avec le ratio de MMP-9/TIMP-1 et leurs effets sur la stabilité de la plaque en cas de syndrome coronarien aigu (SCA).
MÉTHODOLOGIE :
Les auteurs ont analysé la concentration d’adiponectine, de MMP-9, de TIMP-1et d’interleukine-10 à l’aide du test ELISA chez 56 patients consécutifs non sélectionnés divisés en deux groupes : l’angine stable (n=13) et le SCA (n=43), et l’ont comparée à celle de 19 sujets témoins en santé. Les 56 patients ont également subi une étude angiographique et ont été divisés en deux groupes : lésion simple (n=22) et lésion complexe (n=34), d’après la morphologie de la plaque coronaire.
RÉSULTATS :
Le ratio de MMP-9/TIMP-1 a révélé des valeurs beaucoup plus élevées dans le groupe de SCA que dans le groupe témoin (0,22±0,10 par rapport à 0,11±0,03; P<0,001). L’adiponectine était inversement proportionnelle au ratio de MMP-9/TIMP-1 (r=–0,332; P=0,008) et directement proportionnelle à l’interleukine-10 (r=0,651; P=0,001). L’analyse de régression logistique multivariée a révélé que l’adiponectine (P=0,046) et les MMP-9/TIMP-1 (P=0,044) sont des prédicteurs indépendants de SCA, et que les MMP-9/TIMP-1 (P=0,013) sont des prédicteurs indépendants de plaques morphologiques de lésion complexe.
CONCLUSION :
La présente étude a établi que l’adiponectine est inversement proportionnelle au ratio de MMP-9/TIMP-1 chez les patients atteints de SCA et que les MMP-9/TIMP-1 sont des prédicteurs indépendant de la stabilité de la plaque artérioscléreuse et de la gravité de l’artériosclérose coronaire.
Adiponectin is an adipocyte-specific protein abundantly present in human plasma, and has been proposed to play an important role in the development of atherosclerosis (1–4). Low adiponectin has been linked to the presence of coronary artery disease (CAD) (5) and has been shown to be a risk factor for cardiovascular events (6,7). Low adiponectin levels are also independently associated with the progression of coronary artery calcification (8). Reports have shown that the antiatherogenic effect of adiponectin is mediated through its binding ability to collagen, which accumulates in damaged arteries (9).
It is generally accepted that matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) play a crucial role in arteriosclerosis and plaque disruption (10). The balance between MMPs and TIMPs determines actual metalloproteinase activities and controls extracellular matrix degradation. MMP and TIMP plasma levels in premature CAD are linked to clinical presentation and markers of inflammation and metabolic disorders (11), and play a crucial role in the breakdown of the fibrous cap of plaque and subsequent rupture in the pathogenesis of acute coronary syndrome (ACS) (10,12). The MMP family has been identified in the shoulder regions of human atherosclerotic plaque (13) and is more frequently expressed in the coronary plaque of patients with ACS than with stable angina pectoris (14). TIMP-1 is also involved in plaque formation (15), and MMP-9 is mainly released into the coronary circulation from the culprit coronary artery plaque in patients with acute myocardial infarction (AMI) (16).
The decisive role played by MMPs and their inhibiting factors, TIMPs, in ACS is not yet fully understood, and the causes of the abrupt change of a stable plaque to a vulnerable plaque also remain unknown. Previous studies (13–16) have suggested that adiponectin has anti-inflammatory properties and that it may regulate inflammatory responses at atherosclerotic lesions, in which MMPs and TIMPs are abundantly present. Thus, the present study was designed to elucidate the effects of adiponectin and the MMP-9/TIMP-1 ratio on the stability of plaques in ACS and the relationship between them.
METHODS
Study population
A total of 75 consecutive unselected subjects (56 men, 19 women) were divided into three groups: stable angina (n=13), ACS (n=43) and controls (n=19). Patients who had any evidence of concomitant systemic inflammatory process (eg, infections and autoimmune disorders) or diabetes mellitus, and those taking peroxisome proliferator-activated receptor agonists were excluded from the study. Patients who had less than 50% diameter stenosis, as visible on coronary angiography, were also not included. The diagnosis of CAD was confirmed in all patients by coronary angiography showing at least single-vessel disease (50% narrowing of luminal diameter or more). ACS patients included unstable angina patients and AMI patients. The former were defined as having ischemic chest pain at rest within the preceding 48 h or within the past month (Braunwald classes II and III), with transient ST-T segment depression and/or T wave inversion. AMI patients included ST segment elevation and non-ST segment elevation MI patients with typical chest pain persisting for at least 30 min, ST segment elevation greater than 0.2 mV in at least two contiguous leads and increase in serum troponin I (greater than 0.3 μg/L). All patients with stable CAD had stable effort angina of more than six months’ duration. The control group included 19 age- and sex-matched subjects (14 men, five women) who had no evidence of CAD, or any other cardiac or systemic inflammatory processes on examination.
Laboratory immunoassays
Venous blood samples of all 56 patients were obtained in a fasting state on the morning (between 06:30 and 07:30) following the admission day. Thus, the time interval between symptom onset and blood sampling was less than 48 h in all cases (median 29 h). The samples were collected into collection tubes containing EDTA, and the plasma obtained after centrifugation was stored at –80°C for subsequent cytokine analysis. Blood of the 19 control subjects was drawn after an overnight fast, and was collected and stored in a similar way to that mentioned above for cytokine analysis. The concentrations of adiponectin, interleukin (IL)-10, MMP-9 and TIMP-1 were determined using commercially available, solid-phase sandwich ELISA kits (Shanghai Senxiong Biotech Industry/Biosource International, USA). The range of detection was 7.8 pg/mL to 500 pg/mL for IL-10; the minimum detectable level of adiponectin is typically 10–3 μg/mL. The respective inter- and intra-assay variation coefficients were 4.7% or less and 6.9% or less for adiponectin, 5.5% or less and 4.8% or less for IL-10, 4.5% or less and 5.3% or less for MMP-9, and 4.4% or less and 4.7% or less for TIMP-1. All laboratory procedures were conducted according to manufacturers’ instructions by an experienced technician who had no knowledge of the clinical status or the angiographic findings of the subjects. Informed, written consent was obtained from all the subjects.
Angiographic analysis
All catheterizations were performed in the catheterization laboratory at Union Hospital, Wuhan (China). A femoral approach was applied using the Seldinger technique. At least five views of the left coronary artery system and two views of the right coronary artery system were obtained, with the stenotic lesions visualized in two orthogonal views. Lesions with a visual diameter stenosis of 50% or greater were included. The identification of the culprit vessel (ischemia-related artery) was made taking into account the coronary anatomy and/or the localization of the electrocardiographic changes. In cases of single-vessel involvement with multiple lesions, the lesion showing the most severe degree of stenosis or the lesion showing a complex morphological pattern was taken as the culprit lesion. In multivessel involvement, the lesion having the most severe stenosis or that showing complex morphology in the vessel related to the ischemic area as defined by the electrocardiographic changes was regarded as the culprit lesion. When the electrocardiographic changes were not present, the lesion having the most severe degree of stenosis or that showing complex features was considered to be the culprit lesion. All stenotic lesions in the coronary arteries were classified qualitatively into simple or complex lesions, according to the criteria proposed by Ambrose et al (17). Complex lesions included mostly eccentric lesions with overhanging edges, ulcerations or irregular borders, and those having multiple irregularities. Lesions with subtotal occlusion (Thrombolysis In Myocardial Infarction [TIMI] grade 1 flow) or total occlusion were also taken to be complex lesions. The remaining lesions showing concentric or eccentric stenosis with smooth borders and broad bases were classified as simple lesions. All angiographic analyses were independently verified by an experienced cardiologist who had no knowledge of the clinical status or immunological patient parameters.
Statistical analysis
All data showing a normal distribution are expressed as mean ± SD. Normality was tested using the Kolmogorov-Smirnov test. The differences among three groups of the normally distributed continuous variables were analyzed by one-way ANOVA. An unpaired t test was used for comparison between two groups. Spearman’s correlation was used to test correlation between two continuous variables. Univariate and multivariate logistic regression analyses were performed to assess the independent predictors for unstable CAD and complex plaque groups. All categorical and continuous variables were included in the univariate analysis, and variables showing a trend with P<0.20 were included in the multivariate model. In all the tests, P<0.05 was considered to be statistically significant. All data analyses were performed using SPSS 13.0 software (SPSS Inc, USA).
RESULTS
Clinical characteristics of the study groups
The clinical characteristics of each group are listed in Table 1. The prevalence of age, sex, body mass index, smoking history and hypertension were not significantly different in the ACS group compared with the stable angina group and the controls. Also, no difference was observed between the complex and simple lesion groups. Prevalence of low-density lipoprotein cholesterol in the ACS group was significantly higher than in the control group, but the difference between the complex lesion group and the simple lesion group was not significant.
TABLE 1.
Clinical characteristics
| Clinical parameter | Control (n=19) | Stable angina (n=13) | Acute coronary syndrome (n=43) | Simple lesions (n=22) | Complex lesions (n=34) |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 53.21±8.68 | 56±11.38 | 59.3±13.37 | 57.95±12.3 | 58.91±13.46 |
| Sex, n (female/male) (% male) | 5/14 (73.68) | 3/10 (76.92) | 11/32 (74.42) | 6/16 (72.72) | 10/24 (70.59) |
| BMI, kg/m2 (mean ± SD) | 23.5±3.0 | 22±2.8 | 24.7±3.1 | 24.3±5.0 | 24.8±3.7 |
| Hypertension, n (%) | 5 (26.31) | 4 (30.77) | 12 (27.91) | 12 (54.55) | 20 (58.82) |
| Smoking, n (%) | 6 (31.57) | 5 (38.46) | 12 (27.91) | 10 (45.45) | 17 (50) |
| Total cholesterol, mmol/L | 4.49±0.76 | 4.12±0.75 | 4.22±0.73 | 4.21±0.84 | 4.23±0.74 |
| Triglycerides, mmol/L | 1.29±0.48 | 1.80±0.60
(P<0.05*) |
2.08±1.13
(P<0.01*) |
1.87±0.78
(P<0.01*) |
2.10±1.18
(P<0.01*) |
| Low-density lipoprotein cholesterol, mmol/L | 2.91±0.66 | 3.22±0.86 | 3.37±0.86 (P<0.05*) | 3.15±0.89 | 3.45±0.82
(P<0.05*) |
| High-density lipoprotein cholesterol, mmol/L | 1.33±0.35 | 1.05±0.15
(P<0.05*) |
1.09±0.24
(P<0.05*) |
1.033±0.147
(P<0.05*) |
1.041±0.175
(P<0.05*) |
P calculated for group versus control. BMI Body mass index
The study further showed significantly higher triglyceride values in the stable angina group, the ACS group and the simple and complex lesion groups compared with the controls. The study also showed significantly lower high-density lipoprotein values in the stable angina, ACS and the simple and complex lesion groups compared with controls.
Serum levels of various markers in ACS
Table 2 shows the plasma levels of various markers in the different patient groups. Plasma levels of adiponectin in ACS patients (n=43) (6.26±2.20 μg/mL; P<0.001) were significantly lower than in stable angina patients (n=13) (8.74±2.80 μg/mL) and significantly lower in ACS patients than controls (n=19) (9.64±1.97 μg/mL). Plasma levels of adiponectin were also analyzed in 13 patients with stable angina; although the values were lower in this group (8.74±2.80 μg/mL), the difference was not statistically significant compared with the control group.
TABLE 2.
Plasma levels of various markers
| Marker | Control (n=19) | Stable angina (n=13) | Acute coronary syndrome (n=43) | Simple lesions (n=22) | Complex lesions (n=34) |
|---|---|---|---|---|---|
| Adiponectin, μg/mL | 9.64±1.97 | 8.74±2.80 | 6.26±2.20
(P<0.001*) |
7.28±2.88
(P<0.05*) |
6.55±2.32
(P<0.001*) |
| MMP-9, ng/mL | 25.47±12.70 | 30.98±17.5 | 63.22±28.34
(P<0.001*) (P<0.001†) |
33.91±29.82 | 69.86±18.96
(P<0.05*) (P<0.001‡) |
| MMP-9/TIMP-1 ratio | 0.11±0.03 | 0.14±0.11 | 0.22±0.10
(P<0.001*) |
0.11±0.04 | 0.26±0.09
(P<0.001*) (P<0.001‡) |
| TIMP-1, ng/mL | 226.38±51.55 | 240.49±69.39 | 316.63±169.95
(P<0.05*) |
294.97±172.54 | 301.54±145.95
(P<0.05*) |
| Interleukin-10, pg/mL | 9.14±2.55 | 8.51±2.32 | 6.28±3.19
(P<0.001*) (P<0.05†) |
7.17±3.00
(P<0.05*) |
6.57±3.25
(P<0.05*) (P<0.05‡) |
Values are presented as mean ± SD.
P calculated for group versus control;
P calculated for stable angina versus acute coronary syndrome groups;
P calculated for simple lesion versus complex lesion groups. MMP-9 Matrix metalloproteinase-9; TIMP-1 Tissue inhibitor of metalloproteinase-1
The plasma level of MMP-9 was considerably increased in ACS patients compared with stable angina patients and controls (63.22±28.34 ng/mL versus 30.98±17.5 ng/mL; P<0.001, and 63.22±28.34 ng/mL versus 25.47±12.70 ng/mL; P<0.001, respectively). MMP-9 was also increased in stable angina (30.98±17.5 ng/mL; P>0.05), but the difference was not statistically significant. Plasma levels of TIMP-1 were elevated in ACS patients (316.63±169.95 ng/mL; P<0.05) compared with controls (226.38±51.55 ng/mL). The stable angina group also showed increased levels (240.49±69.39 ng/mL; P>0.05) compared with the controls, and decreased levels compared with the ACS group, but the differences were not significant. The MMP-9/TIMP-1 ratio was significantly higher in the ACS group (0.22±0.10 versus 0.11±0.03; P<0.001) than the control group. There was no significant difference observed between the ACS and the stable angina groups, or between the stable angina and control groups.
The anti-inflammatory IL-10 was significantly decreased in ACS patients (6.28±3.19 pg/mL versus 8.51±2.32 pg/mL; P<0.05, and 6.28±3.19 pg/mL versus 9.14±2.55 pg/mL; P<0.001) compared with stable angina patients and controls.
Correlations among the various markers
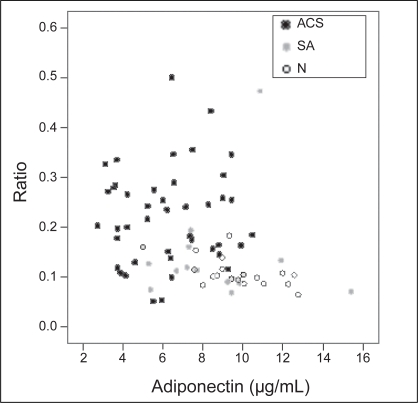
Table 3 shows the correlations of adiponectin with MMP-9, TIMP-1, MMP-9/TIMP-1 and IL-10. There were no significant relationships between adiponectin and MMP-9 or TIMP-1. However, adiponectin was negatively correlated with the MMP-9/TIMP-1 ratio (r=–0.332; P=0.008) (Figure 1) and positively correlated with IL-10 (r=0.651; P=0.001).
TABLE 3.
Correlation of adiponectin with various markers
Correlation coefficient values are shown.
P was calculated for correlation coefficient. MMP-9 Matrix metalloproteinase-9; TIMP-1 Tissue inhibitor of metalloproteinase-1
Figure 1).
Relationship of adiponectin to the ratio of matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1. ACS Acute coronary syndrome; N Control; SA Stable angina
Univariate and multivariate logistic regression analysis
Univariate logistic regression analysis was performed for all the categorical and continuous variables included in the study. A multivariate logistic regression analysis was performed using a model in which variables were included that showed a trend (P<0.20) toward an association with unstable CAD in the univariate logistic regression analysis. The variables included were adiponectin, MMP-9, TIMP-1, MMP-9/TIMP-1 ratio, IL-10, high-density lipoprotein cholesterol and triglycerides. After multivariate logistic regression analysis, it was found that adiponectin (P=0.046) and the MMP-9/TIMP-1 ratio (P=0.044) remained the independent predictors for ACS, and the MMP-9/TIMP-1 ratio (P=0.013), TIMP-1 (P=0.026) and triglycerides (P=0.02) for complex lesions. The results of the multivariate logistic regression analysis are shown in Tables 4 and 5.
TABLE 4.
Multivariate logistic regression analysis with univariate predictive variables in acute coronary syndromes
| Variable | OR (95% CI) | P |
|---|---|---|
| Adiponectin | 0.402 (0.210–0.771) | 0.046 |
| MMP-9 | 0.851 (0.675–1.073) | 0.172 |
| TIMP-1 | 1.037 (0.997–1.078) | 0.067 |
| MMP-9/TIMP-1 | 8E+029 (6.97–9.926E+058) | 0.044 |
| Interleukin-10 | 1.010 (0.67–1.524) | 0.96 |
| Triglycerides | 11.14 (1.14–109.01) | 0.08 |
| High-density lipoprotein cholesterol | 14.022 (0.086–2293.65) | 0.310 |
Adiponectin (P=0.046) and the ratio of matrix metalloproteinase-9 (MMP-9) to tissue inhibitor of metalloproteinase-1 (TIMP-1) (P=0.044) seem to be independent predictors for acute coronary syndrome patients
TABLE 5.
Multivariate logistic regression analysis with univariate predictive variables in the complex lesion group
| Variable | OR (95% CI) | P |
|---|---|---|
| Adiponectin | 0.649 (0.306–1.372) | 0.257 |
| MMP-9 | 0.75 (0.56–1.004) | 0.053 |
| TIMP-1 | 1.055 (1.006–1.107) | 0.026 |
| MMP-9/TIMP-1 | 2E+057 (1.257E+012–2.784E+102) | 0.013 |
| Interleukin-10 | 1.081 (0.634–1.844) | 0.774 |
| Triglycerides | 4.572 (1.272–16.427) | 0.02 |
| High-density lipoprotein cholesterol | 0.238 (0.03~1.906) | 0.176 |
Tissue inhibitor of metalloproteinase-1 (TIMP-1) (P=0.026), the ratio of matrix metalloproteinase-9 (MMP-9) to TIMP-1 (P=0.013) and triglycerides (P=0.02) seem to be independent predictors for complex lesion patients
Culprit lesions and qualitative morphology as visible during coronary angiography
Of the 56 patients included in the stable angina and ACS groups, 34 (60%) had culprit lesions showing complex morphological features; these patients were labelled as the complex lesion group. The remaining 22 patients (39%) had simple lesions and were labelled as the simple lesion group. Only one patient had a culprit lesion in the left main artery, two in the left anterior descending artery, 10 in the left circumflex branch, 15 in the right coronary artery, and the remaining six had the lesion in the other branches of the left coronary artery system.
Various markers and complex lesion morphology
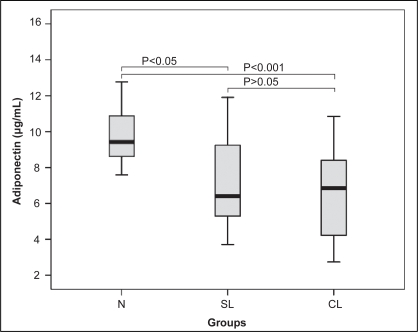
In Table 2, the levels of adiponectin in the complex lesion group (n=34) and the simple lesion group (n=22) were significantly lower compared with the control group (n=19) (6.55±2.32 μg/mL versus 9.64±1.97 μg/mL; P<0.001, and 7.28±2.88 μg/mL versus 9.64±1.97 μg/mL; P<0.05, respectively); however, no statistically significant difference was observed between the complex and simple lesion groups (Figure 2).
Figure 2).
Comparison of adiponectin values among different plaque morphology groups. CL Complex lesion; N Control; SL Simple lesion
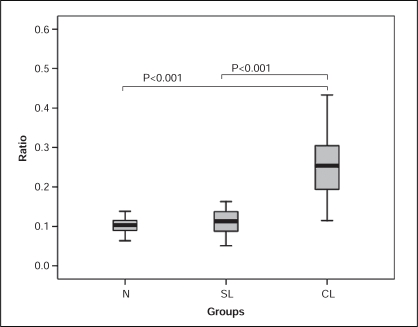
MMP-9 and the MMP-9/TIMP-1 ratio values in the complex lesion group (69.86±18.96 ng/mL and 0.26±0.09 ng/mL, respectively) were significantly higher than in the simple lesion group (33.91±29.82 ng/mL and 0.11±0.04 ng/mL, respectively; P<0.001 for both) and the control group (25.47±12.70 ng/mL; P<0.05, and 0.11±0.03 ng/mL; P<0.001, respectively) (Figure 3). No significant difference was, however, seen between the simple lesion and control groups. The level of TIMP-1 in the complex lesion group (301.54±145.95 ng/mL) was significantly higher than in the control group (226.38±51.55 ng/mL) (P<0.05). No significant difference was seen in the simple lesion group with the control group and the complex group.
Figure 3).
Comparison of the matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio among different plaque morphology groups. CL Complex lesion; N Control; SL Simple lesion
The levels of IL-10 were significantly lower in the complex lesion group (6.57±3.25 pg/mL) and in the simple lesion group (7.17±3.00 pg/mL) than in the control group (9.14±2.55 pg/mL) (P<0.05 for all). The levels of IL-10 in the complex lesion group also tended to be significantly lower than those in the simple lesion group (P<0.05).
DISCUSSION
ACS has a pathophysiological basis of thrombus formation over the disrupted vulnerable atherosclerotic plaques (18). In the present study, a negative correlation was found between adiponectin and the MMP-9/TIMP-1 ratio in ACS, for the first time. This may give an insight to the understanding of pathological change of coronary plaques.
Adiponectin, with its antiatherogenic and anti-inflammatory properties, was observed to be significantly lower in patients with CAD than in age- and body mass index-adjusted control subjects (19). Adiponectin interferes with many monocyte and macrophage functions, including their transition to foam cells (19,20). Adiponectin suppresses the proliferation of vascular smooth muscle cells (4) and suppresses neointimal thickening (21,22). It is related more to the stability of atherosclerotic plaque than to atherosclerotic burden, although a role for adiponectin in the development of atherosclerosis is also likely (23). Reports have shown that the antiatherogenic effect of adiponectin is mediated through its binding ability to collagen, which accumulates in damaged arteries (9). The accumulation of macrophage-derived foam cells in atherosclerotic plaque engenders the local release of MMPs. The activity of MMPs is controlled by TIMPs (24). Furthermore, adiponectin selectively increased TIMP-1 expression in human monocyte-derived macrophages through IL-10 induction. TIMP-1 messenger RNA levels started to increase at 24 h of incubation with adiponectin, suggesting that adiponectin-stimulated TIMP-1 induction is an indirect effect; in addition, IL-10 increases TIMP-1 expression without changing MMP-9 expression in human macrophages (25). Taken together, adiponectin is tightly connected with MMPs and TIMPs. Our results provide the evidence that adiponectin is negatively correlated with MMP-9/TIMP-1, suggesting that the effects of adiponectin on the stability of plaque may be due to the change in the balance of MMP-9/TIMP-1.
Experimental studies using knockout mouse models have demonstrated the importance of the MMP/TIMP balance in atherosclerotic lesion progression (26). The balance between MMPs and TIMPs determines the actual metalloproteinase activities and controls the extracellular matrix degradation; it also plays a crucial role in the breakdown of the fibrous cap of plaque and subsequent rupture in the pathogenesis of ACS (10,12).
Enhancement of the synthesis and secretion of MMPs and/or TIMPs involve potent stimuli of cell activation, which are present in atherosclerotic plaque (27). In some studies, there is a gradient of plasma MMP levels with higher mean values in patients with ACS and the highest levels in patients with AMI compared with those with stable CAD (28,29). TIMP levels may constitute a marker of the local atherosclerotic process occurring in the vascular tree, which is independent of classic inflammatory markers of hepatic origin (30). Together, MMPs and TIMPs play a crucial role in arteriosclerosis and plaque disruption (12). Our results also proved that the MMP-9/TIMP-1 ratio is higher in patients with ACS. Multivariate logistic regression analysis showed that the MMP-9/TIMP-1 ratio is an independent predictor of ACS, and this increased MMP-9/TIMP-1 ratio was an independent predictor of the stability of coronary plaque as a whole, which is characteristic of ACS.
In the present study, we also divided our samples into complex lesion and simple lesion groups, based on coronary angiography results. The MMP-9/TIMP-1 ratio was significantly higher in the complex lesion group than in the simple lesion group. Multivariate logistic regression analysis showed that the MMP-9/TIMP-1 ratio was an independent predictor of complex lesions. This indicates that the MMP-9/TIMP-1 balance affects the stability of the plaque in ACS and reflects the severity of the coronary atherosclerosis.
Our study also found that the levels of anti-inflammatory markers in plasma, adiponectin and IL-10, are significantly decreased in patients with ACS compared with stable angina patients and controls, as well as in complex lesion and simple lesion coronary plaques. Multivariate logistic regression analysis showed that, after adjusting for other factors, adiponectin is an independent predictor of ACS, which has already been proven by previous studies (5,7,19,31). Our study, however, found no significant difference between the stable angina and control groups in terms of various markers.
CONCLUSION
In the present study, we found that adiponectin has a negative relationship with the MMP-9/TIMP-1 ratio in patients with ACS, and that the MMP-9/TIMP-1 ratio is an independent predictor of the stability of atherosclerotic plaque and the severity of coronary atherosclerosis.
Our study has many limitations. We measured serum levels of adiponectin, which were not different among the complex and simple lesion group patients. This result may be due to the small study population or the limited ability of coronary angiography to evaluate plaque morphology. Further studies are needed with a larger study population to clarify this issue. Intravascular ultrasound can be used to study the plaque morphology in greater detail. Also, adiponectin expression needs to be measured locally at the site of coronary plaques; only then the results can be confirmed of whether adiponectin plays an anti-inflammatory role locally at the site of coronary atherosclerotic plaques.
Acknowledgments
The authors are grateful to Guo He Ping, from the Laboratory of Cardiovascular Medicine Union Hospital, Wuhan, China, for his technical support.
REFERENCES
- 1.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κ B signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 3.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–63. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 4.Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–8. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 5.Kumada M, Kihara S, Sumitsuji S, et al. Osaka CAD Study Group Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 6.Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke. 2005;36:1915–9. doi: 10.1161/01.STR.0000177874.29849.f0. [DOI] [PubMed] [Google Scholar]
- 7.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 8.Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–53. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 9.Stejskal D, Bartek J. Adiponectin in patients with various stages of coronary heart disease – comparison of its concentration in coronary arteries and peripheral venous circulation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:161–6. doi: 10.5507/bp.2003.022. [DOI] [PubMed] [Google Scholar]
- 10.Shah PK, Falk E, Badimon JJ, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–9. [PubMed] [Google Scholar]
- 11.Nanni S, Melandri G, Hanemaaijer R, et al. Matrix metalloproteinases in premature coronary atherosclerosis: Influence of inhibitors, inflammation, and genetic polymorphisms. Transl Res. 2007;149:137–44. doi: 10.1016/j.trsl.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 13.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaartinen M, van der Wal AC, van der Loos CM, et al. Mast cell infiltration in acute coronary syndromes: Implications for plaque rupture. J Am Coll Cardiol. 1998;32:606–12. doi: 10.1016/s0735-1097(98)00283-6. [DOI] [PubMed] [Google Scholar]
- 15.Zureik M, Beaudeux JL, Courbon D, Bénétos A, Ducimetière P. Serum tissue inhibitors of metalloproteinases 1 (TIMP-1) and carotid atherosclerosis and aortic arterial stiffness. J Hypertens. 2005;23:2263–8. [PubMed] [Google Scholar]
- 16.Higo S, Uematsu M, Yamagishi M, et al. Elevation of plasma matrix metalloproteinase-9 in the culprit coronary artery in patients with acute myocardial infarction: Clinical evidence from distal protection. Circ J. 2005;69:1180–5. doi: 10.1253/circj.69.1180. [DOI] [PubMed] [Google Scholar]
- 17.Ambrose JA, Winters SL, Stern A, et al. Angiographic morphology and the pathogenesis of unstable angina pectoris. J Am Coll Cardiol. 1985;5:609–16. doi: 10.1016/s0735-1097(85)80384-3. [DOI] [PubMed] [Google Scholar]
- 18.Falk E. Coronary thrombosis: Pathogenesis and clinical manifestations. Am J Cardiol. 1991;68:28B–35B. doi: 10.1016/0002-9149(91)90382-u. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa K, Hori M, Ouchi N, et al. Adiponectin down-regulates acyl-coenzyme A:cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochem Biophys Res Commun. 2004;317:831–6. doi: 10.1016/j.bbrc.2004.03.123. [DOI] [PubMed] [Google Scholar]
- 21.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipovascular axis. J Biol Chem. 2002;277:37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 23.Wolk R, Berger P, Lennon RJ, Brilakis ES, Davison DE, Somers VK. Association between plasma adiponectin levels and unstable coronary syndromes. Eur Heart J. 2007;28:292–8. doi: 10.1093/eurheartj/ehl361. [DOI] [PubMed] [Google Scholar]
- 24.Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: Enhanced biological insights. Atherosclerosis. 2002;160:91–102. doi: 10.1016/s0021-9150(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 25.Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 26.Rouis M, Adamy C, Duverger N, et al. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E-deficient mice. Circulation. 1999;100:533–40. doi: 10.1161/01.cir.100.5.533. [DOI] [PubMed] [Google Scholar]
- 27.Moreau M, Brocheriou I, Petit L, Ninio E, Chapman MJ, Rouis M. Interleukin-8 mediates downregulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: Relevance to stability of atherosclerotic plaque. Circulation. 1999;99:420–6. doi: 10.1161/01.cir.99.3.420. [DOI] [PubMed] [Google Scholar]
- 28.Inokubo Y, Hanada H, Ishizaka H, Fukushi T, Kamada T, Okumura K. Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome. Am Heart J. 2001;141:211–7. doi: 10.1067/mhj.2001.112238. [DOI] [PubMed] [Google Scholar]
- 29.Manginas A, Bei E, Chaidaroglou A, Degiannis D, et al. Peripheral levels of matrix metalloproteinase-9, interleukin-6, and C-reactive protein are elevated in patients with acute coronary syndromes: Correlations with serum troponin I. Clin Cardiol. 2005;28:182–6. doi: 10.1002/clc.4960280405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaudeux JL, Giral P, Bruckert E, Bernard M, Foglietti MJ, Chapman MJ. Serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 as potential markers of carotid atherosclerosis in infraclinical hyperlipidemia. Atherosclerosis. 2003;169:139–46. doi: 10.1016/s0021-9150(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 31.Manigrasso MR, Ferroni P, Santilli F, et al. Association between circulating adiponectin and interleukin-10 levels in android obesity: Effects of weight loss. J Clin Endocrinol Metab. 2005;90:5876–9. doi: 10.1210/jc.2005-0281. [DOI] [PubMed] [Google Scholar]