Abstract
BACKGROUND:
Chronic stable angina (CSA) is a major debilitating health problem in Canada. A paucity of relevant cardiovascular data sets has precluded a detailed examination of the impact of interventions on CSA-related costs and its broader economic burden.
OBJECTIVES:
As part of a larger clinical trial, the authors sought to determine the short-term impact of a standardized self-management training program on CSA-related costs. A secondary objective was to estimate the total annualized cost of CSA per patient from a societal perspective.
METHODS:
Pre- and three-month post-test cost data were collected on 117 participants using the Ambulatory Home Care Record. Mean annualized direct, indirect and system-related CSA costs (2003 to 2005) were estimated; total per-patient CSA costs from a societal perspective were calculated as the sum of these costs.
RESULTS:
The mean (± SD) age of participants was 68±11 years; 80% were male. The program did not impact costs in the short-term. Direct annual out-of-pocket costs, including money paid for health care, travel to appointments, medication, equipment and home support totaled $3,267. Indirect costs, reflecting the value of all unpaid time spent by those engaged in angina-related care, were $12,963. System costs, including costs paid by public and private insurers, were $2,979. Total estimated annual CSA costs from a societal perspective were $19,209 per patient.
CONCLUSIONS:
These data suggest that CSA imposes a major economic burden, comparable with other prevalent conditions such as chronic noncancer pain. Advancements in self-management training research are needed to help reduce the economic burden of CSA in Canada.
Keywords: Angina, Cost-benefit analysis, Education
Abstract
HISTORIQUE :
L’angine chronique stable (ACS) est un trouble de santé débilitant important au Canada. En raison du peu de données cardiovasculaires pertinentes, il a été impossible de procéder à un examen détaillé des répercussions des interventions sur les coûts reliés à l’ACS et sur son fardeau économique plus général.
OBJECTIFS :
Dans le cadre d’un essai clinique plus vaste, les auteurs ont cherché à déterminer les répercussions à court terme d’un programme de formation normalisée en autogestion des soins sur les coûts reliés à l’ACS. Un objectif secondaire consistait à estimer le coût annualisé total de l’ACS par patient, d’un point de vue sociétal.
MÉTHODOLOGIE :
Les données sur les coûts colligées avant l’essai et trois mois plus tard au moyen du dossier des soins ambulatoires à domicile provenaient de 117 participants. Les auteurs ont évalué les coûts annualisés directs, indirects et reliés au système (2003 à 2005) de l’ACS. La somme de ces coûts correspondait aux coûts totaux par patient atteint d’ACS d’un point de vue sociétal.
RÉSULTATS :
Les participants avaient un âge moyen (± ÉT) de 68±11 ans, dont 80 % d’hommes. Le programme n’avait pas de répercussions sur les coûts à court terme .Les coûts généraux annuels directs, y compris l’argent versé pour les soins de santé, les déplacements aux rendez-vous, les médicaments, le matériel et le soutien à domicile, s’élevaient à 3 267 $. Les coûts indirects, reflétant la valeur de toutes les heures impayées que consacraient les personnes participant à des soins relatifs à l’angine, s’élevaient à 12 963 $. Les coûts du système, y compris les coûts payés par les assureurs publics et privés, s’élevaient à 2 979 $. Les coûts annuels totaux estimatifs de l’ACS d’un point de vue sociétal atteignaient 19 209 $ par patient.
CONCLUSIONS :
Ces données indiquent que l’ACS impose un fardeau économique considérable, comparable à celui d’autres maladies prévalentes comme les douleurs non cancéreuses chroniques. Il faut faire progresser les recherches sur la formation en autogestion des soins pour contribuer à réduire le fardeau de l’ACS au Canada.
Chronic stable angina (CSA) is the cardinal symptom of coronary artery disease (CAD), with a major negative impact on health-related quality of life (HRQL), including pain, poor general health status, psychological distress, impaired role functioning, activity restriction and inability to self-manage (1–15). Prevalence data from 1999 to 2002 suggest that more than 6,500,000 Americans are living with CSA (16). Although limitations in current surveillance systems have precluded the direct examination of CSA prevalence in Canada (17), evidence suggests that it is a major clinical problem for this country. For example, 2000/2001 Canadian Community Health Survey data have been used to estimate that among the 5% of Canadians with heart disease (n=1,286,000), 1.9% (n=483,000) live with angina (18). These Canadian Community Health Survey data are likely a conservative estimate of angina prevalence due to reliance on self-report measures. The Laboratory Centre for Disease Control also found that in 1995, 16% of all physician visits related to heart disease in Canada (29.6 million) involved a complaint of angina (unpublished data). As Canadians age, the CSA population may be a major contributor to health care expenditures; these patients are at high risk for acute myocardial infarction (MI), congestive heart failure, atrial fibrillation and stroke (19), as well as cardiovascular-related mortality and hospitalization (men: RR 1.62, women: RR 1.48) (20).
OBJECTIVES
Given the available data on prevalence and impact, angina is a major, debilitating health problem in Canada. To date, however, the paucity of relevant cardiovascular data sets has precluded a detailed examination of the impact of interventions on CSA-related costs and its broader economic burden. The Chronic Angina Self-Management Program (CASMP) was a randomized, controlled trial designed to test the effectiveness of a standardized, six-week, angina self-management training intervention for improving HRQL, self-efficacy and resourcefulness to self-manage symptoms. The study involved 130 CSA outpatients randomly assigned to the CASMP or the three-month wait list usual care group; 117 completed the study. Measures covering two one-month periods were obtained at baseline and three months after baseline (21). The trial’s main findings were that self-management training significantly improved treatment group physical functioning (F=11.75[1.114], P<0.001) and general health (F=10.94[1.114] P=0.001) aspects of generic HRQL. Angina frequency (F=5.60[1.115], P=0.02), angina stability (F=7.37[1.115], P=0.001) and self-efficacy to manage disease (F=8.45[1,115], P=0.004) were also significantly improved in the short term (21).
As a part of this larger trial, the current study sought to determine whether our self-management training program would reduce overall angina-related costs in the short term. A secondary objective was to estimate the total annualized cost of CSA-related illness per patient from a societal perspective.
PATIENTS AND METHODS
Study population, recruitment and design
The CASMP trial was conducted in Toronto, the major urban centre in Ontario, over an 18-month period (2003 to 2005). The target population was CSA patients living in the community. Participants had confirmed diagnoses of CAD and CSA, Canadian Cardiovascular Society (CCS) class I to III angina (22) symptoms for at least six months, and were able to speak, read and understand English. Those who had suffered a MI and/or had undergone a coronary artery bypass graft in the past six months, or had CCS class IV angina and/or a major cognitive disorder that would preclude accurate collection of cost data were excluded (21). One hundred thirty participants were recruited from three large outpatient cardiac programs affiliated with university teaching hospitals, as well as local community physician practices (21).
Participant eligibility was initially assessed by a research assistant via telephone. Willing participants were then interviewed by the research assistant onsite to confirm eligibility and obtain informed consent. On completion of demographic and baseline measures, participants were randomly allocated to either the six-week CASMP group or the three-month wait list control group; this was centrally controlled using a computerized, tamper-proof randomization service (21). Post-test study outcomes were evaluated three months after baseline. The present study (including the economic component) was approved by the research ethics committees of all participating centres, including the University of Toronto Ethics Committee, and CSA patients who participated gave informed consent before enrolling in the trial (21).
Intervention
The CASMP is a standardized self-management training program given in 2 h sessions weekly over a six-week period. As an adaptation of Lorig et al’s (23,24) Chronic Disease Self-Management Program, the goal of the CASMP is to improve HRQL by increasing patients’ day-to-day angina self-management skills. The program integrates strategies known to enhance self-efficacy, including skill mastery, modelling and self-talk, coupled with education about energy conservation, symptom monitoring, decision making about seeking emergency assistance, medication, exercise and diet. Designed to maximize discussion and group problem solving, the program encourages individual experimentation with various cognitive-behavioural self-management techniques and facilitates mutual support, optimism and the self-attribution of success.
Data collection and analysis
Total cost of illness was measured using the Ambulatory and Home Care Record (AHCR). The record is composed of 10 sections that reflect the total cost of illness and is adaptable for diverse populations (25). Four sections of the AHCR pertain to indirect patient and caregiver (formal and informal) time costs; six additional sections capture direct patient and system costs. Using the AHCR, participants were asked by a trained research assistant (blinded to group allocation) to recall all costs incurred over the course of one month before data collection. As is common practice when using this tool, each participant was interviewed according to standardized lists of predetermined possible costs (under each section of the AHCR) to ensure comprehensiveness, maximize recall and enhance the accuracy of cost estimates.
Face validity of the AHCR has been assessed by several health care providers, health economists and administrators who work in the field of ambulatory and home-based care (25–27). The AHCR has been used in several studies to evaluate costs related to a variety of community-based programs for people with chronic and acute health problems (25–27). Reliability of the AHCR has been assessed via the level of agreement between self-reports of cost by 110 cystic fibrosis care recipients and administrative data. Agreement ranged from moderate (kappa = 0.41; 95% CI 0.16 to 0.61) for physician specialist visits to perfect (kappa = 1.0) for physiotherapy visits (25).
Because the AHCR collects comprehensive data on the overall cost of illness, including both direct and indirect costs, economic evaluation can be conducted from a number of perspectives, including those of the patient, caregiver, employer or society. In the present study, the societal perspective was used to determine angina-related costs. The societal perspective includes direct, indirect and system costs, irrespective of payer (28–30).
Derivation of costs
Direct (out-of-pocket) costs: Direct costs included all out-of-pocket costs related to the care and management of angina, or to angina-related disabilities that were incurred by participants, including money paid to health care professionals who came to their homes; to attend health care appointments outside their homes; to have household work done in the home; for angina-related medications; and for supplies or equipment related to heart disease. The cost of attending health care appointments included an allowance of $0.40/km for travel by car. All other direct costs were self-reported; any reported reimbursements (eg, from private insurance) received for out-of-pocket costs were subtracted from the direct costs and were included under system costs (below).
Indirect (time) costs: Indirect costs reflected the estimated value of all unpaid time devoted to caregiving by participants themselves, as well as family members and friends. Inclusion of caregiving time was based on the assumption that time devoted to caregiving may represent forgone opportunities, such as labour, leisure or household work (30,31). This time included time spent directly on patient care (by the patient and by unpaid caregivers), attending angina-related health care appointments, and performing household chores that would be performed by the patient were it not for his/her angina.
Time spent attending health care appointments outside the home was classified in one of two ways – time away from paid employment or time away from leisure. Time away from paid employment consisted of unpaid leave and sick leave, and was assigned a value using the average wage of someone of the same sex and age as the participant or caregiver, and living in the Toronto Census Metropolitan Area (Statistics Canada 2001 Census Public Use Microdata Files). Time spent away from leisure was assigned a value using the estimated wage of a workplace substitute. Derivation of this value assumed that if people valued their leisure time more than the cost of a workplace substitute, they would hire someone for caregiving rather than perform caregiving themselves. The value of leisure time was estimated using the wages of people employed in the child care or home support category of the 2001 Standard Occupational Classification (www.stat-can.ca/english/Subjects/Standard/soc/2001/nocs01-menu.htm), without regard to the age or sex of the provider. All wages were adjusted for inflation, paid vacation, holidays and benefits.
System costs: Health system costs included costs paid by the Ontario Health Insurance Program (OHIP) and private insurers. Costs of physician services were obtained from the OHIP Schedule of Benefits and Fees (Physician Services under the Health Insurance Act, July 1, 2003; Ministry of Health and Long-Term Care). The insured costs of home care were those reported by the Toronto Community Care Access Centre (CCAC), and were adjusted for inflation and CCAC overhead from the Summary Report on Community-Based Services 2000/01 (Ministry of Health and Long-Term Care, Acute Services and Community Health, Finance and Information Management Branch, Information Management Unit; www.mohltcfim.com/cms/upload/a_7665/CBHS_Summary_Report_2000–01.pdf) using the costs reported by the CCAC, and were adjusted for inflation and CCAC overhead. The value of supplies or equipment provided by a CCAC or hospital were also estimated and included under system costs, as well as the costs of prescription drugs and equipment covered by the Ontario Drug Benefit program or private insurers.
Statistical analyses
The impact of the CASMP on cost of illness was then determined in two steps. First, mean changes in costs from baseline to three months (T2 – T1) were calculated. Second, to evaluate the impact of group status (treatment versus usual care) on change in cost, the significance of mean differences in changes in costs between groups was determined using the Wilcoxon rank-sum test (32). Two-tailed P>0.05 was considered to be significant.
RESULTS
Sample derivation and attrition
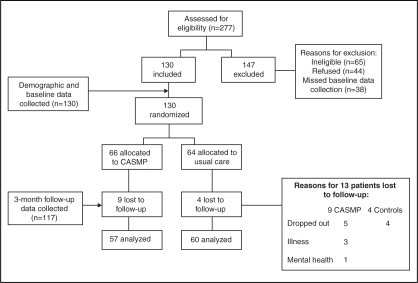
Two hundred seventy-seven potential participants were assessed for inclusion via telephone during an 18-month period from July 2003 to December 2005. Of these, 147 were excluded; 44% did not meet the inclusion criteria, 30% refused, and 26% missed their initial appointment for consent and completion of baseline questionnaires, despite assiduous follow-up (ie, three telephone calls and a follow-up letter). Reasons for refusal included a lack of interest (n=18), too busy to participate (n=15), transportation problems (n=6) and physical limitations that precluded travel (n=5). Those who did not arrive for enrolment procedures were also counted as refusals when determining the acceptance rate. The acceptance rate for enrolment among those eligible was 61%. Of the 130 consenting participants, 66 were randomly assigned to the CASMP and 64 were randomly assigned to the wait list control group (Figure 1).
Figure 1).
Study flow and data collection. CASMP Chronic Angina Self-Management Program
Thirteen participants (treatment group, n=9; usual care group, n=4) did not complete post-test measures, yielding a 10% loss to follow-up rate. Nine participants – five in the treatment group and four in the control group – dropped out of the study without explanation and could not be contacted. Four remaining participants in the treatment group were unable to continue in the study due to hospitalization as a result of natural CAD progression and acute MI (n=2), pneumonia (n=1) and mental health concerns (n=1). Complete baseline and three-month AHCR cost data were obtained from 117 participants (treatment group, n=57; usual care group, n=60) for use in the cost analyses (Figure 1).
Participant characteristics and comparability of groups
Baseline sociodemographic and angina-related characteristics of the treatment and control groups are presented in Table 1. The mean (± SD) age of the sample was 68±11 years, living with angina for 7±7 years. The majority of the sample was male, married or cohabitating, and Caucasian. Individuals of east Indian and Pakistani origin constituted the second largest racial group enrolled. Most were either retired or working full-time. The majority had completed high school and/or had postsecondary education. For both the treatment and control groups, the mean number of previous revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention) was two per person. The majority reported having a comorbid condition, typically a minor medical problem or diabetes. There were no significant differences between groups for any baseline demographic characteristics, comorbid conditions, CCS functional class or number of previous revascularization procedures. Comparisons were also made among all demographic characteristics between those lost to follow-up (n=13) and those who completed (n=117) the study; again, no significant differences were found.
TABLE 1.
Sample characteristics by group
| Treatment (n=66) | Control (n=64) | P | |
|---|---|---|---|
|
Demographics | |||
| Age, years (mean ± SD) | 67±11 | 70±11 | NS |
| Married/cohabitating, n (%) | 44 (67) | 44 (69) | NS |
| Male sex, n (%) | 53 (80) | 50 (78) | NS |
| Working full-time, n (%) | 16 (24) | 15 (23) | NS |
| Retired, n (%) | 46 (70) | 42 (66) | NS |
| High school, n (%) | 59 (89) | 55 (86) | NS |
| Post-secondary education, n (%) | 42 (64) | 44 (69) | NS |
| Caucasian, n (%) | 48 (73) | 54 (84) | NS |
| Black, n (%) | 3 (5) | 0 (0) | NS |
| Latin American, n (%) | 0 (0) | 1 (2) | NS |
| Asian, n (%) | 2 (3) | 1 (2) | NS |
| East Indian/Pakistani, n (%) | 11 (17) | 6 (9) | NS |
| Middle Eastern, n (%) | 3 (5) | 1 (2) | NS |
| Aboriginal, n (%) | 0 (0) | 1 (2) | NS |
|
Angina-related history (mean ± SD) | |||
| Years living with angina, n | 6±6 | 8±8 | NS |
| Previous revascularizations per person including CABG and PCI, n | 2±1 | 2±1 | NS |
|
Canadian Cardiovascular Society functional angina class, n (%) | |||
| Class I | 23 (35) | 19 (30) | NS |
| Class II | 26 (39) | 29 (45) | NS |
| Class III | 17 (26) | 16 (25) | NS |
|
Comorbid conditions, n (%) | |||
| Heart failure | 2 (3) | 5 (8) | NS |
| Asthma | 4 (6) | 2 (3) | NS |
| Diabetes | 18 (27) | 9 (14) | NS |
| Emphysema | 1 (2) | 1 (2) | NS |
| Renal failure | 2 (3) | 1 (2) | NS |
| Peptic ulcer | 1 (2) | 3 (5) | NS |
| Thyroid problem | 3 (5) | 7 (11) | NS |
| Other minor medical problem | 34 (52) | 27 (42) | NS |
|
Medication, n (%) | |||
| ACE inhibitors | 33 (50) | 29 (46) | NS |
| Antiarrythmics | 3 (5) | 2 (3) | NS |
| Anticoagulants | 57 (86) | 48 (73) | NS |
| Beta-blockers | 40 (61) | 38 (59) | NS |
| Calcium channel blockers | 22 (34) | 20 (32) | NS |
| Cholesterol-lowering agents | 49 (74) | 38 (59) | NS |
| Diuretics | 11 (16) | 13 (20) | NS |
| Insulin | 18 (44) | 9 (14) | NS |
ACE Angiotensin-converting enzyme; CABG Coronary artery bypass graft; NS Nonsignificant; PCI Percutaneous coronary intervention
Baseline costs
All costs are expressed in 2004 Canadian dollars. Mean (± SD) baseline costs by group are presented in Table 2. Estimated total costs for the treatment and usual care groups at baseline were $1,865±1,948 and $1,221±1,176, respectively. Indirect costs accounted for over one-half of the total cost of illness for both groups. System costs accounted for approximately two-thirds of the remaining cost, and direct costs were the smallest component. There were no significant baseline differences between groups in direct, indirect or system costs.
TABLE 2.
Estimated baseline costs by group
| Baseline costs | Treatment (n=66) | Control (n=64) |
|---|---|---|
| Direct | $333±907 | $231±522 |
| Indirect | $1,314±1,646 | $810±977 |
| System | $218±217 | $181±195 |
| Total | $1,865±1,948 | $1,221±1,176 |
Values are expressed as mean ± SD
Short-term impact of CASMP on CSA-related costs
Tables 3 and 4 present changes in costs by group and the differences in the change in costs between groups, respectively. No significant differences in change for direct, indirect, system or total costs were found between groups. Therefore, changes in costs were not dependent on treatment group, indicating that the CASMP did not significantly impact cost of illness at three months.
TABLE 3.
Changes (3 months – baseline) in chronic stable angina-related costs by group
| Change in costs (T2 – T1) | Treatment (n=55) | Control (n=62) | Z statistic with continuity correction | P |
|---|---|---|---|---|
| Direct | –$58±1,160 | $7±672 | –0.227 | NS |
| Indirect | $146±2,696 | –$68±1,154 | 0.377 | NS |
| System | $179±992 | $46±318 | –0.355 | NS |
| Total | $268±3,092 | –$15±1,310 | 0.063 | NS |
Values are expressed as mean ± SD. NS Nonsignificant; T1 Time 1; T2 Time 2
TABLE 4.
Differences in change in costs between groups
| Difference in change in costs (ΔT – ΔC) | Value |
|---|---|
| Direct | –$65±933 |
| Indirect | $214±2,029 |
| System | $133±718 |
| Total | $283±2,324 |
Values are expressed as mean ± SD. ΔC Change in control group costs; ΔT Change in treatment group costs
Total annualized cost of CSA-related illness
Given that no previous data on the cost of illness for CSA patients exist, the total annualized cost of illness for CSA was also explored. The total annualized cost of illness was estimated by summing baseline and three-month costs (yielding a two-month cost) and multiplying these costs by six. This method was considered to be most appropriate to determine annualized costs because there were no significant differences between costs at baseline and three months. Table 5 presents the total estimated annualized cost of illness for CSA 2003 to 2005. Total mean (± SD) annualized costs were $19,209±19,193 per person from 2003 through 2005.
TABLE 5.
Estimated annualized cost of illness for chronic stable angina per patient 2003 to 2005
| Annual costs | Value | Range |
|---|---|---|
| Direct costs | $3,267±5,777 | $0 – $40,908 |
| Indirect costs | $12,963±15,187 | $0 – $90,997 |
| System costs | $2,979±4,681 | $0 – $45,132 |
| Total costs | $19,209±19,193 | $0 – $111,309 |
DISCUSSION
We sought to determine the short-term impact of the CASMP on costs associated with CSA-related illness, compared with usual care, at three months from baseline. One-hundred thirty participants were assigned to the six-week CASMP or usual care via centrally controlled, computerized randomization. Cost data were collected using the AHCR. We found that the CASMP did not significantly impact CSA-related costs at three months. Two factors may account for this finding. First, indirect costs were the major contributor to costs, accounting for nearly two-thirds of the total. These indirect costs estimated the value of all unpaid time spent by participants themselves as well as unpaid caregivers (eg, family members, friends or neighbours) engaged in caregiving practices, including direct patient care, attending health care appointments and performing household work that would otherwise be performed by the patient, were it not for his/her angina. Throughout the course of the CASMP, various self-management strategies known to enhance one’s capacity for chronic condition self-management are rehearsed and integrated. At three months, participants would likely still have been experimenting with the various angina pain self-management strategies offered to determine which strategies best fit their lifestyles and short-term goals. More time may therefore be required to observe significant reductions in indirect costs as a function of participants’ enhanced capacity to manage their angina day to day.
A second potential reason for our finding pertains to the makeup of system costs derived. System costs in the present study included those paid by OHIP and the costs borne by private insurers for time spent by health care professionals caring for participants (in either the home or the clinic setting). Over the course of the study, participants attended previously scheduled follow-up visits with their primary care providers and/or cardiologists. A portion of these visits contributed to our cost estimates because they fell within the four-week recall period at follow-up data collection. The CASMP could therefore not have reduced costs related to these prearranged appointments. However, as with indirect costs, there is potential for the CASMP to significantly reduce health service costs in the longer term, which has been demonstrated with other self-management programs. For example, in a four-year examination of an arthritis self-management program in the United States, Lorig et al (33) found that physician visits were reduced by a mean of 40%. Mean cost savings of $648 and $189 per patient were also found (33) for those living with rheumatoid arthritis and osteoarthritis, respectively (33). These significant cost savings occurred over the longer term, once self-management skills taught had been integrated as a routine part of daily life (33).
As a secondary objective, we estimated the total mean annualized cost of illness for CSA patients at $19,209 per person from 2003 to 2005. This annualized cost poses a considerable burden at the individual patient level potentially more so than that of other major chronic, debilitating health problems in Canada. For example, using the AHCR, Guerriere et al (34) estimated the median annualized cost of chronic noncancer pain in Ontario to be $12,700 per person in 2003. The mean age of our angina sample was 68 years, and the majority of patients were retired, with a limited income. Most of the costs incurred were those borne by the patients themselves, along with indirect time costs. Aside from the potential to reduce system-related costs, the CASMP significantly increased HRQL and self-efficacy to manage angina symptoms (21). It could therefore have a critical role to play in helping these patients decrease direct out-of-pocket expenses and reliance on others, reflected by foregone income due to informal caregiving practices.
The overall economic burden of CSA at the societal level in Canada cannot be extrapolated on the basis of our cost estimates due to our limited sample and restriction of data collection procedures to one region of Ontario. Moreover, our annualized estimate of cost burden must be interpreted with caution. Although annualizing cost data can be helpful for discerning yearly fiscal impact, certain costs can become overinflated. For example, 35% of our sample (n=46) reported one cardiologist visit within our two-month data collection period. By virtue of annualizing, these participants may appear to have seen a cardiologist six times over the course of one year. However, this inflation can arguably (but not certainly) be balanced by the fact that those participants who did not report seeing a cardiologist (n=84) within our two-month data collection period appeared as though they had never seen a cardiologist over the course of one year.
Although annualizing can introduce further variation to some cost estimates, the vast majority (two-thirds) of our estimated costs were indirect costs. These costs are less likely to be prone to inflation by annualizing. Indirect costs estimated the value of all unpaid time spent by participants engaged in angina caregiving-related practices. These day-to-day costs are unlikely to have varied significantly from month to month, given that our sample was a stable and chronic illness population. Ideally, a more robust annual estimate of cost burden should be produced in a subsequent, long-term study wherein monthly cost data can be collected for one year.
Despite the potential for our estimated annual costs to inflate the true annual burden of angina, it has been demonstrated that up to 59% of Canadians with heart disease aged over 12 years report activity restriction compared with those without heart disease (17). Given that CSA is a cardinal symptom of ischemic heart disease, the cumulative cost burden of CSA to Canadian society is likely considerable. In the United Kingdom, for example, the direct cost of CSA in 2000, including prescriptions, admissions, outpatient referrals and procedures, was estimated to be £669,000,000, accounting for 1.3% of the total National Health Service expenditure (35).
To our knowledge, our study is the only study to date that has examined CSA-related costs at the patient level. The importance of this contribution lies with the comprehensiveness of our cost estimates using the societal perspective, including direct out-of-pocket costs, indirect time costs and system costs. Although estimates were based on self-report data, previous work by Guerriere et al (25) has demonstrated strong psychometric properties of the AHCR, with almost perfect agreement between participants’ responses and hospital, pharmacy and physician records (kappa = 0.41; 95% CI 0.16 to 0.61). Our current estimate of the economic burden, at $19,209 per annum per patient, makes a compelling case for a large-scale examination of the cost burden of CSA in Canada, beyond the context of the Ontario health care system and our limited data collection period.
With the growing global burden of angina and heart disease, nongovernmental organizations in Canada, the United States and abroad have stressed the need for advancements in cost-effective secondary prevention strategies (17,36,37). Given the magnitude of the per-patient burden that our initial CSA cost-related findings suggest, and given the evidence to suggest that self-management training can reduce costs over the longer term, further research is warranted to evaluate the long-term potential of the CASMP to reduce the cost of illness for CSA patients.
CONCLUSIONS
To our knowledge, the present study is the first to evaluate the cost of illness for CSA patients at the patient level. Our data suggest that CSA imposes a major economic societal burden. Advancements in CSA self-management training research are needed to help reduce the direct, indirect and system-related costs associated with CSA in Canada.
FINANCIAL SUPPORT: This study was supported by the Canadian Institutes of Health Research Fellowship #452639. Dr Coyte is supported by funds from the Canadian Health Services Research Foundation, the Canadian Institutes of Health Research, and the Ontario Ministry of Health and Long-Term Care for his Chair in Health Care Settings and Canadians.
Acknowledgments
The authors are grateful to Dr Kate Lorig, Stanford University Patient Education Research Centre, for permission to adapt the Chronic Disease Self-Management Program, and to Dr Ellen Hodnett and Ms Julie Weston, who supported this trial at the Randomized Controlled Trials Unit, Lawrence Bloomberg Faculty of Nursing, University of Toronto. Portions of the CASMP first appeared in, or are derived from, the Chronic Disease Self-Management Program Master Trainer’s Guide (1999). Those portions are copyright of Stanford University (1999).
REFERENCES
- 1.Stewart S, Inglis S, Hawkes A. Chronic Cardiac Care: A Practical Guide to Specialist Nurse Management. Malden: Blackwell Publishing Ltd; 2006. [Google Scholar]
- 2.Brorsson B, Bernstein SJ, Brook RH, Werko L. Quality of life of chronic stable angina patients four years after coronary angioplasty or coronary artery bypass surgery. J Intern Med. 2001;249:47–57. doi: 10.1046/j.1365-2796.2001.00782.x. [DOI] [PubMed] [Google Scholar]
- 3.Brorsson B, Bernstein SJ, Brook RH, Werko L. Quality of life of patients with chronic stable angina before and 4 years after coronary artery revascularization compared with a normal population. Heart. 2002;87:140–5. doi: 10.1136/heart.87.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caine N, Sharples LD, Wallwork J. Prospective study of health related quality of life before and after coronary artery bypass grafting: Outcome at 5 years. Heart. 1999;81:347–51. doi: 10.1136/hrt.81.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erixson G, Jerlock M, Dahlberg K. Experiences of living with angina pectoris. Nurs Sci Res Nordic Countries. 1997;17:34–8. [Google Scholar]
- 6.Gardner K, Chapple A. Barriers to referral in patients with angina: Qualitative study. BMJ. 1999;319:418–21. doi: 10.1136/bmj.319.7207.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons RA, Lo SV, Littlepage BNC. Comparative health status of patients with 11 common illnesses in Wales. J Epidemiol Community Health. 1994;48:388–90. doi: 10.1136/jech.48.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDermott AF. Living with angina pectoris: A phenomenological study. Eur J Cardiovasc Nurs. 2002;1:265–72. doi: 10.1016/s1474-5151(02)00047-6. [DOI] [PubMed] [Google Scholar]
- 9.Miklaucich M. Limitations on life: Women’s lived experiences of angina. J Adv Nurs. 1998;28:1207–15. doi: 10.1046/j.1365-2648.1998.00828.x. [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ, Henderson RA, Seed P, Treasure T, Hampton J. Quality of life, employment status, and anginal symptoms after coronary artery bypass surgery: 3-year follow-up in the randomized intervention treatment of angina (RITA) trial. Circulation. 1996;94:135–42. doi: 10.1161/01.cir.94.2.135. [DOI] [PubMed] [Google Scholar]
- 11.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–9. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 12.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality of life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789–94. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 13.Wandell PE, Brorsson B, Aberg H. Functioning and well-being of patients with type 2 diabetes or angina pectoris, compared with the general population. Diabetes Metab. 2000;26:465–71. [PubMed] [Google Scholar]
- 14.Andrell P, Ekre O, Wahborg P, Eliasson T, Mannheimer C. Quality of life in patients with refractory angina pectoris. International Association for the Study of Pain 11th World Congress on Pain. (Abst) August 21 to 26, 2005, Sydney. IASP Press; 2005: 200.
- 15.Manuel DC, Leung M, Nguyen K, Tanuseputro P, Johansen H. Burden of cardiovascular disease in Canada. Can J Cardiol. 2003;19:997–1004. [PubMed] [Google Scholar]
- 16.American Heart Association. Heart disease and stroke statistics: 2006 update. Dallas: American Heart Association; 2006. [Google Scholar]
- 17.Heart and Stroke Foundation of Canada. The growing burden of heart disease and stroke in Canada 2003. Ottawa: Heart and Stroke Foundation of Canada; 2003. [Google Scholar]
- 18.Chow C-M, Donovan L, Manuel D, Johansen H, Tu JV for the Canadian Cardiovascular Research Outcomes Team. Regional variation in self-reported heart disease prevalence in Canada. Can J Cardiol. 2005;21:1265–71. [PubMed] [Google Scholar]
- 19.Lampe FC, Whincup PH, Wannamethee SG, et al. The natural history of prevalent ischaemic heart disease in middle-aged men. Eur Heart J. 2000;21:1052–62. doi: 10.1053/euhj.1999.1866. [DOI] [PubMed] [Google Scholar]
- 20.Murphy NF, Stewart S, Hart CL, MacIntyre K, Hole D, McMurray JJ. A population study of the long-term consequences of Rose angina: 20-year follow-up of the Renfrew-Paisley Study. Heart. 2006;92:1739–46. doi: 10.1136/hrt.2006.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGillion MH, Watt-Watson J, Stevens B, LeFort S, Coyte P, Graham A. Randomized controlled trial of a psychoeducation program for the self-management of chronic cardiac pain. J Pain Symptom Manage. 2008;36:126–140. doi: 10.1016/j.jpainsymman.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Campeau L. The Canadian Cardiovascular Society grading of angina pectoris revisited 30 years later. Can J Cardiol. 2002;18:371–9. [PubMed] [Google Scholar]
- 23.Lorig K, Gonzalez V, Laurent D. The Chronic Disease Self-Management Workshop Master Trainer’s Guide. Stanford Patient Education Research Centre; 1999. [Google Scholar]
- 24.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing utilization and costs: A randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Guerriere DN, Ungar WJ, Corey M, et al. Evaluation of the ambulatory and home care record: Agreement between self-reports and administrative data. Int J Technol Assess Health Care. 2006;22:1–8. doi: 10.1017/S0266462306051026. [DOI] [PubMed] [Google Scholar]
- 26.Guerriere DN, Tullis E, Ungar W, et al. Economic burden of ambulatory and home-based care for adults with cystic fibrosis. Treat Respair Med. 2006;5:351–9. doi: 10.2165/00151829-200605050-00006. [DOI] [PubMed] [Google Scholar]
- 27.Stevens B, Guerriere D, McKeever P, et al. Economics of home vs. hospital breastfeeding support for newborns. J Adv Nurs. 2006;53:233–43. doi: 10.1111/j.1365-2648.2006.03720.x. [DOI] [PubMed] [Google Scholar]
- 28.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Toronto: Oxford University Press; 1997. [Google Scholar]
- 29.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 30.Yates BT. Analyzing Costs, Procedures, Processes, and Outcomes in Human Services. Thousand Oaks: Sage Publications; 1997. [Google Scholar]
- 31.Coyte PC, Young W. Applied home care research. Int J Health Care Qual Assur Inc Leadersh Health Serv. 1997;10:i–iv. doi: 10.1108/13660759710159767. [DOI] [PubMed] [Google Scholar]
- 32.Norman GR, Streiner DL. Biostatistics: The bare essentials. 2nd edn. Hamilton: BC Decker Inc; 2000. [Google Scholar]
- 33.Lorig K, Mazonson P, Holman HR. Evidence suggesting that health education for self-management in patients with chronic arthritis has maintained health benefits while reducing health care costs. Arthritis Rheum. 1993;36:439–46. doi: 10.1002/art.1780360403. [DOI] [PubMed] [Google Scholar]
- 34.Guerriere D, LeFort S, Watt-Watson J, Choi BY, Croxford R, Coyte P.Cost-effectiveness of the CPSMP Pain Res Manag 20038Suppl B36B(Abst) [Google Scholar]
- 35.Stewart S, Murphy N, Walker A, McGuire A, McMurray JJ. The current cost of angina pectoris to the National Health Service in the UK. Heart. 2003;89:848–53. doi: 10.1136/heart.89.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA-ASIM guidelines for the management of patients with chronic stable angina: Executive summary and recommendations (a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [Committee on Management of Patients with Chronic Stable Angina]) Circulation. 1999;99:2829–48. doi: 10.1161/01.cir.99.21.2829. [DOI] [PubMed] [Google Scholar]
- 37.British Cardiac Society; British Hypertension Society; Diabetes UK; HEART UK; Primary Care Cardiovascular Society; Stroke Association JBS 2: Joint British Societies’ guidelines on the prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl V):v1–v52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]