Abstract
BACKGROUND:
In randomized trials, paclitaxel-eluting stents (PES) have been shown to be superior to bare metal stents (BMS) in reducing restenosis. However, the effectiveness of PES in patients treated during routine practice has not been fully established.
METHODS:
A retrospective comparison of PES with BMS in consecutive patients undergoing percutaneous coronary intervention (PCI) from April 2003 to March 2004 was conducted. Outcomes included the composite of death, myocardial infarction and target lesion revascularization (TLR) at one year, as well as stent thrombosis.
RESULTS:
A total of 512 patients were treated with PES, and 722 patients were treated with BMS. Patients in the PES group were more likely to receive stents that were 20 mm in length or longer (52.2% versus 33.3%, P<0.0001), 2.5 mm in diameter or smaller (29.1% versus 12.5%, P<0.0001) and implanted in bifurcation positions (15.4% versus 11.6%, P=0.02). At one year, the composite outcome of death, myocardial infarction and TLR was 6.1% in the PES group compared with 10.8% in the BMS group (P=0.004). The one-year rate of stent thrombosis was 0.59% in the PES group compared with 0.28% in the BMS group (P=0.4).
CONCLUSIONS:
Despite being used in higher-risk lesions, there was a lower rate of major cardiac events at one year in patients treated with PES, primarily driven by the reduction in TLR. Thus, the experience with PES in contemporary practice applied to a broader population appears to be consistent with the results reported in randomized trials.
Keywords: Coronary artery disease, Drug-eluting stents, Paclitaxel, Percutaneous coronary intervention, Restenosis, Stent thrombosis
Abstract
HISTORIQUE :
Dans le cadre d’essais aléatoires, les endoprothèses à élution de paclitaxel (EÉP) étaient supérieures aux endoprothèses en métal nu (EMN) pour réduire le risque de resténose. Cependant, l’efficacité des EÉP chez les patients traités dans la pratique quotidienne n’est pas entièrement établie.
MÉTHODOLOGIE :
Les auteurs ont procédé à une comparaison rétrospective des EÉP et des EMN chez des patients consécutifs subissant une intervention coronarienne percutanée (ICP) entre avril 2003 et mars 2004. Les issues incluaient le composite de décès, d’infarctus du myocarde et de revascularisation de lésions cibles (RLC) au bout d’un an, ainsi qu’une thrombose de l’endoprothèse.
RÉSULTATS :
Au total, 512 patients ont été traités par EÉP, et 722, par EMN. Les patients du groupe traités par EÉP étaient plus susceptibles d’avoir reçu une endoprothèse de 20 mm ou plus (52,2, % par rapport à 33,3 %, P<0,0001), de 2,5 mm de diamètre ou moins (29,1 % par rapport à 12,5 %, P<0,0001) et implantée dans des positions de bifurcation (15,4 % par rapport à 11,6 %, P=0,02). Au bout d’un an, l’issue composite de décès, d’infarctus du myocarde et de RLC s’élevait à 6,1 % au sein du groupe traité par EÉP, par rapport à 10,8 % au sein de celui traité par EMN (P=0,004). Le taux de thrombose de l’endoprothèse au bout d’un an était de 0,59 % dans le groupe traité par EÉP, et de 0,28 % dans celui traité par EMN (P=0,4).
CONCLUSIONS :
Même si les EÉP étaient utilisées pour des lésions à plus haut risque, le taux d’événements cardiaques majeurs au bout d’un an était moins élevé dans le groupe traité par ce type d’endoprothèse, surtout attribuable à la diminution des cas de RLC. Ainsi, l’expérience de l’EÉP appliquée à une population plus vaste dans la pratique contemporaine semble en harmonie avec les résultats déclarés dans le cadre des essais cliniques.
Restenosis is a major limitation of percutaneous coronary intervention (PCI) with bare metal stents (BMS). In a number of randomized clinical trials, paclitaxel-eluting stents (PES) have been shown to be superior to BMS in reducing restenosis rates and target lesion revascularization (TLR) (1–4). In the largest of the randomized trials, the TAXUS IV study (3), implantation of PES resulted in a 27% reduction in ischemia-driven TLR compared with BMS at nine months (P<0.001).
Although PES has been shown to be highly effective in randomized clinical trials, their use in patients treated in everyday practice has not been fully established. Randomized trials have generally excluded important subsets of patients treated in routine practice, such as those with multivessel disease, bifurcation lesions, small-diameter vessels, long lesions, vein graft stenoses and acute coronary syndromes. Recently, concerns have also been raised about a possible increased risk of stent thrombosis with DES in long-term follow-up (5–8). Thus, more data are urgently required addressing the safety and effectiveness of PES compared with BMS in complex, unselected patients treated outside of randomized trials.
The purpose of the present study was to report on the one-year clinical outcomes of patients undergoing PCI with PES versus BMS at a tertiary cardiac referral centre in Canada, as well as the rates of stent thrombosis in both groups.
METHODS
Patient population
All patients undergoing revascularization with PCI at the Hamilton General Hospital (Hamilton, Ontario) tertiary cardiac referral centre are entered into a database registry that includes prospectively collected demographic, clinical, procedural, angiographic and in-hospital outcomes details, including death, recurrent nonfatal myocardial infarction (MI), target vessel revascularization (TVR), TLR and in-stent thrombosis.
A retrospective comparison of PES versus BMS was conducted among all consecutive patients at the institution, with at least one stent successfully deployed in de novo lesions over a 12-month period. Patients undergoing primary or rescue PCI, those presenting with cardiogenic shock and those with a dual population of stents (ie, combination of BMS and PES) or presenting with restenosis were excluded from the analyses. All patients provided informed consent to be followed up after their procedure for study purposes, and the protocol was approved by the institution’s research ethics board.
Patients undergoing PCI at the institution during the first six months of the study only had access to BMS. However, in the last six months of the study period, PES (TAXUS Express; Boston Scientific, USA) became available for use with relatively unrestricted access. PES were to be preferentially used in lesions known to be at higher risk for restenosis (ie, bifurcating positions, longer lesions and smaller-diameter vessels), but ultimately, the decision was left to the interventional cardiologist at the time of PCI. During the latter half of the study period, the majority of patients underwent PCI with PES.
Intervention
All procedures were performed according to standardized techniques. The use of periprocedural antithrombotic agents at the time of PCI was at the discretion of the interventional cardiologist. Angiographic success was defined as residual stenosis of less than 20% in the presence of Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow. Recommended postprocedural medications included lifelong acetyl-salicylic acid and clopidogrel 75 mg/day for one year, with a minimum recommendation of six months in the PES cohort and one month in the BMS cohort. Whenever possible, a loading dose of at least 300 mg of clopidogrel was administered before PCI if the patient was not taking clopidogrel already. The 600 mg loading dose of clopidogrel was not used during this study period.
Definitions and follow-up
The primary outcome was defined as a composite of death, recurrent nonfatal MI and TLR at one year. In-hospital MI was defined as recurrent chest pain with ischemic electrocardiogram changes (mainly ST segment elevation), and/or elevation of total creatine kinase to twice the upper limit of normal and a positive troponin T result, as per local standards. A diagnosis of recurrent nonfatal MI after discharge required the presence of two of ischemic symptoms, creatine kinase elevation twice the upper limit of normal with a positive troponin result, or diagnostic Q waves on subsequent electrocardiograms. TLR was defined as clinically motivated repeat PCI or coronary artery bypass graft to treat luminal restenosis of more than 50% within the stent or within the immediate 5 mm of vessel adjacent to the stent.
Secondary outcomes included the individual events of death, MI, TLR, TVR, and in-stent thrombosis both in-hospital and at one year. TVR was defined as clinically motivated repeat PCI or coronary artery bypass graft to the same epicardial vessel in which a stent was deployed. Stent thrombosis was defined as angiographically documented complete occlusion or flow-limiting thrombus of a previously treated artery.
In-hospital outcomes were verified by review of hospital charts. One-year outcomes were verified by telephone calls using standardized questioning techniques. Whenever patients had repeat hospitalizations for chest pain or cardiac catheterization, details of the admission were obtained for verification of outcomes. At one year, all patients included in the study were cross-referenced with the hospital’s computerized medical records database and the PCI database to ensure that no repeat interventions had been missed.
Statistical analysis
Data from a locked database were analyzed using SAS version 8.2 (SAS Institute, USA). Descriptive statistics were provided for baseline demographics and procedural characteristics. Logistic regression models were developed using SAS Proc Logistic (SAS Institute). Univariate logistic models were examined for each baseline explanatory variable.
Multivariable logistic regression models were developed to describe the relationship between the composite end point and a set of baseline explanatory variables using a forward selection method. This method started with an empty model into which variables were entered if P for the variables was less than the prespecified alpha level of 0.05. The process was then repeated until none of the remaining variables met the specified alpha level for entry. A final multivariable model included all variables with P<0.05. The Kaplan-Meier method was used to estimate the time to TLR or TVR.
A time period analysis was also undertaken, evaluating baseline characteristics and clinical outcomes as above in all consecutive patients undergoing PCI with at least one stent inserted in the first six months of the study period (almost exclusively BMS) compared with those in the last six months of the study period (after the introduction of PES).
A secondary logistic analysis was performed using the propensity score approach to estimate the exposure to PES and BMS as a function of prespecified covariates, and to further estimate the impact of the exposure on the outcome by introducing the propensity score as a covariate.
RESULTS
A total of 1234 patients (512 PES, 722 BMS) who underwent PCI for de novo lesions between April 2003 and March 2004, and who met inclusion criteria for the present study were eligible for analysis.
Table 1 shows the baseline characteristics of the study patients. The mean age was 64.1 years and 26.3% had diabetes. Overall, 56.3% of patients underwent PCI for acute coronary syndromes. A significantly higher proportion of patients in the BMS cohort had a history of dyslipidemia and previous MI. There was a trend toward more multivessel disease and previous revascularization procedures (both PCI and coronary artery bypass graft) in the PES group. There were no significant differences with respect to other baseline characteristics.
TABLE 1.
Baseline clinical characteristics of patients treated with paclitaxel-eluting stents (PES) and those treated with bare metal stents (BMS)
| PES (n=512) | BMS (n=722) | P | |
|---|---|---|---|
| Age, years (mean ± SD) | 63±2 | 65±12 | N/A |
| Female sex, n (%) | 150 (29) | 224 (31) | 0.5 |
| Diabetes, n (%) | 138 (27) | 186 (26) | 0.6 |
| Non-insulin-dependent | 92 (18) | 122 (17) | 0.62 |
| Insulin-dependent | 46 (9) | 64 (9) | 0.9 |
| Hypertension, n (%) | 348 (68) | 505 (70) | 0.5 |
| Current cigarette smoking, n (%) | 115 (22) | 160 (22) | 0.9 |
| Dyslipidemia, n (%) | 390 (76) | 584 (81) | 0.05 |
| Previous myocardial infarction, n (%) | 297 (58) | 488 (68) | 0.001 |
| Previous PTCA, n (%) | 78 (15) | 88 (12) | 0.1 |
| Previous CABG, n (%) | 64 (13) | 83 (12) | 0.6 |
| Multivessel disease, n (%) | 269 (53) | 353 (49) | 0.2 |
| Inpatient referral, n (%) | 272 (53) | 422 (59) | 0.1 |
| Clinical presentation, n (%) | |||
| Stable angina | 205 (40) | 219 (30) | 0.0004 |
| Unstable angina/NSTEMI | 307 (60) | 503 (70) |
CABG Coronary artery bypass grafting; N/A Not applicable; NSTEMI Non-ST segment elevation myocardial infarction; PTCA Percutaneous transluminal coronary angioplasty
Key angiographic and procedural characteristics appear in Table 2. The periprocedural use of glycoprotein IIb/IIIa inhibitors and the proportion of patients receiving stents for lesions in the left anterior descending artery were similar in both groups. However, significantly more patients in the PES group received stents that were 2.5 mm in diameter or smaller, 20 mm in length or longer, and in bifurcation positions.
TABLE 2.
Angiographic and procedural characteristics
| PES (n=512) | BMS (n=722) | P | |
|---|---|---|---|
| Stented coronary vessel*, n (%) | 0.33 | ||
| Left main | 8 (1) | 9 (1) | |
| Left anterior descending | 225 (37) | 293 (35) | |
| Circumflex | 161 (27) | 209 (25) | |
| Right | 190 (31) | 276 (33) | |
| Coronary artery bypass graft | 20 (0.03) | 45 (5) | |
| Lesion type†, n (%) | 0.02 | ||
| Type A | 16 (2) | 26 (3) | |
| Type B1 | 142 (20) | 245 (26) | |
| Type B2 | 375 (52) | 451 (46) | |
| Type C | 143 (20) | 219 (23) | |
| Periprocedural glycoprotein IIb/IIIa inhibitors, n (%) | 157 (31) | 225 (31) | 0.9 |
| Bifurcation stenting†, n (%) | 110 (15) | 113 (12) | 0.02 |
| Implanted stents/patient, n (mean ± SD) | 1.4±0.7 | 1.4±0.7 | 0.09 |
| Stent diameter ≤2.5 mm‡, n (%) | 187 (27) | 112 (11) | <0.0001 |
| Stent length ≥20 mm‡, n (%) | 304 (43) | 275 (27) | <0.0001 |
| Post-PCI TIMI grade 3 flow§, n (%) | 704 (99) | 939 (100) | 0.1 |
*n=604 for paclitaxel-eluting stent (PES) group, n=832 for bare metal stent (BMS);
n=714 for PES, n=971 for BMS;
n=701 for PES, n=1036 for BMS;
n=714 for PES, n=939 for BMS. PCI Percutaneous coronary intervention; TIMI Thrombolysis In Myocardial Infarction
Medical therapy at discharge included prescriptions for acetylsalicylic acid 81 mg/day, clopidogrel 75 mg/day for six months in the PES group and 30 days in the BMS group, a statin and an angiotensin-converting enzyme (ACE) inhibitor. Acetylsalicylic acid, clopidogrel and statin use exceeded 90% in both groups (Table 3).
TABLE 3.
Medication at discharge
| PES (n=512) | BMS (n=722) | P | |
|---|---|---|---|
| Acetylsalicylic acid, n (%) | 505 (99) | 721 (100) | 0.02 |
| Clopidogrel, n (%) | 510 (100) | 721 (100) | 0.4 |
| Oral anticoagulant, n (%) | 16 (3) | 27 (4) | 0.6 |
| Angiotensin-converting enzyme inhibitor, n (%) | 399 (78) | 568 (79) | 0.8 |
| Beta-blocker, n (%) | 407 (80) | 585 (81) | 0.5 |
| Statin, n (%) | 488 (95) | 683 (95) | 0.6 |
BMS Bare metal stent; PES Paclitaxel-eluting stent
There were no in-hospital deaths or repeat interventional procedures in either group. The in-hospital MI rate was 0.8% in the PES group and 1.4% in the BMS group (P=0.32).
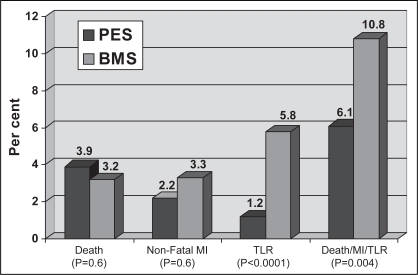
At one year, there was 100% follow-up in both groups. One-year clinical outcomes are presented in Figure 1. There was a significant reduction in the primary outcome of combined death, MI and TLR at one year for PES compared with BMS. The composite event rate was 6.1% in the PES group versus 10.9% in the BMS group (P=0.004).
Figure 1).
End points at one year. BMS Bare metal stent; MI Myocardial infarction; PES Paclitaxel-eluting stent; TLR Target lesion revascularization
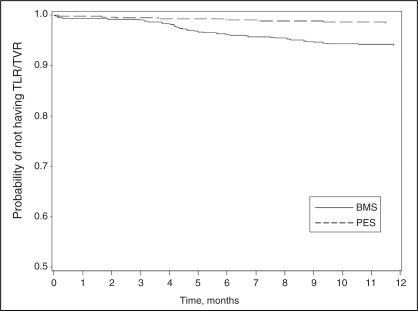
There were no significant differences in death and MI between the two groups at one year. However, treatment with PES was associated with a significant reduction in both TLR and TVR compared with BMS. At one year, the rate of clinically driven TLR was 1.2% in the PES group compared with 5.8% in the BMS group (P<0.0001). Similarly, the one-year rate of clinically driven TVR was 1.6% in the PES group compared with 6.2% in the BMS group (P<0.0001) (Figure 2). The overall rates of stent thrombosis did not vary significantly and were 0.59% in the PES group versus 0.28% in the BMS group (P=0.4).
Figure 2).
Survival curve for time to target vessel revascularization (TVR). BMS Bare metal stent; PES Paclitaxel-eluting stent; TLR Target lesion revascularization
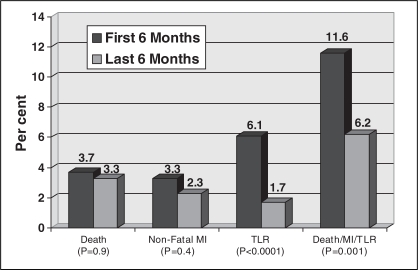
Time period analysis comparing the first six months of the study period (95% BMS) versus the last six months (76% PES) demonstrated no significant differences between these groups (Table 4) compared with the baseline characteristics of the BMS and PES groups (Table 1). There was also a statistically significant reduction in clinically driven TLR and TVR at one year following the introduction of PES in the latter half of the study period, as shown in Figure 3.
TABLE 4.
Baseline clinical characteristics of patients treated in the first 6 months and those treated in the last 6 months after the introduction of paclitaxel-eluting stents (PES)
| First 6 months (n=602) | Last 6 months (n=632) | P | |
|---|---|---|---|
| Age, years (mean ± SD) | 63±11 | 62±12 | N/A |
| Female sex, n (%) | 194 (32) | 180 (29) | 0.2 |
| Diabetes, n (%) | 151 (25) | 173 (27) | 0.4 |
| Non-insulin-dependent | 95 (16) | 119 (19) | 0.2 |
| Insulin-dependent | 56 (9) | 54 (9) | 0.6 |
| Hypertension, n (%) | 423 (70) | 430 (68) | 0.4 |
| Current cigarette smoking, n (%) | 122 (20) | 153 (24) | 0.1 |
| Dyslipidemia, n (%) | 492 (82) | 482 (76) | 0.02 |
| Previous myocardial infarction, n (%) | 409 (68) | 376 (60) | 0.002 |
| Previous PTCA, n (%) | 80 (13) | 86 (14) | 0.9 |
| Previous CABG, n (%) | 69 (12) | 78 (12) | 0.6 |
| Multivessel disease, n (%) | 293 (49) | 329 (52) | 0.2 |
| Inpatient referral, n (%) | 339 (56) | 355 (56) | 0.9 |
| Clinical presentation, n (%) | |||
| Stable angina | 185 (31) | 234 (37) | 0.004 |
| Unstable angina/NSTEMI | 412 (68) | 398 (63) |
CABG Coronary artery bypass grafting; N/A Not applicable; NSTEMI Non-ST segment elevation myocardial infarction; PTCA Percutaneous transluminal coronary angioplasty
Figure 3).
Clinical one-year end points of patients treated in the first six months and those treated in the last six months after the introduction of paclitaxel-eluting stents. MI Myocardial infarction; TLR Target lesion revascularization
Multivariate analysis was performed to identify independent predictors of the primary outcome of death, MI and TLR at one year (Table 5). The following variables were identified as significant predictors of the composite outcome: stent placement in the proximal LAD (OR=2.7, 95% CI 1.7 to 4.5, P<0.01), insertion of multiple stents (OR=2.1, 95% CI 1.3 to 3.2, P≤0.01) and diabetes (OR=1.7, 95% CI 1.1 to 2.0, P=0.03). Treatment with PES was associated with a significantly reduced risk of the composite outcome at one year (OR=0.59, 95% CI 0.37 to 0.95, P=0.03). When a logistic analysis using the propensity score approach was used to estimate the exposure to PES and BMS, and further estimate the impact of the exposure on the outcome, treatment with PES was again associated with significantly reduced risk (OR=0.505, 95% CI 0.322 to 0.793, P<0.003).
TABLE 5.
Multivariate predictors of the combined end point of death, myocardial infarction and target lesion revascularization
| OR | 95% CI | P | |
|---|---|---|---|
| Paclitaxel-eluting stent | 0.59 | 0.37–0.95 | 0.03 |
| Individual vessel length ≥20 mm | 0.96 | 0.62–1.49 | 0.85 |
| Individual vessel diameter ≤2.5 mm | 1.27 | 0.75–2.16 | 0.38 |
| Proximal left anterior descending artery stenting | 2.73 | 1.65–4.51 | <0.01 |
| More than one stent | 2.05 | 1.30–3.22 | <0.01 |
| Age | 1.01 | 0.99–1.03 | 0.15 |
| Multivessel disease | 1.33 | 0.85–2.08 | 0.22 |
| Female sex | 1.28 | 0.82–2.01 | 0.28 |
| Diabetes | 1.65 | 1.06–2.01 | 0.03 |
| Previous myocardial infarction | 0.97 | 0.61–1.54 | 0.89 |
| Clinical presentation (unstable angina/NSTEMI versus stable angina) | 1.49 | 0.91–2.44 | 0.11 |
| Clopidogrel use | 0.70 | 0.45–1.07 | 0.10 |
NSTEMI Non-ST segment elevation myocardial infarction
DISCUSSION
Although PES have been shown to be highly effective in reducing restenosis in randomized trials, their safety and effectiveness in everyday practice remains largely unknown. Most of the randomized trials have generally enrolled noncomplex patients referred for elective interventions. The purpose of our study was to determine the safety and effectiveness of PES compared with BMS in patients treated in routine practice. Given that most registry data on PES are derived from outside Canada, the information presented in our study is useful for Canadian physicians.
In the current study, PES were used in significantly more patients with bifurcation lesions, small-diameter vessels and longer lesions compared with BMS. Despite being used in lesions associated with higher risk for restenosis, treatment with PES resulted in a significant reduction in the primary outcome of the composite of death, MI and TLR at one year compared with BMS. This reduction in the composite outcome was largely driven by the reduction in TLR at one year. Use of PES resulted in an almost 80% RR reduction in TLR compared with BMS during the study period, which is similar to the benefits observed in the randomized trials with both PES and sirolimus-eluting stents (SES). The incidence of death and MI alone was not significantly different between the two groups, which is also consistent with the results from the randomized trials.
In multivariate analysis, the following variables were identified as significant predictors of the composite outcome: more than one stent, proximal left anterior descending artery lesion and diabetes, variables that are associated with a higher risk for restenosis with BMS alone.
The rate of TLR in the present study was lower than that observed in the literature (3,9). Our rate of TLR might have been lower for several reasons. First, our rate of TLR was clinically driven, and not angiographically driven. Second, all patients were on excellent medical therapy, with the use of antiplatelet, statin and ACE inhibitor therapy exceeding rates reported in various registries (10–12).
Although the reduction in TLR with PES was accomplished without any statistically significant increase in stent thrombosis, it was still almost twice that of the BMS group (three of 512 [0.59%] for PES versus two of 722 [0.28%] for BMS; P=0.4). The rate of stent thrombosis in the present study was similar to that reported for BMS and SES in the literature. For example, in the e-Cypher registry, the largest registry of SES in worldwide practice to date, the rate of stent thrombosis at one year for SES was 0.87% (13). Similarly, the rate of stent thrombosis was reported to be 1.2% for BMS in a pooled analysis of various multicentre clinical trials (14). These findings are reassuring, given the recent concerns regarding the possibility of excess rates of stent thrombosis with DES raised with the pooled meta-analysis of DES (15). However, the present study was not designed to detect a difference in thrombosis rates between BMS and PES. Larger randomized trials and registries with long-term follow-up are required to clarify the concern regarding increased stent thrombosis with DES.
A number of studies have already confirmed the safety and efficacy of SES in unselected patients treated in daily practice. In 2003, Lemos et al (16) reported that the unrestricted use of SES was safe and effective in reducing both repeat revascularizations and major cardiac events at one year compared with BMS. Recently, the e-Cypher registry (13) demonstrated the safety of SES in a very large cohort of patients treated worldwide. One retrospective cohort study demonstrated the safety and efficacy of PES compared with BMS in the setting of ST segment elevation MI (17). However, the present study is the first, to our knowledge, to report the safety and efficacy of PES versus BMS in routine practice. The results of our study complement the findings of the Taxus-Stent Evaluated At Rotterdam Cardiology Hospital (T-SEARCH) registry (9), which compared the unrestricted use of PES with SES in unselected patients and showed no difference in major adverse cardiac events after adjusting for differences in baseline characteristics (9).
Data from the current study and the T-SEARCH registry suggest that the routine clinical use of PES results in favourable outcomes at one year, and results from randomized, controlled trials can be replicated in everyday practice despite the inclusion of patients with a variety of higher-risk clinical and angiographic characteristics. This awaits further confirmation from more large-scale registries that are currently underway.
Limitations
Although the present study showed a significant benefit from the implantation of PES versus BMS in routine daily practice, it had some important limitations. First, the use of PES versus BMS was not randomized, and as such, there were some differences in the baseline patient characteristics. However, in multivariate analysis, none of these factors appeared to be predictive of the composite outcome.
Second, the present study involved a retrospective analysis of prospectively collected observational data. Although a retrospective analysis can introduce bias, this design enabled a real-world evaluation of PES versus BMS in contrast to the ideal patients and setting of a randomized clinical trial.
Third, the final stent choice was left to the discretion of the interventional cardiologist, which could have introduced bias into the study. As shown in Table 2, however, PES appeared to be used in significantly more patients with higher-risk lesions (small-diameter vessels, long lesions, bifurcation positions). These would be expected to increase the incidence of complications in PES-treated patients, and might have resulted in an underestimate of the true treatment effect.
Fourth, the two study groups were not evaluated concurrently, possibly introducing bias and a difference in management between the two groups based on temporal trends in medicine. However, all patients were enrolled within one year, limiting discrepancies in medical therapies between the two groups. Also, time period analysis was undertaken to evaluate the effect of PES on one-year clinical outcomes, following their introduction into everyday practice. Without a significant difference in baseline characteristics when compared with the PES and BMS groups, following the introduction of PES, there was a preserved significant reduction in clinically driven TLR.
Finally, the duration of combined antiplatelet therapy likely differed between the two groups. As per local practice patterns during the study period, patients in the BMS group were treated with dual antiplatelet therapy for a minimum of one month, while those in the PES group were treated for a minimum of six months. However, whenever possible, all patients were encouraged to remain on dual anti-platelet therapy for one year.
CONCLUSIONS
PES implantation resulted in a statistically significant reduction in major cardiac events at one year compared with BMS, primarily driven by the reduction in TLR. The rate of stent thrombosis at one year was 0.59% and did not vary significantly from the BMS cohort. Thus, the experience with PES in contemporary practice applied to a broader population appears to be consistent with the results reported in randomized trials.
Acknowledgments
The authors thank the interventional cardiologists at the Heart Investigation Unit of Hamilton General Hospital, Hamilton, Ontario.
REFERENCES
- 1.Grube E, Silber S, Hauptmann KE, et al. TAXUS I: Six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- 2.Colombo A, Drzewiecki J, Banning A, et al. TAXUS II Study Group. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stent for coronary artery lesions. Circulation. 2003;108:788–94. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Ellis SG, Cox DA, et al. TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting stent: The TAXUS-IV trial. Circulation. 2004;109:1942–7. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 5.Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: A pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103:1967–71. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 6.Bavry AA, Kumbhani DJ, Helton T, et al. What is the risk of stent thrombosis associated with the use of paclitaxel eluting stents for percutaneous coronary intervention? J Am Coll Cardiol. 2005;45:941–6. doi: 10.1016/j.jacc.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 7.Moreno R, Fernandez C, Hernandez R, et al. Drug eluting stent thrombosis: Results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005;45:954–9. doi: 10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 8.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 9.Ong TL, Serruys PW, Aoki J, et al. The unrestricted use of paclitaxel-versus sirolimus-eluting stents for coronary artery disease in an unselected population: One-year results of the Taxus-Stent Evaluated at Rotterdam Cardiology Hospital (T-SEARCH) registry. J Am Coll Cardiol. 2005;45:1135–41. doi: 10.1016/j.jacc.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.EUROASPIRE II Study Group: Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; Principal results from EUROASPIRE II Euro Heart Survey Program. Eur Heart J. 2001;22:554–72. doi: 10.1053/euhj.2001.2610. [DOI] [PubMed] [Google Scholar]
- 11.Hasdai D, Behar S, Wallentin L, et al. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin. The Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS) Eur Heart J. 2002;23:1190. doi: 10.1053/euhj.2002.3193. [DOI] [PubMed] [Google Scholar]
- 12.Steg PG, Goldberg RJ, Gore JM, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 13.Urban P, Gershlick AH, Guagliumi G, et al. One-year follow-up of the e-Cypher registry. Circulation. 2006;113:1434–41. doi: 10.1161/CIRCULATIONAHA.104.532242. [DOI] [PubMed] [Google Scholar]
- 14.Kereiakes DN, Choo JK, Young JJ, et al. Thrombosis and drug-eluting stents: A critical appraisal. Rev Cardiovasc Med. 2004;5:9–15. [PubMed] [Google Scholar]
- 15.Bavry AA, Kumbhani DJ, Helton TJ, et al. Late thrombosis of drug-eluting stents: A meta-analysis of randomized clinical trials. Am J Med. 2006;119:1056–61. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Lemos PA, Serruys PW, van Domburg RT, et al. Unrestricted utilization of sirolimus-eluting stents compared with conventional bare stent implantation in the ‘real world’: The Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation. 2004;109:190–5. doi: 10.1161/01.CIR.0000109138.84579.FA. [DOI] [PubMed] [Google Scholar]
- 17.Schwalm JD, Ahmad M, Velianou JL, Natarajan MK. Primary and rescue percutaneous coronary intervention with paclitaxel-eluting stent implantation in ST-elevation myocardial infarction. Am J Cardiol. 2006;97:1308–10. doi: 10.1016/j.amjcard.2005.11.071. [DOI] [PubMed] [Google Scholar]