Abstract
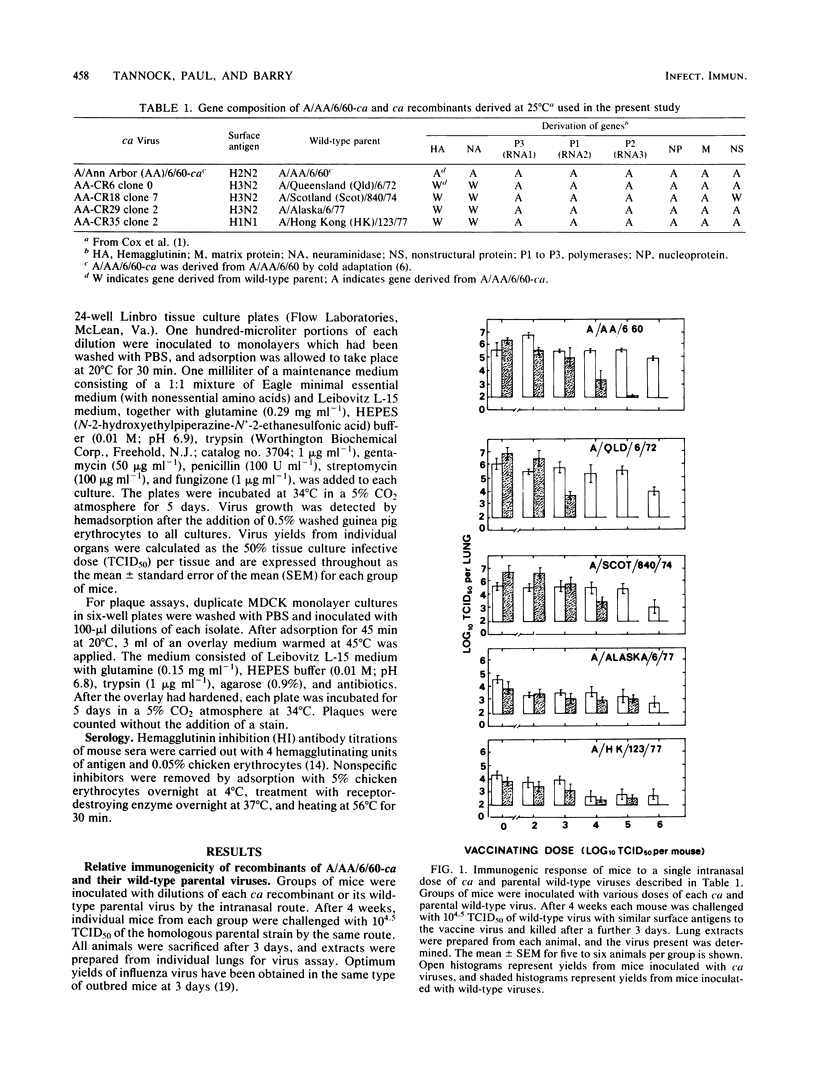
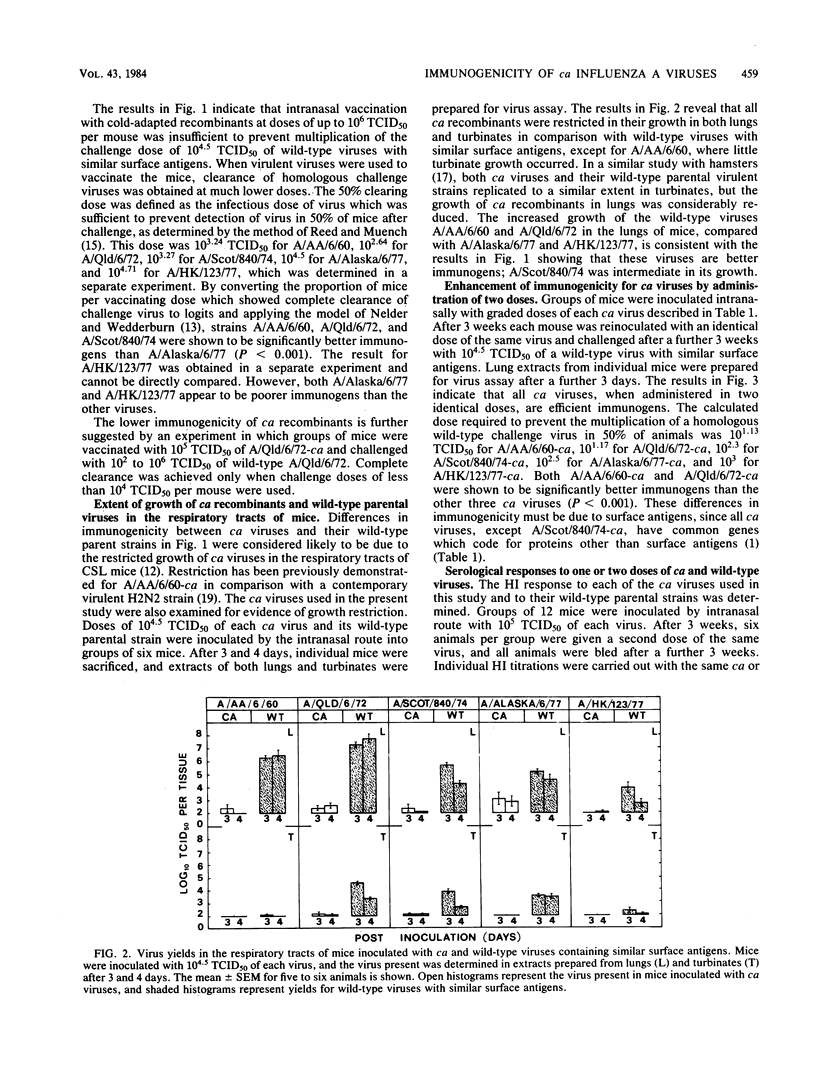
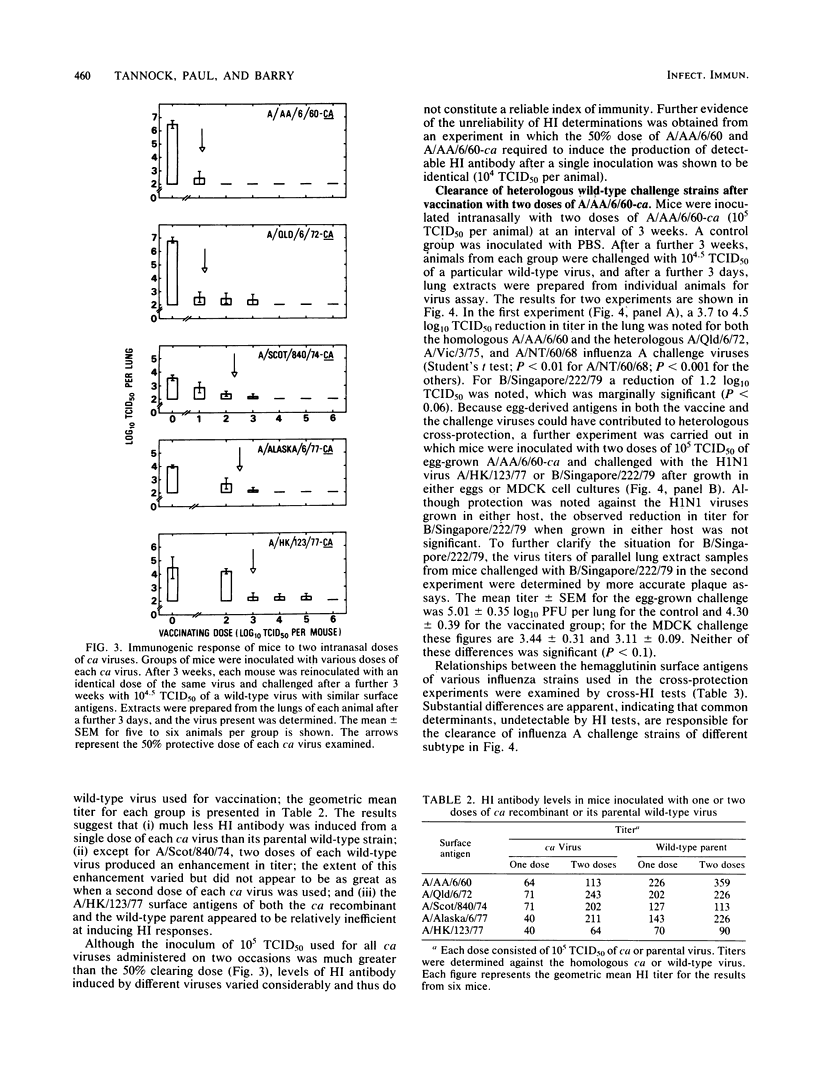
The immunogenicity of several cold-adapted (ca) viruses was compared in CSL mice with that of wild-type parental viruses with similar surface antigens, according to the vaccinating dose required to clear a challenge consisting of 10(4.5) 50% tissue culture infective doses of the wild-type virus. All ca viruses were less immunogenic than their wild-type parental strains by a factor of 10(1.3) to 10(3.4), probably due to the restricted capacity of ca viruses to replicate in the respiratory tracts of mice. However, their immunogenicity was considerably enhanced when two quite small doses were administered 3 weeks apart. The immunogenicity of ca viruses when administered in two doses and wild-type viruses when administered as a single dose varied according to their surface antigens. It was highest for viruses with the H2N2 A/Ann Arbor/6/60 and H3N2 A/Queensland/6/72 surface antigens and lowest for those with H1N1 A/HK/123/77 surface antigens. When two doses consisting of 10(5.0) 50% tissue culture infective doses of A/Ann Arbor/6/60-ca were administered at an interval of 3 weeks, solid immunity was induced against the wild-type A/Ann Arbor/6/60 parental virus, two heterologous H3N2 strains, and an H1N1 strain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox N. J., Maassab H. F., Kendal A. P. Comparative studies of wild-type and cold-mutant (temperature-sensitive) influenza viruses: nonrandom reassortment of genes during preparation of live virus vaccine candidates by recombination at 25 degrees between recent H3N2 and H1N1 epidemic strains and cold-adapted A/An Arbor/6/60. Virology. 1979 Aug;97(1):190–194. doi: 10.1016/0042-6822(79)90386-6. [DOI] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P. W., Murphy A. M. Naturally acquired immunity to influenza type A: a clinical and laboratory study. Med J Aust. 1976 Aug 28;2(9):329–333. doi: 10.5694/j.1326-5377.1976.tb130219.x. [DOI] [PubMed] [Google Scholar]
- Hope-Simpson R. E. Protection against Hong Kong influenza. Br Med J. 1972 Nov 25;4(5838):490–491. doi: 10.1136/bmj.4.5838.490-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. Y., Askonas B. A. Cross-reactivity for different type A influenza viruses of a cloned T-killer cell line. Nature. 1980 Nov 13;288(5787):164–165. doi: 10.1038/288164a0. [DOI] [PubMed] [Google Scholar]
- Maassab H. F. Biologic and immunologic characteristics of cold-adapted influenza virus. J Immunol. 1969 Mar;102(3):728–732. [PubMed] [Google Scholar]
- Mahmud M. I., Maassab H. F., Jennings R., Potter C. W. Influenza virus infection of a newborn rats: virulence of recombinant strains prepared from a cold-adapted, attenuated parent. Arch Virol. 1979;61(3):207–216. doi: 10.1007/BF01318055. [DOI] [PubMed] [Google Scholar]
- Mak N. K., Zhang Y. H., Ada G. L., Tannock G. A. Humoral and cellular responses of mice to infection with a cold-adapted influenza A virus variant. Infect Immun. 1982 Oct;38(1):218–225. doi: 10.1128/iai.38.1.218-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiz A. J., Kunz C., Hofman H., Liehl E., Reeve P., Maassab H. F. Studies with a cold-recombinant A/Victoria/3/75 (H3N2) virus. II. Evaluation in adult volunteers. J Infect Dis. 1980 Dec;142(6):857–860. doi: 10.1093/infdis/142.6.857. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Holley H. P., Jr, Berquist E. J., Levine M. M., Spring S. B., Maassab H. F., Kendal A. P., Chanock R. M. Cold-adapted variants of influenza A virus: evaluation in adult seronegative volunteers of A/Scotland/840/74 and A/Victoria/3/75 cold-adapted recombinants derived from the cold-adapted A/Ann Arbor/6/60 strain. Infect Immun. 1979 Feb;23(2):253–259. doi: 10.1128/iai.23.2.253-259.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Maassab H. F., Kendal A. P., Murphy B. R., Chanock R. M. Cold adapted variants of influenza A. II. Comparison of the genetic and biological properties of ts mutants and recombinants of the cold adapted A/AA/6/60 strain. Arch Virol. 1977;55(3):233–246. doi: 10.1007/BF01319909. [DOI] [PubMed] [Google Scholar]
- Tannock G. A., Wark M. C., Smith L. E., Sutherland M. M. A clearance test in mice using non-adapted viruses to determine the immunogenicity of influenza strains. Arch Virol. 1981;70(2):91–101. doi: 10.1007/BF01315003. [DOI] [PubMed] [Google Scholar]
- Wark M. C., Tannock G. A., Sutherland M. M., Smith L. E. A murine model for assessment of living attenuated influenza A vaccines. Aust J Exp Biol Med Sci. 1980 Dec;58(6):615–626. doi: 10.1038/icb.1980.64. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Okabe N., McKee K. T., Jr, Maassab H. F., Karzon D. T. Cold-adapted recombinant influenza A virus vaccines in seronegative young children. J Infect Dis. 1982 Jul;146(1):71–79. doi: 10.1093/infdis/146.1.71. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Thompson J., Karzon D. T. Differing virulence of H1N1 and H3N2 influenza strains. Am J Epidemiol. 1980 Dec;112(6):814–819. doi: 10.1093/oxfordjournals.aje.a113053. [DOI] [PubMed] [Google Scholar]


