Abstract
BACKGROUND:
Although rosiglitazone may offer vascular benefits beyond lowering glucose, recently, concern has been raised that this drug may paradoxically increase cardiovascular risk.
OBJECTIVE:
To assess the effects of rosiglitazone compared with standard oral hypoglycemic therapies on adipokines, and inflammatory and fibrinolytic markers in subjects with type 2 diabetes.
METHODS:
A 12-week, randomized, open-label, parallel-group study will be conducted on 100 type 2 diabetic subjects with suboptimal glycemic control (glycosylated hemoglobin 0.075 or greater) despite management with lifestyle alone (drug-naive) or with monotherapy (either metformin or sulfonylurea). Drug-naive patients will be randomly assigned to receive either rosiglitazone (4 mg/day to 8 mg/day) or metformin (500 mg/day to 2000 mg/day). Patients on pre-existing monotherapy will be randomly assigned to the addition of rosiglitazone (4 mg/day to 8 mg/day), or to either metformin (500 mg/day to 2000 mg/day) or glyburide (5 mg/day to 20 mg/day) (depending on background treatment). The primary end point of the study is the change in adiponectin level (from baseline to 12 weeks) in the rosiglitazone versus metformin or sulfonylurea arms. Secondary end points include changes in leptin, high-sensitivity C-reactive protein, interleukin-6, tumour necrosis factor-alpha, matrix metalloproteinase-9, vascular cell adhesion molecule-1, plasminogen activator inhibitor type 1, insulin sensitivity, glycosylated hemoglobin and lipid levels. Additionally, all patients will be required to be treated with an inhibitor of the renin-angiotensin system, namely an angiotensin receptor antagonist, as per national diabetes treatment guidelines, to a target systolic blood pressure of less than 130 mmHg and a diastolic blood pressure of less than 80 mmHg, or for the optimal suppression of microalbuminuria.
CONCLUSION:
The present study will further elucidate the potential beneficial metabolic and cardiovascular effects of rosiglitazone in optimally treated diabetic patients.
Keywords: Cardiometabolic risk, Inflammation, Thiazolidinediones, Type 2 diabetes
Abstract
HISTORIQUE :
Même si la rosiglitazone peut comporter des bienfaits vasculaires en plus de réduire la glycémie, on s’est récemment inquiété du fait que ce médicament pourrait paradoxalement accroître le risque cardiovasculaire.
OBJECTIF:
Évaluer les effets de la rosiglitazone par rapport à des thérapies hypoglycémiques orales standard sur les adipokines, et les marqueurs inflammatoires et fibrinolytiques chez des personnes atteintes de diabète de type 2.
MÉTHODOLOGIE :
Les auteurs effectueront une étude aléatoire ouverte de 12 semaines auprès de groupes parallèles de 100 diabétiques de type 2 dont le contrôle glycémique est sous-optimal (hémoglobine glycosylée de 0,75 ou plus) malgré une prise en charge par le mode de vie seulement (pas de médicaments) ou par une monothérapie (metformine ou sulfonylurée). Les patients qui ne prenaient pas de médicaments seront répartis au hasard entre un traitement à la rosiglitazone (4 mg/jour à 8 mg/jour) ou à la metformine (500 mg/jour à 2 000 mg/jour). Les patients déjà sous monothérapie seront répartis au hasard entre l’ajout de rosiglitazone (4 mg/jour à 8 mg/jour) ou soit de metformine (500 mg/jour à 2 000 mg/jour), soit de glyburide (5 mg/jour à 20 mg/jour) (selon le traitement de fond). Le point de virage primaire de l’étude correspond au changement du taux d’adiponectine (du début jusqu’à 12 semaines) dans les branches de rosiglitazone par rapport à celles de metformine ou de sylfonurée. Les points de virage secondaires incluent des changements aux taux de leptine, de protéine C réactive de haute sensibilité, d’interleukine-6, de facteur de nécrose tumorale alpha, de matrice métalloprotéinase-9, des molécules d’adhésion des cellules vasculaires 1, de l’inhibiteur de l’activateur du plastimogène de type 1, de la sensibilité à l’insuline, de hémoglobine glycosylée et de lipides. De plus, tous les patients devront recevoir un inhibiteur du système rénine-angiotensine, soit un antagoniste des récepteurs de l’angiotensine, conformément aux lignes directrices nationales sur le traitement du diabète, afin de cibler une tension systolique inférieure à 130 mmHg et une tension diastolique inférieure à 80 mmHg, ou d’assurer la suppression optimale des microalbuminuries.
CONCLUSION :
La présente étude permettra de mieux comprendre les effets métaboliques et cardiovasculaires bénéfiques potentiels de la rosiglitazone chez des patients diabétiques profitant d’un traitement optimal.
Type 2 diabetes is a chronic and progressive disease that is strongly associated with all-cause and cardiovascular mortality (1). The United Kingdom Prospective Diabetes Study (UKPDS) (2) demonstrated that glycemic control alone only modestly reduces the risk of macrovascular disease among type 2 diabetes patients. Insulin resistance, which has been identified as an important underlying or associated factor in the pathogenesis of type 2 diabetes, is the main mechanism proposed to be responsible for the accelerated atherosclerosis noted in this population (3,4).
Evidence continues to accumulate to support the role of inflammation as a central component in the development of atherosclerosis, insulin resistance and type 2 diabetes (5–7). Particularly, C-reactive protein (CRP), interleukin-6 (IL-6), tumour necrosis factor-alpha and markers of endothelial dysfunction have been shown to be independent predictors of both diabetes and cardiovascular risk (6,8–10). Furthermore, inhibition of fibrinolysis, which is attributable to elevated concentrations of plasminogen activator inhibitor type 1 (PAI-1), is associated with insulin resistance and type 2 diabetes, and predicts myocardial infarction and stroke (11,12).
More recently, the visceral adipocyte has been recognized to produce a number of metabolically and hormonally active substances, which are collectively called adipokines (13). Adiponectin is an adipokine that may have antiatherogenic and anti-inflammatory properties (13–15). High levels of adiponectin seem to be associated with protection against type 2 diabetes and atherosclerosis via anti-inflammatory pathways (16). Unlike adiponectin, leptin seems to be associated with the development of both atherosclerosis and insulin resistance (17,18).
Partial or complete thrombotic occlusion of a coronary artery, secondary to rupture of an atherosclerotic plaque, is the key event in the development of acute coronary syndromes (19). Macrophage-derived foam cells in vulnerable shoulder regions of atherosclerotic plaques produce matrix metalloproteinases (MMPs), which mediate extracellular matrix degradation and consequent plaque destabilization (20). MMP-9 is one of the MMPs that are highly expressed in the vulnerable regions of atherosclerotic plaques. It has been implicated in the remodelling processes associated with atherogenesis and plaque rupture (21,22). A strong association between baseline MMP-9 levels and future risk of cardiovascular mortality has been demonstrated in patients with documented coronary artery disease (23), suggesting that MMP-9 might constitute a novel biomarker for characterizing individuals at higher cardiovascular risk.
Rosiglitazone is a thiazolidinedione drug that is approved for the treatment of type 2 diabetes. As a nuclear peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone reduces insulin resistance, thereby sensitizing the liver, muscle and adipose tissue to the actions of circulating insulin (24). Treatment with rosiglitazone has been demonstrated to reduce coronary events following percutaneous coronary intervention (25,26) and to retard atherosclerosis disease progression (27,28). These effects have been, at least partially, attributed to the favourable effects of rosiglitazone on inflammatory biomarkers, insulin sensitivity and endothelial dysfunction (29–32).
Diabetes and hypertension coexist in approximately 75% of patients and this combination synergistically augments cardiovascular risk (33). In fact, blood pressure control seems to have greater importance in the prevention of macrovascular disease than glycemic control (34,35). Furthermore, the presence of diabetic nephropathy is associated with increased cardiovascular mortality (36). There is accumulating evidence that activation of the renin-angiotensin system (RAS) is associated with hypertension and end-organ damage, including renal and cardiovascular disease. Therefore, RAS inhibitors have been considered as a first-line therapy for renal and vascular protection in diabetic patients, even in the absence of overt hypertension.
The rationale of the present study is to compare the effects of rosiglitazone with the effects of usual oral hypoglycemic therapy on adipokine levels, inflammatory and fibrinolytic markers, and insulin sensitivity in diabetic patients with suboptimal glycemic control. We hypothesize that diabetic patients receiving rosiglitazone will experience greater reductions in vascular inflammation and fibrinolytic dysfunction, associated with increased adiponectin levels, compared with a metformin or sulfonylurea regimen, and that these benefits will occur, in part, as a result of greater improvements in insulin sensitivity in the rosiglitazone group.
METHODS
Study design
A 12-week, randomized, single-centre, open-label, parallel-group study of 100 patients with type 2 diabetes and suboptimal glycemic control (glycosylated hemoglobin [HbA1c] 0.075 or greater) will be conducted. The rationale of using a 12-week study period is based on previous data (30,31), which suggest that changes in inflammatory biomarkers with rosiglitazone treatment can be observed within this time period.
The protocol consists of a screening visit, a random assignment visit, two-week and eight-week telephone visits, a four-week clinic visit and a 12-week close-out visit. Study subjects will undergo a detailed medical history, including cardiovascular status, risk factors and medication use, physical examination (including waist and hip circumference measurements), and collection of blood and urine in the fasting state (Table 1). The study protocol has been approved by an independent ethics review board and all participants will provide written informed consent.
TABLE 1.
Summary of study visits and procedures
| Procedure | Visit 1, screening | Visit 2, random assignment | Visit 3, telephone, 2 weeks | Visit 4, 4 to 6 weeks | Visit 5, telephone, 8 weeks | Visit 5, 12 weeks |
|---|---|---|---|---|---|---|
| Review of inclusion and exclusion criteria | X | X | – | – | – | – |
| Informed consent | X | – | – | – | – | – |
| Medical history | X | – | – | – | – | – |
| Review concomitant medications | X | X | X | X | X | X |
| Physical | X* | X† | – | X* | – | X† |
| Physician physical | X | – | – | – | – | X |
| Fasting blood work | X‡ | X§ | – | X¶ | – | X‡§ |
| Study drug dispensing | – | X | – | X | – | – |
| Study drug compliance | – | – | X | X | X | X |
| Review of adverse events | – | – | X | X | X | X |
Blood pressure and pulse measurements taken
Blood pressure, pulse, height, weight, and waist and hip circumference measurements taken
Total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, creatinine, urea, fasting plasma glucose, glycosylated hemoglobin, potassium, sodium, chloride, complete blood count and albumin to creatinine ratio measurements taken
Insulin, high-sensitivity C-reactive protein, vascular cell adhesion molecule-1, interleukin-6, tumour necrosis factor-alpha, plasminogen activator inhibitor type 1, matrix metalloproteinase-9, adiponectin and leptin measurements taken
Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, creatinine, urea, fasting plasma glucose, potassium, sodium, chloride and complete blood count measurements taken. X Procedure will be performed
Study population
Inclusion criteria: Male or female patients with type 2 diabetes, who are 40 to 80 years of age will be included in the study if they have an HbA1c score of 0.075 or greater despite management with diet and exercise alone (drug-naive) or with oral monotherapy (either met-formin or sulfonylurea, at 50% or more of the maximum effective daily dose, or at the maximum tolerated dose).
To be enrolled in the trial, subjects must also already be receiving an angiontensin-receptor blocker (ARB) for hypertension and/or diabetic nephropathy, or have an indication for such therapy. The present study’s design will allow us to evaluate whether rosiglitazone has an impact on inflammatory biomarkers over and above background therapy with an inhibitor of the RAS system.
Exclusion criteria: Patients will be considered to be ineligible for inclusion in the study for any of the following reasons during screening: clinical signs of congestive heart failure or known left ventricular ejection fraction of less than 40%; hemodynamically significant valvular heart disease or hypertrophic obstructive cardiomyopathy; hepatic disease, or aspartate aminotransferase or alanine aminotransferase greater than 1.5 times the upper limit of normal; renal dysfunction (serum creatinine concentration greater than or equal to 1.8 times the upper limit of normal); use of insulin, rosiglitazone or pioglitazone; history of systemic inflammatory disease (rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus), myositis or myopathic process; known malignancy; use of steroids or chemotherapy drugs within the past year; chronic use of nonsteroidal anti-inflammatory drugs other than acetylsalicylic acid (use for two or more weeks within the past year); HIV; use of potassium-sparing diuretics; known hypersensitivity to rosiglitazone or ARBs; and women who are pregnant, breastfeeding or not using a reliable method of contraception.
Treatment
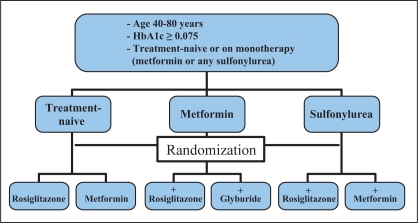
Treatment-naive diabetic patients with suboptimal glycemic control (HbA1c 0.075 or greater) will be randomly assigned to receive either rosiglitazone or metformin. Subjects inadequately controlled with existing sulfonylurea monotherapy will be randomly assigned to add-on therapy with either rosiglitazone or metformin. Patients inadequately controlled with existing metformin monotherapy will be randomly assigned to add-on therapy with either rosiglitazone or glyburide (Figure 1).
Figure 1).
A summary of the study treatment algorithm. HbA1c Glycosylated hemoglobin
For patients assigned to receive rosiglitazone therapy, the initial dose will be 4 mg/day titrated to 8 mg/day (for those on existing sulfonylurea, rosiglitazone will be used at a maximum dose of 4 mg/day as per the updated version of the Canadian product monograph for Avandia [GlaxoSmithKline Canada]). For patients assigned to receive metformin therapy, the initial dose will be 500 mg/day titrated to 1 g/day (for those whose HbA1c is between 0.075 and 0.080) or 1 g/day titrated to 2 g/day (for those whose HbA1c is greater than 0.080). For patients assigned to receive glyburide therapy, the initial dose will be 5 mg/day titrated to 10 mg/day (for those whose HbA1c is between 0.075 and 0.080 or those older than 60 years of age) or 10 mg/day titrated to 20 mg/day (for those whose HbA1c is greater than 0.080).
Dose titration, as required, will occur at visit 4 (four weeks after random assignment) if home capillary glucose readings suggest persisting hyperglycemia, depending on tolerability. Dosages can be titrated down if poorly tolerated during the course of the study.
Eligible patients who are not receiving treatment with any RAS inhibitor will be started on telmisartan 80 mg/day at the time of random assignment if they have hypertension (systolic blood pressure 130 mmHg or greater, and/or diastolic blood pressure 80 mmHg or greater) or diabetic nephropathy (albumin to creatinine ratio (ACR) greater than 2.0 mg/mmol in men or greater than 2.8 mg/mmol in women) (37). Patients already taking an ARB or angiotensin-converting enzyme inhibitor will be maintained on their baseline drug, although the dose may be increased or a second RAS blocker may be added throughout the study if blood pressure or ACR targets have not been achieved.
The patients will be asked to continue with all other previous medications, diet plans and physical activity for the duration of the study.
Methods of evaluation
An outline of the study assessments at each clinic visit is provided in Table 1.
Fasting blood will be collected at the time of random assignment and after 12 weeks of treatment for the measurement of adipokines, inflammatory and fibrinolytic markers, and insulin. These assays will be performed centrally in the research laboratories at St Michael’s Hospital, University of Toronto (Toronto, Ontario).
Standard screening and laboratory safety parameters tests will be performed in local laboratories using standard assays. Concentrations of total cholesterol, triglycerides and high-density lipoprotein (HDL) cholesterol will be measured enzymatically. Low-density lipoprotein (LDL) cholesterol will be calculated using Friedewald’s formula:
Urinary albumin will be measured by nephelometry on the Dade Behring automated immunoassay system (Dade Behring Diagnostics, Germany). Urinary creatinine will be measured by the kinetic Jaffe colorimetric reaction on the Roche COBAS Integra assay system (Roche Diagnostics, USA). The urinary ACR will be calculated based on these two results. HbA1c will be measured by turbidimetry using an automated immuoassay on the Roche COBAS Integra system.
High-sensitivity CRP (hs-CRP) will be quantified by immunonephelometry on a Behring Nephelometer II (Dade Behring Diagnostics). Insulin sensitivity in the fasting state will be estimated by the homeostasis model assessment and calculated using the following formula (38):
Plasma insulin levels, adipokines (adiponectin, leptin), inflammatory markers (IL-6, tumour necrosis factor-alpha, vascular cell adhesion molecule-1, MMP-9) and PAI-1 will be measured by luminometry (LINCOplex: Multiplexed Biomarker Immunoassays; Millipore, USA).
Efficacy end points
The primary end point of the present study will be the change in adiponectin level (from baseline to 12 weeks) in the rosiglitazone-treated versus metformin- or sulfonylurea-treated subjects.
Secondary end points include changes in leptin, hs-CRP, IL-6, MMP-9, vascular cell adhesion molecule-1, PAI-1, insulin sensitivity (homeostasis model assessment), glucose control and lipid levels (from baseline to 12 weeks) in the rosiglitazone versus metformin- or sulfonylurea-treated subjects.
Safety assessments
Changes in findings on physical examination, vital signs, clinical laboratory tests and participant reports will be assessed through the course of the study. If alanine aminotransferase levels increase to more than three times the upper limit of the reference range levels on two consecutive occasions, the study drug will be discontinued, although patients will continue to be followed throughout the study. Patients who become pregnant will also be withdrawn from the study drug.
Statistical considerations
The expected change in adiponectin levels in the rosiglitazone versus the metformin or sulfonylurea group is conservatively anticipated to be approximately 10% (Δ0.2). There is an expected approximate 5% to 7% variance of the measurement in the range of biomarkers and insulin sensitivity. Assuming a Δ0.2±0.07, a two-sided alpha of 0.05 and a beta of 0.2, a sample size of approximately 100 (n=50 per group) will be required for the primary end point of per cent change in adiponectin. The data will be expressed as mean ± SD. Because inflammatory biomarkers often have a rightward skewed distribution, natural logarithmic transformed values will be used to compare between groups. Multivariate analysis will be performed with a background, stepwise logistic regression model to control for known confounders and cardiovascular risk factors. We will use χ2 tests for bivariate analyses and Student’s t tests to compare the geometric means between groups. Furthermore, a Cochran-Armitage trend test will be used to evaluate trends. All analyses will be performed using SAS software version 8.0 (SAS Institute Inc, USA) and P<0.05 will be considered significant.
DISCUSSION
Type 2 diabetes is associated with an accelerated development of atherosclerosis and increased cardiovascular morbidity and mortality (39). Thiazolidinediones, such as rosiglitazone, have been shown to retard atherosclerosis disease progression. In a 12-month study (27), treatment with rosiglitazone led to a significant beneficial effect on the progression of carotid intima-media thickness in people with type 2 diabetes mellitus. In another study (28), rosiglitazone reduced common carotid intima-media thickness progression in nondiabetic subjects with coronary artery disease.
The PROspective pioglitAzone Clinical Trial In macroVascular Events (PROACTIVE) (40) demonstrated that pioglitazone, another drug of the thiazolidinedione class, did not improve the primary composite outcome in more than 5000 high-risk patients with type 2 diabetes. However, a predefined composite secondary end point of all-cause mortality, nonfatal myocardial infarction and stroke, was significantly reduced. Although the pioglitazone-treated group had better control of glucose, HDL cholesterol and triglyceride levels, and blood pressure, the improvements in these traditional risk factors only partially explained the favourable outcomes in the pioglitazone-treated patients. Previous studies (29–32) have demonstrated improvements in both insulin resistance and vascular inflammation with glitazones, possible mechanisms by which pioglitazone resulted in clinical benefit in PROACTIVE.
Recently, the potential cardiovascular profile of rosiglitazone has come under scrutiny. A meta-analysis of 42 trials (41) examined the effects of rosiglitazone on cardiovascular morbidity and mortality, and demonstrated a 43% increased risk of myocardial infarction (P=0.03) in those taking rosiglitazone compared with controls, and a nonsignificant 64% increase in the risk of cardiovascular death (P=0.06). Subsequent analyses by other groups have both supported and refuted these findings, and regulators have recommended that ongoing clinical trials continue, and that the current available evidence does not support either an increased or a decreased risk of ischemic events on rosiglitazone (42–45).
Further research is required to determine whether rosiglitazone has a favourable, neutral or detrimental effect on cardiovascular outcomes, and the potential mechanisms behind these effects. Although high levels of adiponectin have been associated with reduced cardiovascular risk, recent studies have suggested that high levels of plasma adiponectin were shown to be a predictor of mortality in patients with chronic heart failure, coronary artery disease and chronic kidney disease (46–49), and in a population of older men without heart failure or cardiovascular disease (50). Therefore, the relationship between adiponectin concentrations and vascular morbidity and mortality remains unclear and warrants further study. Although hard cardiac events, such as myocardial infarction and death are not expected to be frequent in our study and therefore are not primary end points, adjustments for cardiovascular status and risk factors will be performed, providing additional insight into the recent paradoxical findings regarding adiponectin.
Additionally, our study will investigate the effects of rosiglitazone on an extensive panel of markers, including other adipokines, and markers of endothelial and fibrinolytic dysfunction, which may provide clarification of the potential anti-inflammatory actions of rosiglitazone in type 2 diabetic patients.
SUMMARY
The present study will evaluate the effects of rosiglitazone on adipokine levels, inflammatory and fibrinolytic markers, and insulin resistance, compared with standard oral therapies for diabetes. The results of the present study may allow for a better understanding of the apparent cross-talk between inflammatory-signalling pathways and insulin-signalling pathways in type 2 diabetes, cross-talk that may predispose to cardiovascular disease. Most importantly, the present study may further elucidate the potential mechanisms by which rosiglitazone exerts its cardiovascular effects.
Acknowledgments
The present study is supported by an unrestricted investigator-initiated grant from GlaxoSmithKline Canada to Dr Milan Gupta and Dr Subodh Verma.
REFERENCES
- 1.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21:1167–72. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reaven GM. Banting lecture 1988: Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–22. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 5.Saito I, Folsom AR, Brancati FL, Duncan BB, Chambless LE, McGovern PG. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81–91. doi: 10.7326/0003-4819-133-2-200007180-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 7.Festa A, D’Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin-6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 11.Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: A potential factor predisposing to thrombosis and its persistence. Circulation. 1998;97:2213–21. doi: 10.1161/01.cir.97.22.2213. [DOI] [PubMed] [Google Scholar]
- 12.Hamsten A, de Faire U, Walldius G, et al. Plasminogen activator inhibitor in plasma: Risk factor for recurrent myocardial infarction. Lancet. 1987;2:3–9. doi: 10.1016/s0140-6736(87)93050-9. [DOI] [PubMed] [Google Scholar]
- 13.Shuldiner AR, Yang R, Gong DW. Resistin, obesity and insulin resistance – the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001;345:1345–6. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 14.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 16.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-KappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 17.Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: A novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–24. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108:1533–40. doi: 10.1172/JCI13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuster V, Stein B, Ambrose JA, Badimon L, Badimon JJ, Chesebro JH. Atherosclerotic plaque rupture and thrombosis. Circulation. 1990;82(II):47–59. [PubMed] [Google Scholar]
- 20.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res. 2002;90:251–62. [PubMed] [Google Scholar]
- 22.Shah PK, Falk E, Badimon JJ, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques: Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–9. [PubMed] [Google Scholar]
- 23.Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–85. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 24.Stumvoll M, Haring HU. Glitazones: Clinical effects and molecular mechanisms. Ann Med. 2002;34:217–24. [PubMed] [Google Scholar]
- 25.Wang G, Wei J, Guan Y, Jin N, Mao J, Wang X. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces clinical inflammatory responses in type 2 diabetes with coronary artery disease after coronary angioplasty. Metabolism. 2005;54:590–7. doi: 10.1016/j.metabol.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Cao Z, Zhou YJ, Zhao YX, Liu YY, Guo YH, Cheng WJ. Rosiglitazone could improve clinical outcomes after coronary stent implantation in nondiabetic patients with metabolic syndrome. Chin Med J (Eng) 2006;119:1171–5. [PubMed] [Google Scholar]
- 27.Hedblad B, Zambanini A, Nilsson P, Janzon L, Berglund G. Rosiglitazone and carotid IMT progression rate in a mixed cohort of patients with type 2 diabetes and the insulin resistance syndrome: Main results from the Rosiglitazone Atherosclerosis Study. J Intern Med. 2007;261:293–305. doi: 10.1111/j.1365-2796.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- 28.Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2004;24:930–4. doi: 10.1161/01.ATV.0000124890.40436.77. [DOI] [PubMed] [Google Scholar]
- 29.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–84. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 30.Sidhu JS, Cowan D, Kaski JC. The effects of rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, on markers of endothelial cell activation, C-reactive protein, and fibrinogen levels in non-diabetic coronary artery disease patients. J Am Coll Cardiol. 2003;42:1757–63. doi: 10.1016/j.jacc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Marx N, Frochlich J, Siam L, et al. Antidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:283–8. doi: 10.1161/01.atv.0000054195.35121.5e. [DOI] [PubMed] [Google Scholar]
- 32.Ghanim H, Dhindsa S, Aljada A, Chaudhuri A, Viswanathan P, Dandona P. Low-dose rosiglitazone exerts an antiinflammatory effect with an increase in adiponectin independently of free fatty acid fall and insulin sensitization in obese type 2 diabetics. J Clin Endocrinol Metab. 2006;91:3553–8. doi: 10.1210/jc.2005-2609. [DOI] [PubMed] [Google Scholar]
- 33.Hypertension in Diabetes Study Group HDS 1: Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11:309–17. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ. 2000;321:412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 36.Gerstein HC, Mann JFE, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–6. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 37.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2003 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2003;27(Suppl 2):S1–141. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 39.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 40.Dormandy JA, Charbonnel B, Eckland DJA, PROactive Investigators et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 41.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:1–15. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 42.Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med. 2007;147:578–81. doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- 43.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 44.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 45.Home PD, Pocock SJ, Beck-Nielsen H, et al. RECORD Study Group Rosiglitazone evaluated for cardiovascular outcomes – an interim analysis. N Eng J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 46.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 47.George J, Patal S, Wexler D, et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92:1420–4. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–9. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 49.Pilz S, Mangge H, Wellnitz B, et al. Adiponectin and mortality in patients undergoing coronary angiography. J Clin Endocrinol Metab. 2006;91:4277–86. doi: 10.1210/jc.2006-0836. [DOI] [PubMed] [Google Scholar]
- 50.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–7. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]