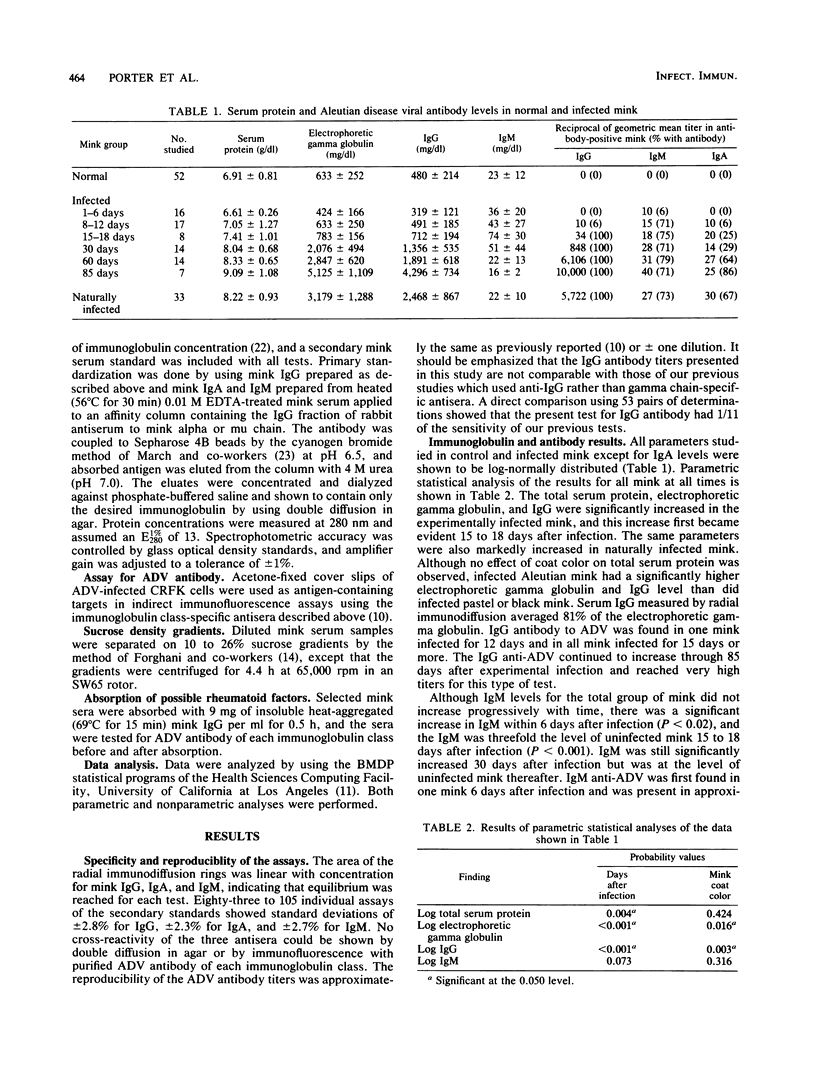
Abstract
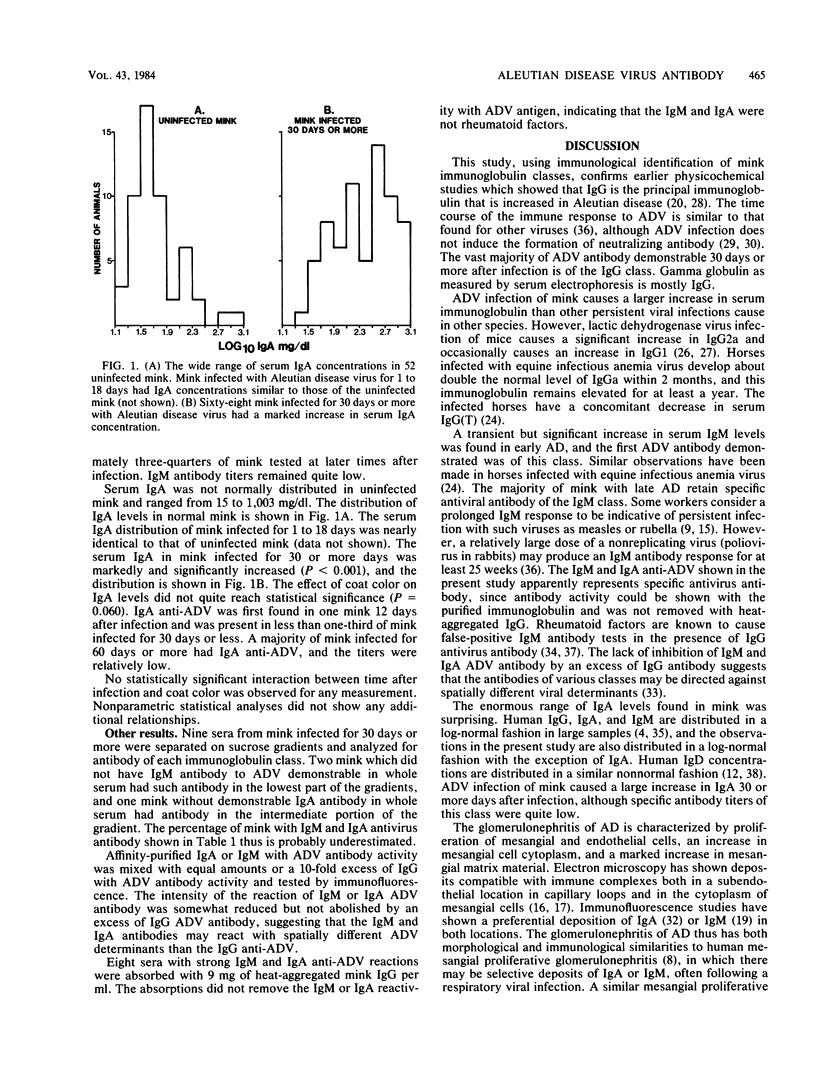
Aleutian disease virus (ADV) persistently infects mink and causes marked hypergammaglobulinemia. Immunoglobulin class-specific antisera were used to define the total immunoglobulin of each class by radial immunodiffusion and the immunoglobulin class of ADV-specific antibody by immunofluorescence in experimentally and naturally infected mink. Electrophoretic gamma globulin closely reflects the immunoglobulin G (IgG) level in mink, and the majority of the increased immunoglobulin and ADV antibody in infected mink is IgG. IgM becomes elevated within 6 days after infection, reaches peak levels by 15 to 18 days, and returns to normal by 60 days after infection. The first ADV antibody demonstrable is IgM, and most mink have virus-specific IgM antibody for at least 85 days postinfection. Serum IgA levels in normal mink are not normally distributed, and ADV infection causes a marked elevation of IgA. Low levels of ADV-specific IgA antibody can be shown throughout the course of infection. Failure of large amounts of virus-specific IgG antibody to inhibit the reaction of virus-specific IgM and IgA antibodies suggests that the various classes of antibodies are directed against spatially different antigenic determinants. The IgM and IgA were shown not to be rheumatoid factors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Hadlow W. J., Chesebro B. Aleutian disease of mink: the antibody response of sapphire and pastel mink to Aleutian disease virus. J Immunol. 1975 Oct;115(4):1034–1037. [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley C. E., 3rd, Dorsey F. C. Serum immunoglobulin levels throughout the life-span of healthy man. Ann Intern Med. 1971 Nov;75(5):673–682. doi: 10.7326/0003-4819-75-5-673. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Ingram D. G. Antigen and antibody in Aleutian disease in mink. II. The reaction of antibody with the Aleutian disease agent using immunodiffusion and immunoelectroosmophoresis. Can J Comp Med. 1973 Jul;37(3):217–223. [PMC free article] [PubMed] [Google Scholar]
- Coe J. E., Hadlow W. J. Studies on immunoglobulins of mink: definition of IgG, IgA and IgM. J Immunol. 1972 Feb;108(2):530–537. [PubMed] [Google Scholar]
- Coe J. E. Studies on immunoglobulins of mink: definition of two populations of light chains. Immunochemistry. 1972 Feb;9(2):147–151. doi: 10.1016/0019-2791(72)90035-3. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Haire M., Hadden D. S. Measles immunoglobulins in subacute sclerosing panencephalitis. Br Med J. 1971 Jan 2;1(5739):23–25. doi: 10.1136/bmj.1.5739.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford T. B., McGuire T. C., Porter D. D., Cho H. J. A comparative study of detection methods for Aleutian disease viral antibody. J Immunol. 1977 Apr;118(4):1249–1251. [PubMed] [Google Scholar]
- Dunnette S. L., Gleich G. J., Miller R. D., Kyle R. A. Measurement of IgD by a double antibody radioimmunoassay: demonstration of an apparent trimodal distribution of IgD levels in normal human sera. J Immunol. 1977 Nov;119(5):1727–1731. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Demonstration of rubella IgM antibody by indirect fluorescent antibody staining, sucrose density gradient centrifugation and mercaptoethanol reduction. Intervirology. 1973;1(1):48–59. doi: 10.1159/000148832. [DOI] [PubMed] [Google Scholar]
- Haire M., Hadden D. S. Immunoglobulin responses in rubella and its complications. Br Med J. 1970 Jul 18;3(5715):130–132. doi: 10.1136/bmj.3.5715.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. B., Gorham J. R., Tanaka Y. Renal glomerular ultrastructure in mink affected by Aleutian disease. Lab Invest. 1967 Aug;17(2):123–139. [PubMed] [Google Scholar]
- Isaacs K. L., Miller F. Role of antigen size and charge in immune complex glomerulonephritis. Lab Invest. 1982 Aug;47(2):198–205. [PubMed] [Google Scholar]
- Johnson M. I., Henson J. B., Gorham J. R. The influence of genotype on the development of glomerular lesions in mink with Aleutian disease virus. Am J Pathol. 1975 Nov;81(2):321–336. [PMC free article] [PubMed] [Google Scholar]
- KENYON A. J., TRAUTWEIN G., HELMBOLDT C. F. Characterization of blood serum proteins from mink with Aleutian disease. Am J Vet Res. 1963 Jan;24:168–173. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B., Gorham J. R. Aleutian disease of mink: detection of large quantities of complement-fixing antibody to viral antigen. J Immunol. 1971 Nov;107(5):1481–1482. [PubMed] [Google Scholar]
- McGuire T. C. Suppression of synthesis of an IgG subclass in a persistent viral infection. Immunology. 1976 Jan;30(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Michaelides M. C., Simms E. S. Immune responses in mice infected with lactic dehydrogenase virus. I. Antibody response to DNP-BGG and hyperglobulinaemia in BALB/c mice. Immunology. 1977 Jun;32(6):981–988. [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Rizzo A. A., Scheele C., Waldmann T. A. Elevated gamma-globulin and increased antibody production in mice infected with lactic dehydrogenase virus. J Exp Med. 1966 Feb 1;123(2):347–364. doi: 10.1084/jem.123.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER D. D., DIXON F. J., LARSEN A. E. METABOLISM AND FUNCTION OF GAMMA GLOBULIN IN ALEUTIAN DISEASE OF MINK. J Exp Med. 1965 Jun 1;121:889–900. doi: 10.1084/jem.121.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Cox N. A., Porter H. G., Suffin S. C. Isolation of Aleutian disease virus of mink in cell culture. Intervirology. 1977;8(3):129–144. doi: 10.1159/000148888. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. Aleutian disease of mink. Adv Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., Coe J. E. Deposition of IgA in renal glomeruli of mink affected with Aleutian disease. Am J Pathol. 1979 Jul;96(1):227–236. [PMC free article] [PubMed] [Google Scholar]
- Riggs J. L., Cremer N. E. Differentiation of cytomegalovirus antigens by their reactivity with various classes of human antibodies in the indirect fluorescent antibody test. J Clin Microbiol. 1980 Jan;11(1):88–93. doi: 10.1128/jcm.11.1.88-93.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. I. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN KINETICS AND ANTIGEN DOSE REQUIREMENT FOR INDUCTION. J Exp Med. 1964 Jan 1;119:1–19. doi: 10.1084/jem.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodaria P. V., Fraser K. B., Stanford F. Secondary fluorescent staining of virus antigens by rheumatoid factor and fluorescein-conjugated anti-IgM. Ann Rheum Dis. 1973 Jan;32(1):53–57. doi: 10.1136/ard.32.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop J. W., Zegers B. J., Sander P. C., Ballieux R. E. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol. 1969 Jan;4(1):101–112. [PMC free article] [PubMed] [Google Scholar]
- Torfason E. G., Diderholm H. False RIA IgM titres to herpes simplex virus and cytomegalovirus: factors causing them, and their absorption by protein A-Sepharose/IgG-protein A-Sepharose. J Med Virol. 1982;10(3):157–170. doi: 10.1002/jmv.1890100302. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Kunkel H. G. The correlation of serum IgD concentration with Gm allotype. J Immunol. 1974 Jul;113(1):274–278. [PubMed] [Google Scholar]


