Abstract
Neutrophilic inflammation in acute exacerbations of asthma tends to be resistant to treatment with glucocorticoids. This may be related to decreased activity and expression of histone deacetylase-2 (HDAC2), which down-regulates expression of proinflammatory genes via recruitment to the glucocorticoid receptor complex. We assessed airway inflammation and response to steroid treatment in a novel mouse model of an acute exacerbation of chronic asthma. Systemically sensitized mice received low-level challenge with aerosolized ovalbumin for 4 weeks, followed by a single moderate-level challenge to induce enhanced inflammation in distal airways. We assessed the effects of pre-treatment with dexamethasone on the accumulation of inflammatory cells in the airways, airway responsiveness to methacholine, expression and enzymatic activity of nuclear proteins including histone acetyl transferase (HAT) and HDAC2, and levels of transcripts for neutrophil chemoattractant and survival cytokines. Dexamethasone suppressed inflammation associated with eosinophil and T-lymphocyte recruitment, but did not prevent neutrophil accumulation or development of airway hyperresponsiveness. Increased activity of HAT was suppressed by steroid treatment, but the marked diminution of HDAC2 activity and increased activity of nuclear factor-κB were not reversed. Correspondingly, elevated expression of mRNA for TNF-α, granulocyte-macrophage colony-stimulating factor, IL-8, and p21waf were also not suppressed by dexamethasone. Levels of lipid peroxidation and protein nitration products were elevated in the acute exacerbation model. We conclude that impaired nuclear recruitment of HDAC2 could be an important mechanism of steroid resistance of the neutrophilic inflammation in exacerbations of asthma. Oxidative stress may contribute to decreased HDAC2 activity.
Keywords: airway inflammation, cytokines, dexamethasone, histone deacetylase-2
CLINICAL RELEVANCE
In a model of an exacerbation of asthma, neutrophil recruitment and enhanced cytokine expression were resistant to dexamethasone. This correlated with decreased histone deacetylase-2 expression, which may be an important mechanism of resistance to steroid therapy.
Asthma is characterized by intermittent airflow obstruction, which develops on a background of chronic inflammation of the airways driven by CD4+ T-lymphocytes. Superimposed upon this are episodes of acute inflammation, typically dominated by eosinophils (1). In general, this inflammation is well controlled by inhaled glucocorticoids, although in a subset of patients these drugs are less effective. Persistent asthma may be associated with the presence of significant neutrophilic inflammation (2), and predominant neutrophilic inflammation has been linked to steroid resistance in individuals with stable asthma (3). Neutrophil recruitment is a prominent feature of acute exacerbations of chronic asthma (4), in which it may be related to respiratory tract infections, especially by viruses (5, 6). Similarly, in patients with difficult-to-control asthma, neutrophils rather than eosinophils often predominate (7). Again, exacerbations in these patients are often relatively resistant to treatment with inhaled steroids (8).
Histone acetyl transferase (HAT) and histone deacetylase (HDAC) are key enzymes involved in modifying the expression of inflammatory genes in airway diseases (9). The anti-inflammatory actions of glucocorticoids are at least in part related to the recruitment by activated glucocorticoid receptors (GR) of HDAC2, which inhibits the activation of inflammatory genes by the transcription factor nuclear factor-κB (NF-κB) (10). We have previously demonstrated that in individuals with mild stable asthma, HAT activity in tissues is increased, and is suppressed by treatment with glucocorticoids. Conversely, HDAC2 activity is decreased, but this is only partially reversed by steroid therapy (11). In peripheral blood cells from individuals with severe asthma, reduced HDAC2 activity correlates with in vitro steroid resistance of cytokine secretion (12). Furthermore, we have shown that reduced HDAC activity correlates with increased disease severity in chronic obstructive pulmonary disease, potentially accounting for the steroid unresponsiveness of the airway inflammation in these patients (13, 14). Whether decreased HDAC2 activity might be a key contributor to steroid resistance in acute exacerbations of asthma, in association with enhanced recruitment of neutrophils, is unknown.
To investigate this, we employed a novel murine model of an acute exacerbation of chronic asthma, in which systemically sensitized mice receive chronic low-level challenge with aerosolized ovalbumin (OVA) for 4 weeks, followed by a single moderate-level challenge. The low-level challenge elicits eosinophil recruitment on a background of chronic inflammation localized to the airway wall, without significant parenchymal inflammation, and is accompanied by changes of structural remodeling of the airways (15). The subsequent moderate-level challenge induces a rapidly developing, exaggerated inflammatory response in distal airways, associated with airway hyperresponsiveness (AHR) of peripheral origin (16). The model thus uniquely replicates key features of asthmatic exacerbations in patients.
We report that in this model, an exacerbation could be induced in a setting of prior treatment with dexamethasone. Under these circumstances, inflammation associated with recruitment of eosinophils and lymphocytes was suppressed, but neutrophil accumulation was resistant to glucocorticoid therapy, analogous to the findings in patients with asthma. Induction of an acute exacerbation was associated with a significant decrease in HDAC2 expression, as well as enhanced expression of NF-κB, proinflammatory cytokines, and markers of severe asthma. All of these were relatively resistant to dexamethasone treatment. This suggests that impaired nuclear recruitment of HDAC2 could be an important mechanism of steroid resistance of the neutrophilic inflammation in exacerbations of asthma. Furthermore, levels of lipid peroxidation and protein nitration products were elevated in lung tissues in the acute exacerbation model, suggesting that oxidative stress may contribute to the observed decrease in HDAC2 activity.
MATERIALS AND METHODS
Experimental Model
Specific pathogen–free female BALB/c mice aged 8 weeks were obtained from the Animal Resources Centre (Perth, Australia). All experimental procedures were approved by the Animal Care and Ethics Committee of the University of New South Wales (ref. no. 04/06).
As previously described (16), animals were sensitized by intraperitoneal injection of 50 μg of chicken egg ovalbumin (Grade V, ≥ 98% pure; Sigma, St Louis, MO [unless otherwise specified, all chemicals were obtained from this source]) adsorbed to 1 mg of aluminum hydroxide, 21 and 7 days before the commencement of inhalational challenges. Mice were exposed to aerosolized ovalbumin in a whole-body inhalation exposure chamber (Unifab Corporation, Kalamazoo, MI). During the exposure, the animals were held in wire flow-through cage racks and filtered air was drawn through the 0.5 m3 inhalation chamber at a flow rate of 250 L/minute. A solution of 2.5% ovalbumin in normal saline was aerosolized by delivery of compressed air to a sidestream jet nebulizer (Trimed, Sydney, Australia) and injected into the airstream entering the chamber. Chronic low-level challenge involved exposure to ≈3 mg/m3 aerosolized ovalbumin for 30 minutes/day on 3 days/week for 4 weeks. At the end of this period, a single moderate-level challenge with exposure to ≈30 mg/m3 of ovalbumin for 30 minutes was used to induce the acute exacerbation. Particle concentration within the breathing zone of the mice was continuously monitored using a DustTrak 8520 instrument (TSI, St. Paul, MN).
Drug Treatment
At 24 and 2 hours before the single moderate-level challenge, animals received either dexamethasone (1 mg/kg, cyclodextrin compound in saline; Sigma), or vehicle alone by gavage. Controls were either sham-challenged, vehicle-treated mice exposed to ≈30 mg/m3 of saline aerosol for 30 minutes, or nonsensitized animals that were not exposed to aerosol challenge. Experimental groups comprised 6 to 8 animals each for assessment of airway inflammation, hyperresponsiveness, and protein/mRNA levels.
Airway Responsiveness
Airway responsiveness to inhaled β-methacholine was determined in mice 4 hours after the final aerosol challenge. Transpulmonary resistance (RL) and dynamic compliance (Cdyn) were assessed as described (16, 17). Animals were anesthetized with ketamine-xylazine, tracheostomized, and mechanically ventilated within a plethysmograph chamber. Volume changes due to thoracic expansion and alterations in tracheal pressure were measured in response to challenge with saline, followed by increasing concentrations of β-methacholine (6.25, 12.5, 25, and 50 mg/ml). Peak values were taken as the maximum response to the concentration of methacholine being tested, and were expressed as the percentage change relative to the saline control.
Bronchoalveolar Lavage and Histochemical/Immunohistochemical Staining
Mice were killed 4 hours after the final aerosol challenge, the lungs were perfused with saline, and the tracheae were cannulated to perform bronchoalveolar lavage (BAL) with 2 × 1 ml phosphate-buffered saline. Counts were performed on at least 300 cells in a Giemsa-stained smear.
Eosinophils were identified in 5-μm frozen sections from the mid-zone of the single-lobed left lung, on the basis of staining for cyanide-resistant peroxidase (18). Immunostaining for T cells used a rat anti-CD3 monoclonal antibody, reactive across multiple species (NCL-CD3–12; Novocastra, Newcastle, UK). Staining for neutrophils used rat anti–Gr-1 (RB6–8C5; PharMingen, San Diego, CA). Detection was with a rabbit anti-rat bridging antibody (DakoCytomation, Glostrup, Denmark) followed by the Envision biotin-free detection system (DakoCytomation), which provided high sensitivity. Morphometric assessment was performed according to previously established protocols (19).
Nuclear Protein Extraction and Assays
Tissues were ground under liquid nitrogen using a pestle and mortar. Hypotonic buffer (1×; Active Motif, Rixensart, Belgium) with one complete protease inhibitor cocktail tablet (Roche Diagnostics, Burgess Hill, UK) was added, followed by the nonionic detergent NP-40 (Sigma), and samples were vortexed to release nuclei. The nuclear-rich fraction was used for measurement of glucocorticoid receptor (GR) activity using a TransAM GR kit (Active Motif), expressed as the absorbance per 10 μg protein. Similarly, HDAC activity was measured using an assay kit based on a fluorescent derivative of ɛ-acetyl lysine (Biomol, Plymouth Meeting, PA) and was expressed as μM of fluorescent substrate standard per 10 μg protein. HAT was measured using an enzyme-linked immunosorbent assay kit (Millipore, Billerica, MA) and was also expressed as the absorbance per 20 μg protein. NF-κB activity was measured using a modified TransAM p65 colorimetric assay kit (Active Motif) and was similarly expressed as the absorbance per 10 μg protein.
SDS-PAGE and Western blotting were used to quantify immunoreactive HDAC2 in nuclear-rich extracts, using mouse monoclonal HDAC2 antibody (Sigma) and enhanced chemiluminescence detection (ECL; Amersham, Little Chalfont, UK). Expression was normalized to lamin A/C protein as a standard.
RT-QPCR
Total RNA extraction and reverse transcription were performed using an RNeasy kit (Qiagen, Crawley, UK) and an Omniscript RT kit (Qiagen). Gene transcript levels of HDAC2, TNF-α, GM-CSF, KC (the murine counterpart of IL-8), p21waf (a cyclin-dependent kinase inhibitor), and GAPDH, as a housekeeping gene, were quantified by real-time PCR using commercially available primers and a Taqman system (Applied Biosystems, Warrington, UK) on a Rotor-Gene 3000 (Corbett Research, Mortlake, NSW, Australia).
Oxidative Modification of Cytoplasmic Lipids and Proteins
The cytoplasmic fraction of the tissue extracts was clarified by microcentrifugation and the protein concentration of each sample was measured using a Bradford Protein Assay kit (Bio-Rad, Hemel Hempstead, UK) with bovine serum albumin as a standard. Lipid peroxidation in lung tissue was assessed using a colorimetric assay kit (BIOXYTECH LPO-586; Oxis International, Portland, OR) based on the reaction between a chromogenic reagent (N-methyl-2-phenylindole) and the peroxidation products malonaldehyde and 4-hydroxyalkenal, which yields a stable chromophore with maximal absorbance at 586 nm. The assay was performed according to the manufacturer's instructions, using 100 μg of lung tissue extract per sample. Protein nitration was assessed by measuring levels of nitrotyrosine in lung tissue using a chemiluminescence enzyme linked immunosorbent assay (Millipore) and the value was expressed as micrograms of nitrated bovine serum albumin equivalent.
Statistical Analysis
Data are presented as arithmetic means ± SEM for each experimental group. For comparison between groups, analysis was performed using one-way ANOVA followed by Tukey's multiple comparison test. The software package GraphPad Prism 5.01 (GraphPad Software, San Diego, CA) was used for all data analysis and preparation of graphs.
RESULTS
Airway Inflammation
In BAL fluid, the total numbers of cells and the numbers of eosinophils, lymphocytes, and neutrophils were significantly increased in vehicle-treated animals compared with naïve mice (Table 1). Numbers of macrophages were also increased, although this was not statistically significant. Pretreatment with dexamethasone did not decrease total cell numbers or the numbers of macrophages in BAL fluid, but completely suppressed eosinophil and lymphocyte recruitment. However, drug treatment only partially suppressed neutrophils in BAL fluid (Table 1).
TABLE 1.
BRONCHOALVEOLAR LAVAGE CELL COUNTS
| Naïve | Vehicle | Dexamethasone | |
|---|---|---|---|
| Total number of cells (×10−3) | 31.50 ± 4.45 | 64.33 ± 7.15* | 80.67 ± 10.23† |
| Macrophages (×10−3) | 29.92 ± 4.54 | 52.77 ± 6.82 | 76.76 ± 9.97† |
| Lymphocytes (×10−3) | 0.31 ± 0.13 | 5.72 ± 1.33† | 0.76 ± 0.13‡ |
| Eosinophils (×10−3) | 0.01 ± 0.01 | 0.63 ± 0.18† | 0.00 ± 0.00‡ |
| Neutrophils (×10−3) | 1.26 ± 0.14 | 5.22 ± 1.02§ | 3.15 ± 0.86 |
Values are expressed as mean ± SEM.
P < 0.05, significant difference compared with naïve animals.
P < 0.001, significant difference compared with naïve animals.
P < 0.001, significant difference between vehicle-treated and dexamethasone-treated animals.
P < 0.01, significant difference compared with naïve animals.
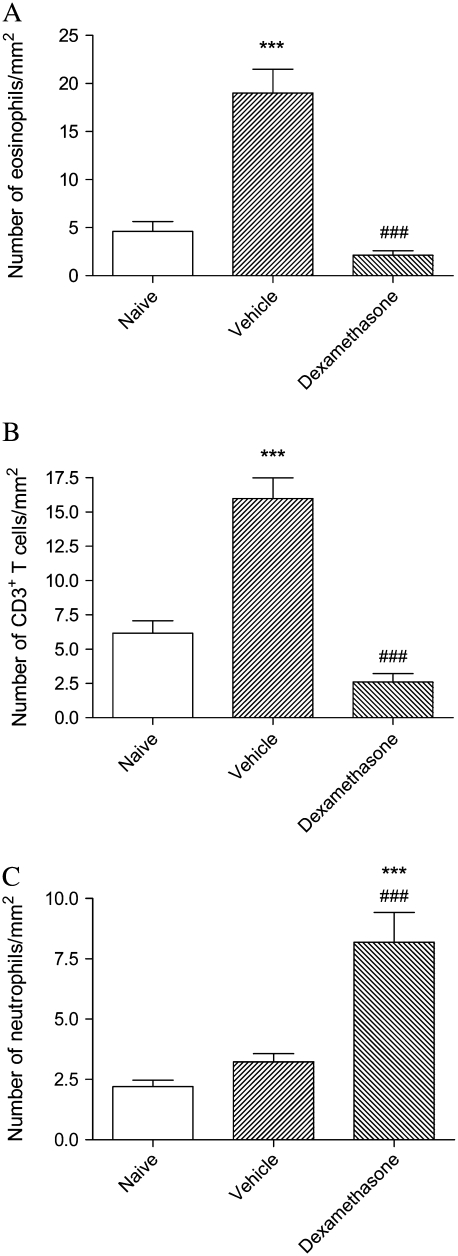
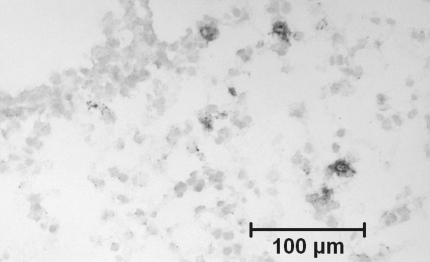
Similar to its effects on BAL cells, dexamethasone completely suppressed the increase in the number of eosinophils and CD3-positive T lymphocytes in lung tissue that was observed in vehicle-treated mice (Figures 1A and 1B). In contrast, whereas the number of neutrophils in lung tissue did not rise appreciably in the vehicle-treated group after moderate-level allergen challenge, numbers were strikingly increased after treament with dexamethasone (Figure 1C). Microscopically, these cells appeared to be entirely within airway or alveolar walls rather than in the alveolar lumen (Figure 2).
Figure 1.
Profile density of (A) eosinophils, (B) CD3+ T cells, and (C) neutrophils, in lung tissue of mice from the acute exacerbation model treated with vehicle or with dexamethasone, compared with naïve animals. Significant differences compared with naïve animals shown as ***P < 0.001 and compared with mice treated with vehicle shown as ###P < 0.001.
Figure 2.
Immunostaining for Gr-1+ neutrophils in a frozen section of lung tissue of an animal from dexamethasone-treated group, demonstrating the location of the cells within the airway and alveolar walls. Immunoperoxidase, methyl green counterstain.
AHR
Vehicle-treated mice exhibited significant AHR, both in terms of increased transpulmonary resistance and decreased dynamic compliance. This was not suppressed by treatment with dexamethasone (Table 2).
TABLE 2.
AIRWAY HYPERRESPONSIVENESS
| Naïve | Vehicle | Dexamethasone | |
|---|---|---|---|
| Transpulmonary resistance (percent of saline control at 25 mg/ml methacholine) | 155 ± 19 | 389 ± 65* | 408 ± 62* |
| Dynamic compliance (percent of saline control at 25 mg/ml methacholine) | −35.3 ± 3.9 | −73.6 ± 3.9† | −68.2 ± 5.4† |
Values are expressed as mean ± SEM.
P < 0.05, significant difference compared with naïve animals.
P < 0.001, significant difference compared with naïve animals.
Nuclear Protein Assays
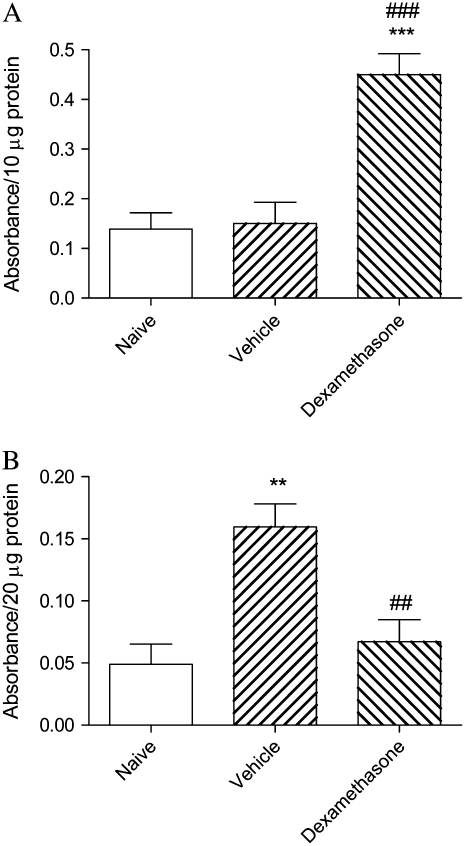
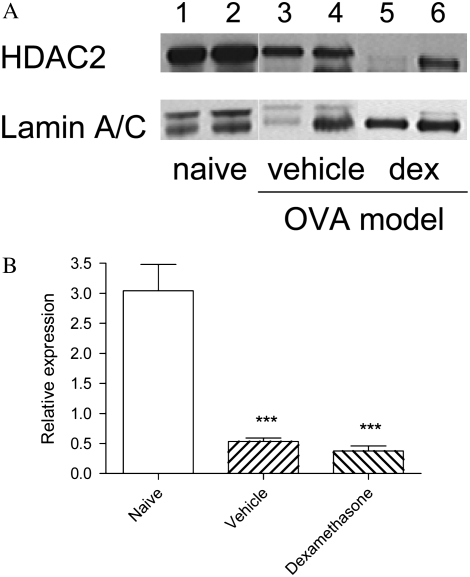
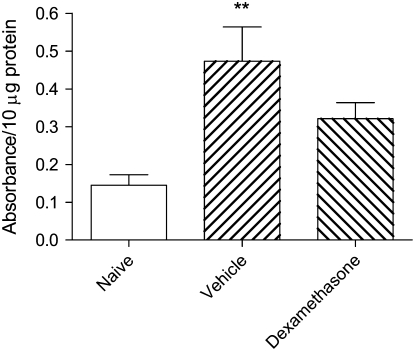
Levels of GR activity in nuclear extracts were elevated over 3-fold after dexamethasone treatment, confirming the effectiveness of drug therapy (Figure 3A). HAT activity in nuclear extracts of lung tissue was strikingly increased in vehicle-treated animals, but was suppressed to levels comparable with those of naïve mice in animals treated with dexamethasone (Figure 3B). Total activity of all HDAC isoforms was similar in all three groups (not shown), but levels of HDAC2 protein as assessed by Western blotting (Figure 4A) were markedly reduced in vehicle-treated animals (Figure 4B), even though mRNA expression for this isoform was unchanged (HDAC2/GAPDH 0.040 ± 0.025 in naïve mice, 0.040 ± 0.026 in animals treated with vehicle, and 0.037 ± 0.024 in those treated with dexamethasone). In dexamethasone-treated mice, levels of HDAC2 protein remained as low as in vehicle-treated mice (Figure 4B). NF-κB activity was markedly elevated in vehicle-treated animals, but this was only partially suppressed by dexamethasone treatment (Figure 5).
Figure 3.
(A) GR activity and (B) HAT activity in nuclear extracts of lung tissue of mice from the acute exacerbation model treated with vehicle or with dexamethasone, compared with naïve animals. Significant differences compared with naïve animals shown as **P < 0.01, ***P < 0.001 and compared with mice treated with vehicle shown as ##P < 0.01, ###P < 0.001.
Figure 4.
(A) Western blotting of nuclear extracts of lung tissue of mice for HDAC2 and the reference protein lamin A/C. Lanes 1 and 2 are samples from individual naïve animals; lanes 3 and 4 are from individual vehicle-treated animals; lanes 5 and 6 are from dexamethasone-treated animals. (B) Relative expression of HDAC2 protein in nuclear extracts of lung tissue of mice from the three groups. Significant differences compared with naïve animals shown as ***P < 0.001.
Figure 5.
NF-κB activity in nuclear extracts of lung tissue of mice from the acute exacerbation model treated with vehicle or with dexamethasone, compared with naïve animals. Significant differences compared with naïve animals shown as **P < 0.01.
mRNA Expression
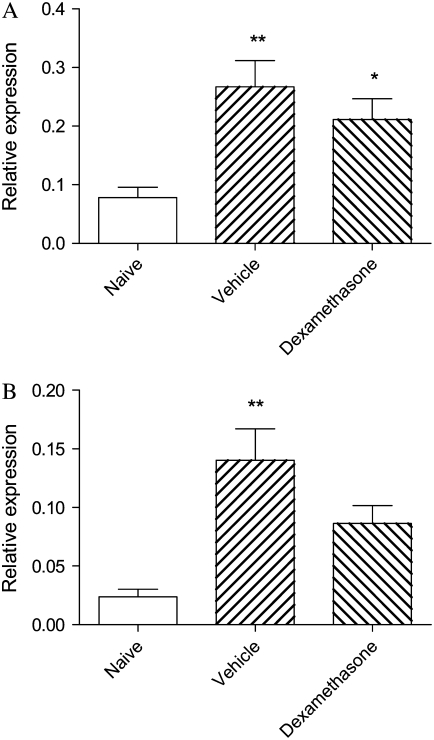
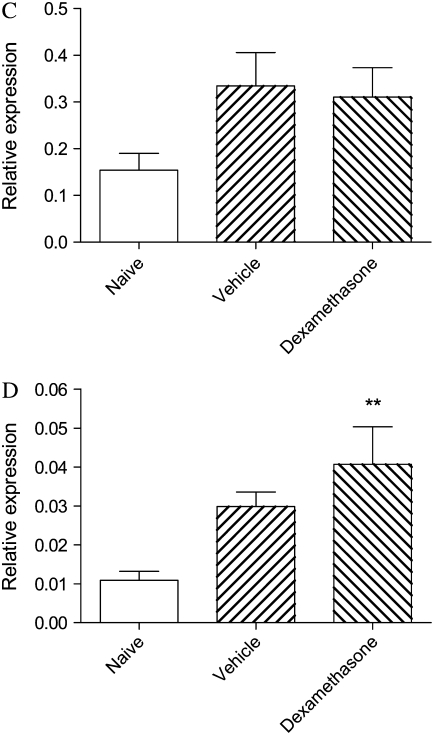
Relative levels of mRNA for TNF-α were significantly elevated in lung tissues from vehicle-treated mice, and these levels remained increased after dexamethasone treatment (Figure 6A). Levels of mRNA for GM-CSF were similarly increased and were only modestly diminished after drug treatment (Figure 6B). Relative levels of mRNA for both KC and p21waf were also elevated more than 2-fold in vehicle-treated animals, although these increases were not statistically significant. The elevated expression of KC and p21waf was not suppressed by dexamethasone (Figures 6C and 6D).
Figure 6.
Relative expression of mRNA for (A) TNF-α; (B) GM-CSF; (C) KC, the murine functional homolog of IL-8; and (D) p21waf, a marker of severe asthma, in nuclear extracts of lung tissue of mice from the acute exacerbation model treated with vehicle or with dexamethasone, compared with naïve animals. Significant differences compared with naïve animals shown as *P < 0.05, **P < 0.01.
Markers of Oxidative Stress
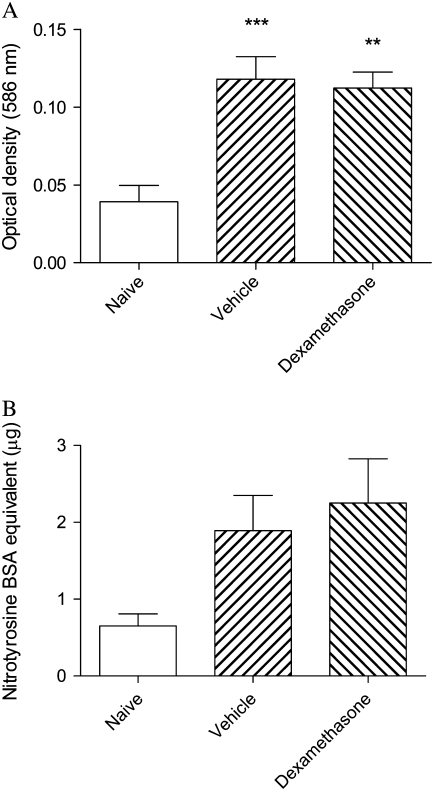
In the acute exacerbation model, levels of lipid peroxidation products were significantly increased and were unaffected by treatment with dexamethasone (Figure 7A). Levels of protein nitrotyrosine were similarly elevated more than 2-fold, although these changes were not statistically significant (Figure 7B). Other changes of oxidative stress (e.g., carbonylation of proteins, increased levels of 4-hydroxynonenal) were not detectable in these samples (data not shown).
Figure 7.
Relative levels of (A) lipid peroxidation products and (B) nitrotyrosine in cytoplasmic extracts of lung tissue of mice from the acute exacerbation model treated with vehicle or with dexamethasone, compared with naïve animals. Significant differences compared with naïve animals shown as **P < 0.01, ***P < 0.001.
DISCUSSION
In this study, we have demonstrated that in a mouse model of an acute exacerbation of chronic asthma, there was recruitment into the airways not only of eosinophils and CD3+ T-lymphocytes but also of neutrophils, with parallel development of AHR. In lung tissue, significantly increased HAT activity and decreased levels of HDAC2 protein were evident, accompanied by significantly increased activity of NF-κB and elevated expression of mRNA for TNF-α and GM-CSF. In addition, levels of mRNA for KC, the murine functional equivalent of IL-8 (20) and p21waf, a cyclin-dependent kinase inhibitor that is elevated in severe asthma (21), were both increased.
Pretreatment with dexamethasone suppressed eosinophilic and lymphocytic inflammation in this model, but did not suppress numbers of macrophages and only partially suppressed neutrophils in BAL fluid. Remarkably, this therapy increased the numbers of the latter cells in tissues. Dexamethasone treatment had no effect on AHR. At a dose that significantly enhanced GR activity in lung tissue, dexamethasone completely suppressed HAT activity, but NF-κB activity and relative levels of mRNA for TNF-α and GM-CSF were only partially suppressed, while levels of mRNA for KC and p21waf were undiminished. Importantly, dexamethasone treatment failed to restore HDAC2 levels in the tissues.
The pattern of inflammation in this mouse model of an asthmatic exacerbation closely simulates that in patients. Although exacerbations in individuals with asthma are most often triggered by viral infections (22), allergen exposure synergizes with infection, eliciting a more severe inflammatory response (23, 24). In our model of an allergen-induced acute exacerbation, background lesions of mild asthma are induced by chronic low-level allergen challenge (15), and animals are then exposed for a 30-minute period to a single moderate-level challenge with a 10-fold higher mass concentration of aerosolized antigen. This is still a much lower mass concentration than is used by most investigators in models of allergic airway inflammation, with 10- to 100-fold higher concentrations being typical in uncontrolled exposure chambers. Thus, although our model of an allergen-induced acute exacerbation is associated with more marked airway inflammation that extends further distally than in our chronic challenge model, the inflammatory response remains relatively mild and the numbers of eosinophils, lymphocytes, and neutrophils in BAL fluid are modest (16). Despite this, the animals develop a pattern of AHR distinct from that seen in the chronic challenge model, reflecting more distal airway involvement.
Treatment of the experimental acute exacerbation with dexamethasone clearly had different effects on the accumulation of eosinophils and lymphocytes as compared with macrophages and neutrophils. Consistent with the well-established effects of glucocorticoids on eosinophil and T lymphocyte recruitment and survival (25), dexamethasone almost completely suppressed the increased numbers of these cells. However, the drug did not diminish the number of macrophages in BAL fluid (indeed, these were somewhat increased, although this was not statistically significant) and had only a moderate effect on the number of neutrophils in BAL. Moreover, treatment with dexamethasone was associated with an increased number of neutrophils in lung tissue. This observation, which is consistent with results previously reported in subjects with asthma who received oral prednisolone (26), emphasizes the need to assess both lavage and tissue findings in airway inflammatory diseases. In both settings, however, neutrophilic inflammation in this model was relatively steroid resistant, thus resembling the clinical situation.
The high numbers of neutrophils visible in lung sections are unlikely to be the result of increased recruitment, given that in other respects, the inflammatory response is suppressed by treatment with glucocorticoids. Instead, it may be related to mechanisms such as increased intravascular trapping or enhanced survival of the cells—this is consistent with the observation that the cells appeared to be within alveolar walls rather than in the alveolar lumen. Numerous noncirculating neutrophils are normally sequestered in the pulmonary capillaries (27, 28) and numbers of such cells are increased in pulmonary inflammation, apparently because of the reduced deformability of neutrophils induced by inflammatory mediators (27, 29). It is possible that trapped or marginated neutrophils might exhibit prolonged survival in tissues of dexamethasone-treated animals because of the capacity of glucocorticoids to inhibit neutrophil apoptosis (30, 31). In addition, these cells may have failed to be recruited into the airways—thus accounting for their reduced number in BAL fluid—and therefore have accumulated in the tissue.
The relative lack of effect of dexamethasone treatment on neutrophilic inflammation in this model of an acute exacerbation may be related to inability of the drug to suppress signals for recruitment and survival of these cells. We found that mRNA levels for TNF-α, GM-CSF, and the murine counterpart of IL-8 were either only modestly suppressed or unaffected by treatment with the glucocorticoid. As the expression of these cytokines is driven by NF-κB, this lack of suppression is consistent with the failure of dexamethasone treatment to effectively suppress the increased activity of NF-κB in the acute exacerbation model. In the context of the observed modest increase in number of macrophages in BAL fluid, it is also possible that an increased number of inflammatory macrophages might contribute to enhanced levels of mRNA for these cytokines. In other experiments, we have shown that alveolar macrophages express markedly increased levels of mRNA for these and other proinflammatory cytokines in this model of an acute exacerbation of chronic asthma (unpublished data C. Herbert, J.S. Siegle, and R.K. Kumar).
It appears likely that the steroid-resistant neutrophilic inflammation is a consequence of the low levels of HDAC2 protein, given our finding that glucocorticoid treatment fails to induce HDAC2 protein, despite the clear evidence of increased nuclear GR activity and suppression of HAT activity. These results are consistent with our previous findings in patients with mild asthma, in whom elevated levels of HAT activity were directly suppressed by glucocorticoids but diminished HDAC activity was only partially restored by treatment (11). They are also consistent with our recent evidence that reduced HDAC activity, at least as assessed in peripheral blood mononuclear cells, correlates with steroid insensitivity in severe asthma (12). Thus the findings in this animal experimental study integrate data from various studies in patients and have allowed us to relate all of the relevant changes in a single setting.
In previous studies, we have shown that oxidative stress is a potentially important mechanism for reduction of HDAC2 activity (32). Together with direct activation of NF-κB, oxidative stress mechanisms may thus contribute to enhanced production of proinflammatory cytokines in chronic obstructive pulmonary disease (reviewed in Ref. 33). The inflammatory environment of asthma is similarly known to be associated with enhanced production of reactive oxygen species, which correlates with disease severity (34). Therefore, we examined whether there was evidence of increased oxidative stress in this animal model of an acute exacerbation of chronic asthma. These experiments yielded evidence of both lipid peroxidation and protein nitration in lung tissues, thus providing a plausible mechanism for the reduction in HDAC2 activity.
In this model, levels of expression of mRNA for p21waf, identified as a marker of severe asthma (21), were also elevated and resistant to steroid therapy. Glucocorticoids such as dexamethasone may be able to induce transcription of this gene (35), so the lack of suppression after drug treatment is not altogether surprising.
We found that AHR in this model, as estimated by transpulmonary resistance and dynamic compliance, was also resistant to steroid therapy. Previously, we have shown that AHR in these animals is not dependent on the recruitment of eosinophils (16). Whether the AHR is related to the neutrophilic inflammation is, however, unclear. Other mechanisms of AHR that may be important include early airway closure as a consequence of thickening of the inflamed airway walls, together with surfactant inactivation as a consequence of inflammatory exudation (36, 37). These processes might also be expected to be steroid resistant.
In humans, TNF-α is able to induce both AHR and neutrophil recruitment (38). In the context of our findings in this animal model, it seems possible that TNF-α might play a key role in the manifestations of an acute exacerbation of asthma. This inference is consistent with evidence that inhibition of this cytokine is of benefit both in severe asthma and in preventing exacerbations of moderate asthma (39, 40).
Collectively, the results of this animal experimental study support the concept that steroid-resistant neutrophilic inflammation in an acute exacerbation of asthma may be related to impaired nuclear recruitment of HDAC2, leading to continuing enhanced expression of neutrophil chemoattractant and survival factors. Our findings suggest that therapeutic agents that can suppress neutrophilic inflammation, such as low-dose theophylline, which restores HDAC activity (41), and long-acting β agonists, which reduce neutrophil recruitment in asthma (42), may be of particular relevance in the context of an asthmatic exacerbation.
This work was supported by grants from the National Health and Medical Research Council of Australia and The Wellcome Trust.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0028OC on May 12, 2008
Conflict of Interest Statement: K.I. has received research funding ($50,000 from Boehringer Ingelheim over 2003–2006, $30,000 from Mitsubishi WelPharma) and salary from GlaxoSmithKline ($500,000 over 2002–2007). C.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.S.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.S.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.J.B. has received research funding, lecture fees, and has served on Scientific Advisory Boards for GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, ALTANA Pharma, and Pfizer, all of whom have an interest in new therapies for asthma. R.K.K. received €25,000 in 2006 and €8,000 in 2007 from ALTANA Pharma for studies on roflumilast, currently under investigation for treatment of asthma.
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola A. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. [DOI] [PubMed] [Google Scholar]
- 2.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 2001;119:1329–1336. [DOI] [PubMed] [Google Scholar]
- 3.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002;57:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, Tonnel AB. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med 1998;157:394–402. [DOI] [PubMed] [Google Scholar]
- 5.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol 1995;95:843–852. [DOI] [PubMed] [Google Scholar]
- 6.Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J 2002;19:68–75. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999;160:1001–1008. [DOI] [PubMed] [Google Scholar]
- 8.in't Veen JC, Smits HH, Hiemstra PS, Zwinderman AE, Sterk PJ, Bel EH. Lung function and sputum characteristics of patients with severe asthma during an induced exacerbation by double-blind steroid withdrawal. Am J Respir Crit Care Med 1999;160:93–99. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Transcription factors in airway diseases. Lab Invest 2006;86:867–872. [DOI] [PubMed] [Google Scholar]
- 10.Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 2006;203:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K, Caramori G, Lim S, Oates T, Chung KF, Barnes PJ, Adcock IM. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med 2002;166:392–396. [DOI] [PubMed] [Google Scholar]
- 12.Hew M, Bhavsar P, Torrego A, Meah S, Khorasani N, Barnes PJ, Adcock I, Chung KF. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med 2006;174:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967–1976. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol 2007;119:1055–1062. (quiz 1063–1054). [DOI] [PubMed] [Google Scholar]
- 15.Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax 1998;53:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegle JS, Hansbro N, Herbert C, Yang M, Foster PS, Kumar RK. Airway hyperreactivity in exacerbation of chronic asthma is independent of eosinophilic inflammation. Am J Respir Cell Mol Biol 2006;35:565–570. [DOI] [PubMed] [Google Scholar]
- 17.Ameredes BT. Cardiac activity during airway resistance alterations with intravenous and inhaled methacholine. Respir Physiolo Neurobiol 2004;139:281–292. [DOI] [PubMed] [Google Scholar]
- 18.Korsgren M, Erjefalt JS, Korsgren O, Sundler F, Persson CGA. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J Exp Med 1997;185:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar RK. Quantitative immunohistologic assessment of lymphocyte populations in the pulmonary inflammatory response to intratracheal silica. Am J Pathol 1989;135:605–614. [PMC free article] [PubMed] [Google Scholar]
- 20.Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol 1995;17:103–108. [DOI] [PubMed] [Google Scholar]
- 21.Puddicombe SM, Torres-Lozano C, Richter A, Bucchieri F, Lordan JL, Howarth PH, Vrugt B, Albers R, Djukanovic R, Holgate ST, et al. Increased expression of p21(waf) cyclin-dependent kinase inhibitor in asthmatic bronchial epithelium. Am J Respir Cell Mol Biol 2003;28:61–68. [DOI] [PubMed] [Google Scholar]
- 22.Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, Whitehead BF, Gibson PG. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med 2005;172:433–439. [DOI] [PubMed] [Google Scholar]
- 23.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ 2002;324:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proud D, Chow CW. Role of viral infections in asthma and chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2006;35:513–518. [DOI] [PubMed] [Google Scholar]
- 25.Bentley AM, Hamid Q, Robinson DS, Schotman E, Meng Q, Assoufi B, Kay AB, Durham SR. Prednisolone treatment in asthma: reduction in the numbers of eosinophils, T cells, tryptase-only positive mast cells, and modulation of IL-4, IL-5, and interferon-gamma cytokine gene expression within the bronchial mucosa. Am J Respir Crit Care Med 1996;153:551–556. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen LT, Lim S, Oates T, Chung KF. Increase in airway neutrophils after oral but not inhaled corticosteroid therapy in mild asthma. Respir Med 2005;99:200–207. [DOI] [PubMed] [Google Scholar]
- 27.Selby C, MacNee W. Factors affecting neutrophil transit during acute pulmonary inflammation: Minireview. Exp Lung Res 1993;19:407–428. [DOI] [PubMed] [Google Scholar]
- 28.Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome—how neutrophils migrate into the lung. Crit Care 2004;8:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida K, Kondo R, Wang Q, Doerschuk CM. Neutrophil cytoskeletal rearrangements during capillary sequestration in bacterial pneumonia in rats. Am J Respir Crit Care Med 2006;174:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils: separation of survival and activation outcomes. J Immunol 1995;154:4719–4725. [PubMed] [Google Scholar]
- 31.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol 1996;156:4422–4428. [PubMed] [Google Scholar]
- 32.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun 2004;315:240–245. [DOI] [PubMed] [Google Scholar]
- 33.Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, Rahman I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 2008;10:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood LG, Garg ML, Simpson JL, Mori TA, Croft KD, Wark PA, Gibson PG. Induced sputum 8-isoprostane concentrations in inflammatory airway diseases. Am J Respir Crit Care Med 2005;171:426–430. [DOI] [PubMed] [Google Scholar]
- 35.Cha HH, Cram EJ, Wang EC, Huang AJ, Kasler HG, Firestone GL. Glucocorticoids stimulate p21 gene expression by targeting multiple transcriptional elements within a steroid responsive region of the p21waf1/cip1 promoter in rat hepatoma cells. J Biol Chem 1998;273:1998–2007. [DOI] [PubMed] [Google Scholar]
- 36.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol 2004;96:2019–2027. [DOI] [PubMed] [Google Scholar]
- 37.Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 2004;114:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 1995;152:76–80. [DOI] [PubMed] [Google Scholar]
- 39.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med 2006;354:697–708. [DOI] [PubMed] [Google Scholar]
- 40.Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, Zacharasiewicz AS, Turner J, Barnathan ES, Kon OM, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med 2006;174:753–762. [DOI] [PubMed] [Google Scholar]
- 41.Ito K, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, Barnes PJ. A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci USA 2002;99:8921–8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maneechotesuwan K, Essilfie-Quaye S, Meah S, Kelly C, Kharitonov SA, Adcock IM, Barnes PJ. Formoterol attenuates neutrophilic airway inflammation in asthma. Chest 2005;128:1936–1942. [DOI] [PubMed] [Google Scholar]