Abstract
Rationale: Accurate pleural fluid pH and glucose measurement is a key component in the diagnosis and management of patients with pleural effusion. Standardized methods of pleural fluid collection have not been defined.
Objectives: To assess the effect of common clinical factors that may distort measurement accuracy of pleural fluid pH and glucose.
Methods: Ninety-two exudative pleural aspirates were collected in commercially available blood gas syringes.
Measurements and Main Results: Samples were analyzed immediately using a blood gas analyzer. The effects of residual air, lidocaine, heparin, and delay in analysis (24 h) on pH and glucose measurement accuracy were assessed. Pleural fluid pH was significantly increased by residual air (mean ± SD, 0.08 ± 0.07; 95% confidence interval [CI], 0.06 to 0.09; P < 0.001) and significantly decreased by residual lidocaine (0.2 ml; mean change in pH, −0.15 ± 0.09; 95% CI, −0.13 to −0.18; P < 0.001) and residual heparin (mean change in pH, −0.02 ± 0.05; 95% CI, −0.01 to −0.04; P = 0.027). Pleural fluid pH was stable at room temperature for 1 hour and significantly increased at 4 (mean ± SD, 0.03 ± 0.07; 95% CI, 0.01 to 0.04; P = 0.003) and 24 hours (0.05 ± 0.12; 95% CI, 0.03 to 0.08; P < 0.001). Pleural fluid glucose concentration was not clinically significantly altered by residual air, lidocaine (up to 0.4 ml), or 24-hour analysis delay.
Conclusions: Accuracy of measured pleural pH is critically dependent on sample collection method. Residual air, lidocaine, and analysis delay significantly alter pH and may impact on clinical management. Pleural fluid glucose concentration is not significantly influenced by these factors. Protocols defining appropriate sampling and analysis methods are needed.
Keywords: pleural fluid, pH, glucose, measurement
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Pleural fluid pH and glucose measurement are important components in managing patients with pleural disease. A standardized method of accurate pleural pH measurement has not been defined.
What This Study Adds to the Field
This study demonstrates that the accuracy of pleural fluid pH measurement (but less so with glucose) is critically dependent on sample collection and handling and influenced by variations likely to occur in clinical practice.
Approximately 300,000 pleural effusions require diagnostic assessment each year in the United States and United Kingdom (1, 2). Near patient testing of pleural fluid pH is an important component of this assessment for the diagnosis of pleural infection (including tuberculosis), rheumatoid pleural effusion, and esophageal rupture (3), and the prognostic assessment of malignant effusion (1, 4–7). Measurement of pleural fluid pH is advocated in the literature and in management guidelines from professional societies (including the American and British Thoracic Societies [3, 8–11]) as part of first-line pleural fluid analysis.
A low pleural fluid pH implies a high metabolic activity in the pleural space. A reduced pH (typically <7.20 [12]) has been shown to be a robust marker for instituting intercostal tube drainage in patients with suspected pleural infection (13, 14), and is used as an entry criterion for clinical studies of pleural infection (15, 16). In malignant pleural effusion, low pleural fluid pH correlates with a poor prognosis (7) and predicts pleurodesis failure (6). To maximize diagnostic accuracy in individual patients requires that any measurement inaccuracies that cancel out in larger samples be minimized. Previous studies advocate pH measurement using a blood gas analyzer (17), within 1–2 hours (18, 19) of collection, but beyond this there have been no systematic studies to define the sampling algorithm that best reduces variation during measurement, and there is likely to be substantial variation in collection methods (20). There have been no comparisons of the effects of such sampling strategies on pleural fluid glucose, which closely reflects pleural fluid pH (21) and which is advocated as a suitable substitute for pH measurement (12).
We hypothesized that pleural fluid pH and/or glucose may be significantly altered by commonly observed variations in collecting practice. Admixture of fluid with air, residual lidocaine or heparin (which are of acidic pH), or analysis delay could produce clinically important changes in measured pH, with implications for patient care. Equally, omission of heparin could allow fibrin clot formation, risking blood gas analyzer damage, or distorted analysis results. This study was designed to describe the effects of these factors.
METHODS
Inclusion Criteria
Consecutive patients presenting to a tertiary referral respiratory center with a pleural effusion requiring thoracentesis, chest drain insertion, or thoracoscopy were included between October 2006 and September 2007. To increase representation of samples from patients with suspected pleural infection (in whom the pleural fluid pH is particularly important), patients were specifically recruited with suspected parapneumonic pleural effusions for a 2-month period (February to April 2008, 12 further cases added).
Exclusion Criteria
If sufficient pleural fluid beyond the requirement for clinical investigations was not available, the patient was excluded from the study. The presence of frankly purulent pleural fluid was considered a contraindication to blood gas machine analysis.
Pleural Fluid Collection
Pleural fluid was collected using a 20-ml syringe and 21-gauge needle before other pleural procedures and before the administration of supplementary oxygen or sedation. Where local anesthetic was given before thoracentesis, a new syringe and needle were used.
Fluid was immediately introduced into pre-prepared syringes (Portex Arterial Blood Sampling System, CE 98/79/EC; Smiths Medical International, Watford, UK), which have a maximum volume of 3 ml and contain preloaded heparin sodium (∼8 IU) as follows:
Standard: The syringe was cleared of preloaded heparin before fluid (3 ml) was drawn into the syringe. All air was expelled and an airtight seal placed on the syringe. The sample was analyzed immediately—this sample was the “standard” with which other samples were compared.
Effects of air and lidocaine: Syringes were cleared of preloaded heparin and any residual air. A fixed volume of air (1.0 ml) or 2% lidocaine (Antigen Pharmaceuticals Ltd, Roscrea, Ireland) (0.2, 0.4, or 1.0 ml) was added to the syringe. Pleural fluid was subsequently introduced into each syringe to a total volume of 3 ml. Care was taken not to agitate the syringes once air/fluid was inoculated.
Effects of heparin: Syringes were cleared of air, but not the preloaded heparin (∼0.4 mL). Pleural fluid was subsequently introduced into the syringe to a total volume of 3 ml.
Pleural fluid samples were analyzed within 10 minutes of collection by a blood gas machine (ABL 700 series; Radiometer, Copenhagen, Denmark). During each analysis, 195 μl of fluid were withdrawn for measurements of pleural fluid pH, partial pressure of O2 and CO2, and glucose concentration. The machine conducted an autocalibration protocol on a 4-hourly basis and in addition was manually calibrated by a technician three times a day according to the manufacturer's instructions.
Stability of pleural fluid pH.
The “standard” syringe was analyzed 1, 4, and 24 hours after initial analysis, during which time the sample was kept at room temperature, with air excluded from the syringe following each analysis and the syringe capped. If fibrinous clots formed within the sample over time, the syringe was gently agitated without removing the seal immediately before sample analysis.
Pleural fluid glucose analysis.
Glucose concentration in the samples were assayed using the blood gas analyzer (method as described above), and separately by standard laboratory methods (glucose hexokinase method [22, 23]); ADVIA 2400 Chemistry System; Bayer Healthcare [now Siemens, Camberley, Surrey, UK]). For the laboratory samples, pleural fluid was inoculated into two fluoride/oxalate tubes (containing 5.0 mg sodium fluoride and 4.0 mg potassium oxalate) (Vacutainer; BD Diagnostics, Oxford, UK). One tube was sent immediately to the laboratory for glucose quantification, and the second was sent 24 hours later, during which time it was kept at room temperature.
All pleural fluid samples were sent to the laboratory for lactate dehydrogenase (LDH) and total protein quantification. Additional tests (e.g., cytology, microbiology) were performed as clinically indicated.
To assess the contribution of cellular contents in pleural fluid to any observed changes in pH (e.g., release of cell metabolites), supernatant of pleural fluid was obtained by centrifugation of the fluid at 3,000 rpm for 10 minutes (5702R Centrifuge; Eppendorf, Cambridge, UK), or by passing the pleural fluid through a 0.2-μM filter—both methods serving to remove cellular material from the sample. The supernatants and paired untreated pleural fluid samples (i.e., not centrifuged or filtered) were placed in identical containers with all air expelled, and their pH at Time 0 (just after centrifugation period) and at 24 hours was measured as described above in a subset of samples.
Statistical Methods
Changes in pleural fluid parameters were compared using two tailed paired t tests. Correlations between variables were described with Pearson's correlation. All analyses were performed using a computer software program (SPSS version 12.0; SPSS, Inc., Chicago, IL). A P value less than 0.05 for a two-tailed paired t test was considered statistically significant. A pH difference of 0.05 or more and a glucose difference of 1.0 mmol/L or greater (≥18 mg/dl) were considered as thresholds of clinical significance, because this degree of change may alter clinical management. Results are presented as mean ± SD, unless otherwise specified.
RESULTS
A total of 92 pleural fluid samples from 81 patients were obtained. All samples were used to assess pleural fluid pH and glucose stability over time (see below). In a subset of 52 samples, the effects of exposure to air, residual lidocaine or heparin on pH, and glucose analyses were assessed.
Baseline characteristics of the 92 samples are presented in Table 1. Mean pleural fluid pH at baseline was 7.27 ± 0.17.
TABLE 1.
BASELINE CHARACTERISTICS OF THE PATIENTS AND PLEURAL FLUID SAMPLES
| Parameter | Measurement |
|---|---|
| Demographics | |
| Age, yr (SD) | 68 (15) |
| Male:female, % | 65:35 |
| Diagnoses of samples | |
| Malignant | |
| Mesothelioma | 17 |
| Adenocarcinoma | 15 |
| Other | 18* |
| Total malignant | 50 |
| Benign | |
| Benign fibrous pleurisy | 13 |
| Infective causes (clinically suspected/diagnosis) | 26 |
| Parapneumonic effusions | 21 |
| TB pleural effusions | 5 |
| Other | 3 |
| Total benign | 42 |
| Pleural fluid baseline characteristics | |
| Protein, g/L (SD) | 42 (11) |
| Glucose, mmol/L (SD) | 4.4 (2.7) |
| LDH, IU/L (SD) | 530 (1,168) |
| pH (SD) | 7.27 (0.17) |
| Po2 (SD) | 10.16 (4.61) kPa, 76.2 (34.6) mm Hg |
| Pco2 (SD) | 7.12 (2.76) kPa, 53.4 (20.7) mm Hg |
| Procedure, n (%) | |
| Thoracoscopy | 48 (52) |
| Thoracentesis | 18 (20) |
| Drain insertion | 26 (28) |
Definition of abbreviation: LDH = lactate dehydrogenase.
Other cancer diagnoses included squamous cell carcinoma, melanoma, small cell lung carcinoma, angiosarcoma, clear cell carcinoma, and spindle cell carcinoma.
Presence of Air on pH
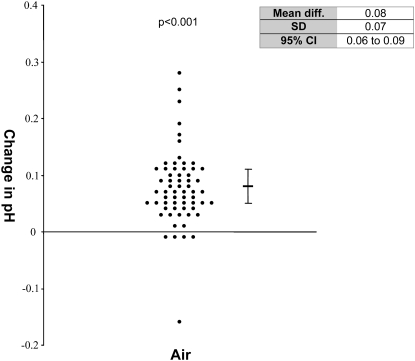
The presence of air significantly increased pleural fluid pH by 0.08 ± 0.07 (95% confidence interval [CI], 0.06 to 0.09; P < 0.001) (Figure 1). In 71% of samples, presence of air in the collection syringe resulted in a clinically significant change in pleural fluid pH of greater than 0.05 (Table 2). The change in pH was not related to the baseline pH (data not shown). Change in CO2 and change in pH were correlated in each air-exposed sample, giving a Pearson's correlation coefficient of −0.42 (P = 0.001).
Figure 1.
Effect of the addition of air on pleural fluid pH. Change in pH from baseline is shown. Mean and 95% confidence intervals (95% CI) are shown. P values represent the paired t test compared with pH of pleural fluid at baseline without added air.
TABLE 2.
PROPORTION OF SAMPLES THAT ALTER MORE THAN THE PRESPECIFIED CLINICALLY SIGNIFICANT THRESHOLD BY COLLECTION/HANDLING PARAMETER
| Proportion of Samples Altered by More than the Clinically Significant Threshold* (%)
|
||
|---|---|---|
| Collection/Handling Variation | pH | Glucose |
| Presence of air | 71 | 0 |
| Lidocaine | ||
| 0.2 ml | 94 | 9 |
| 0.4 ml | 96 | 19 |
| 1.0 ml | 100 | 52 |
| Heparin | 21 | 31 |
| Analysis delay | ||
| 1 h | 13 | 0 |
| 4 h | 27 | 0 |
| 24 h | 68 | 6 |
0.05 for pH and 1.0 mmol/L for glucose concentration.
Presence of Residual Lidocaine on pH
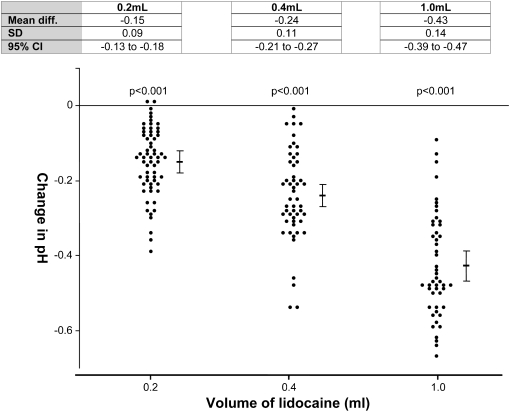
The pH of lidocaine used was 5.40 ± 0.05 (average of three independent measurements). A significant reduction in pleural fluid pH was observed in the presence of even minimal volumes (0.2 ml) of residual lidocaine (mean change of pH, −0.15 ± 0.09; 95% CI, −0.13 to −0.18; P < 0.001). A dose-dependent reduction of pH with lidocaine was observed with 0.4 ml lidocaine (mean change of pH, −0.24 ± 0.11; 95% CI, −0.21 to −0.27; P < 0.001) and with 1.0 ml lidocaine (mean change of pH, −0.43 ± 0.14; 95% CI, −0.39 to −0.47; P < 0.001) (Figure 2).
Figure 2.
Effect of the addition of 2% lidocaine on pleural fluid pH. Change in pH from baseline is shown with increasing volumes of lidocaine. Mean and 95% confidence intervals (95% CI) are shown. P values represent the paired t test compared with pH of pleural fluid pH at baseline with no lidocaine added.
The presence of residual lidocaine induced a clinical significant reduction in pH in nearly all samples: 94, 96, and 100% of samples in the presence of 0.2, 0.4, and 1.0 ml of lidocaine, respectively (Table 2). The change in pH in cases of suspected pleural infection were not different from those with other diagnoses (Table 3).
TABLE 3.
ANALYSIS OF CHANGE OF pH IN PATIENTS WITH SUSPECTED PLEURAL INFECTION (n = 26) COMPARED WITH ALL NONINFECTIVE SAMPLES (n = 66)
| Observed Change in Parameter
|
|||||
|---|---|---|---|---|---|
| Collection/Handling Variation | Noninfective
|
Suspected Infection
|
|||
| Mean Difference | 95% CI | Mean Difference | 95% CI | P Value* | |
| Presence of air | 0.08 | 0.06 to 0.09 | 0.07 | 0.02 to 0.12 | 0.62 |
| Lidocaine | |||||
| 0.2 ml | −0.16 | −0.13 to −0.19 | −0.13 | −0.10 to −0.18 | 0.38 |
| 0.4 ml | −0.25 | −0.22 to −0.29 | −0.21 | −0.15 to −0.28 | 0.21 |
| 1.0 ml | −0.45 | −0.40 to −0.49 | −0.39 | −0.31 to −0.48 | 0.20 |
| Heparin | −0.14 | −0.00 to 0.03 | −0.04 | 0.00 to −0.07 | 0.18 |
| Analysis delay | |||||
| 1 h | 0.01 | 0.01 to 0.03 | 0.02 | 0.01 to 0.03 | 0.39 |
| 4 h | 0.02 | 0.01 to 0.04 | 0.04 | 0.02 to 0.06 | 0.20 |
| 24 h | 0.05 | 0.01 to 0.08 | 0.08 | 0.03 to 0.12 | 0.28 |
Definition of abbreviation: CI = confidence interval.
P value represents significance of unpaired t test comparing change between infected and non-infected samples in each case.
Presence of Heparin on pH
Retaining preloaded heparin within the blood gas syringe resulted in a small but statistically significant decrease in pH (mean change of pH, −0.02 ± 0.05; CI, −0.01 to −0.04; P = 0.027) (Figure 3). The pH of the heparin in the blood gas syringes was 6.49 ± 0.16 (based on three independent measurements).
Figure 3.
Effect of the addition of heparin on pleural fluid pH. Change in pH from baseline is shown. Mean and 95% confidence intervals (95% CI) are shown. P values represent the paired t test compared with pH of pleural fluid at baseline with heparin added.
Effect of Delayed Analysis on pH
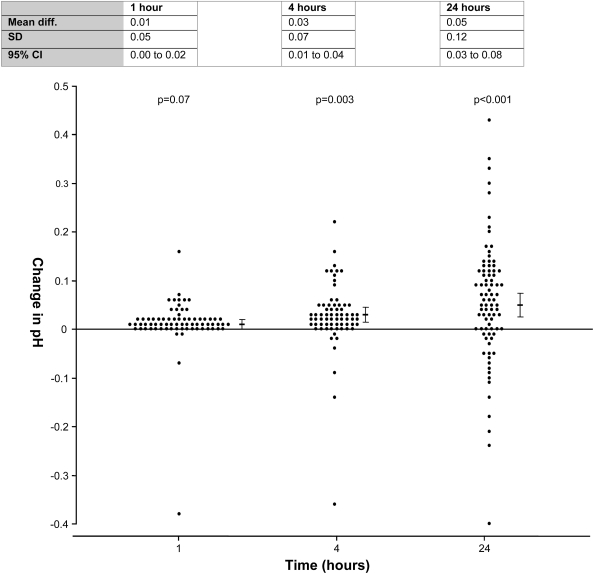
Pleural fluid pH measurements changed significantly from baseline over time, and the direction of change (increase or decrease) was variable among samples, although an overall rise in pH over time was observed. Statistically, no significant mean difference was observed in pleural fluid pH measured at baseline and at 1 hour (mean change in pH from baseline, 0.01 ± 0.05; 95% CI, −0.00 to 0.02; P = 0.07). The changes in pH became significant at 4 and 24 hours (4 h: mean change in pH, 0.03 ± 0.07; 95% CI, 0.01 to 0.04; P = 0.003; 24 h: 0.05 ± 0.12; 95% CI, 0.03 to 0.08; P < 0.001) (Figure 4). At 1 hour, 13% of samples were altered beyond the clinically significant limit (>0.05), compared with 26% at 4 hours and 68% at 24 hours (Table 2). Change in pH was plotted against baseline pH, and no significant correlation was demonstrated.
Figure 4.
Pleural fluid pH stability over time. Change in pH from baseline is shown at different time points. Mean and 95% confidence intervals are shown (95% CI). P values represent the paired t test compared with pleural fluid pH at Time 0.
The Po2 level in samples showed a consistent increase in samples over time (Po2 rise after 1 h: 3.20 ± 2.75 kPa [24.0 ± 20.6 mm Hg]; 4 h: 3.98 ± 3.76 kPa [29.9 ± 28.2 mm Hg]; 24 h: 5.25 ± 5.55 kPa [39.4 ± 41.6 mm Hg]).
pH and Pco2
To investigate possible mechanisms of pH change over time, the correlation between change in pH and change in Pco2 within the same sample was assessed. There was a significant negative correlation between change in pH and change in Pco2 at each time point, and greater correlation was seen with increased time. The Pearson's correlation coefficients at 1, 4, and 24 hours were −0.68 (95% CI, −0.52 to −0.79), −0.79 (95% CI, −0.67 to −0.86), and −0.91 (95% CI, −0.86 to −0.94), respectively (P < 0.001 for all analyses), between the change in CO2 and change in pH.
To assess the contribution of cellular content (e.g., via metabolism or release of metabolic products) to changes in pleural fluid pH, pleural fluid samples were centrifuged or filtered to remove cells, and were compared with a paired untreated sample at both 0 and 24 hours. The sample centrifugation or filtration process resulted in a significant change in pleural fluid pH compared with untreated samples (mean difference for both = 0.06), and precluded further analysis.
Glucose Measurement by Blood Gas Analyzer
Glucose values were measured using both blood gas analyzer and a laboratory (glucose hexokinase [22, 23]) method at Time 0. There was little difference between laboratory-measured and blood gas–analyzed glucose concentration (mean difference, 0.29 mmol/L [5.22 mg/dl]; SD, 0.34 mmol/L; 95% CI, 0.16 to 0.41 mmol/L) with a close correlation (Pearson's correlation coefficient, 0.993; P < 0.001) and a line of linearity defined by the following equation: blood gas machine value = 0.96 × laboratory measured value.
Some of the minor variation observed between the two measurements is likely to be due to rounding up of results below 1.0 mmol/L to 1.0 in the lab sample, which did not occur with the blood gas machine.
Presence of Air, Lidocaine, and Heparin on Pleural Fluid Glucose Concentration
Glucose concentration was increased slightly but not significantly by the presence of air (mean difference, 0.05 mmol/L [0.9 mg/dl]; SD, 0.18 mmol/L; 95% CI, −0.01 to 0.10; P = 0.11) (Figure 5A).
Figure 5.
(A–D) Effect of variables on pleural fluid glucose. The graph represents change in individual samples. P values represent the paired t test compared with baseline pleural fluid glucose at Time 0. A single result has been removed from the 0.2-ml 2% lidocaine graph due to aberrant values.
The addition of small volumes of lidocaine (0.2 ml) caused a statistically but not clinically significant decrease in the pleural fluid glucose concentration (mean difference, −0.41 mmol/L [10.8 mg/dl]; SD, 0.60 mmol/L; 95% CI, −0.24 to −0.60; P < 0.001) (Figure 5B). More pronounced reductions in glucose concentration were seen with larger volumes of lidocaine, and were likely due to dilution effect (0.4 ml lidocaine: mean difference, −0.51 mmol/L [9.18 mg/dl]; SD, 0.49 mmol/L; 95% CI, −0.33 to −0.68; P < 0.001; 1.0 ml lidocaine: mean difference, −1.19 mmol/L [21.4 mg/dl]; SD, 0.99 mmol/L; 95% CI, −0.83 to −1.56; P < 0.001).
The addition of heparin caused a statistically but not clinically significant decrease in pleural fluid glucose concentration (mean difference, −0.66 mmol/L [11.88 mg/dl]; SD, 0.52 mmol/L; 95% CI, −0.47 to −0.85; P < 0.001) (Figure 5C). Comparable reductions in glucose concentrations with similar volumes of heparin and lidocaine were observed, suggesting that the observed reduction in glucose concentration was the result of dilution.
Effect of Delayed Analysis on Glucose Measurements
Stability of glucose concentrations over time was assessed using both the stored oxalate tube (lab analyzed) and the sealed arterial blood gas syringe (machine analyzed) kept at room temperature for 24 hours. Syringe-stored pleural fluid glucose levels did not significantly change over 1 and 4 hours (mean difference from baseline after 1 h: −0.03 mmol/L [0.54 mg/dl]; SD, 0.08 mmol/L; 95% CI, −0.01 to 0.06; P = 0.11; after 4 h: −0.05 mmol/L [0.9 mg/dl]; SD, 0.09 mmol/L; −0.00 to −0.10; P = 0.07). At 24 hours there was a statistically but not clinically significant decrease in glucose concentration (mean difference, −0.24 mmol/L [4.32 mg/dl]; SD, 0.31 mmol/L; 95% CI −0.13 to −0.35; P < 0.001) (Figure 5D). The proportion of samples altering by the prespecified clinically significant limit was 0, 0, and 6% at 1, 4, and 24 hours, respectively (Table 2).
Measurements of pleural fluid glucose stored in oxalate tubes for 24 hours were highly consistent with those analyzed immediately (mean difference, −0.03 mmol/L [0.54 mg/dl]; SD, 0.16 mmol/L; 95% CI, −0.08 to 0.03; P = 0.33).
DISCUSSION
This study has demonstrated that the accuracy of measured pleural pH is critically dependent on the method of sample collection. There is currently no standardized protocol for pleural fluid collection for pH/glucose measurement and it is therefore likely that the factors studied here are common influences on measurement in day-to-day clinical practice. These data demonstrate that presence of air or residual lidocaine in the collection syringe results in clinically significant changes in pH. Heparin produces a statistically detectable pH change that is of little clinical importance. In contrast, pleural fluid glucose measurements, analyzed by either blood gas machine or conventional laboratory assays, are less susceptible to variations in collection practice or delay in analysis.
These data are the first to show that even small amounts of air in the pleural fluid collection syringe result in a significant increase in pleural fluid pH. This finding applies to all samples, regardless of the baseline pH, and shows that air in pleural fluid is capable of artificially elevating the pH sufficiently to change clinical management—for example, by deferring the drainage of an acidic parapneumonic effusion. The mechanism of the increase in pH with air has not been explored. We hypothesize that the gradient between the partial pressures of CO2 in pleural fluid (mean Pco2: 7.12 kPa, 53.4 mm Hg, in our samples) and atmospheric air results in rapid CO2 diffusion through the fluid–air interface, raising the pH.
Lidocaine, administered as a local anesthetic before a pleural procedure, appears clinically important in its potential effect on pH measurement. Even the presence of a minute amount of residual lidocaine (0.2 ml), compatible with the dead space of fine-bore needles, causes a clinically significant drop in pH in 94% of samples, and this effect becomes more marked in a dose-dependent manner. This helps explain previous data showing reduction in pleural fluid pH in samples collected after infiltration of intercostal tissue with a local anesthetic (24).
Heparin in the sample produces a statistically significant, but clinically insignificant change in pH. This finding is in contrast with one previous smaller study (25). Fibrinous clots often develop in pleural fluid samples and addition of a small volume of anticoagulant (e.g., heparin) to thoracentesis specimens, to avoid clotting before laboratory analysis, has been advocated, especially for leukocyte differential counts (18). Commercially available blood gas syringes, which are commonly used for pleural fluid analysis, are often preheparinized. The presented results confirm that heparin is acidic (pH 6.49) and the preloaded heparin in commercially prepared blood gas syringes produces a statistically significant reduction in pH. Reassuringly, this change was not clinically significant.
Delay after sample collection does not seem to substantially alter measured pH over 1 hour, although delays of 4 hours or longer produce clinically significant alterations. A delay in analysis of 24 hours produced clinically significant inaccuracies in pH readings in over two-thirds of samples. A close correlation was observed between increase in pH and decrease in pleural fluid Pco2 over 24 hours. This may suggest that the mechanism of pH change is related to CO2 diffusing out of pleural fluid with time. The Po2 level consistently increases in these samples over 24 hours, supporting the suggestion that constant gas exchange occurs between atmospheric air and pleural fluid, even in capped containers. Changes in pH over time may also be a result of metabolism of cells or bacteria (in infected samples).
The pH and glucose levels in pleural fluids are strongly correlated (21). The presented data show that pleural fluid glucose measurements are less susceptible to inaccuracy induced by the presence of air, lidocaine, or heparin, except where the volume of additive is sufficient to cause a dilutional effect.
This study highlights the need for a standardized protocol for pleural pH sampling and analysis, both for clinical practice and for future research studies. Most existing literature on pleural pH did not specify details of its measurement, which may explain some of the conflicting data in this field. Clinicians must realize that, although existing literature/guidelines often use definitive thresholds of pH to guide management (e.g., a cutoff pH of 7.20 determines need for chest tube drainage of parapneumonic effusions), measurements of pH are vulnerable to variations in processing protocols. This adds to recent observations that pH may vary in different locules within a septated pleural effusion (26). Decisions on clinical management should not be governed by pleural pH alone, and must be made in conjunction with information from other investigations.
There are limitations to this study. No data exist on what is the “gold standard” of pleural pH measurement, and we compared samples with an assumed “standard” collection (samples analyzed immediately with blood gas machine in the absence of air and other additives).
In conclusion, pleural fluid pH measurements are subject to substantial variability during collection, which could be minimized by the systematic application of a simple collection protocol. Pleural fluid should be collected avoiding inclusion of air or other additives with the sample—particularly lidocaine—and analyzed in less than 1 hour using a blood gas analyzer. Pleural fluid glucose is an alternative to pH and its measurements are less vulnerable to changes in collection method.
Supported by a Medical Research Council Training Fellowship (N.M.R.), the British Lung Foundation (H.E.D.), and the Medical Research Council (Y.C.G.L.).
Originally Published in Press as DOI: 10.1164/rccm.200801-062OC on June 12, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Light RW, Lee YC. Textbook of pleural diseases, 2nd ed. London: Arnold Press; 2008.
- 2.Light RW. Pleural diseases, 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2007.
- 3.Maskell NA, Butland RJ. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003;58:ii8–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 2000;117:79–86. [DOI] [PubMed] [Google Scholar]
- 5.Heffner JE, Heffner JN, Brown LK. Multilevel and continuous pleural fluid pH likelihood ratios for evaluating malignant pleural effusions. Chest 2003;123:1887–1894. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Panadero F, Lopez MJ. Low glucose and pH levels in malignant pleural effusions: diagnostic significance and prognostic value in respect to pleurodesis. Am Rev Respir Dis 1989;139:663–667. [DOI] [PubMed] [Google Scholar]
- 7.Sahn SA, Good JT Jr. Pleural fluid pH in malignant effusions: diagnostic, prognostic, and therapeutic implications. Ann Intern Med 1988;108:345–349. [DOI] [PubMed] [Google Scholar]
- 8.Antony VB, Loddenkemper R, Astoul P, Boutin C, Goldstraw P, Hott J, Rodriguez PF, Sahn SA. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987–2001. [DOI] [PubMed] [Google Scholar]
- 9.Antony VB, Loddenkemper R, Astoul P, Boutin C, Goldstraw P, Hott J, Rodriguez PF, Sahn SA. Management of malignant pleural effusions. Eur Respir J 2001;18:402–419. [DOI] [PubMed] [Google Scholar]
- 10.Antunes G, Neville E, Duffy J, Ali N. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58:ii29–ii38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies CW, Gleeson FV, Davies RJ. BTS guidelines for the management of pleural infection. Thorax 2003;58:ii18–ii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heffner JE, Brown LK, Barbieri C, DeLeo JM. Pleural fluid chemical analysis in parapneumonic effusions: a meta-analysis. Am J Respir Crit Care Med 1995;151:1700–1708. [DOI] [PubMed] [Google Scholar]
- 13.Light RW, MacGregor MI, Ball WC Jr, Luchsinger PC. Diagnostic significance of pleural fluid pH and PCO2. Chest 1973;64:591–596. [DOI] [PubMed] [Google Scholar]
- 14.Light RW, Girard WM, Jenkinson SG, George RB. Parapneumonic effusions. Am J Med 1980;69:507–512. [DOI] [PubMed] [Google Scholar]
- 15.Maskell NA, Batt S, Hedley EL, Davies CW, Gillespie SH, Davies RJ. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 2006;174:817–823. [DOI] [PubMed] [Google Scholar]
- 16.Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, Gabe R, Rees GL, Peto TE, Woodhead MA, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865–874. [DOI] [PubMed] [Google Scholar]
- 17.Cheng DS, Rodriguez RM, Rogers J, Wagster M, Starnes DL, Light RW. Comparison of pleural fluid pH values obtained using blood gas machine, pH meter, and pH indicator strip. Chest 1998;114:1368–1372. [DOI] [PubMed] [Google Scholar]
- 18.Sarodia BD, Goldstein LS, Laskowski DM, Mehta AC, Arroliga AC. Does pleural fluid pH change significantly at room temperature during the first hour following thoracentesis? Chest 2000;117:1043–1048. [DOI] [PubMed] [Google Scholar]
- 19.Haro-Estarriol M, Baldo-Padro X, Lora-Diez M, Rubio-Garay M, Rubio-Goday M, Sebastian-Quetglas F. Changes in the acid-base equilibrium of pleural fluid during the first 2 hours after thoracentesis [in Spanish]. Arch Bronconeumol 2005;41:612–617. [DOI] [PubMed] [Google Scholar]
- 20.Ross Hill A. Avoiding air in pleural fluid pH. Chest 1998;113:1729. [DOI] [PubMed] [Google Scholar]
- 21.Potts DE, Taryle DA, Sahn SA. The glucose-pH relationship in parapneumonic effusions. Arch Intern Med 1978;138:1378–1380. [PubMed] [Google Scholar]
- 22.Tietz NW. Clinical guide to laboratory tests, 3rd ed. Philadelphia: W.B. Saunders; 1995. pp. 268−269.
- 23.Tietz NW. Textbook of clinical chemistry, 3rd ed. Philadelphia: W.B. Saunders; 1999. pp. 777–778.
- 24.Jimenez CD, Diaz G, Perez-Rodriguez E, Prieto E, Yusen RD. Modification of pleural fluid pH by local anesthesia. Chest 1999;116:399–402. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein LS, McCarthy K, Mehta AC, Arroliga AC. Is direct collection of pleural fluid into a heparinized syringe important for determination of pleural pH? A brief report. Chest 1997;112:707–708. [DOI] [PubMed] [Google Scholar]
- 26.Maskell NA, Gleeson FV, Darby M, Davies RJ. Diagnostically significant variations in pleural fluid pH in loculated parapneumonic effusions. Chest 2004;126:2022–2024. [DOI] [PubMed] [Google Scholar]