Abstract
Rationale: Overproduction of mucus is a contributory factor in the progression of chronic obstructive pulmonary disease (COPD). The polymeric mucins are major macromolecules in the secretion. Therefore, we hypothesized that the polymeric mucin composition or properties may be different in the sputum from individuals with COPD and smokers without airflow obstruction.
Objectives: To determine the major polymeric mucins in COPD sputum and whether these are different in the sputum from individuals with COPD compared with that from smokers without airflow obstruction.
Methods: The polymeric mucin composition of sputum from patients with COPD and smokers without airflow obstruction was analyzed by Western blotting analysis. The tissue localization of the mucins was determined by immunohistochemistry, and their size distribution was analyzed by rate–zonal centrifugation.
Measurements and Main Results: MUC5AC and MUC5B were the major mucins. MUC5AC was the predominant mucin in the smoker group, whereas MUC5B was more abundant from the patients with COPD, with a significant difference in the ratio of MUC5B to MUC5AC (P = 0.004); this ratio was correlated with FEV1 in the COPD group (r = 0.63; P = 0.01). The lower-charged glycosylated form of MUC5B was more predominant in COPD (P = 0.012). No significant associations were observed with respect to sex, age, or pack-year history. In both groups, MUC5AC was produced by surface epithelial cells and MUC5B by submucosal gland cells. Finally, there was a shift toward smaller mucins in the COPD group.
Conclusions: Our data indicate that there are differences in mucin amounts and properties between smokers with and without COPD. Further studies are needed to examine how this may impact disease progression.
Keywords: chronic obstructive pulmonary disease, mucus, mucin, pathophysiology
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Smoking is a major risk factor in the development of chronic obstructive pulmonary disease (COPD). Chronic hypersecretion of mucin is thought to contribute to the progression of disease. However, little is known about the mucin composition of mucus in COPD.
What This Study Adds to the Field
We show that MUC5AC was the predominant mucin in smokers without airflow obstruction, whereas MUC5B was more abundant from the patients with COPD. Furthermore, there was a shift towards smaller mucins in the COPD group.
Chronic obstructive pulmonary disease (COPD) is a major cause of illness and death throughout the world, with approximately 2.7 million deaths attributed to COPD each year (1). Smoking is a major risk factor in the development of COPD; however, not all smokers go on to develop COPD (2, 3). One of the features of COPD is chronic hypersecretion of mucus, and this mucus is thought to contribute to the progression of disease and eventually contribute to morbidity (4). Surprisingly little is known about the composition of mucus in COPD. Mucus is composed mostly of water, mucins, other proteins, and salt. The correct balance of these components is essential for the protective function of the mucus layer. Mucins are a large group of glycoproteins (17 members identified to date) split into two subcategories: secreted mucins and membrane-tethered mucins, which are both characterized by a high substitution with O-glycans (5, 6). A subgroup of the secreted mucins, MUC2, MUC5AC, MUC5B, MUC6, and MUC19, are known as gel-forming or polymeric mucins, and are essential for mucus gel formation (5). In the airways, MUC2, MUC5AC, MUC5B, and MUC19 mRNA expression has been reported (7–9).
Respiratory mucus contains MUC5AC and MUC5B, and these polymeric glycoproteins, 5–50 MDa, are believed to be the main contributors to the biophysical properties of the mucus in the healthy airway (5, 10, 11). However, in hypersecretory disease, other macromolecules also contribute to the physical properties of the gel (12–14). Previous studies have shown that airways mucus is a heterogeneous mixture of these two glycoproteins, and their relative amounts are different in sputum from healthy and diseased airways (11). More recently, variation in MUC5AC and MUC5B mucin gene expression and the amount of intracellular stores of these two mucins has been demonstrated within the surface epithelium of nonsmokers and smokers with or without airflow obstruction (15–17). No data were presented on the amounts of these mucins stored within mucous cells in the submucosal glands or, more importantly, on their amounts in the secreted mucus gel that may obstruct the airways. However, based on immunoreactivity, it was suggested that MUC5B was the predominant mucin in the intraluminal mucus from patients with COPD (15).
At present it is not known how the mucin composition of mucus affects development and progression of disease. Change in mucin composition may alter the transport properties of mucus, and it is possible that different mucins retain bacteria and other antigens differently in the airways, thus affecting immune response in the airways. We therefore hypothesized that differences in mucin composition and properties may determine risk of COPD, and, in this study, our aim was to investigate whether mucin composition and properties of the sputum from smokers with no airflow obstruction is different from that found in patients with COPD.
METHODS
Study Subjects
Fifteen patients with COPD, diagnosed according to current Global Initiative for Chronic Obstructive Lung Disease guidelines based on clinical history and spirometric criteria (FEV1/FVC ratio < 0.7), were recruited. We also sampled a group of 17 smokers with no history of respiratory disease and no airflow obstruction. Characteristics of patients with COPD and smoker control subjects are shown in Table 1. Subjects were excluded if they had experienced a respiratory tract infection or exacerbation of COPD in the preceding 6 weeks. All subjects provided written informed consent, and the local ethics committee approved the study.
TABLE 1.
BASELINE CHARACTERISTICS OF PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE AND SMOKING CONTROL SUBJECTS
| Characteristics | Patients with COPD (n = 15) | Smoking Control Subjects (n = 17) |
|---|---|---|
| Median age (range), yr | 67 (58–81) | 56 (43–70) |
| Male/female ratio | 13/2 | 8/9 |
| Current smokers, % | 40 | 100 |
| Median pack-years (range) | 37 (12–73) | 32 (12–55) |
| Median post-bronchodilator FEV1 % predicted (range) | 66 (45–92) | 97 (85–127) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Sputum Collection
Saliva was collected and then a spontaneous sputum sample was obtained (n = 23). In those subjects not able to expectorate (n = 9: three with COPD and six smokers), sputum was induced by inhalation of nebulized saline (3, 4, and 5% saline) using an ultrasonic nebulizer (Ultraneb 2000; Medix, Harlow, UK). Immediately after collection, samples were weighed and a 10× protease inhibitor cocktail added (0.1 M ethylenediaminetetraacetic acid, 1 M aminohexanoic acid, 0.05 M benzamidine, 0.1 M N-ethylmaleimide (NEM), 100 μg/ml trypsin inhibitor, 10 μg/ml each of leupeptin, aprotinin, chymostatin, pepstatin A, and antipain). Samples were then stored at 4°C before separation into two phases: samples which are able to support their own weight when separated by tweezers (“gel”) and remaining sample, which were more liquid in nature (“sol”). Samples were then frozen at −20°C until analyses were performed.
Semiquantitative Analysis of Mucins
For each subject, gel, sol, and saliva were solubilized in 6 M guanidinium chloride (GdmCl)/5 mM NEM; saliva and sol samples were solubilized in 30 vol of GdmCl and gel samples with 75 vol (in each case, the volume of GdmCl added was per weight of sample). Samples were gently rotated for 7 days at 4°C. Quantitation of polymeric, gel-forming mucins (MUC5B, MUC5AC, and MUC2) was performed as described previously (11). Briefly, aliquots of the solubilized samples (gel, sol, and saliva from each individual) were reduced, carboxymethylated, and electrophoresed alongside purified, reduced, and carboxymethylated MUC5AC, MUC5B, and MUC2 mucins of known concentrations on 0.7% (wt/vol) agarose gels. Mucin was detected after Western blotting using MAN-5BI, MAN-5ACI, and MAN-2I antisera (11). MUC5B (1.95 mg/ml used at 866, 520, 312, 208, and 156 ng) standard was purified from human saliva, MUC5AC (138 μg/ml used at 16.9, 10.5, 7.03, 4.22, and 2.81 ng) from HT29-A1 cell media, and MUC2 from human adenocarcinoma cells in culture (200 ng used at 20, 32, 42, 85, 125, and 170 ng). Purification of the standards was as described previously (11). Staining was visualized with the chromogenic substrate NBT/BCIP, and staining intensity measured by reflectance densitometry using a GS-800 calibrated densitometer (Bio-Rad, Hercules, CA).
Data Analysis
Mucin calibration curves were accepted if they fit the criteria determined previously (11). Calibration curves were used to determine the concentration of mucins in each sample, again using previously determined criteria (11). Between-group mucin concentration data were analyzed for statistical significance with a Mann-Whitney U-test using the SIMFIT program (http://www.simfit.man.ac.uk/), and correlations were analyzed using SPSS software (SPSS Inc., Chicago, IL).
Histological and Immunohistochemical Staining
Fresh lung tissues, as far distal from tumor as possible, were collected from healthy smokers and patients with COPD undergoing lung resection surgery for suspected or confirmed lung cancer. These were immediately fixed in formalin. After dehydration through a graded series of alcohol (50, 90, then 100% vol/vol) and then xylene. The samples were then embedded in wax and 4-μm sections cut. Sections from each tissue sample were stained with H&E and periodic acid Schiff reagent and alcian blue (PAS-AB; pH 2.5). For immunohistochemical staining, sections were dewaxed and microwaved for 5 minutes at 500 W in antigen retrieval solution (10 mM sodium citrate, pH 6). For staining with the EU-MUC5Bb antibody, the sections were reduced with 10 mM dithiotreitol and carboxymethylated with 25 mM iodoacetamide in Tris-HCl, pH 8; each treatment was for 30 minutes. Endogenous peroxidase activity of all sections was quenched with 3% (vol/vol) hydrogen peroxide in methanol for 30 minutes at room temperature. The tissue sections were then blocked with 10% (vol/vol) donkey serum and 1% (wt/vol) bovine serum albumin in phosphate-buffered saline (blocking solution) for 1 hour before incubation with the monoclonal antibody, EU-MUC5Bb (18), or the polyclonal antiserum, MAN5ACI, diluted 1:500 in the blocking solution for 1 hour at room temperature. Sections were then incubated for 30 minutes with biotin-labeled rabbit anti-donkey IgG or mouse anti-donkey IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), diluted 1:250 in the blocking solution, and then treated with Elite Vector stain ABC Kit (Vector Laboratories, Burlingame, CA) for 30 minutes before detection with Diaminobenzidine (Sigma, St. Louis, MO). All sections were counterstained with Harris's hematoxylin, and representative pictures were taken using an Axiovision imaging system, version 4.2, on a Zeiss microscope with a 20× objective (Carl Zeiss-Welwyn Garden City, Herts., U.K.).
Characterization of Mucin Size Distribution
The size distributions of MUC5B and MUC5AC were determined by rate–zonal centrifugation (19). In brief, solubilized gel samples were layered onto preformed 6–8 M GdmCl gradients and spun at 40,000 rpm for 2.75 hours at 15°C in an SW40 rotor (Beckman, High Wycombe, Bucks., U.K.). Tubes were emptied from the top into 24 fractions and, after transfer to nitrocellulose by slot blotting, the mucin distributions were analyzed by immunodetection using MAN-5BI and MAN-5ACI.
RESULTS
The baseline characteristics of patients are shown in Table 1. Of the 15 patients with COPD, three had stage-1, 11 stage-2, and one stage-3 COPD; eight patients were on inhaled corticosteroids. Patients with COPD were significantly older than control subjects.
Mucins Present in Sputum Samples
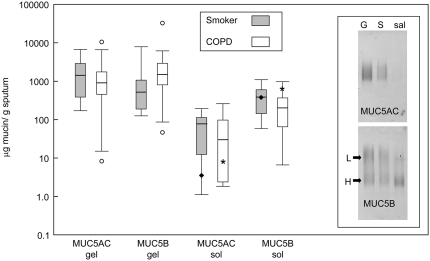
Using mucin-specific antisera, MUC5AC and MUC5B were observed in all samples; MUC2 was also present in some samples (two COPD sample and one smoker sample), but only at very low levels. Unlike MUC5AC, MUC5B showed evidence of different glycoforms (Figure 1, inset). The saliva samples contained predominantly MUC5B, with varying amounts of the two glycoforms; no MUC2 was detected.
Figure 1.
Amount of MUC5AC and MUC5B mucins in sputum and saliva collected from study subjects. The concentration of MUC5AC and MUC5B in the sol and gel phases of sputum, and saliva, were determined by quantitative Western blotting (see Methods). Data are presented as μg/g of total sputum or saliva for chronic obstructive pulmonary disease (COPD) (open bars) and smokers with no airflow obstruction (gray bars). The data are presented as box and whisker plots; the median (horizontal line inside bars) is shown, as well as a box showing the interquartile ranges (25th and 75th percentile); individual outlier points beyond the 10th and 90th percentile are indicated (open circles, only seen in patients with COPD).The asterisk and the solid diamond on the data shown for the sol indicate the median value for MUC5AC and MUC5B in the saliva from the two groups. Inset shows an example of agarose gel separation of MUC5AC and MUC5B mucins from gel (G) and sol (S) phases of sputum and saliva (sal) of a single individual. The different glycoforms of MUC5B are highlighted; low charge (L) and high charge (H). The gel shown was loaded with different amounts of sample to highlight the different mucins present and was not used for quantitative analysis.
Semiquantitative Analysis of Mucins
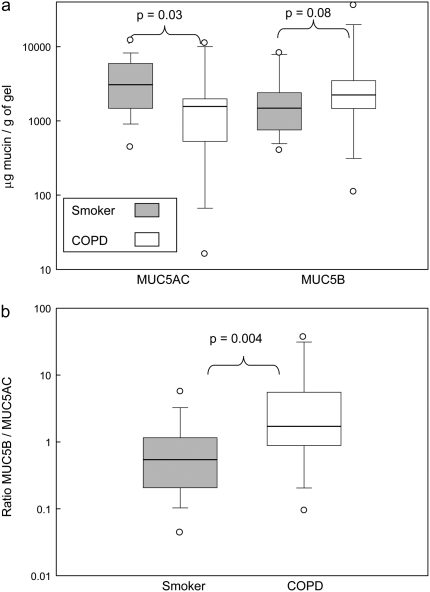
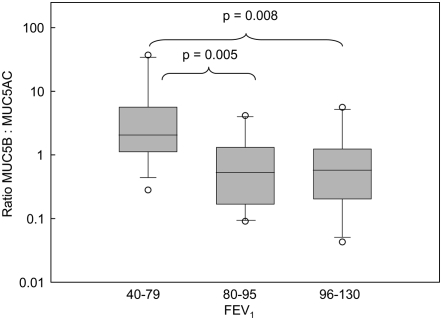
Figure 1 shows the amount of the mucins in different components of the samples (the numerical values are in Table E1 in the online supplement). The data showed no overall significant change in mucin between the two clinical groups, although there was a trend toward more MUC5B in the gel phase of COPD sputum. These data also show that the levels of MUC5B in saliva were comparable to that in the sol phase of the sputum samples. Therefore, to ensure the mucins analyzed were from the respiratory tract and not from salivary contamination, subsequent analyses were performed solely on the mucins in the gel component of the sputum. There was no significant difference between the total mucin content of the gels from the patients with COPD and the smoker control subjects (data not shown). When the data are expressed as the amount of MUC5AC and MUC5B mucins relative to the weight of gel, a difference can be seen between the two groups (Figure 2A). MUC5AC predominates in the gel phase of the sputum from the smoker group (P = 0.03), whereas MUC5B seems the more abundant mucin in gel phase of the sputum from the patients with COPD (P = 0.08) (Figure 2A). The difference is more apparent when the relative ratio of the two mucins (MUC5B:MUC5AC) in the gel phase of the sputum from the two groups is determined (P = 0.004) (Figure 2B). Moreover, when data were analyzed with respect to post-bronchodilator FEV1, sputum with increased amounts of MUC5B over MUC5AC correlates with lower FEV1 (Figure 3) and post-bronchodilator FEV1 % predicted was significantly correlated with the MUC5B/MUC5AC ratio (r = 0.63; P = 0.01). Mucin contents did not vary between current and former smokers.
Figure 2.
Amount of MUC5AC and MUC5B mucins in the gel phase of sputum collected from study subjects. The concentration of MUC5AC and MUC5B in the gel phase of sputum was determined by Western blotting (see Methods). (A) Data for each mucin are presented as μg/g of gel, and in (B) the ratio of the mucins (MUC5B/MUC5AC) is shown; COPD (open bar), smokers with no airflow obstruction (gray bar). The median (horizontal line inside bar) is shown, as well as a box showing the interquartile ranges (25th and 75th percentile); individual outlier points beyond the 10th and 90th percentile are indicated (open circles).
Figure 3.
Ratio of MUC5B to MUC5AC mucins in the gel phase as a function of FEV1. The ratio of the mucins (MUC5B/MUC5AC) in the gel phase of sputum is plotted against post-bronchodilator FEV1. The median (horizontal line inside bar) is shown as well as a box showing the interquartile ranges (25th and 75th percentile); individual outlier points beyond the 10th and 90th percentile are indicated (open circles).
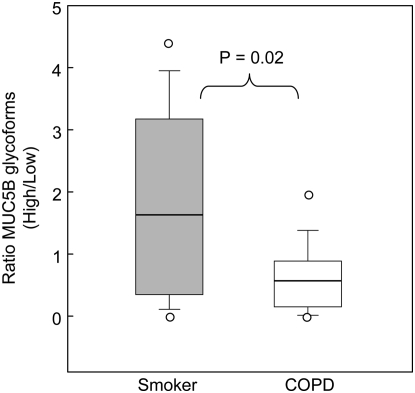
Figure 1 showed that MUC5B was present in different glycoforms. Analysis of the relative amounts of these glycoforms present in the sputum from the two groups revealed a further difference between the “healthy” smoker group and patients with COPD (Figure 4). A significant difference in the distribution of high- and low-charge glycoforms was observed; the low-charge glycoform was more predominant in patients with COPD (P = 0.02).
Figure 4.
Ratio of the glycoforms of the MUC5B mucin in the gel phase of sputum collected from study subjects. The ratio of the concentration of the MUC5B glycoforms in the gel phase of sputum was determined by Western blotting (see Methods) for COPD (open bar) and smokers with no airflow obstruction (gray bar). The median (horizontal line inside bars) is shown, as well as a box indicating the interquartile ranges (25th and 75th percentile); individual outlier points beyond the 10th and 90th percentile are indicated (open circles).
Analysis of the samples with respect to sex, age, and pack-year history showed no statistical difference; also, there were no differences between samples from patients with COPD treated with or without inhaled corticosteroids.
Mucin Localization
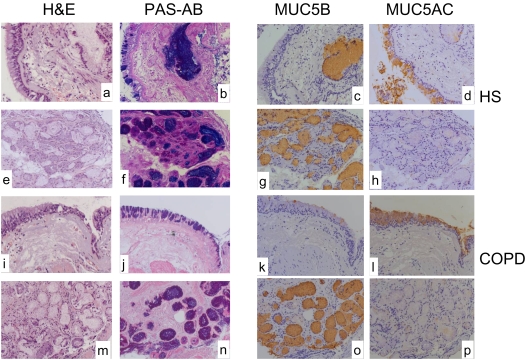
PAS-AB staining of tissue sections from healthy smokers and patients with COPD showed intense staining of cells in the epithelial surface and in the submucosal glands (Figures 5B, 5D, 5J, and 5L). In both subject groups, MUC5AC-immunoreactivity was predominantly in cells of the epithelial surface (Figures 5F and 5N), whereas MUC5B immunoreactivity was mainly in submucosal gland cells (Figures 5E, 5G, and 5O). In healthy smokers and patients with COPD some minor MUC5AC staining was observed in the submucosal glands (Figures 5H and 5P). MUC5B staining was not observed in the surface epithelium in healthy smokers (Figure 5E); in contrast, MUC5B-immunoreactivity was clearly visible in some epithelial surface cells in the patients with COPD (Figure 5M). Comparison of the PAS-AB and the immunostaining of the submucosal glands (Figure 5B, 5D, 5E, 5G, 5L, and 5O) with the H&E staining (Figures 5A, 5C, and 5I) shows that the gland lumen also contains mucins, that was predominantly MUC5B.
Figure 5.
Comparison of mucin content in bronchial biopsies from a healthy smoker and a patient with COPD. Representative sections from a healthy smoker (A–H) and a patient with COPD (I–P). Staining with H&E, PAS-alcian blue (PAS-AB), EuMUC5B (MUC5B), and MAN-5ACI (MUC5AC) at the surface epithelium (A–D and I–L), and in the submucosal glands (E–H and M–P) are shown. Sections from two other patients with COPD and one healthy smoker gave similar staining patterns.
Analysis of Mucin Properties
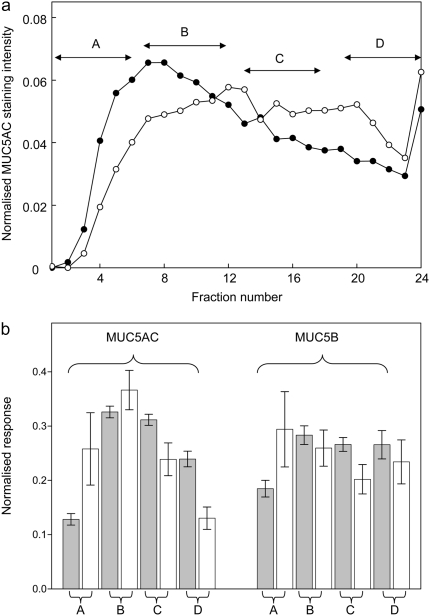
The size distribution of the mucins in the sputum samples was analyzed by measuring their sedimentation behavior by rate–zonal centrifugation (Figure 6). A typical sedimentation profile is shown in Figure 6A for MUC5AC solubilized from the sputum of an individual with COPD and a smoker. There was a difference in the size distribution of MUC5AC and MUC5B between the COPD and control group; with a shift toward smaller mucins in the COPD samples (Figure 6B). This was more evident for MUC5AC.
Figure 6.
Analysis of the size distributions of MUC5AC and MUC5B mucins in the gel phase of sputum collected from study subjects. (A) A typical example of the separation of the MUC5AC mucins in the sputum from an individual with COPD (filled circles) and a smoker (open circles) after rate–zonal centrifugation. (B) For each of the samples analyzed—smokers (n = 14) and those with COPD (n = 11)—the resultant MUC5AC and MUC5B distributions were normalized and the distribution split into four sections (fractions 1–6, section A; 7–12, section B; 13–18, section C; and 19–24, section D), representing different mucin sizes from smallest (section A) to largest (section D). The proportion of mucin in each fraction was then summed for all the smoker and COPD samples, and these data are shown for MUC5AC (left-hand bars) and MUC5B (right-hand bars). Gray bars represent smokers and open bars represent patients with COPD. Error bars represent ± 1 SEM.
DISCUSSION
Previous studies have investigated whether there is a difference in the amounts of the polymeric mucins, MUC5AC and MUC5B, in the airways of individuals with COPD and those from smokers without airway obstruction (15–17). To address this issue, these studies analyzed mucin gene expression of airways epithelial cells or the mucin content of epithelial goblet cells. No quantitative analysis of the mucins in the secreted mucus gel that may obstruct the airways was reported, nor was any information presented on the other source of mucin production: the submucosal gland mucous cells. Here, for the first time we have compared the polymeric mucins present in the sputum from individuals with COPD with those from smokers without airway obstruction. MUC5AC and MUC5B are the major polymeric mucins in these sputum samples and their relative levels showed a significant difference between the two groups. MUC5B was more prominent in the COPD sputum, and increasing levels of this mucin correlated with a lower level of lung function. We have previously reported that MUC5B was the major polymeric mucin in purified mucin preparations from COPD sputum (11). However, in that study, we did not control for salivary contamination (see subsequent text), and there were no patient details, such as FEV1, age, pack-years, or current medication.
At first glance, our compositional data are at odds with the mucin gene expression and immunohistochemical data that have indicated an increase in MUC5AC (15–17) and a decrease in MUC5B (16) in the epithelial cells in COPD airways. However, these studies did not analyze mucin production by submucosal glands, which have been shown to undergo hypertrophy in COPD. Our immunohistochemical data indicate that MUC5B is mainly being produced by submucosal glands, and thus we suggest that, with increasing severity of disease, the glandular secretions are making a greater contribution to the mucin composition of the gel. In support of this, immunoreactivity of intraluminal mucus in patients with COPD suggested that MUC5B was the predominant mucin (15).
In the two groups, smokers without airway obstruction and COPD, MUC5AC is present at levels above that found previously in induced sputum from healthy individuals (11). These data are consistent with the reported increase in MUC5AC mRNA expression and stored mucin in the airways of smokers with airflow obstruction, and increased MUC5AC correlated with FEV1 (16). Here we show that increasing amounts of MUC5B in sputum, in conjunction with already high levels of MUC5AC, correlates with level of FEV1. Moreover, the lower-charged glycoform of MUC5B is increased in the COPD sputum. This is in agreement with previous analyses of MUC5B isolated from COPD sputum, which demonstrated a decrease in “acidity” of the mucin compared with that from normal subjects (11, 20). Whether this is due to a gross change in glycosylation machinery of MUC5B-secreting cells or production from a specific population of MUC5B-secreting cells is not currently known. However, immunohistochemical data using a carbohydrate-specific probe suggest that the different MUC5B glycoforms may be the products of different cells (21).
How the changes in mucin composition of sputum relate to the pathobiology of COPD is not known. One might speculate that the increased levels of mucin alone might alter the transport properties of the mucus gel, resulting in reduced lung function and increased incidence of infection. On top of this, the change in glycosylation (increased amounts of low-charge MUC5B) could also affect the protective properties of the gel. For example, low–charge density MUC5B mucins might form a tighter, less expanded gel network that is less efficiently transported. In this regard, mucus plugging the airways of an individual in status asthmaticus was enriched in the low-charged form of MUC5B (22). Altered glycosylation of mucins has previously been reported in airways disease (11, 20, 23). Because mucin glycans are known receptors for microorganisms, changes in glycosylation might favor bacterial colonization, increasing the risk of exacerbation and infection (24, 25).
Entanglement of polymeric mucin chains is an important mechanism for mucus gel formation (5), and the extent of mucin polymerization might be expected to impact the rheological properties of the mucus gel. Solubilization of the mucins in the highly denaturing solvent, 6 M GdmCl, and subsequent analysis of their sedimentation behavior revealed a shift toward smaller mucins in COPD, which was more pronounced for MUC5AC. Whether this difference arises as part of the synthetic process, or after secretion in the mucus, is not known. However, MUC5AC and MUC5B have been shown to be degraded in cystic fibrosis mucus, and it was suggested that MUC5AC may be more susceptible to proteolytic degradation than MUC5B (26). Although the size of the polymeric mucins is important, one cannot rule out that the amounts of other components of the mucus gel that might influence either the physical or antibacterial properties of the gel are altered. For example, the trefoil peptide, TFF3, has been shown to be raised in COPD (27), and other studies have suggested that these peptides can interact with mucins to alter the rheological properties of mucus (28, 29).
Initial analysis of the data referenced mucin content to total sputum weight (sol plus gel) and showed no overall significant change in total mucin between the two clinical groups. However, we could not rule out that mucin present in saliva, a MUC5B-containing secretion (30), was present in the expectorated sputum samples. If saliva were present, then one would reasonably expect that this watery secretion would most likely contaminate the sol samples. Indeed, the MUC5B levels in the sol samples were very similar to those of saliva. Further evidence to indicate that expectorated sputum contains saliva was recently demonstrated in a proteomic analysis of induced sputum (31). Therefore, in subsequent analysis, we only looked at the gel component of the sputum, as it was more solid-like in nature and could be removed from the liquid-like sol, and was thus unlikely to suffer major contamination by saliva.
It is important to consider some of the limitations of the methods used here. First, there are a number of potential pitfalls associated with using Western blotting to determine the amounts of MUC5AC and MUC5B, and these arise due to the complex nature of these glycoproteins. For example, the antisera used are raised against peptidic epitopes in cysteine regions of the polypeptides that are supposed to be nonglycosylated. However, this has not been proven, and glycosylation could therefore affect antibody binding. Furthermore, proteolytic cleavage of the mucins could destroy epitopes. In addition, the mucin standards used were purified from nonrespiratory secretions, and could be modified differently to their airways counterparts. However, even after taking these potential problems into account, this is currently the best method available to analyze specific mucin gene products from sputum. Another issue to consider is whether there is a difference between induced and spontaneous mucus in terms of mucin composition, as we were unable to obtain spontaneous sputum from all subjects. Inspection of the data showed that there was no obvious difference in the nine samples produced by induction, and these data points were effectively indistinguishable from the spontaneous samples. Moreover, there are no published reports of differences between spontaneous and induced sputum regarding mucin composition.
In conclusion, we have found differences in mucin properties that could contribute to progression of COPD. Further studies are needed to see if altered mucus transport or binding properties contribute to the bacterial colonization and infection of the lower airways often seen in more severe disease.
Supplementary Material
Supported by a grant from the Wellcome Trust.
This article has an online supplement, which is accessible from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200803-391OC on September 5, 2008
Conflict of Interest Statement: S.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. U.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.V. has been reimbursed by GlaxoSmithKline for presenting at various meetings, receiving fees of $12,000 in 2005, $12,000 in 2006, and $10,000 in 2007, and received $4,000 from GlaxoSmithKline for taking part in an advisory board; for similar activities, he received $2,000 in 2005, 2006, and 2007 from Boehringer-Ingelheim, $2,000 in 2006 and $1,500 in 2007 from AstraZeneca, $2,000 in 2006 from Kamada, and $2,000 in 2006 from Hoffmann-La Roche; he received $450,000 in 2006, and expects to receive $400,000 per year in 2007 and 2008 as research grants for participating in multi- and single-center clinical studies; J.V.'s wife is an employee of AstraZeneca, Denmark; neither J.V. nor his wife owns shares in any pharmaceutical company. D.J.T. received $36,000 from Novartis for a Ph.D. studentship (2005–2008) to study the properties of mucus secreted from human primary airways cells in culture, and received $600 from Novartis for a research talk in 2005.
References
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive Summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 2.Anto JM, Vermeire P, Vestbo J, Sunyer J. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J 2001;17:982–994. [DOI] [PubMed] [Google Scholar]
- 3.Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006;61:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestbo J, Hogg JC. Convergence of the epidemiology and pathology of COPD. Thorax 2006;61:86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 2008;70:5.1–5.28 [DOI] [PubMed] [Google Scholar]
- 6.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol 2007;70:7.1–7.27. [DOI] [PubMed] [Google Scholar]
- 7.Buisine MP, Devisme L, Copin MC, Durand-Reville M, Gosselin B, Aubert JP, Porchet N. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol 1999;20:209–218. [DOI] [PubMed] [Google Scholar]
- 8.Vinall LE, Fowler JC, Jones AL, Kirkbride HJ, de Bolos C, Laine A, Porchet N, Gum JR, Kim YS, Moss FM, et al. Polymorphism of human mucin genes in chest disease: possible significance of MUC2. Am J Respir Cell Mol Biol 2000;23:678–686. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, Ann DK, Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol 2004;30:155–165. [DOI] [PubMed] [Google Scholar]
- 10.Thornton DJ, Davies JR, Kraayenbrink M, Richardson PS, Sheehan JK, Carlstedt I. Mucus glycoproteins from ‘normal’ human tracheobronchial secretion. Biochem J 1990;265:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J 2002;361:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhaskar KR, O'Sullivan DD, Seltzer A, Rossing TH, Drazen TM, Reid LM. Density gradient study of bronchial mucus aspirates from healthy volunteers (smokers and non-smokers) and from patients with tracheostomy. Exp Lung Res 1985;9:289–308. [DOI] [PubMed] [Google Scholar]
- 13.Kater A, Henke MO, Rubin BK. The role of DNA and actin polymers on the polymer structure and rheology of cystic fibrosis sputum and depolymerization by gelsolin or thymosin β 4. Ann N Y Acad Sci 2007;1112:140–153. [DOI] [PubMed] [Google Scholar]
- 14.Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am J Respir Cell Mol Biol 2004;31:86–91. [DOI] [PubMed] [Google Scholar]
- 15.Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology 2004;45:477–484. [DOI] [PubMed] [Google Scholar]
- 16.Innes AL, Woodruff PG, Ferrando MA, Donnelly S, Dolganov GM, Lazarus, SG, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 2006;130:1102–1108. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell RA, Richter A, Ward J, Angco G, Mehta A, Rousseau K, Swallow DM, Holgate ST, Djukanovic R, Davies DE, et al. Expression of Erb receptors and mucins in the airways of long term current smokers. Thorax 2004;59:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousseau K, Wickstrom C, Whitehouse DB, Carlstedt I, Swallow DM. New monoclonal antibodies to non-glycosylated domains of the secreted mucins MUC5B and MUC7. Hybrid Hybridomics 2003;5:293–299. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan JK, Thornton DJ. Methods for the analysis of the heterogeneity of mucins. Methods Mol Biol 2000;125:77–85. [DOI] [PubMed] [Google Scholar]
- 20.Davies JR, Hovenberg HW, Lindén CJ, Howard R, Richardson PS, Sheehan JK, Carlstedt I. Mucins in airway secretions from healthy and chronic bronchitic subjects. Biochem J 1996;313:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickstrom C, Davies, JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J 1998;334:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehan JK, Howard M, Richardson PS, Longwill T, Thornton DJ. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem J 1999;338:507–513. [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz BL, Sloane AJ, Robinson LJ, Prasad SS, Linder RA, Robinson M, Bye PT, Nielson DW, Harry JL, Packer N, et al. Glycosylation of sputum mucins is altered in cystic fibrosis patients. Glycobiology 2007;17:698–712. [DOI] [PubMed] [Google Scholar]
- 24.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev 2001;14:336–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedzicha JA, Hurst JR. Structural and functional co-conspirators in chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc 2007;4:602–605. [DOI] [PubMed] [Google Scholar]
- 26.Davies JR, Svitacheva N, Lannefors L, Kornfält R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J 1999;344:321–330. [PMC free article] [PubMed] [Google Scholar]
- 27.Chari R, Lonergan KM, Ng RT, MacAulay C, Lam WL, Lam S. Effect of active smoking on human bronchial epithelium transcriptome. BMC Genomics 2007;8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest 2002;32:519–527. [DOI] [PubMed] [Google Scholar]
- 29.Kjellev S, Nexø E, Thim L, Poulsen SS. Systemically administered trefoil factors are secreted into the gastric lumen and increase the viscosity of gastric contents. Br J Pharmacol 2006;149:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornton DJ, Khan N, Howard M, Veerman E, Packer NH, Sheehan JK. Salivary mucin MG1 is comprised almost entirely of differently glycosylated populations of the MUC5B gene product. Glycobiology 1999;3:293–302. [DOI] [PubMed] [Google Scholar]
- 31.Nicholas B, Skipp P, Mould R, Rennard S, Davies DE, O'Connor CD, Djukanovic R. Shotgun proteomic analysis of human-induced sputum. Proteomics 2006;6:4390–4401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.