Abstract
Rationale: Tuberculosis-associated immune reconstitution inflammatory syndrome (TB–IRIS) induced by combination antiretroviral therapy (cART) has been attributed to dysregulated expansion of tuberculin PPD–specific IFN-γ–secreting CD4+ T cells.
Objectives: To investigate the role of type 1 helper T cell expansions and regulatory T cells in HIV–TB IRIS.
Methods: Longitudinal and cross-sectional studies of Mycobacterium tuberculosis–specific IFN-γ enzyme-linked immunospot responses and flow cytometric analysis of blood cells from a total of 129 adults with HIV-1–associated tuberculosis, 98 of whom were prescribed cART.
Measurements and Main Results: In cross-sectional analysis the frequency of IFN-γ–secreting T cells recognizing early secretory antigenic target (ESAT)-6, α-crystallins 1 and 2, and PPD of M. tuberculosis was higher in patients with TB–IRIS than in similar patients treated for both HIV-1 and tuberculosis who did not develop IRIS (non-IRIS; P ≤ 0.03). The biggest difference was in the recognition of α-crystallin molecules: peptide mapping indicated a polyclonal response. Flow cytometric analysis indicated equal proportions of CD4+ and CD8+ cells positive for activation markers HLA-DR and CD71 in both patients with TB–IRIS and non-IRIS patients. The percentage of CD4+ cells positive for FoxP3 (Forkhead box P3) was low in both groups (TB–IRIS, 5.3 ± 4.5; non-IRIS, 2.46 ± 2.46; P = 0.13). Eight weeks of longitudinal analysis of patients with tuberculosis who were starting cART showed dynamic changes in antigen-specific IFN-γ–secreting T cells in both the TB–IRIS and non-IRIS groups: the only significant trend was an increased response to PPD in the TB–IRIS group (P = 0.041).
Conclusions: There is an association between helper T-cell type 1 expansions and TB–IRIS, but the occurrence of similar expansions in non-IRIS brings into question whether these are causal. The defect in immune regulation responsible for TB–IRIS remains to be fully elucidated.
Keywords: tuberculosis, HIV, immune reconstitution inflammatory syndrome, FoxP3, type 1 helper T cells
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Little is known of the pathogenesis of the HIV–TB immune reconstitution inflammatory syndrome (TB–IRIS), although preliminary analyses have attributed it to dysregulated helper T-cell type 1 (Th1) expansion.
What This Study Adds to the Field
Dynamic Mycobacterium tuberculosis–specific Th1 responses associate with TB–IRIS, although the presence of such responses in patients who did not develop the syndrome suggests that other regulatory factors are involved.
HIV-1–associated tuberculosis is a major cause of mortality in sub-Saharan Africa (1). The widespread introduction of combination antiretroviral therapy (cART) has, however, been associated with a decrease of between 50 and 80% in the annual risk of tuberculosis in HIV-1–infected persons (2–4). cART partially and beneficially restores pathogen-specific immunity (5), but is also associated under some circumstances with paradoxical deterioration of existing, or unmasking of, unapparent infection (6, 7). This phenomenon is presumed to reflect the simultaneous restoration of immunopathological as well as protective immunity during antitubercular and cART. Deterioration of mycobacterial disease was recognized early as one of the forms of aberrant immune restoration or immune reconstitution inflammatory syndrome (IRIS) (8, 9). A number of case series of tuberculosis–IRIS (TB–IRIS) have been reported (reviewed by Dhasmana and coworkers [10]) with recognized risk factors being disseminated tuberculosis, a low nadir CD4+ cell count, high viral load, and short interval between starting antituberculosis chemotherapy and cART. Initially reported from specialized centers (11, 12), TB–IRIS is now a frequent diagnosis in areas of the world where tuberculosis and HIV frequently coexist (13, 14).
The diverse clinical presentations of TB–IRIS have been characterized (10), but little is known of its immunopathogenesis. It has been related to expansion of tuberculin purified protein derivative (PPD)–specific interferon-γ–secreting CD4+ T cells during cART (8, 9, 15). In the most comprehensive analysis to date, expansion of PPD-specific T cells was suggested as the cause of the syndrome: the maximal median value in 7 patients who developed TB–IRIS being higher than the maximal median values obtained from 12 similar patients who did not experience IRIS (16). In addition, T-cell reactivity to the species-specific and immunodominant early secretory antigenic target (ESAT)-6 protein was reported as low in patients with IRIS, suggesting that TB–IRIS may be associated with specific recognition of components of PPD. These findings in turn have led to the suggestion that regulatory T cells that secrete transforming growth factor-β or IL-10 may not expand at an equal rate during cART, thereby setting up conditions favorable to immunopathology (17, 18). This study was therefore performed to investigate the role of helper T-cell type 1 (Th1) expansions and regulatory cells in much larger numbers of patients than has hitherto been possible. We chose a range of previously well-characterized antigens of Mycobacterium tuberculosis including the species-specific, secreted and immunodominant antigen ESAT-6, the 38-kD cell wall antigen, and two cytoplasmic heat shock proteins, α-crystallin-1 (Acr1) and Acr2, to address the possibility that the immune response underlying TB–IRIS is antigen specific. Some of the results of these studies have been previously reported in the form of an abstract (19, 20).
METHODS
Subjects
The University of Cape Town (Cape Town, South Africa) Research Ethics Committee approved the study (REC references 337/2004 and 173/2005). In a cross-sectional analysis 35 cases of paradoxical TB–IRIS (see Table E1 in the online supplement for the case definition and also Meintjes and coworkers [21] for the consensus definition) were compared with 29 persons treated for HIV-associated tuberculosis with similar baseline CD4+ cell count and duration of combination antiretroviral treatment (cART) who did not develop IRIS (non-IRIS). A third group consisted of 31 HIV-infected persons with tuberculosis sampled before antitubercular treatment and who were not receiving cART (untreated HIV+TB). Tuberculosis was diagnosed primarily on the basis of smear or culture positivity. Where this was negative or unavailable, diagnosis was in line with guidelines for smear-negative or extrapulmonary tuberculosis in HIV-infected persons (22, 23). TB–IRIS was diagnosed according to case definitions shown in Table E1. New TB cases were treated with 6 months duration of first-line therapy (isoniazid, rifampin, pyrazinamide, and ethambutol [HRZE] for 2 months followed by HR for 4 months [2HRZE/4HR]). The retreatment regimen added streptomycin (S) as follows: 2HRZES/1HRZE/5HRE. All non-IRIS patients and 28 of 35 patients with TB–IRIS were prescribed the cART combination of stavudine (d4T), lamivudine (3TC), and efavirenz (EFZ). Six patients with TB–IRIS were prescribed zidovudine (AZT), 3TC, and EFZ and one was prescribed AZT, 3TC, and nevirapine (NVP). No patient had received prior cART.
Longitudinal analysis was of a prospective cohort of 63 adults who started cART in primary care while undergoing TB treatment between January 2006 and September 2007. Participants were monitored four times for a period of 2 months after initiation of cART. The main outcome was TB–IRIS, with suspected cases being referred to secondary care for investigation and further management.
Immunological Analysis
Blood was drawn for IFN-γ enzyme-linked immunospot (ELISpot) analysis and peptide mapping as previously described (24, 25). For flow cytometric analysis peripheral blood mononuclear cells (PBMCs) were separated by standard protocols, and plated at 106/ml per well in 24-well plates in RPMI−10% fetal calf serum (FCS). Heat-killed M. tuberculosis H37Rv was added to the wells at a multiplicity of infection of 1:15 (hkH37Rv:PBMC) and the plates were incubated overnight at 37°C in 5% CO2. Cells were then surface stained for 20 minutes on ice, using the following fluorescent antibodies (all from BD Biosciences, San Jose, CA): CD3–allophycocyanin (APC), CD4–peridinin chlorophyll protein (PerCP), CD8–PerCP, HLA-DR–fluorescein isothiocyanate (FITC), and CD71–phycoerythrin (PE). After washing, stained cells were fixed in phosphate-buffered saline (PBS)−2% FCS containing 1.6% paraformaldehyde and acquired with a FACSCalibur flow cytometer (BD Biosciences). Intranuclear staining for FoxP3 (Forkhead box P3) was performed with the PE-conjugated anti-human FoxP3 staining set from eBioscience (San Diego, CA). Cells were stimulated as described previously for 2 hours, after which brefeldin A was added at a concentration of 5 μg/ml (an unstimulated control well was also included). Cells were first surface stained with anti-CD3–APC and anti-CD4–PerCP, followed by incubation for 45 minutes on ice in eBioscience Fix/Perm buffer. After washing in permeabilization buffer, cells were incubated for 15 minutes on ice in 2% normal rat serum, followed by incubation for 30 minutes on ice in 10 μl of anti-human FoxP3 (clone PCH101) antibody. The cells were then washed again, fixed in PBS−2% FCS containing 1.6% paraformaldehyde, and acquired. Analysis was performed with FlowJo software (Tree Star, Ashland, OR). First, the lymphocyte population was gated on the basis of forward scatter and side scatter, and analyzed for CD4 and FOXP3. For the activation and proliferation markers HLA-DR and CD71, CD3+ cells were selected out of the lymphocyte population, followed by CD4+/CD71+ or CD4+/HLA-DR+ cells. Serum C-reactive protein was determined by analysis with a SYNCHRON CX system (Beckman Coulter, Fullerton, CA).
Statistical Analysis
The normality of data was assessed by the D'Agostino and Pearson omnibus normality test. Medians are given with the interquartile range (IQR) and means are presented with the standard deviation (SD). Paired parametric data were analyzed by Student paired t test or repeated-measures analysis of variance, and nonparametric paired data were analyzed by Wilcoxon matched-pairs test or by the Friedman test. Unpaired parametric variables were assessed by Student unpaired t test and nonparametric variables were assessed by Mann-Whitney U test.
RESULTS
We first compared the frequency of M. tuberculosis antigen–specific IFN-γ spot-forming cells (SFCs) cross-sectionally in TB–IRIS at presentation, non-IRIS after 2 weeks of cART, and untreated HIV+TB groups (Table 1). The TB–IRIS and non-IRIS groups were similar in age, tuberculosis disease form, duration of cART at sampling, baseline CD4+ cell count, and sex (see Table 1). They did, however, differ in the median interval between starting TB treatment and cART, which was shorter in the TB–IRIS group (55 d, IQR 30–82 vs. 80 d, IQR 44–141; P = 0.011). In the untreated HIV+TB group more TB diagnoses were microbiologically confirmed and the baseline CD4+ cell count was higher than for the both TB–IRIS and non-IRIS groups (see Table 1). In addition the median age of the untreated HIV+TB group was lower than that of the non-IRIS group (28 yr, IQR 25–36 vs. 35.6 yr, IQR 30–41; P = 0.018). In 25 of 30 (83%) patients with TB–IRIS for whom a result was available the convalescent (i.e., post-IRIS episode) viral load was below the detection limit. In the patients with a detectable load, no value exceeded 1,000 copies/ml. In addition, an increase in CD4+ cell count occurred in 31 of 33 (94%) patients, with a median increase of 130 cells/μl (IQR 63–209).
TABLE 1.
BASELINE CHARACTERISTICS OF PATIENTS WITH TUBERCULOSIS-ASSOCIATED IMMUNE RECONSTITUTION INFLAMMATORY SYNDROME AND COMPARATOR NON-IRIS AND UNTREATED HIV+TB GROUPS
| TB–IRIS | Non-IRIS | Untreated HIV+TB | |
|---|---|---|---|
| N | 35 | 29 | 31 |
| Median age, yr (IQR) | 31.4 (25.8–36.2) | 35.6 (30.3–41.0) | 28 (25–36)* |
| Baseline CD4+ cell count, cells/μl (IQR) | 51 (29–106) | 45 (23–122) | 195 (111–331)† |
| TB treatment before cART, d | 55 (30–82) | 78 (38–132)‡ | N/A |
| Days of cART to IRIS or to sample | 14 (8–21) | 14 (14, 15) | N/A |
| Females, n (%) | 23 (66) | 19 (66) | 18 (58) |
| Previous TB, n (%) | 12 (34) | 4 (14) | 7 (23) |
| TB disease form, n (%) | |||
| Pulmonary or pleural | 21 (60) | 22 (76) | 31 (100) |
| Disseminated | 11 (31) | 4 (14) | |
| Lymphadenopathic | 3 (9) | 3 (10) | |
| Smear or culture confirmed? | 19 (54) | 16 (55) | 30 (97)§ |
Definition of abbreviations: cART = combination antiretroviral therapy; N/A = not applicable; TB = tuberculosis; TB–IRIS = tuberculosis-associated immune reconstitution inflammatory syndrome.
Significantly lower than non-IRIS: P = 0.018.
Significantly higher than TB–IRIS and non-IRIS: P < 0.0001.
Significantly more than TB–IRIS: P = 0.011.
Significantly more than TB–IRIS and non-IRIS: P < 0.0001.
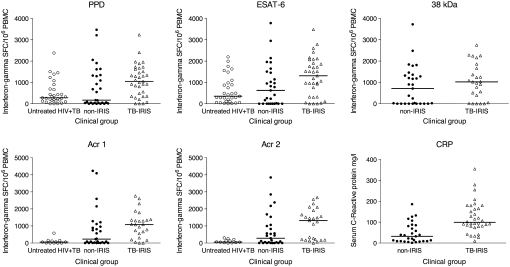
The antigens assayed included the secreted and species-specific ESAT-6 molecule (26, 27); a 38-kD cell wall antigen (28, 29); two small cytoplasmic heat shock proteins, Acr1 and Acr2 (25, 30); and PPD. The response to the 38-kD antigen was not assayed in the untreated HIV+TB group. Overall responses were highly heterogeneous with strong positive responses to some antigens in some non-IRIS patients and a lack of response to some antigens in some patients with TB–IRIS (Figure 1). However, the median frequency of IFN-γ SFCs was higher in the TB–IRIS group than in the non-IRIS group, with the exception of the response to the 38-kD antigen (ESAT-6: 1,312, IQR 322–1,908 vs. 624, IQR 0–1,481 [P = 0.024]; Acr1: 1,080, IQR 432–1,363 vs. 228, IQR 7–1,037 [P = 0.019]; Acr2: 1,313, IQR 196–1,785 vs. 281, IQR 0–1,032 [P = 0.027]; 38-kD: 1,026, IQR 222–1,761 vs. 710, IQR 0–1,340 [P = 0.25]; PPD: 1,047, IQR 347–1,620 vs. 168, IQR 34–1,333 [P = 0.047]). When the TB–IRIS group was compared with the untreated HIV+TB group (median ESAT-6: 353, IQR 99–977 [P = 0.004]; Acr1: 57, IQR 0–139 [P = 0.0001]; Acr2: 58, IQR 22–189 [P = 0.0002]; PPD: 279, IQR 130–693 [P = 0.001]) the higher response of patients with TB–IRIS was even more pronounced. By contrast, there were no significant differences between the non-IRIS and untreated HIV+TB groups in response to any antigen. An alternative to effector T cells as a marker of immune activation is the serum C-reactive protein (CRP). We therefore determined CRP concentrations in the TB–IRIS and non-IRIS groups. The median CRP concentration was three times higher in the TB–IRIS group than in the non-IRIS group (99 mg/L, IQR 80–169 vs. 32 mg/L, IQR 9–83; P < 0.0001). Elevation of CRP above the reference laboratory range (10 mg/L) was invariable in the patients with TB–IRIS, but it was also elevated in 20 of 29 (69%) of the non-IRIS patients. Although this difference in proportions is significant (P = 0.0004) these data again reflect considerable immune activation in the non-IRIS group in the absence of reported symptoms or signs of TB–IRIS.
Figure 1.
Frequency of Mycobacterium tuberculosis antigen–specific IFN-γ spot-forming cells (SFCs) in HIV-infected patients with tuberculosis-associated immune reconstitution inflammatory syndrome (TB–IRIS). Two comparator groups were patients with HIV-associated tuberculosis before treatment and not receiving combination antiretroviral therapy (cART) (untreated HIV+TB), and patients with treated HIV-associated TB also receiving cART for a similar duration to the patients with TB–IRIS (non-IRIS). The frequency of IFN-γ SFCs was higher in patients with TB–IRIS than in non-IRIS patients (P ≤ 0.03), with the exception of the response to 38-kD cell wall antigen. The frequency of IFN-γ SFCs was also higher in patients with TB–IRIS than in patients with untreated HIV+TB in every instance (P ≤ 0.004). By contrast, the frequency of IFN-γ SFCs did not differ between the non-IRIS and untreated HIV+TB groups in response to any antigen.
The high response to several classes of M. tuberculosis antigens in the TB–IRIS group implies polyclonal, rather than oligoclonal, T-cell expansion. To further test this hypothesis we peptide-mapped responses to the two heat shock proteins Acr1 and Acr2, antigens that showed the greatest fold difference in recognition between the TB–IRIS and non-IRIS groups (see Figure 1). PBMCs from 13 patients with TB–IRIS known to respond to either Acr1, Acr2, or both were selected for this purpose. The median number of Acr1 peptides recognized per donor was 10 (IQR 3–14) and 6 (IQR 3–10) for Acr2. Immunodominant determinants (defined by a response exceeding 30 IFN-γ SFCs per 106 PBMCs) were distributed widely across both molecules (see Figure E1 in the online supplement). Taken together, these findings strongly suggest a polyclonal response.
It has been suggested that defective restoration of regulatory T-cell function may contribute to the pathogenesis of TB–IRIS (10, 18). This was examined by flow cytometric analysis of PBMCs from a subset of TB–IRIS (n = 11) and non-IRIS (n = 8) patients for whom sufficient cells were available. The percentage of unstimulated CD4+ cells positive for the transcription factor FoxP3, although higher in the TB–IRIS group, did not significantly differ between the groups (TB–IRIS: mean, 5.3 ± 4.5; vs. non-IRIS: mean, 2.46 ± 2.46 [P = 0.13]; Figure 2). hkH37Rv restimulation of PBMCs for 24 hours did not appreciably change the detection frequency in the TB–IRIS group or make the difference between groups significant (TB–IRIS: mean, 5.6 ± 4.6; vs. non-IRIS: mean, 2.9 ± 2.3 [P = 0.15]; see Figure 2). In these subjects we also evaluated lymphocyte activation as determined by CD71 (transferrin receptor) and HLA-DR expression (see Figure 2). The percentage of activated CD4+ cells did not differ between groups (TB–IRIS: mean CD4+CD71+, 26.6 ± 17.2; vs. non-IRIS: mean CD4+CD71+, 29.9 ± 11.2 [P = 0.64]; TB–IRIS: mean CD4+ DR+, 45.2 ± 22.3 vs. non-IRIS: mean CD4+ DR+, 38.0 ± 15.0 [P = 0.44]). There was also evidence of a similar degree of CD8+ cell activation (TB–IRIS: mean CD4+ DR+, 28.8 ± 18.9 vs. non-IRIS: mean CD4+ DR+, 22.4 ± 9.6 [P = 0.40]; TB–IRIS: mean CD8+ DR+, 32.4 ± 8.6 vs. non-IRIS: mean CD8+ DR+, 37.0 ± 12.1 [P = 0.35]), but again this did not differentiate the TB–IRIS group from the non-IRIS group.
Figure 2.
FoxP3 and T-cell activation markers in peripheral blood mononuclear cells (PBMCs) of patients with tuberculosis-associated immune reconstitution inflammatory syndrome (TB–IRIS). PBMCs from 11 TB–IRIS and 8 non-IRIS patients were analyzed. Although the proportion of activated T cells (both CD4+ and CD8+) was high in both groups, there were no significant differences between clinical groups. For FoxP3 analysis cells were either unstimulated (unstim) or restimulated (stim) with PPD for 24 hours. Again, there were no significant differences between TB–IRIS and non-IRIS groups. Error bars show the SD.
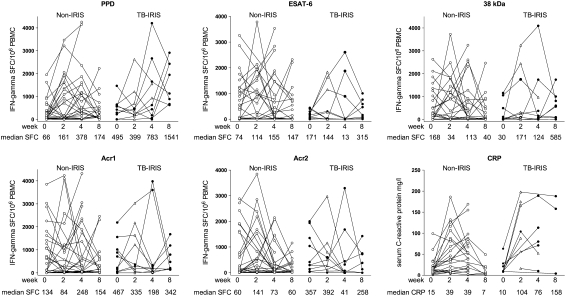
Cross-sectional analyses only provide partial insight and we were therefore interested to determine whether our findings could be repeated in a prospective longitudinal analysis of patients with TB commencing cART. In the prospective analysis 8 of 62 participants had insufficient clinical follow-up to exclude IRIS, 1 was withdrawn because of drug-induced hepatitis, 1 declined further study involvement after Week 1, and 1 died of Escherichia coli septicemia 12 days after starting cART. Ten of 51 (19.6%) of those with sufficient follow-up developed TB–IRIS according to our case definitions (see Table E1 and Table 2). ELISpot data gathered at three or more time points was available for 39 participants, all patients with IRIS, and 29 of 41 non-IRIS patients. Responses showed marked dynamic changes within both groups (Figure 3). Within-category (non-IRIS and TB–IRIS) data in the longitudinal study were first assessed by repeated measures analysis (Friedman). The only significant trend was the increase in response to PPD in the TB–IRIS group over 8 weeks (P = 0.041). Within-category analysis by less stringent two–time point paired testing confirmed the increase in median PPD response in the TB–IRIS group, from 495 SFCs per 106 PBMCs (IQR 169–630) to 1,549 SFCs per 106 PBMCs (IQR 725–2432) between Weeks 0 and 8, to be significant (P = 0.008). In addition, the increase in PPD response in the non-IRIS group between Week 0 (66 SFCs per 106 PBMCs [IQR 10–358]) and both Week 2 (161 SFCs per 106 PBMCs [IQR 5–1414]; P = 0.04) and Week 4 (376 SFCs per 106 PBMCs [IQR 37–1140]; P = 0.01) was significant. Expansion of PPD-specific SFCs to more than twice baseline at any time was observed in 8 of 10 (80%) patients with TB–IRIS and in 18 of 29 (62%) non-IRIS patients (P = 0.45). No other significant intragroup changes occurred, despite the highly dynamic nature of these responses. When comparing IRIS and non-IRIS groups the only significant difference was between the 8-week response to PPD in the non-IRIS group (174 SFCs per 106 PBMCs; IQR 87–556) and the group that developed TB–IRIS (1,541 SFCs per 106 PBMCs; IQR 725–2432) (P = 0.001). Two or more CRP results were also available from all 10 TB–IRIS cases and 29 of 41 non-IRIS cases (see Figure 3). Repeated-measures tests did not confirm overall trends, partially because of data gaps at some time points that reduced the overall power of the analysis. Within-category analysis by two–time point paired testing showed the increase in median CRP response in the TB–IRIS group, from 10 mg/L (IQR 9–25) to 104 mg/L (IQR 56–175) between Weeks 0 and 2, to be significant (P = 0.031). In addition, the increase in CRP in the non-IRIS group between Week 0 (15 mg/L, IQR 7–20) and both Week 2 (39 mg/L, IQR 9–87; P = 0.004) and Week 4 (39 mg/L, IQR 12–78; P = 0.006) was significant. When comparing IRIS and non-IRIS groups significant differences were found between the 2-week response in the non-IRIS group (39 mg/L, IQR 9–87) and the group that developed TB–IRIS (104 mg/L, IQR 56–175) (P = 0.027). Of the 10 TB–IRIS patients shown in Figure 3, 4 were prescribed corticosteroid therapy and 2 were randomized to a double-blind trial of prednisone versus placebo for mild to moderate TB–IRIS. No obvious trend with respect to the effect of corticosteroid therapy on the Th1 expansions was observed.
TABLE 2.
CLINICAL CHARACTERISTICS OF TB–IRIS THAT AROSE DURING LONGITUDINAL FOLLOW-UP OF HIV-INFECTED PATIENTS WITH TUBERCULOSIS WHO RECEIVED COMBINATION ANTIRETROVIRAL THERAPY
| Patient no. | Sex | Age (yr) | CD4+ Cell Count Nadir (cells/μl) | Presenting Form of TB | Days of cART to TB–IRIS | Presentation of IRIS |
|---|---|---|---|---|---|---|
| B13 | F | 38 | 92 | Culture-positive PTB | 13 | Developed new right cervical lymphadenopathy |
| B20 | M | 36 | 92 | Clinical PTB | 28 | Transient worsening of pulmonary symptoms |
| B28 | M | 46 | 30 | Disseminated | 7 | Worsening pulmonary infiltrate, hepatomegaly, and tachycardia. |
| B31 | F | 37 | 71 | Culture-positive disseminated | 13 | Hepatic enlargement and cholestatic LFT derangement: liver biopsy showed necrotizing granulomas |
| B30 | F | 42 | 42 | Clinical PTB | 14 | Anemia and new cervical lymphadenopathy |
| B33 | M | 28 | 5 | Culture-positive pleural | 14 | Worsening pulmonary infiltrate |
| B34 | M | 30 | 13 | Culture-positive PTB | 15 | Pleurisy, cough, and high fever. |
| B38 | M | 37 | 10 | Disseminated, smear positive | 7 | Fever, confusion, and lymphocytic meningitis |
| B49 | F | 36 | 113 | Disseminated, smear positive | 12 | New headache and fever, found to have meningitis and a tuberculoma |
| B52 | F | 20 | 48 | Disseminated | 28 | New bilateral cervical lymphadenopathy |
Definition of abbreviations: cART = combination antiretroviral therapy; F = female; LFT = liver function test; M = male; PTB = pulmonary tuberculosis; TB–IRIS = tuberculosis-associated immune reconstitution inflammatory syndrome.
Figure 3.
Longitudinal analysis of the frequency of M. tuberculosis antigen–specific IFN-γ spot-forming cells in response to five antigens and C-reactive protein (CRP) in HIV-TB patients receiving combination antiretroviral therapy (cART). An open triangle in the TB–IRIS group indicates the point at which tuberculosis-associated immune reconstitution inflammatory syndrome (TB–IRIS) was diagnosed. Repeated measures analysis demonstrated a significant increase in PPD response only in the TB–IRIS group (P = 0.041). Within-category analysis confirmed the increase in median PPD response in the TB–IRIS group between Weeks 0 and 8 to be significant (P = 0.008). In addition, the increase in PPD response in the non-IRIS group between Week 0 and both Weeks 2 and 4 was significant (P ≤ 0.04). When comparing IRIS and non-IRIS groups the only significant difference was between the 8-week response to PPD (P = 0.001). The increase in median CRP response in the TB–IRIS group from between Weeks 0 and 2 was significant (P = 0.031). In addition, the increase in CRP in the non-IRIS group between Week 0 and both Weeks 2 and 4 (P ≤ 0.006) was significant. When comparing IRIS and non-IRIS groups significant differences in CRP were found between the 2-week response in the non-IRIS group when compared with those who developed TB–IRIS (P = 0.027).
DISCUSSION
Cape Town has a high tuberculosis case notification rate (>1,000/100,000 per annum in the area in which this study was conducted), rising adult HIV prevalence (antenatal seroprevalence exceeds 30% in some areas where the study was conducted), and rapidly increasing access to cART with roll-out to more than 20,000 people in the city so far. This triple coincidence has led to a substantial increase in cases of TB–IRIS presenting to our service: we have been referred nearly 400 cases in the last 3 years. This situation is mirrored in other large urban areas of sub-Saharan Africa. TB–IRIS is an opportunity to better understand aberrant immunopathological immunity in tuberculosis and thereby not only to treat TB–IRIS more effectively but also to reduce the tissue damage that is characteristic of tuberculosis in both HIV-infected and uninfected persons.
Cross-sectional analysis clearly confirmed that TB–IRIS is associated with high frequencies of M. tuberculosis antigen–specific IFN-γ–secreting T cells, as previously reported (16). These cells seem likely to contribute to the inflammatory reactions observed clinically. The biggest difference in recognition between TB–IRIS and non-IRIS was in the recognition of the small cytoplasmic heat shock proteins Acr1 and Acr2 (see Figure 1). Both are upregulated by M. tuberculosis during conditions of stress (31, 32), and it is possible that the combined stresses of antituberculosis chemotherapy and an improving immune response lead to such upregulation, thereby rendering these molecules prominent antigenic targets. In this respect the general principle of antigenic specificity implied by the poor recognition of ESAT-6 in the patients with TB–IRIS studied by Bourgarit and coworkers is borne out (16). However, and contrary to those findings, we observed ESAT-6 to be a significant antigenic target in patients with TB–IRIS. This difference may relate to ethnicity, disease extent, or length of antituberculosis therapy, as the response to ESAT-6 tends to decline during successful treatment (33). We have also previously documented a high frequency of recognition of ESAT-6 (60–90%) in HIV-infected persons with both active and latent tuberculosis in this population (24, 34, 35).
Our flow cytometric analysis (see Figure 2) indicated that both CD4+ and CD8+ cells from patients with TB–IRIS (and non-IRIS patients) are activated, with a high percentage of cells expressing HLA-DR or CD71. We attribute the CD8+ cell activation to the restoration of effector responses to HIV-1 itself. Expression of the transcription factor FoxP3 has been associated with depression of M. tuberculosis antigen–specific effector T-cell responses (36–38), leading to the hypothesis that delayed cART-mediated restoration of such presumed regulatory T-cell responses underlies TB–IRIS (18). We found only low levels of FoxP3-expressing CD4+ T cells, perhaps in keeping with this idea but, if anything, their level was actually higher in patients with TB–IRIS than in non-IRIS patients. Flow cytometry was performed on a relatively small subset of 11 patients with TB–IRIS and 8 non-IRIS subjects; this, given the heterogeneous responses, limits power. However, we cannot readily ascribe the difference between clinical groups to CD4+ T cells that express FoxP3.
In the longitudinal analysis 10 patients developed TB–IRIS (see Figure 3). These data, however, illustrate an important point that was partially apparent from the cross-sectional analysis (see Figure 1). Dynamic expansion (and reductions) of M. tuberculosis antigen–specific T cells also occurred in the majority of non-IRIS patients, as did elevation of CRP. This had the consequence that stringent tests for trend (with the exception of the response to PPD in the TB–IRIS group) did not yield significant results. Overall, the highly variable patterns of fluctuation observed did not differ between persons who did and did not develop TB–IRIS. Although an association between dynamic T-cell expansions and TB–IRIS therefore exists, these data bring into question whether an explosion of tuberculin-specific Th1 responses is the cause of this syndrome (16). Our data favor the interpretation that during early cART in HIV-1–infected patients with tuberculosis, effector responses are highly dynamic, most likely reflecting a complex mixture of recirculation, immune restoration, and a variably falling mycobacterial antigen and HIV-1 viral load. The clinical heterogeneity of the syndrome (see Table 2) also cautions against drawing conclusions from limited numbers of patients.
In additional to clinical heterogeneity, a limitation of all studies of TB–IRIS is the selection of control subjects. We chose to study similar HIV-1–infected patients with tuberculosis sampled after a similar duration of cART (14 d, the characteristic time to onset of TB–IRIS) (see Table 1), but who did not develop the condition during 2 months of follow-up. However, the median duration of antituberculosis treatment was longer in the non-IRIS group (see Table 1). It is recognized that a risk factor for TB–IRIS is a short interval between the initiation of antituberculosis therapy and cART. As prescribing practice trends toward the earlier initiation of cART in patients with tuberculosis because of likely mortality benefits (7), we can expect the incidence and thus clinical importance of TB–IRIS to increase. Another limitation is that the study was conducted under program conditions and thus World Health Organization clinical case definitions of HIV-1–associated tuberculosis (23) and a clinical case definition of TB–IRIS (see Table E1) that excluded documentation of increased CD4+ cell count and a fall in HIV-1 viral load were in use. However, in patients with TB–IRIS for whom results were available 83% had complete HIV suppression in samples taken after the IRIS episode and 94% showed an increase in CD4+ cell count: highly consistent with general program conditions in which the HIV-1 viral load was undetectable in 88.1% persons at 3 months and the median increase in CD4+ cell count was 134 cells/μl at 6 months (39). A final limitation is that, in keeping with many studies of the immunology of tuberculosis, we studied blood cells as a readily accessible compartment. It would be of interest to conduct similar analyses on cells from disease sites.
TB–IRIS is associated with high frequencies of M. tuberculosis antigen–specific IFN-γ–secreting T cells and invariably with CRP elevation, both indicative of vigorous cART-mediated immune restoration. However, dynamic expansion of M. tuberculosis antigen–specific T cells also occurred in the majority of non-IRIS patients, as did CRP elevation. The frequency of CD4+ cells expressing FoxP3 did not differ between persons who developed TB–IRIS and those who did not. Therefore the differences in immune regulation that mark orderly or dysregulated cART-mediated immune restoration in the context of antituberculosis treatment in HIV-infected persons remain to be fully elucidated.
Supplementary Material
Acknowledgments
Willem Hanekom is thanked for advice on flow cytometry.
Supported by the Wellcome Trust of Great Britain, which provided personal awards to R.J.W., M.P.N., G.M., K.v.V., and M.X.R. (072070, 072065, 081667, 083226, and 084670). K.A.W. and R.J.W. are supported by the Medical Research Council (UK). M.X.R. is a European and Developing Countries Clinical Trials Partnership (EDCTP) Intermediate Fellow. The Medical Research Council of South Africa provided additional support in the form of a self-initiated project grant to G.M., G.M., and R.J.W.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200806-858OC on August 28, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Maartens G, Wilkinson RJ. Tuberculosis. Lancet 2007;370:2030–2043. [DOI] [PubMed] [Google Scholar]
- 2.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002;359:2059–2064. [DOI] [PubMed] [Google Scholar]
- 3.Brinkhof MW, Egger M, Boulle A, May M, Hosseinipour M, Sprinz E, Braitstein P, Dabis F, Reiss P, Bangsberg DR, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis 2007;45:1518–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn S, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS 2005;19:2109–2116. [DOI] [PubMed] [Google Scholar]
- 5.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 1997;277:112–116. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Wilkinson RJ. Immune reconstitution disease associated with parasitic infections following antiretroviral treatment. Parasite Immunol 2006;28:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med 2008;177:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med 1998;158:157–161. [DOI] [PubMed] [Google Scholar]
- 9.French MA, Mallal SA, Dawkins RL. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS 1992;6:1293–1297. [DOI] [PubMed] [Google Scholar]
- 10.Dhasmana DJ, Dheda K, Ravn P, Wilkinson RJ, Meintjes G. Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: pathogenesis, clinical manifestations and management. Drugs 2008;68:191–208. [DOI] [PubMed] [Google Scholar]
- 11.Breen RA, Smith CJ, Bettinson H, Dart S, Bannister B, Johnson MA, Lipman MC. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax 2004;59:704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratnam I, Chiu C, Kandala N, Easterbrook P. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1–infected cohort. Clin Infect Dis 2006;42:418–427. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS 2007;21:335–341. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS 2008;22:601–610. [DOI] [PubMed] [Google Scholar]
- 15.Hengel RL, Allende MC, Dewar RL, Metcalf JA, Mican JM, Lane HC. Increasing CD4+ T cells specific for tuberculosis correlate with improved clinical immunity after highly active antiretroviral therapy. AIDS Res Hum Retroviruses 2002;18:969–975. [DOI] [PubMed] [Google Scholar]
- 16.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006;20:F1–F7. [DOI] [PubMed] [Google Scholar]
- 17.Lim A, D'Orsogna L, Price P, French MA. Imbalanced effector and regulatory cytokine responses may underlie mycobacterial immune restoration disease. AIDS Res Ther 2008;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruhwald M, Ravn P. Immune reconstitution syndrome in tuberculosis and HIV-co-infected patients: Th1 explosion or cytokine storm? AIDS 2007;21:882–884. [DOI] [PubMed] [Google Scholar]
- 19.Meintjes G, Wilkinson K, Rangaka M, Abrahams M, Pepper D, Seldon R, Skolimowska K, van Veen K, Maartens G, Wilkinson RJ. Aberrant immunity: immunological analysis of the HIV–tuberculosis immune reconstitution inflammatory syndrome (TB–IRIS) [abstract]. Presented at the 4th meeting of the International AIDS Society, Sydney, Australia, 2007.
- 20.Meintjes G, Wilkinson K, van Veen K, Rebe K, Pepper D, Skolimowska K, Rangaka M, Seldon R, Maartens G, Wilkinson RJ. M. tuberculosis–specific T cell expansions and the pathogenesis of the HIV–TB-associated immune reconstitution inflammatory syndrome. Poster abstract 1006 presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2008.
- 21.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008;8:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis 2003;3:288–296. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Improving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings. Geneva: World Health Organization; 2006.
- 24.Rangaka MX, Diwakar L, Seldon R, van Cutsem G, Meintjes GA, Morroni C, Mouton P, Shey MS, Maartens G, Wilkinson KA, et al. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin Infect Dis 2007;44:1639–1646. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson KA, Stewart GR, Newton SM, Vordermeier HM, Wain JR, Murphy HN, Horner K, Young DB, Wilkinson RJ. Infection biology of a novel α-crystallin of Mycobacterium tuberculosis: Acr2. J Immunol 2005;174:4237–4243. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun 1995;63:1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 2004;170:65–69. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson RJ, Hasløv K, Rappuoli R, Giovannoni F, Narayanan PR, Desai CR, Vordermeier M, Paulsen J, Pasvol G, Ivanyi J, et al. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J Clin Microbiol 1997;35:553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson RJ, Vordermeier HM, Wilkinson KA, Sjölund A, Moreno C, Pasvol G, Ivanyi J. Peptide specific response to M. tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J Infect Dis 1998;178:760–768. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson RJ, Wilkinson KA, De Smet KAL, Haslov K, Pasvol G, Singh M, Svarcova I, Ivanyi J. Human T and B cell reactivity to the 16 kDa α crystallin protein of Mycobacterium tuberculosis. Scand J Immunol 1998;48:403–409. [DOI] [PubMed] [Google Scholar]
- 31.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc Natl Acad Sci USA 2001;98:7534–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart GR, Newton SM, Wilkinson KA, Humphreys IR, Murphy HN, Robertson BD, Wilkinson RJ, Young DB. The stress-responsive chaperone α-crystallin 2 is required for pathogenesis of Mycobacterium tuberculosis. Mol Microbiol 2005;55:1127–1137. [DOI] [PubMed] [Google Scholar]
- 33.Nicol MP, Pienaar D, Wood K, Eley B, Wilkinson RJ, Henderson H, Smith L, Samodien S, Beatty D. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin Infect Dis 2005;40:1301–1308. [DOI] [PubMed] [Google Scholar]
- 34.Connell TG, Shey MS, Seldon R, Rangaka MX, van Cutsem G, Simsova M, Marcekova Z, Sebo P, Curtis N, Diwakar L, et al. Enhanced ex vivo stimulation of Mycobacterium tuberculosis–specific T cells in HIV-infected persons via antigen delivery by the Bordetella pertussis adenylate cyclase vector. Clin Vaccine Immunol 2007;14:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, Mouton P, Diwakar L, Connell TG, Maartens G, et al. Effect of HIV-1 infection on T-cell–based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med 2007;175:514–520. [DOI] [PubMed] [Google Scholar]
- 36.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med 2006;173:803–810. [DOI] [PubMed] [Google Scholar]
- 37.Hougardy JM, Place S, Hildebrand M, Drowart A, Debrie AS, Locht C, Mascart F. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med 2007;176:409–416. [DOI] [PubMed] [Google Scholar]
- 38.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of FoxP3-expressing T regulatory cells during tuberculosis. J Exp Med 2007;204:2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, Reuter H, Ntwana N, Goemaere E. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS 2004;18:887–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.