Abstract
Stem cells divide asymmetrically, leading to self-renewal and the production of a daughter cell committed to differentiation. This property has engendered excitement as to the use of these cells for treatments. The majority of the work with stem cells has used the relatively accessible and well-characterized adult bone marrow stem cell compartment. Initially the focus of this research was on the potential for these stem cells to repair damaged organs by differentiating into epithelial cells to replace the injured areas. More recently it has become clear that engraftment of these stem cells as epithelial tissue is a rare event with perhaps limited clinical significance. Despite this, stem cells appear to have the ability to home to and be specifically recruited to areas of inflammation and injured tissues often characterized by excessive extracellular matrix deposition. As a consequence they are intimately involved in regions of physiological and pathological repair. Coupled with this, autologous hematopoietic stem cells, or the relatively immunoprivileged mesenchymal stem cells, can be expanded and engineered ex vivo and reintroduced without immunomodulation. The prospect of using such cells clinically as a cellular therapy holds much promise for many conditions and organ pathologies. Here we address the evidence for the incorporation of bone marrow stem cells into areas of stroma formation as a prelude to possible future treatment options for common lung diseases.
Keywords: stem cell, vector, gene therapy, bone marrow, chemokine
Recent research has suggested that bone marrow derived cells can incorporate into various tissues and in some cases take on characteristics of the tissue to which they have homed. The use of these cells to regenerate organs has been suggested and studies continue both in vitro and in vivo. Alternatively, allogenic bone marrow cells or genetically manipulated autologous cells can “replace” mutant genes in genetic deficiencies, and animal and small clinical studies have shown potential with published data in osteogenesis imperfecta (1, 2) and lysosomal storage diseases (3). Conceivably, if bone marrow cell engraftment was high enough, this approach could be used for the treatment of inherited lung diseases such as cystic fibrosis and α1-antitrypsin deficiency. A third use for these cells, however, is now being investigated. That is to use their capacity to home to and engraft in areas of damage to deliver a disease-modifying agent. It is the formation of tissue stroma by bone marrow cells that is the focus of this review, and the possibility that we could use these cells to modify the pathogenesis of common lung diseases and their clinical outcomes.
Lung cancer and idiopathic pulmonary fibrosis (IPF) are two such conditions for which new treatments are desperately needed. Lung cancer is the cancer associated with the greatest mortality in the world today, with limited therapeutic options and a survival rate of approximately 15% (4, 5). IPF is characterized by a progressive debilitating disorder with a 30% 5-year survival from diagnosis (6). In recent years it has been determined that bone marrow–derived stem cells are actively recruited to both of these lesions, suggesting a role as a treatment modality either by modulating their role in disease or by using them as a vector for treatment delivery.
STEM CELLS
Stem cells are cells that have unlimited self-renewal, meaning that they divide asymmetrically, both renewing themselves and producing a more differentiated daughter cell. Stem cells are traditionally divided into embryonic and adult stem cells. Embryonic stem cells are derived from the inner cell mass of the blastocyst of a developing embryo and are able to produce progeny of all cell lineages (ectoderm, mesoderm, endoderm). In contrast to the pluripotency of embryonic stem cells, the progeny of adult stem cells are classically thought to be lineage restricted. Adult stem cells are found in discrete niches within adult tissues and divide infrequently in the steady state, but have the potential to repair damaged tissues by replacing specific, specialized cells. The best-characterized adult stem cells are bone marrow–derived stem cells (BMSCs). BMSCs consist of hematopoietic stem cells (HSCs), which produce progenitors for all types of mature blood cells, and mesenchymal stem cells (MSCs), which differentiate into mature cells of the stromal tissue including fat, bone, and cartilage (7) (Table 1).
TABLE 1.
DEFINITIONS OF CELL TYPES
| Stem cell | Cells with unlimited self renewal, dividing asymmetrically to produce an identical daughter cell and a more differentiated progenitor. |
| Bone marrow stem cell | Comprises hematopoietic and mesenchymal stem cells. |
| Hematopoietic stem cell | Stem cell able to form all cells of the blood lineage. |
| Mesenchymal stem cell | Stromal stem cell able to produce supporting cells including bone, fat, and cartilage. |
| Fibroblast | Main mature cell type involved in the production of the extracellular matrix and collagen of tissues. |
| Myofibroblast | Fibroblasts can be activated (e.g., by transforming growth factor β) to form these cells, which produce extracellular matrix but also have the ability to contract. Stain for α-smooth muscle actin. |
| Fibrocytes | Circulating peripheral cells of bone marrow origin. Often described as blood-derived fibroblasts. Express a characteristic pattern of markers including the leukocyte common antigen CD45, the hematopoietic marker CD34, and collagen 1. |
The reparative potential and unlimited survival properties of stem cells has led to research in harnessing this potential to improve tissue repair. The primitive nature and pluripotent potential of embryonic stem cells would appear to make them a good candidate for future therapies with the ability to produce any differentiated cell necessary. The limited potency of adult stem cells would seem to restrict them to repair of cells of a specific lineage, for example the restoration of the immune system after bone marrow transplantation. However, the use of embryonic cells has met with moral, ethical, and political objections. Furthermore, embryonic stem cells have a greater tumorigenic potential than adult stem cells (8).
Adult stem cells, meanwhile, can be manipulated ex vivo and the cells used can be autologous, thus reducing the risk of immune rejection. Ethical objections are also not valid with the use of these cells. In addition, several studies over the last decade suggest that adult stem cells may have a greater potential than first realized, with BMSCs in particular able to produce differentiated cells not restricted to their lineage. Adult bone marrow cells have produced a variety of nonhematopoietic cells both in vitro and in vivo (9–13). This ability of adult cells to produce progeny crossing lineage barriers, adopting the phenotypes of other tissues, is termed “plasticity.”
PLASTICITY OF ADULT STEM CELLS AND CONTRIBUTION TO TISSUE STROMA
Many adult organs have limited regenerative capacity, and attempts were initially made to harness the potential of the bone marrow stem cell plasticity to mediate epithelial repair in injured organs. Experiments suggested that after transplantation, a single bone marrow stem cell had the potential to engraft as epithelial cells in many organs, including 20% of type 2 pneumocytes in the lung (9). Further studies demonstrated a reduction in injury after bone marrow stem cell administration (14, 15). However, the last few years have seen a re-evaluation, and there is an appreciation that the significant contribution of bone marrow cells to epithelial repair is maybe a function of methodological flaws and artifacts (16–18).
Despite the reassessment of the contribution of bone marrow–derived cells to the epithelial compartment in damage models, there remains strong evidence as to its contribution to areas of both physiological and pathological extracellular matrix deposition including wound healing, tissue stroma, and organ fibrosis. The fibroblasts that proliferate within fibrotic lesions were classically thought to be of resident tissue origin. Models describing the pathophysiology of fibrosis have developed to include other contributions to the fibroblast and myofibroblast communities within these fibrotic lesions. These include the possibility of epithelial to mesenchymal transition (EMT) and the significant contribution of fibroblasts and myofibroblasts from the bone marrow (19). Several bone marrow cell types have been suggested to contribute. Circulating fibrocytes (Table 1) have been described and shown to be important in both physiological and pathological repair (20–23). Chimeric mice with transplanted labeled bone marrow have demonstrated that bone marrow contributes to over 30% of the fibroblasts in a skin wound healing model (24, 25). In a bleomycin mouse model of lung fibrosis, 80% of type 1 collagen-expressing fibroblasts at the sites of lung fibrosis were shown to be of bone marrow origin (25, 26), and similar results have been found with paracetamol-induced lung injury (24). Finally, injection of exogenous, GFP-labeled, mesenchymal stem cells have also been shown to be recruited to irradiation-induced lung fibrosis, contributing as fibroblasts (27).
This bone marrow recruitment to areas of pathological fibrosis and wound healing may provide novel ways for treating these disorders. In addition, their involvement in areas of pathological repairs suggests the possible use of bone marrow–derived cells as vectors for directed treatments or gene therapy.
CONTRIBUTION TO TUMOR STROMA
Contrary to acting solely as a supporting structure, tumor stroma is integral to the behavior of the tumor (28), including cancer spread and growth. It is composed of fibroblasts and myofibroblasts, which produce extracellular matrix and the “desmoplastic reaction,” including endothelial cells involved in angiogenesis, and inflammatory cells (29–31). Myofibroblasts in the tumor stroma secrete growth factors and proteolytic enzymes that influence tumor invasion and progression (32). In some situations the presence of a tumor capsule has been shown to be protective, leading for example to improved prognosis in human hepatocellular carcinoma (33). Conversely, increased stroma and myofibroblast numbers have been associated with a worse prognosis (34–37), with the proliferative activity of stromal fibroblasts correlated to breast cancer metastasis (38). Further, in an in vitro study, myofibroblasts and fibroblasts, activated by irradiation, led to an increased invasiveness of pancreatic cancer cells in co-culture experiments (39).
As with repair and fibrosis, bone marrow–derived stem cells contribute to a desmoplastic response in the form of myofibroblasts and fibroblasts. Experiments tracking the fate of labeled bone marrow–derived cells after bone marrow transplant have shown BM-derived myofibroblasts and endothelial cells in a murine xenograft pancreatic tumor model (40), and an endogenous murine pancreatic cancer model in which up to 25% of the myofibroblasts were bone marrow derived (41). These results have been repeated in a range of xenograft tumor models, with the amount of tumor stroma and BM-derived cell contribution related to both the tumor cell type and the site of implantation (42). Furthermore, these bone marrow–derived cells appear to be functional with the demonstration of collagen production (43).
Tumor neovasculogenesis is one of the hallmarks of cancer, and a contribution of bone marrow–derived stem cells to the angiogenesis of tumors has also been demonstrated (44). Bone marrow cells (Sca1+) labeled and injected intravenously were shown to incorporate as endothelial-like cells into the periphery of a glioma (45). The importance of this contribution was illustrated by a decrease in tumor size and an increase in apoptosis when these bone marrow cells were transduced with the suicide gene (HSV-tk) (46). In contrast, other studies have only shown a minimal contribution of bone marrow cells to the newly formed tumor endothelium (47).
Evidence in humans of bone marrow contribution to tumors comes from sex-mismatched bone marrow transplants. Colorectal adenomas diagnosed 2 months after bone marrow transplantation consist of 1 to 4% bone marrow–derived cells displaying features of neoplastic colonic adenoma cells. A similar pattern, with up to 20% of the neoplastic cells of bone marrow origin, was found in a patient who developed lung cancer 4 years after transplant (48). A contribution to the tumor vasculature has also been demonstrated (49).
In addition to the incorporation of bone marrow–derived cells after whole bone marrow transplantation, mesenchymal stem cells alone have also been shown to have an ability to specifically target tumor tissue. In vitro migration studies have demonstrated a enhanced migration of MSCs toward tumor cells, in addition to just the conditioned medium from tumor cells (50–52), suggesting the role of soluble chemokines. Possible candidates include platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and stromal cell–derived factor 1α (SDF1 α), which have all demonstrated enhanced MSC migration in vitro (50). A variety of tumor models have also shown the ability of MSCs to incorporate into and proliferate within tumor stroma in vivo. Kaposi's sarcoma (53), colorectal cancer (51), glioma (50), breast metastases (54), and melanoma metastases (52, 54, 55) have all been used and showed consistent MSC incorporation when MSCs were delivered systemically. The incorporation of MSCs has been shown both in established tumors and in some cases when delivered coincidentally to the tumor cells (53). However, some authors have suggested that established tumors are necessary for the development of the neovascularization and the stromal-derived cytokines and growth factors that are essential to attract the circulating MSCs (56).
HOMING MEDIATORS
It is likely that the mechanism responsible for the homing of adult hematopoietic stem cells to injured tissue involves chemokine ligands and receptors in a similar fashion to the recruitment of leukocytes to areas of inflammation. The importance of the chemokine CXCL12 (SDF-1α) and its receptor CXCR4 has been well established for hematopoietic stem cells (57, 58). The chemokines responsible for homing and migration of mesenchymal stem cells are, however, less well characterized. Multiple authors have attempted to describe a definitive account of the functional chemokine receptors that are present on human MSCs and the chemokines and growth factors that have the greatest influence on MSC migration (59–61). There has been a large variability between the reports, which may be explained by the heterogeneity of the cell population. Despite this, the general consensus is that MSCs express a number of chemokine receptors likely to be involved in their homing capabilities (62), possibly with combination of growth factors and chemokines necessary for the maximal effect (63). Other studies have shown adhesion molecules enable extravasation in a fashion similar to that of leukocytes (64). It is important to note that the ability of MSCs to home and migrate appears to decrease during in vitro expansion in relation to their loss of surface expression of chemokine receptors (61, 65).
The contribution of the CXCL12 (SDF-1α)/CXCR4 axis to the recruitment of bone marrow–derived stem cells in lung fibrosis has been demonstrated in a number of studies. Increased CXCL12 (SDF-1α) levels and numbers of cells expressing CXCR4 have been shown in lung tissue samples of patients with idiopathic pulmonary fibrosis. In vitro, the migration of MSCs toward lung lysates exposed to bleomycin was blocked by a CXCR4 antagonist that was also able to reduce the amount of fibrosis in vivo (66). A similar murine bleomycin model of lung fibrosis was used to show that the number of bone marrow–derived fibrocytes in the injured lung and the resulting fibrosis could be reduced by the inhibition of CXCL12 (23). This study also, however, suggested the importance of other chemokine/receptor combinations, while in other studies secondary lymphoid chemokine (SLC)/CCR7 (26) and CCR2 (67) have been implicated in bone marrow cell homing to mouse models of lung fibrosis.
The cytokine CXCL12 (SDF-1α) may also be an important mediator of bone marrow cell recruitment to tumors, although the precise mechanism is unclear. The stroma surrounding breast cancer is a rich source of this chemokine (32). One in vitro study examined the differences in gene expression profiles between MSCs exposed to conditioned medium from tumor cells and bone marrow cells. It appeared that the CXCL12 (SDF-1α)/CXCR4 axis was important, but that the MSCs produced the chemokine, which then acted in an autocrine manner (51).
ADULT STEM CELLS AS THE PERFECT VECTOR? THE ADVANTAGES OF MESENCHYMAL STEM CELLS
The ability of these bone marrow–derived cells to specifically home to a wide range of pathological conditions such as organ fibrosis and tumors and then to incorporate into these areas suggest that they may be perfect vectors to deliver anti-fibrotic or oncological therapies. As a subgroup of the adult bone marrow stem cells, MSCs have several properties in addition to their homing capabilities that incline them toward a role as a vector. MSCs can be relatively easily transduced and expanded in culture for many passages, while retaining their growth and multi-lineage potential. They also seem to be relatively immunoprivileged due to their expression of major histocompatibility complex (MHC)1, but lack of MHC2, and the costimulatory molecules CD80, CD86, CD40 (68). This property may allow the delivery of allogeneic MSCs without prior immunomodulation.
Studies have demonstrated the potential of this approach. Human MSCs, engineered to express interferon β (IFN-β), have been used to provide targeted delivery of this potent antiproliferative and proapoptotic agent to gliomas (50) and metastatic breast (54) and melanoma models (54, 55). MSC-delivered IFN-β results in an increased survival in all these models. MSCs have also been transduced to express interleukin-12 (IL-12), with the rationale of improving the anti-cancer immune surveillance by activating cytotoxic lymphocytes, natural killer cells, and producing IFN-γ. In this model the IL-12–expressing MSCs were used before tumor inoculation and prevented the development of subcutaneous melanomas, hepatomas, and lung cancers (69). A similar approach was also used to reduce the metastastic load caused by the intravenous delivery of melanoma and colon cancer cell lines. In this case MSCs were transduced to express the immunostimulatory chemokine CX3CL1 (fractalkine) (52).
DIRECT EFFECTS OF STEM CELLS
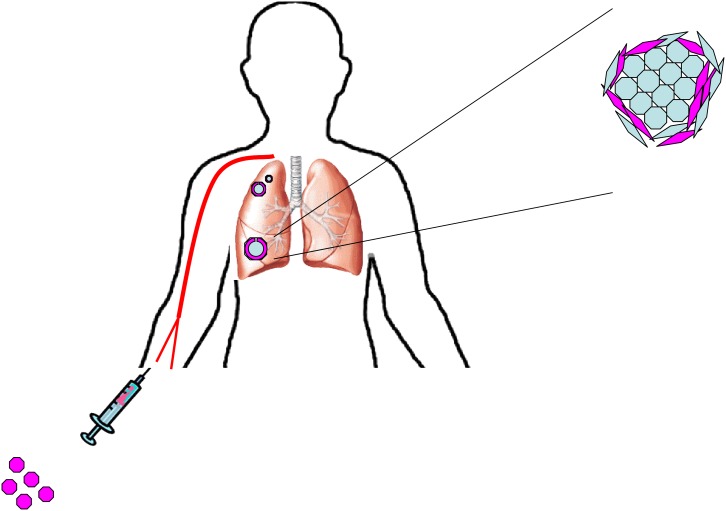
The use of exogenous MSCs as vectors for targeted delivery of therapies and genes in tumors and areas of fibrosis is promising (Figure 1); however, it is important to consider the roles of these cells in disease beyond their function as an inert vehicle.
Figure 1.
Bone marrow stem cells can be engineered to express antineoplastic drugs and then be delivered intravenously. These cells have been shown to home to tumors and incorporate into the tumor stroma. Manipulating this ability may provide new vectors for drugs in cancer therapy.
This is particularly true in cancer where, as described above, the tissue stroma is thought to have a direct influence on tumor progression. The addition of mesenchymal stem cells to human breast carcinoma cells in a mouse subcutaneous xenograft model led to an increased rate of metastasis. This was shown to be secondary to the secretion of CCL5 from the MSCs, promoting an increase in the motility, invasion, and metastasis of the breast cancer cells (70). A similar result demonstrating increased tumor progression was also shown with colonic tumor cells (71). MSCs have also been shown to have immunosuppressive effects, which may favor tumor growth in vivo, as demonstrated in a murine melanoma model (56). In contrast, MSCs have also been shown to have intrinsic antineoplastic properties, with an improvement in a Kaposis's sarcoma model secondary to the inhibition of Akt activity (53).
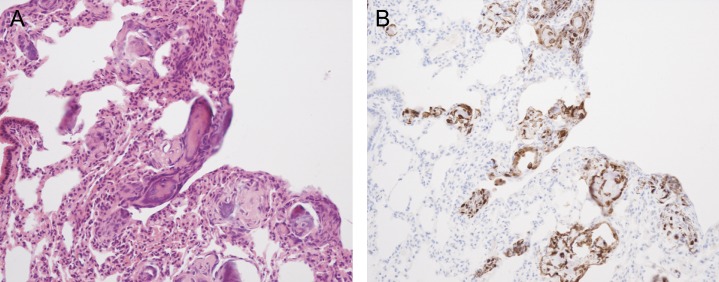
Furthermore, as well as affecting the behavior of cancer cells, there is some concern that these stem cells may themselves have malignant potential. Stem cells have the ability for self-renewal and unlimited proliferation, making them attractive candidates for malignant change. In vitro passaging of bone marrow stem cells has demonstrated the potential for the development of karyotype abnormalities (72), and systemically delivered murine MSCs have produced sarcomas (73) and osteosarcomas (74) (Figure 2). Malignant change of bone marrow–derived stem cells has also been implicated in a murine gastric carcinoma model. Helicobacter felis was used to create a chronic gastric injury, within which a carcinoma developed from bone marrow–derived cells (75).
Figure 2.
Murine mesenchymal stem cells (MSCs) generating tumors after intravenous injection. (A) Hematoxylin and eosin and (B) green fluorescent protein (GFP) imunostaining of mouse lungs 14 days after intravenous injection of murine MSCs expressing GFP showing the generation of osteosarcoma-like lesions. The murine MSCs were found to have karyotype abnormalities after only four in vitro passages.
The contribution of bone marrow–derived stem cells to the pathogenesis of organ fibrosis is equally as confused. A reduction in the recruitment of these bone marrow cells to areas of fibrosis by the removal of the chemotactic gradient demonstrated a reduction in the amount of fibrosis (23). Conversely, suppression of the bone marrow with busulphan led to a worsening in mice subjected to such insults (15), while systemic MSCs appear able to alleviate bleomycin lung fibrosis (14). Further studies examining precise cell types and chemotactic factors are imperative to dissect these issues.
CONCLUSIONS
The last few years have seen a re-evaluation of the potential of adult bone marrow stem cells as a future clinical treatment. Although the prospect of using them for direct epithelial repair now appears distant, their involvement in areas of injury and pathogenesis may still allow their use in disease. With the realization that caution is needed, the possibility of the use of bone marrow–derived cells as a cellular therapy in conditions such as lung cancer and idiopathic pulmonary fibrosis is exciting.
M.R.L. is an MRC Clinical Training Fellow. S.M.J. is an MRC Clinician Scientist.
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001;97:1227–1231. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999;5:309–313. [DOI] [PubMed] [Google Scholar]
- 3.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002;30:215–222. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 5.Giaccone G. Clinical impact of novel treatment strategies. Oncogene 2002;21:6970–6981. [DOI] [PubMed] [Google Scholar]
- 6.Perez A, Rogers RM, Dauber JH. The prognosis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S19–S26. [PubMed] [Google Scholar]
- 7.Bonnet D. Biology of human bone marrow stem cells. Clin Exp Med 2003;3:140–149. [DOI] [PubMed] [Google Scholar]
- 8.Orkin SH, Morrison SJ. Stem-cell competition. Nature 2002;418:25–27. [DOI] [PubMed] [Google Scholar]
- 9.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 10.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 11.Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci 2004;117:5655–5664. [DOI] [PubMed] [Google Scholar]
- 12.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249–252. [DOI] [PubMed] [Google Scholar]
- 13.Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 2004;172:1266–1272. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc 2006;3:193–207. [DOI] [PubMed] [Google Scholar]
- 17.Loebinger MR, Aguilar S, Janes SM. Therapeutic potential of stem cells in lung disease: progress and pitfalls. Clin Sci (Lond) 2008;114:99–108. [DOI] [PubMed] [Google Scholar]
- 18.Loebinger MR, Janes SM. Stem cells for lung disease. Chest 2007;132:279–285. [DOI] [PubMed] [Google Scholar]
- 19.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol 2007;39:666–671. [DOI] [PubMed] [Google Scholar]
- 20.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–7562. [DOI] [PubMed] [Google Scholar]
- 21.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389. [DOI] [PubMed] [Google Scholar]
- 23.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells 2003;21:514–520. [DOI] [PubMed] [Google Scholar]
- 25.Ishii G, Sangai T, Sugiyama K, Ito T, Hasebe T, Endoh Y, Magae J, Ochiai A. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells 2005;23:699–706. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:213–224. [DOI] [PubMed] [Google Scholar]
- 28.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol 2003;200:429–447. [DOI] [PubMed] [Google Scholar]
- 30.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol 2004;48:509–517. [DOI] [PubMed] [Google Scholar]
- 31.Direkze NC, Alison MR. Bone marrow and tumour stroma: an intimate relationship. Hematol Oncol 2006;24:189–195. [DOI] [PubMed] [Google Scholar]
- 32.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121:335–348. [DOI] [PubMed] [Google Scholar]
- 33.Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma: a pathologic study of 189 cases. Cancer 1992;70:45–49. [DOI] [PubMed] [Google Scholar]
- 34.Cardone A, Tolino A, Zarcone R, Borruto Caracciolo G, Tartaglia E. Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med 1997;39:174–177. [PubMed] [Google Scholar]
- 35.Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A. CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch 2002;440:128–133. [DOI] [PubMed] [Google Scholar]
- 36.Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R. CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch 2002;440:298–303. [DOI] [PubMed] [Google Scholar]
- 37.Barth PJ, Ramaswamy A, Moll R. CD34(+) fibrocytes in normal cervical stroma, cervical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cervix uteri. Virchows Arch 2002;441:564–568. [DOI] [PubMed] [Google Scholar]
- 38.Hasebe T, Sasaki S, Imoto S, Ochiai A. Proliferative activity of intratumoral fibroblasts is closely correlated with lymph node and distant organ metastases of invasive ductal carcinoma of the breast. Am J Pathol 2000;156:1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohuchida K, Mizumoto K, Murakami M, Qian LW, Sato N, Nagai E, Matsumoto K, Nakamura T, Tanaka M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res 2004;64:3215–3222. [DOI] [PubMed] [Google Scholar]
- 40.Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun 2003;309:232–240. [DOI] [PubMed] [Google Scholar]
- 41.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res 2004;64:8492–8495. [DOI] [PubMed] [Google Scholar]
- 42.Sangai T, Ishii G, Kodama K, Miyamoto S, Aoyagi Y, Ito T, Magae J, Sasaki H, Nagashima T, Miyazaki M, et al. Effect of differences in cancer cells and tumor growth sites on recruiting bone marrow-derived endothelial cells and myofibroblasts in cancer-induced stroma. Int J Cancer 2005;115:885–892. [DOI] [PubMed] [Google Scholar]
- 43.Direkze NC, Jeffery R, Hodivala-Dilke K, Hunt T, Playford RJ, Elia G, Poulsom R, Wright NA, Alison MR. Bone marrow-derived stromal cells express lineage-related messenger RNA species. Cancer Res 2006;66:1265–1269. [DOI] [PubMed] [Google Scholar]
- 44.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 2001;7:1194–1201. [DOI] [PubMed] [Google Scholar]
- 45.Anderson SA, Glod J, Arbab AS, Noel M, Ashari P, Fine HA, Frank JA. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood 2005;105:420–425. [DOI] [PubMed] [Google Scholar]
- 46.Ferrari N, Glod J, Lee J, Kobiler D, Fine HA. Bone marrow-derived, endothelial progenitor-like cells as angiogenesis-selective gene-targeting vectors. Gene Ther 2003;10:647–656. [DOI] [PubMed] [Google Scholar]
- 47.Dwenger A, Rosenthal F, Machein M, Waller C, Spyridonidis A. Transplanted bone marrow cells preferentially home to the vessels of in situ generated murine tumors rather than of normal organs. Stem Cells 2004;22:86–92. [DOI] [PubMed] [Google Scholar]
- 48.Cogle CR, Theise ND, Fu D, Ucar D, Lee S, Guthrie SM, Lonergan J, Rybka W, Krause DS, Scott EW. Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells 2007;25:1881–1887. [DOI] [PubMed] [Google Scholar]
- 49.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med 2005;11:261–262. [DOI] [PubMed] [Google Scholar]
- 50.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 2005;65:3307–3318. [DOI] [PubMed] [Google Scholar]
- 51.Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, Mayer-Kuckuk P, Glod J, Banerjee D. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells 2007;25:520–528. [DOI] [PubMed] [Google Scholar]
- 52.Xin H, Kanehira M, Mizuguchi H, Hayakawa T, Kikuchi T, Nukiwa T, Saijo Y. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells 2007;25:1618–1626. [DOI] [PubMed] [Google Scholar]
- 53.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med 2006;203:1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 2004;96:1593–1603. [DOI] [PubMed] [Google Scholar]
- 55.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 2002;62:3603–3608. [PubMed] [Google Scholar]
- 56.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:3837–3844. [DOI] [PubMed] [Google Scholar]
- 57.Chute JP. Stem cell homing. Curr Opin Hematol 2006;13:399–406. [DOI] [PubMed] [Google Scholar]
- 58.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999;283:845–848. [DOI] [PubMed] [Google Scholar]
- 59.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007;25:1737–1745. [DOI] [PubMed] [Google Scholar]
- 60.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem 2007;101:135–146. [DOI] [PubMed] [Google Scholar]
- 61.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006;24:1030–1041. [DOI] [PubMed] [Google Scholar]
- 62.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 63.Ozaki Y, Nishimura M, Sekiya K, Suehiro F, Kanawa M, Nikawa H, Hamada T, Kato Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev 2007;16:119–129. [DOI] [PubMed] [Google Scholar]
- 64.Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006;108:3938–3944. [DOI] [PubMed] [Google Scholar]
- 65.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003;17:160–170. [DOI] [PubMed] [Google Scholar]
- 66.Xu J, Mora A, Shim H, Stecenko A, Brigham KL, Rojas M. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol 2007;37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol 2004;32:414–425. [DOI] [PubMed] [Google Scholar]
- 69.Chen XC, Wang R, Zhao X, Wei YQ, Hu M, Wang YS, Zhang XW, Zhang R, Zhang L, Yao B, et al. Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis 2006;27:2434–2441. [DOI] [PubMed] [Google Scholar]
- 70.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557–563. [DOI] [PubMed] [Google Scholar]
- 71.Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 2006;80:267–274. [DOI] [PubMed] [Google Scholar]
- 72.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res 2005;65:3035–3039. [DOI] [PubMed] [Google Scholar]
- 73.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 2007;25:371–379. [DOI] [PubMed] [Google Scholar]
- 74.Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes SM. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells 2007;25:1586–1594. [DOI] [PubMed] [Google Scholar]
- 75.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science 2004;306:1568–1571. [DOI] [PubMed] [Google Scholar]