Abstract
BACKGROUND:
In older persons with heart failure (HF), body composition may influence physical function and treatment effectiveness. There is a lack of research concerning the associations between waist circumference (WC) or body mass index (BMI) and physical function in this population.
OBJECTIVE:
To determine whether BMI and WC are associated with physical function in older men and women with HF.
METHODS:
Seventy-one men and 36 women 65 years of age and older living with HF completed two surveys spaced three months apart. Height, weight, WC, time since diagnosis, edema, comorbidities and physical function were self-reported at baseline and follow-up. Physical function was determined using the physical component score of the Short Form-12 and the physical limitation domain (PLD) of the Kansas City Cardiomyopathy Questionnaire. Multivariate linear regression and analysis of covariance were used to evaluate the relationships between WC and BMI, as well as cross-classifications of WC and BMI with physical function, after adjusting for confounders and interactions.
RESULTS:
The cross-sectional and short-term follow-up analyses did not detect an association between WC or BMI and physical function, with the exception of changes in the PLD, which were significantly different across WC categories. Persons with a moderate WC experienced the greatest improvement in function. The physical component and PLD scores were lower than those reported by Canadians 75 years of age and older and stable HF patients, respectively. Women reported lower physical function scores than men.
CONCLUSION:
Findings from the present study indicate that older persons with HF, especially women, have poor physical functioning regardless of their WC or BMI.
Keywords: Body mass index, Elderly, Heart failure, Physical function, Waist circumference
Abstract
HISTORIQUE :
Chez les personnes âgées atteintes d’insuffisance cardiaque (IF), la composition corporelle peut influer sur la fonction physique et l’efficacité du traitement. Il n’y a pas assez de recherches sur les associations entre le tour de taille (TT) ou l’indice de masse corporelle (IMC) et la fonction physique au sein de cette population.
OBJECTIF :
Déterminer si l’IMC et le TT s’associent à la fonction physique chez les hommes et les femmes âgés atteints d’IF.
MÉTHODOLOGIE :
Soixante et onze hommes et 36 femmes de 65 ans et plus atteints d’IF ont rempli deux sondages à trois mois d’intervalle. Ils ont fourni leur taille, leur poids, leur TT, la période depuis le diagnostic, l’œdème, leurs comorbidités et leur fonction physique au début de l’étude et au suivi. Les auteurs ont déterminé leur fonction physique à l’aide de l’indice d’élément physique du formulaire court de 12 questions et du domaine de limitations physiques (DLP) du questionnaire sur la myocardiopathie de Kansas City. Ils ont utilisé la régression linéaire multivariée et l’analyse de covariance pour évaluer le lien entre le TT et l’IMC, ainsi que les classifications croisées du TT et de l’IMC avec la fonction physique après rajustement des variables confusionnelles et des interactions.
RÉSULTATS :
Les analyses transversales et à court terme n’ont permis de déceler aucune association entre le TT ou l’IMC et la fonction physique, à l’exception des changements de DLP, dont les différences étaient significatives selon les catégories de TT. Les personnes au TT modéré présentaient la plus grande amélioration de la fonction physique. Les indices d’élément physique et de DLP étaient plus faibles que ceux déclarés par des Canadiens de 75 ans et plus et par des patients stables atteints d’IF, respectivement. Les femmes ont déclaré des indices de fonction physique plus faibles que les hommes.
CONCLUSION :
D’après les observations de la présente étude, les personnes âgées atteintes d’IC, notamment les femmes, ont une mauvaise fonction physique, quel que soit leur TT ou leur IMC.
In Canada, the proportion of elderly (65 years of age and older) individuals is rising, with a projected increase of 14% by the year 2011 (1). With the growth in the proportion of elderly individuals, the prevalence of chronic illness, such as heart disease, diabetes and stroke, will increase. Heart failure (HF) currently affects approximately 400,000 Canadians (2). Older persons with chronic illness such as HF are at a greater risk for functional decline due to factors associated with their disease and the natural aging process. Those with HF often have symptoms that limit performance of regular activities of daily living and generally have a poor health-related quality of life.
Advancing age is associated with gains in fat mass and losses in skeletal muscle mass. In the elderly, a large waist circumference (WC) (3,4) and a high body mass index (BMI) (5) are independently associated with decreased physical function. For older persons with chronic health conditions such as HF, body composition may influence their level of function and treatment effectiveness. Obesity and muscle loss may precede or compound HF, and HF may, in turn, confound the normal decline in muscle tissue that occurs with aging (6,7).
Current evidence is emerging with regard to the relationships between body composition and physical function as they pertain to older persons with heart disease. In a longitudinal study of postmenopausal women with coronary artery disease (including HF) (8), it was found that after accounting for both WC and BMI, a large WC was associated with an increased risk of mortality, while a high BMI was associated with a decreased risk. It was subsequently postulated that because BMI is a measure of total body mass, after adjusting for WC, it may be an indicator of lean body mass (8–10). A number of cross-sectional studies have shown that older men and women with low muscle mass or high fat mass experience lower levels of physical function than those with normal muscle and fat mass (11–13). There has been little longitudinal evidence to establish temporality between the onset of muscle loss and fat gain and the decline of physical function. Results have been conflicting, with a causal relationship identified in some (14) and not seen in others (15).
Sex differences exist with regard to the amount and distribution of skeletal muscle mass and fat mass, as well as the effects on physical function. Women with high fat mass and/or low muscle mass generally experience lower levels of physical function than their male peers. As a result, low muscle mass and obesity are hypothesized to be greater public health concerns in women due to a ‘survivor effect’, because women generally tend to live longer, have higher rates of disability, have lower lifetime levels of physical activity, and because older men represent a selectively healthier group (3,16).
To date, there have been very few studies examining the association between low muscle mass or low BMI combined with high fat mass or large WC and physical functioning in older persons. The literature has generally reinforced the idea that a combination of a loss in muscle mass and strength and the gain of excess fat mass reinforce each other and act synergistically to create greater reductions in physical function than either condition alone (11,17). Individuals with HF can experience rapid changes in their physical function (18). Few studies have examined the combined effects of WC and BMI longitudinally in the elderly population and none have looked at its effects on physical function in older persons with HF.
Optimizing functional well-being and quality of life is a treatment goal for HF; however, there is little understanding of the factors that influence physical function in this population. It was hypothesized that a large WC and a high WC to BMI ratio are associated with low levels of physical function, and that short-term changes, if they occur, correspond to declines in function. As a result, the objectives of the present study were to cross-sectionally determine the associations between BMI and WC with physical function, and determine whether the changes in HF symptoms and physical function, if present, were influenced by BMI and WC over a short-term (three-month) follow-up in a cohort of older persons living with HF.
METHODS
Study population
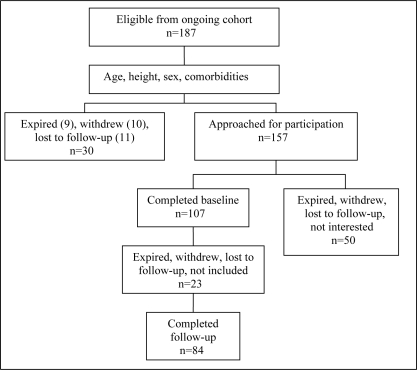
Participants in the present study included adults 65 years of age and older with a diagnosis of HF. In brief, 187 men and women were recruited from five emergency departments in southeastern Ontario following an exacerbation of their HF symptoms. Eligible participants were noninstitutionalized, able to communicate in English, have access to a phone and able to provide informed consent. The diagnosis of HF was validated using the Framingham criteria for the clinical diagnosis of HF (19) or a documented ejection fraction of less than 40%. Of the 187 eligible to participate in the present study, 107 completed the baseline questionnaire and 84 completed the three-month follow-up. Figure 1 illustrates the participant flow in the study. The Queen’s Health Sciences Research Ethics Board approved the project and each study site received ethics approval through their respective board. Written, informed consent was obtained from all participants.
Figure 1).
Flow diagram of study participant selection
Exposure variables
WC:
WC was used as a measure of obesity, analyzed as a continuous variable and categorized using Lean et al’s (20) WC action levels: small WC (less than 94 cm in men and less than 80 cm in women); moderate WC (94 cm to 101 cm in men and 80 cm to 87 cm in women); and large WC (102 cm or more in men, and 88 cm or more in women). Participants received written instruction on how to measure their WC, including an illustration and a MyoTape tape measure (AccuFitness, USA). The MyoTape forms a complete loop and lies firmly around the waist using a spring-lock mechanism, making it appropriate for frail individuals and those with large waists. Instructions for waist measurement directed participants to measure their WC to the closest one-quarter inch at one-half the distance between the last rib and the iliac crest. Measurements were later converted to cm. Participants self-measured their WC for seven days in the morning before breakfast, and an average of the seven measurements was used.
BMI:
BMI was calculated using self-reported weight divided by height squared; height was self-reported at baseline. Weight was collected at baseline and follow-up. Participants were asked to weigh themselves in the morning before breakfast for seven days in a row and record this information in a log sheet. An average of the seven days was used to calculate BMI. BMI was analyzed as a continuous variable and categorized according to the following World Health Organization (21) and Health Canada (22) categories: underweight (less than 18.5 kg/m2), normal weight (18.5 kg/m2 to 24.9 kg/m2), overweight (25.0 kg/m2 to 29.9 kg/m2) and obese (30 kg/m2 or more). The relationship between BMI and physical function was also examined using tertiles of BMI. On its own, BMI is a recognized correlate of total fat and measure of obesity status (22). However, after controlling for WC, it has been postulated by a number of researchers (9,10) that BMI is a marker of lean mass and skeletal muscle content. In the present study, a lower BMI was considered to represent less muscle mass than a high BMI after controlling for WC.
Cross-classification of BMI and WC:
Cross-classifications of WC action levels and BMI categories (eg, high-risk WC and normal weight BMI) were performed. Cross-classifications provide a means of comparing individuals who possess greater central obesity relative to their total body mass (a large WC with a normal BMI) with those who had lower central obesity relative to their total body mass (low-risk WC and overweight BMI).
Outcome measures
The physical component scale (PCS) of the Short Form-12 (SF-12) and the physical limitation domain (PLD) of the Kansas City Cardiomyopathy Questionnaire (KCCQ) were collected as measures of physical function. The SF-12 provides a generic measure of physical function that provides a basis of comparison with other nonclinical populations, and the KCCQ is an HF-specific measure sensitive to changes in clinical status. During the baseline and follow-up examinations, participants completed the brief, self-administered SF-12 and KCCQ.
PCS of the SF-12:
The SF-12 measures functioning and well- being in two health status domains – physical functioning and emotional functioning (23,24). Items from the physical function domain were scored to form the PCS. The PCS of the SF-12 has been validated and shown to be a reliable measure of physical function (25). The PCS of the SF-12 is transformed into scores of 0 to 100, with an average score for the Canadian population previously identified as 42 in those 75 years of age and older (26). The score was analyzed as a continuous variable, and a change score was calculated as the difference between baseline and follow-up. A change in the PCS of greater than two points in either direction was considered meaningful (24).
Eight participants were missing items from the SF-12, the majority missing one or two items. Missing item-level data and PCS scores were imputed using a single imputation and the Markov chain Monte Carlo method (27). Imputation fills in missing data with a set of plausible values that represent the uncertainty about the correct value. Several imputations were analyzed and it was deemed that a single imputation gave very similar results to multiple imputations. This is a valid means of imputation when six items or fewer are missing from the SF-12 (28).
PLD of the KCCQ:
The KCCQ consists of 23 items that quantify the following domains: physical limitation, symptoms, quality of life, social interference and self-efficacy (29). The PLD of the KCCQ is a valid and reliable measure of physical function (29). KCCQ responses are transformed into scores from 0 to 100, a higher score indicating better physical function. A Canadian norm has not been identified; however, in stable HF patients, the mean domain scores have been reported to be 64 (29). The domain was analyzed as a continuous variable, and a change score was calculated using the difference between the follow-up and baseline scores. A clinically significant change is defined as a change greater than five points in either direction (30). One participant was missing baseline items and two were missing follow-up items; those missing more than three items from the domain were not used in the analyses.
Covariates
Variables that were independently associated with the exposure and outcome measures in the literature were analyzed as potential confounding variables and effect modifiers.
Age and sex:
Age was included in the multivariate analysis as a continuous variable. Sex was coded as 0 for men and 1 for women.
Time since diagnosis:
Time since diagnosis was self-reported at baseline and categorized as ‘less than one month’, ‘greater than one month but less than six months’, ‘greater than six months but less than one year’, ‘one to five years’ or ‘greater than five years’.
Edema:
Edema was self-assessed using a self-report descriptor method and recorded using a seven-day log to control for the effects of edema on weight and WC. The descriptor method uses a scale adopted by the Canadian Congestive Heart Failure Clinics Network (31) and uses a 0 to 4 scale, with 0 representing no edema and 4 representing edema above the thigh (increased severity).
Comorbidity:
Comorbid diagnoses were obtained by a self-reported functional comorbidity index (FCI) designed to predict physical functional capacity (32,33). The FCI contains diagnoses associated with physical function and has been validated on a cross-sectional database of 9423 Canadian adults, using the SF-36 physical function subscale as the outcome (32). An FCI score of 0 indicates no comorbidity and increases up to 18 with the addition of each comorbidity. BMI higher than 30 kg/m2 was not used in the score.
Smoking:
At baseline, participants were asked to classify themselves as a ‘current smoker’, a ‘recent quitter’ (more than four weeks, but less than five years), a ‘remote quitter’ (five years or more) or ‘never a smoker’.
Statistical analysis
All analyses were conducted using SAS version 9.1 (SAS Institute Inc, USA). Baseline and change score descriptive statistics were performed. Paired t tests were used to identify significant changes in the anthropometric measures and physical function scores.
Multivariate linear regression was used to assess relationships between continuous baseline BMI and WC, and the PCS and PLD at baseline (n=107). Potential confounders were identified through their associations with the dependent and independent variables of interest. Stepwise selection was performed using a significance level of 0.25. Age and sex showed colinearity with many of the anthropometric variables, and were subsequently centred to increase the predictive ability of the models. Parameter estimates and P values were calculated for each of the independent variables, and R2 was calculated for each model.
Analysis of covariance (ANCOVA) was used to assess whether the relationships between the anthropometric measurements and physical function differed across BMI (controlling for WC) and WC at baseline (n=107) and whether differences between the categories were present for the PCS or PLD (n=84). Two-way ANCOVA was used to assess the cross-classification of WC and BMI and was also performed using cross-sectional data and follow-up changes in the PCS and PLD. Effect modifiers were identified by significant interaction terms. Potential confounders were identified by their ability to change the regression slopes by a minimum of a 10%. Respective confounders and effect modifiers were tested for in each ANCOVA model. P values for each category were calculated to determine whether statistical differences in physical function scores occurred between categories. Changes in WC and BMI were not examined because a preliminary study in this population reported large limits of agreement between self-measured and technician-measured BMI (34) and WC (35), suggesting that self-report of these variables is not sensitive enough for monitoring small, individual changes.
RESULTS
Participant characteristics
Baseline sample characteristics for the 107 participants are shown in Table 1. The age range was 65 to 93 years, and participants reported between one and 10 comorbidities. The most reported comorbidities included visual impairment (53%), arthritis (51%), angina (51%), hearing impairment (36%) and diabetes (36%). Those who participated in the baseline study did not differ significantly from those who did not participate by age (mean of 77.9 years versus 78.8 years; P=0.41) or ejection fraction (43% versus 45%; P=0.43). However, those who did participate were more likely to be male (34% versus 23%; P=0.04). Respondents and nonrespondents of the short-term, three-month follow-up were not statistically significantly different by age, sex, weight, comorbidity, smoking status, WC, BMI, PCS or PLD. However, respondents were more likely to engage in current physical activity than nonrespondents (P=0.03). Only two participants were enrolled in cardiac rehabilitation, and of those who did participate in physical activity, almost all were doing so on their own. One individual was placed in the underweight BMI category, 37 (35%) were considered to be of normal weight, 41 (38%) were overweight and 27 (25%) were obese. As a result of the small cell size, the underweight category was not further examined in the present study. Using the WC action levels, 30 participants (28%) at low risk, 26 participants (24%) at moderate risk and 51 participants (48%) at high risk were identified. The mean PCS was well below the Canadian norm of 42 for those 75 years of age and older (26), and the mean PLD was below 64, a previously reported mean in a group of stable HF patients (29). Mean physical function scores were significantly different between men and women; men reported higher levels of physical function than women using the PCS (34.6 versus 29.6, respectively; P=0.01) and the PLD (60.0 versus 48.2, respectively; P=0.03).
TABLE 1.
Baseline participant characteristics
| Characteristics | Total (n=107) |
|---|---|
| Male sex, n (%) | 71 (66) |
| Age, years | 78.5±6.7 |
| Weight, kg | 78.8±18.3 |
| Body mass index, kg/m2 | 27.3±5.6 |
| Waist circumference, cm | 98.4±15.1 |
| Functional Comorbidity Index score (range) | 5.2±2.1 (1–10) |
| Level of edema (range) | 0.6±1.1 (0–4) |
| Physical component score of SF-12 (range) | 32.9±9.6 (15.3–55.5) |
| Physical limitation domain of KCCQ* (range) | 56.0±26.7 (0–100) |
| Smoking status, n (%) | |
| Current smoker | 2 (2) |
| Quit recently (>4 weeks, <5 years) | 4 (4) |
| Quit remotely (≥ 5 years) | 64 (60) |
| Never a smoker | 37 (34) |
| Prediagnosis physical activity level, n (%) | |
| Much more active than friends | 24 (22) |
| Slightly more active than friends | 24 (22) |
| Same as friends | 39 (37) |
| Slightly less active than friends | 8 (8) |
| Much less active than friends | 12 (11) |
| Current exercise program, n (%) | |
| Organized exercise program | 2 (2) |
| Cardiac rehabilitation | 2 (2) |
| On their own ≥3 times per week | 48 (45) |
| None | 55 (51) |
| Time since diagnosis, n (%) | |
| <1 month | 8 (8) |
| 1 to 6 months | 24 (22) |
| >6 months to <1 year | 13 (12) |
| 1 to 5 years | 31 (29) |
| >5 years | 31 (29) |
Data are presented as mean ± SD unless otherwise stated. *n=106. KCCQ Kansas City Cardiomyopathy Questionnaire; SF-12 Short Form-12
Cross-sectional (baseline) analyses
Bivariate results of continuous BMI and WC variables showed no significant associations between BMI or WC, or the PCS and the PLD, suggesting that the relationships may not be linear. Bivariate results for BMI and WC categories demonstrate the variation in the PCS and PLD, although not significant, between cross-classification categories. Results from the multivariate linear regression analyses identified no significant associations between WC or BMI (adjusting for WC), or the PCS and the PLD, confirming that the relationships are not linear. Power was calculated based on the multivariate linear regression model using WC as the key exposure of interest and the PCS as the outcome of interest. This model achieved 8% power to detect a linear association between baseline WC and PCS scores.
Results from ANCOVA (Table 2) identified no statistically significant differences in the PCS or PLD across BMI (controlling for WC) or WC categories. Two-way ANCOVA results showed no statistical differences between BMI and WC cross-classifications (Table 3). It is noteworthy that the PCS and PLD were lower than the mean scores reported for older Canadians (26) and HF patients (29), respectively, irrespective of BMI and WC categories.
TABLE 2.
Mean physical function scores at baseline and change scores across body mass index categories and waist circumference action levels
| Mean PCS at baseline (n=107) | P* | Mean PlD at baseline (n=106) | P* | Mean change in PCS (n=84) | P* | Mean change in PlD (n=78) | P* | |
|---|---|---|---|---|---|---|---|---|
| Body mass index category† | ||||||||
| Normal | 34.0±10.6 | 55.1±30.8 | –0.02±8.4 | 8.1±20.3 | ||||
| Overweight | 33.0±9.3 | 0.72 | 59.9±24.3 | 0.39 | 1.4±8.0 | 0.62 | –0.73±21.4 | 0.26 |
| Obese | 31.4±9.1 | 51.7±24.6 | –0.3±7.2 | –1.1±19.3 | ||||
| Waist circumference action level | ||||||||
| Low | 33.1±9.2 | 62.1±24.4 | –0.1±7.5 | –0.4±20.1 | ||||
| Moderate | 34.0±9.7 | 0.99 | 50.7±33.4 | 0.36 | 0.3±9.1 | 0.67 | 12.9±25.8 | 0.01 |
| High | 32.3±9.7 | 55.1±23.8 | 1.5±7.7 | –2.1±13.7 | ||||
*P for independent variable category from one-way analysis of covariance models;
†Controlling for waist circumference. PCS Physical component score; PLD Physical limitation domain
TABLE 3.
Mean physical function scores at baseline and change scores across body mass index and waist circumference cross-classification categories
| Body mass index category (kg/m2)
|
P
|
|||||
|---|---|---|---|---|---|---|
| Normal | Overweight | Obese | Waist circumference | Body mass index | P* | |
| Mean physical component score at baseline (n=106) | ||||||
| Low | 33.82±10.17 | 30.27±4.83 | N/A | |||
| Moderate | 34.68±12.53 | 34.20±6.56 | 23.64† | 0.54 | 0.3 | 0.47 |
| High | 31.68±7.29 | 32.95±11.33 | 31.66±9.11 | |||
| Mean physical limitation domain at baseline (n=105) | ||||||
| Low | 62.68±25.99 | 63.20±19.62 | N/A | |||
| Moderate | 42.71±37.22 | 61.09±27.45 | 12.50† | 0.75 | 0.99 | 0.9 |
| High | 41.67±23.57 | 58.30±24.38 | 53.23±23.71 | |||
| Mean change in physical component score (n=84) | ||||||
| Low | 2.16±8.08 | 0.62±6.94 | –0.71±8.01 | |||
| Moderate | –3.99±8.42 | 4.44±8.45 | 0.65† | 0.56 | 0.58 | 0.15 |
| High | 0.32† | 0.19±7.98 | –0.38±7.36 | |||
| Mean change in physical limitation domain (n=78) | ||||||
| Low | 0.69±14.09 | –10.97±10.01 | –0.94±15.80 | |||
| Moderate | 23.70±23.66 | 3.25±24.77 | N/A | 0.01 | 0.09 | 0.73 |
| High | N/A | 0.35±21.97 | –1.14±19.29 | |||
*Interaction between waist circumference action levels and body mass index categories from two-way analysis of covariance models;
†SD data for these participants were not available (N/A)
Short-term follow-up analyses
Mean changes from baseline to follow-up were not significantly different for BMI (0.04±0.67 kg/m2), WC (0.29±3.84 cm), the PCS (0.29±7.89) or the PLD (2.74±20.01). The majority of participants experienced either an absolute increase or decrease in their physical function scores; one individual experienced no change in PCS and 11 experienced no change in PLD. Seventy-one per cent of participants experienced a clinically significant change in the PCS and 69% experienced a clinically significant change in the PLD. Of those who experienced a clinically significant change in either physical function score, the sample was split evenly, with one-half experiencing an increase and one-half experiencing a decrease.
Multivariate linear regression results indicated no associations between baseline BMI or WC and changes in either of the physical function scores after adjusting for confounders and effect modifiers. Results using baseline categories of the anthropometric measures and mean change scores for physical function saw no statistically significant differences across BMI or WC and BMI cross-classification categories (Tables 2 and 3). However, there was a difference in PLD change scores across the WC action levels. Individuals with a moderate-risk WC saw a 20-point greater increase in their PLD over the three months than those with a low-risk WC (P<0.01). Those with a high-risk WC did not, however, see a significant increase over the three months compared with the low-risk group (P=0.63). Controlling for age, comorbidity, changes in exercise, prediagnosis physical activity, time since diagnosis and average edema levels did not impact the differences between the levels (P=0.01).
Results were consistent for the cross-sectional and follow-up analyses when tertiles of BMI and WC were held in place of predefined BMI and WC categories.
DISCUSSION
Our sample consisted of older men and women with HF for whom physical function is an important component of daily living. We assessed the influences of WC and BMI on physical function in this group as a key outcome for individuals living with the condition. The primary finding of the present study was that older persons with HF experience poor physical function irrespective of their body composition, with women reporting lower levels than men. Most participants were considered to be overweight or obese based on either BMI (64%) or WC (72%), and experienced clinically significant changes in physical function over the short study period. Furthermore, in this group, a moderate WC was associated with an increase in physical function above those with a low WC.
The prevalence of obesity in our sample was higher than that reported for age-similar Canadians (48% versus 24%) (36). This difference may be partially explained by the biological relationship between obesity and the development of HF, as well as the use of WC action levels in lieu of BMI to identify obesity. In the elderly, WC may be better able to diagnose obesity as a result of an age-related redistribution of fat from the periphery to the abdomen (37). Therefore, the use of WC for detection, in combination with the fact that obesity is a risk factor for the development of HF (38,39), might have resulted in the higher rates seen in this sample.
Both the cross-sectional and follow-up analyses did not detect a relationship between WC or BMI and physical function when examining WC and BMI continuously or using categories. In general, previous cross-sectional and longitudinal studies have reported that older adults with a high WC (40,41) or those with an obese BMI (30 kg/m2 or more) (5,41,42) are at a two to three times greater risk of functional impairment. The findings of the present study suggest that in older persons with HF, the effects of obesity may not influence physical function to the same degree as in older adults without this chronic illness. High body fat is likely an important factor, but the symptoms and disease condition of HF may overshadow their effects.
Our findings also identified that individuals with a moderate WC had significantly greater improvements in their PLD than those with a low WC; this relationship was absent in those with a large WC. It is likely that we were able to see a difference in the PLD and not the PCS because it is a disease-specific measure of physical function and is therefore more sensitive to changes related to HF. This suggests that HF symptoms, such as shortness of breath and fatigue, may be more sensitive to changes in body composition, although this requires further study. Findings in the present study differ from those reported by Lean et al (43), who found a linear negative relationship between WC action levels and physical function. Differences between study findings may be attributed to the differences in the study samples. The previous study involved participants who were younger than 60 years of age, whereas our sample consisted of older persons with HF.
We used BMI while controlling for WC as a marker of lean body mass content and observed no significant relationships with physical function. Our findings are consistent with those previously reported using data from the Cardiovascular Health Study (15), which identified that the lowest quintile of muscle mass was not associated with an increased likelihood of reporting difficulty in instrumental activities of daily living in cross-sectional and longitudinal analyses. However, there is also a large contingency of literature reporting that older men and women with low skeletal muscle mass experience lower levels of physical function compared with those with a normal muscle mass (11,13,14,44,45). Discrepancies between study findings may largely be explained by differences in definitions and measurement of lean mass and physical function, as well as the populations studied. In the present sample of older persons with HF, we estimated lean body mass using a combination of BMI and WC. The use of BMI, while accounting for WC, does not accurately account for lean body mass, but provides a raw estimate. Therefore, our method may have introduced misclassification as a result of the inaccuracy of BMI after controlling for WC as a measure of lean mass.
Previous studies generally support the hypothesis that low muscle mass combined with high fat mass is associated with decreased physical function (11,17). However, the cross-sectional and follow-up analyses of the present study found no differences in physical function scores across WC and BMI cross-classification categories. The present findings may be accounted for by the fact that very few participants were identified as having a low lean-to-fat mass content, thereby not allowing us to see any associations that might have existed.
Our results generally showed a lack of association between BMI or WC and physical function, which is contrary to what is reported in the literature. The study sample consisted of elderly persons with HF that self-reported low physical function compared with similar populations. It is postulated that obesity, which might have preceded the development of HF, had the potential to lower physical function, and HF, with its clinical manifestations, led to further losses in this already compromised group. Given the low scores in our study, it would have been difficult for BMI and/or WC to have an additional effect. We saw a significant increase in HF-related physical function in the moderate WC group compared with those with a low WC. At baseline, this group possessed the lowest PLD score and had the greatest room for improvement. Our findings identified that in this older group of persons with HF, the impact of body composition on physical function is questionable, perhaps due to the low levels of function experienced as a result of their chronic condition. A further explanation for our findings, and a very concerning trend, was the negligible participation in cardiac rehabilitation – a program aimed at increasing physical function in cardiac populations. It is important to realize that a number of other factors that were not measured here (eg, other health behaviours, nutrition, inflammatory markers, disease severity and access to resources) can alter the associations between WC, BMI and physical function.
Limitations
Our study has limitations that should be recognized. First, the study’s sample size was likely too small to identify possible differences between the anthropometric categories and physical function scores. Second, because we relied on self-measured anthropometric variables and self-reported physical function, the variables are subject to error. However, self-measured WC and body weight have been shown to be both reliable and valid methods in epidemiological settings (unpublished data; 46), and the physical function measures have shown to be accurate reflections of measured physical function (25,29). Third, our method for defining lean mass and fat mass body content involved some interpretation. Due to the nature of the population, we used simple anthropometric measures to estimate body composition. WC has been shown to be a better measure of visceral adipose tissue than BMI (10), and with age, fat redistributes to the abdominal region, rendering BMI a suggested indicator of lean mass rather than fat mass after controlling for WC (9,10). In addition, the sample used to develop the WC action levels that were applied consisted of individuals 25 to 74 years of age and potentially limits the application of these categories in our cohort. Finally, we were not able to see any significant changes in mean physical function scores over the follow-up, which is likely due to the short time between assessments.
CONCLUSION
Results from the present study identified that in older men and women with HF, the relationships between fat and muscle mass and physical function are nonlinear, and a moderate WC may predict improvements in HF-related physical function. Further larger and longer-term studies to assess the associations between body composition and physical function in older persons with HF are needed. Findings identified that older persons with HF, especially women, have poor physical functioning regardless of their body composition.
Acknowledgments
The authors are grateful to all participants. Support for this study was partially provided by the Canadian Institute of Health Research, grant number MOP-68891. S Prince received support from an Ontario Graduate Scholarship.
REFERENCES
- 1.Moore EG, Rosenberg MW, Fitzgibbon SH. Activity limitation and chronic conditions in Canada’s elderly, 1986–2011. Disabil Rehabil. 1999;21:196–210. doi: 10.1080/096382899297620. [DOI] [PubMed] [Google Scholar]
- 2.Congestive heart failure – an epidemic we can avoid. Heart and Stroke Foundation of Canada, 2002. <ww2.heartandstroke.ca/Page.asp?PageID=33&ArticleID=1664&Src=news&From=SubCategory> (Version current at June 11, 2008).
- 3.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–9. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 4.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins KR. Obesity’s effects on the onset of functional impairment among older adults. Gerontologist. 2004;44:206–16. doi: 10.1093/geront/44.2.206. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85:51–66. doi: 10.1016/s0167-5273(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 7.Roubenoff R, Castaneda C. Sarcopenia – understanding the dynamics of aging muscle. JAMA. 2001;286:1230–1. doi: 10.1001/jama.286.10.1230. [DOI] [PubMed] [Google Scholar]
- 8.Kanaya AM, Vittinghoff E, Shlipak MG, et al. Association of total and central obesity with mortality in postmenopausal women with coronary heart disease. Am J Epidemiol. 2003;158:1161–70. doi: 10.1093/aje/kwg271. [DOI] [PubMed] [Google Scholar]
- 9.Bigaard J, Tjønneland A, Thomsen BL, Overvad K, Heitmann BL, Sørensen TI. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res. 2003;11:895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 10.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–8. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner RN. Body composition in healthy aging. Ann NY Acad Sci. 2000;904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 12.Rolland Y, Lauwers-Cances V, Cournot M, et al. Sarcopenia, calf circumference, and physical function of elderly women: A cross-sectional study. J Am Geriatr Soc. 2003;51:1120–4. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I. Influence of sarcopenia on the development of physical disability: The Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 15.Visser M, Langlois J, Guralnik JM, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: The Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–90. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R, Hughes VA. Sarcopenia: Current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–24. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 18.Hauptman PJ, Masoudi FA, Weintraub WS, Pina I, Jones PG, et al. Variability in the clinical status of patients with advanced heart failure. J Card Fail. 2006;10:397–402. doi: 10.1016/j.cardfail.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee DS, Tran C, Flintoft V, Grant FC, Liu PP, Tu JV. CCORT/CCS quality indicators for congestive heart failure care. Can J Cardiol. 2003;19:357–64. [PubMed] [Google Scholar]
- 20.Han TS, van Leer EM, Seidell JC, Lean EJ. Waist circumference action levels in the identification of cardiovascular risk factors: Prevalence study in a random sample. BMJ. 1995;311:1401–5. doi: 10.1136/bmj.311.7017.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Body Mass Index World Health Organization Nutrition and Food Security 2006. <www.euro.who.int/nutrition/20030507_1> (Version current at June 11, 2008).
- 22.Health Canada . Catalogue no: H49-179/2003E. Ottawa: Minister of Public Works and Government Services Canada; 2003. Canadian guidelines for body weight classification in adults. [Google Scholar]
- 23.SF-36.org The SF-12: An even shorter health survey The SF Community 2005. <www.sf-36.org/tools/sf12.shtml> (Version current at June 11, 2008).
- 24.Ware JE, Kosinki M, Keller SD. 2nd edn. Boston: The Health Institute, New England Medical Centre; 1995. SF-12: How to score the SF-12 physical and mental health summary scales. [Google Scholar]
- 25.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Hopman WM, Towheed T, Anastassiades A, et al. Canadian normative data for the SF-36 health survey. CMAJ. 2000;163:265–71. [PMC free article] [PubMed] [Google Scholar]
- 27.SAS Institute Inc The Markov chain Monte Carlo method for arbitrary missing data. 2003.
- 28.Liu H, Hays RD, Adams JL, et al. Imputation of SF-12 health scores for respondents with partially missing data. Health Serv Res. 2005;40:905–21. doi: 10.1111/j.1475-6773.2005.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman S.KCCQ Interpretability Cardiovascular Outcomes Inc 2004. <www.cvoutcomes.org/topics/one-page?page_id=833&topic=The%20Kansas%20City%20Cardiomyopathy%20Questionnaire&page_topic_id=1318> (Version current at June 11, 2008).
- 31.Staples P. Edema rating scale for patients with heart failure used in the Canadian Congestive Heart Failure Clinic Network database. Canadian Congestive Heart Failure Clinic Network. 2005.
- 32.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 33.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: A critical review of available methods. J Clin Epidemiol. 2003;56:221–9. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 34.Lawlor DA, Bedford C, Taylor M, Ebrahim S. Agreement between measured and self-reported weight in older women. Results from the British Women’s Heart and Health Study. Age Ageing. 2002;31:169–74. doi: 10.1093/ageing/31.3.169. [DOI] [PubMed] [Google Scholar]
- 35.Prince SA, Janssen I, Tranmer JE. Self-measured waist circumference in older patients with heart failure: A study of validity and reliability using a MyoTape. J Cariopulm Rehabil Prev. 2008;28:43–7. doi: 10.1097/01.HCR.0000311508.39096.a5. [DOI] [PubMed] [Google Scholar]
- 36.Tjepkema M, Shields M. Catalogue no: 82-620-MWE. Ottawa: Statistics Canada; 2005. Measured Obesity: Adult obesity in Canada. [Google Scholar]
- 37.Harris TB. Invited commentary: Body composition in studies of aging: New opportunities to better understand health risks associated with weight. Am J Epidemiol. 2002;156:122–4. doi: 10.1093/aje/kwf024. [DOI] [PubMed] [Google Scholar]
- 38.Coviello JS, Nyström KV. Obesity and heart failure. J Cardiovasc Nurs. 2003;18:360–6. doi: 10.1097/00005082-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Lavie CJ, Mehra MR, Milani RV. Obesity and heart failure prognosis: Paradox or reverse epidemiology? Eur Heart J. 2005;26:5–7. doi: 10.1093/eurheartj/ehi055. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Bermúdez OI, Tucker KL. Waist circumference and weight change are associated with disability among elderly Hispanics. J Gerontol A Biol Sci Med Sci. 2002;57A:M19–25. doi: 10.1093/gerona/57.1.m19. [DOI] [PubMed] [Google Scholar]
- 41.Bannerman E, Miller MD, Daniels LA, et al. Anthropometric indices predict physical function and mobility in older Australians: The Australian Longitudinal Study of Ageing. Public Health Nutr. 2002;5:655–62. doi: 10.1079/PHN2002336. [DOI] [PubMed] [Google Scholar]
- 42.Deschamps V, Astier X, Ferry M, et al. Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. Eur J Clin Nutr. 2002;56:305–12. doi: 10.1038/sj.ejcn.1601311. [DOI] [PubMed] [Google Scholar]
- 43.Lean ME, Han TS, Seidell JC. Impairment of health and quality of life in people with large waist circumference. Lancet. 1998;351:853–6. doi: 10.1016/s0140-6736(97)10004-6. [DOI] [PubMed] [Google Scholar]
- 44.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 45.Newman AB, Kupelian V, Visser M, et al. Health ABC Study Investigators. Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–9. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 46.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: Findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc. 2001;101:28–34. doi: 10.1016/S0002-8223(01)00008-6. [DOI] [PubMed] [Google Scholar]