Abstract
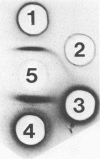
The alkali-soluble, water-soluble cell wall antigen of Coccidioides immitis (C-ASWS) mycelia and spherules was shown to react with anti-Coccidioides immunoglobulin M (IgM) precipitin antibody, both in the classical tube precipitin test and in the immunodiffusion assay for tube precipitin antibody (IDTP). The reactions obtained between C-ASWS and reference IgM precipitin antibody were identical to the reaction obtained when reference coccidioidin (CDN) was used. Definitive proof that C-ASWS extracts contain antigenic determinants that are reactive with IgM tube precipitin antibody was obtained by solid-phase immunoadsorption. Elution of reference IDTP antiserum over a column containing mycelium C-ASWS coupled to Sepharose 4B completely adsorbed precipitin antibody; i.e., reactivity in the IDTP was demonstrable in the column eluate but not in the column effluent fraction. The antigenic composition of C-ASWS extracts was evaluated and compared with that of CDN by two-dimensional immunoelectrophoresis against burro anti-CDN. The results established that both mycelium and spherule C-ASWS contain antigenic determinants in common with only one antigen present in CDN. The latter, designated antigen 2, is a large polymer which is predominant among the antigenic components in CDN. On a dry weight comparison, antigen 2 determinants were most concentrated in spherule C-ASWS, followed by mycelium C-ASWS and reference IDTP antigen. The finding that C-ASWS extracts are reactive with IgM tube precipitin antibody and are antigenically identical to antigen 2 in CDN suggests that antigen 2 is the biologically active component of CDN in tube precipitin assays.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Wheat R. W., Conant N. F. Fractionation and composition studies of skin test-active components of sensitins from Coccidioides immitis. Appl Microbiol. 1971 Sep;22(3):294–299. doi: 10.1128/am.22.3.294-299.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Arnold D. R. Immunoglobulin E in coccidioidomycosis. J Immunol. 1979 Jul;123(1):194–200. [PubMed] [Google Scholar]
- Cox R. A., Brummer E., Lecara G. In vitro lymphocyte responses of coccidioidin skin test-positive and -negative persons to coccidioidin, spherulin, and a coccidioides cell wall antigen. Infect Immun. 1977 Mar;15(3):751–755. doi: 10.1128/iai.15.3.751-755.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. Cross-reactivity between antigens of Coccidioides immitis, Histoplasma capsulatum and Blastomyces dermatitidis in lymphocyte transformation assays. Infect Immun. 1979 Sep;25(3):932–938. doi: 10.1128/iai.25.3.932-938.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Mead C. G., Pavey E. F. Comparisons of mycelia- and spherule-derived antigens in cellular immune assays of Coccidioides immitis-infected guinea pigs. Infect Immun. 1981 Feb;31(2):687–692. doi: 10.1128/iai.31.2.687-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R. Spectrum of in vivo and in vitro cell-mediated immune responses in coccidioidomycosis. Cell Immunol. 1977 Jun 1;31(1):130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Krasnow I., Vukovich K. R., Sun S. H., Rice E. H., Kutner L. J. Comparison of coccidioidin and spherulin in complement fixation tests for coccidioidomycosis. J Clin Microbiol. 1977 Jul;6(1):33–41. doi: 10.1128/jcm.6.1.33-41.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG Y. C., LEVINE H. B., SMITH C. E. Immunogenic properties of nondisrupted and disrupted spherules of Coccidioides immitis in mice. Sabouraudia. 1963 Feb;2:131–142. [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B., KONG Y. C., SMITH C. IMMUNIZATION OF MICE TO COCCIDIOIDES IMMITIS: DOSE, REGIMEN AND SPHERULATION STAGE OF KILLED SPHERULE VACCINES. J Immunol. 1965 Jan;94:132–142. [PubMed] [Google Scholar]
- Landay M. E. Spherules in the serology of Coccidioides immitis. II. Complement fixation tests with human sera. Mycopathol Mycol Appl. 1973 Jan 31;49(1):45–52. doi: 10.1007/BF02057446. [DOI] [PubMed] [Google Scholar]
- Lecara G., Cox R. A., Simpson R. B. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect Immun. 1983 Jan;39(1):473–475. doi: 10.1128/iai.39.1.473-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPAGIANIS D., LINDSEY N. J., SMITH C. E., SAITO M. T. ANTIBODIES IN HUMAN COCCIDIOIDOMYCOSIS: IMMUNOELECTROPHORETIC PROPERTIES. Proc Soc Exp Biol Med. 1965 Jan;118:118–122. doi: 10.3181/00379727-118-29773. [DOI] [PubMed] [Google Scholar]
- PAPPAGIANIS D., PUTMAN E. W., KOBAYASHI G. S. Polysaccharide of Coccidioides immitis. J Bacteriol. 1961 Nov;82:714–723. doi: 10.1128/jb.82.5.714-723.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappagianis D., Hector R., Levine H. B., Collins M. S. Immunization of mice against coccidioidomycosis with a subcellular vaccine. Infect Immun. 1979 Jul;25(1):440–445. doi: 10.1128/iai.25.1.440-445.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. F., Scheer E. R., Wheat R. W. Characterization of 3-O-methylmannose from Coccidioides immitis. Infect Immun. 1971 Nov;4(5):660–661. doi: 10.1128/iai.4.5.660-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., BEARD R. R., KEPP R. M., CLARK R. W., EDDIE B. U. Serological tests in the diagnosis and prognosis of coccidioidomycosis. Am J Hyg. 1950 Jul;52(1):1–21. doi: 10.1093/oxfordjournals.aje.a119404. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T. Histoplasmin sensitivity and coccidioidal infection; occurrence of cross-reaction. Am J Public Health Nations Health. 1949 Jun;39(6):722–736. doi: 10.2105/ajph.39.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., SIMONS S. A. Pattern of 39,500 serologic tests in coccidioidomycosis. J Am Med Assoc. 1956 Feb 18;160(7):546–552. doi: 10.1001/jama.1956.02960420026008. [DOI] [PubMed] [Google Scholar]
- Sawaki Y., Huppert M., Bailey J. W., Yagi Y. Patterns of human antibody reactions in coccidioidomycosis. J Bacteriol. 1966 Jan;91(1):422–427. doi: 10.1128/jb.91.1.422-427.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. R., Jr, Cox R. A., Schmitt J. A., Jr, Huppert M., Sun S. H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975 Nov;12(5):1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat R. W., Su Chung K. S., Ornellas E. P., Scheer E. R. Extraction of skin test activity from Coccidioides immitis mycelia by water, perchloric acid, and aqueous phenol extraction. Infect Immun. 1978 Jan;19(1):152–159. doi: 10.1128/iai.19.1.152-159.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]