Abstract
RANTES (CC chemokine ligand 5) contributes to airway inflammation through accumulation of eosinophils, but the exact role of RANTES (CCL5) is not defined. C57BL/6 mice, sensitized by injection of ovalbumin (OVA) on Days 1 and 14, were challenged with OVA on Days 28, 29, and 30 (3 challenges, short-term–challenge model) or on Days 28, 29, 30, 36, 40, 44, and 48 (7 challenges, repeated–challenge model) and evaluated 48 h later. Anti-mouse RANTES was given intravenously, and recombinant mouse RANTES or PBS was given intratracheally. These reagents were given on Days 28, 29, and 30 in the short-term–challenge study and on Days 44 and 48 in the repeated-challenge study. After short-term challenge, there were no effects after administration of anti-RANTES or RANTES. In the repeated-challenge study, although control mice showed a decrease in airway hyperresponsiveness, administration of anti-RANTES sustained and enhanced airway hyperresponsiveness and increased goblet cell numbers. In contrast, administration of RANTES normalized airway function but reduced goblet cell numbers. IL-12 and IFN-γ levels in BAL decreased in the anti-RANTES group and increased in the RANTES group. IFN-γ–producing CD4 T cells in lung, and IFN-γ production from lung T cells in response to OVA in the anti-RANTES group, were significantly decreased but were increased in the RANTES group. Anti–IFN-γ, administered with RANTES, decreased the effects of RANTES on AHR after repeated challenge. These data indicate that RANTES plays a role in the regulation of airway function after repeated allergen challenge, in part through modulation of levels of IFN-γ and IL-12.
Keywords: airway hyperresponsiveness, IFN-γ, IL-12, RANTES (CCL5)
Bronchial asthma is a chronic inflammatory airway disease with the features of reversible airway obstruction and nonspecific airway hyperresponsiveness (AHR). Elevated serum IgE levels and inflammatory cell infiltration, especially of eosinophils, mast cells, and CD4+ T cells, seems to be involved in the pathogenesis of the disease (1–3). In animal models of acute allergic inflammation, Th2-cell–derived cytokines, notably IL-4, IL-5, IL-9, and IL-13 and C-C chemokine family members such as RANTES (regulated upon activation, normal T-cell expressed and secreted) and eotaxin-1, are thought to play important roles in the induction of eosinophilic airway inflammation, antigen-specific IgE production, and AHR (4). Although airway inflammation and Th2 cytokines and chemokines are a cornerstone of asthma, the asthmatic response is complex, and the relative importance of some of these contributors may vary with stage or duration of disease.
RANTES (CCL5) belongs to the CC chemokine family and induces leukocyte migration by binding to specific receptors in the G protein–coupled receptor family (5, 6). RANTES (CCL5) has been shown in vitro to mediate eosinophil, lymphocyte, neutrophil, and monocyte chemotaxis (7–10), and it initiates several other proinflammatory events, such as integrin activation, lipid mediator biosynthesis, and degranulation (11, 12). RANTES production, which is generated predominantly by CD8+ T cells, epithelial cells, fibroblasts, and platelets, is associated with airway inflammation (8, 13–16). In vivo, RANTES is constitutively expressed in the lungs of patients with asthma (17, 18), and increased levels are detected in the BAL fluid (BALF) of patients with asthma (18, 19). However, the exact role of RANTES in airway allergic inflammation has been somewhat controversial. Several studies have shown the potent chemoattractant activity of RANTES for eosinophils in airway allergic inflammation. For example, treatment with methylated RANTES, which is a RANTES antagonist and retains binding to the receptor without activation, decreased eosinophilia after antigen challenge (20). On the other hand, RANTES has been shown to modulate cytokine production, inducing a switch from Th2-type to Th1-type cytokines (21). RANTES also induces the upregulation of Th1 cytokines (IL-2 and IFN-γ) and Th2 cytokines (IL-5) (22). The contribution of RANTES to the development of AHR has not been well defined. We previously reported that after repeated allergen challenge in sensitized mice, RANTES is upregulated in parallel with a decline in AHR (23). Kline and colleagues similarly demonstrated that administration of CpG oligodeoxynucleotides reduced AHR and airway eosinophilia and was associated with a significant increase in the levels of transcription for RANTES (24).
In this study, we defined the role of RANTES in allergen-induced airway responses after repeated antigen challenged by neutralization of RANTES with specific antibody or administration of exogenous recombinant RANTES.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice were purchased at 8–12 wk of age from Jackson Laboratories (Bar Harbor, ME) and housed under specific pathogen–free conditions. The animals were maintained on an ovalbumin (OVA)-free diet. Experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the National Jewish Medical and Research Center.
Sensitization and Airway Challenge
Mice were sensitized at 8 wk of age by intraperitoneal injection of 20 μg of OVA (grade V; Sigma, St. Louis, MO) emulsified in 2.25 mg of Al(OH)3 (Pierce, Rockford, IL) in a total volume of 100 μl on Days 0 and 14. After sensitization, animals were challenged with nebulized OVA (1% in saline) for 20 min on Days 28, 29, and 30 (three challenges, short-term–challenge model) or continued to receive airway challenges on Days 36, 40, 44, and 48 (seven challenges, repeated–challenge model). Forty-eight hours after the last OVA challenge (Day 32 or 50), AHR was assessed, and BAL, serum, and tissues were obtained for further analysis. Controls were nonsensitized but challenged with OVA.
Treatment Protocol for RANTES
Anti-mouse RANTES antibody (R&D Systems, Minneapolis, MN) was administered intravenously (100 μg in 100 μl of PBS) 30 min before each antigen challenge on Days 28, 29, and 30 (in the short-term–challenge model) or on Days 44 and 48 (in the repeated-challenge model). Control animals received the same amount of rat IgG (Sigma-Aldrich, St. Louis, MO) on the same days.
Recombinant mouse RANTES (Pepro Tech, Rocky Hill, NJ) was given via the trachea (1 μg in 20 μl of PBS) 4 h before each antigen challenge on Days 28, 29, and 30 (short-term challenge) or on Days 44 and 48 (repeated challenge). The same volume of PBS containing 0.1% BSA was administered as a control.
Anti–IFN-γ antibody (R&D Systems) was administered intravenously (100 μg in 100 μl of PBS) 30 min before each antigen challenge on Days 44 and 48 (repeated challenge) in some animals that also received RANTES or PBS through the trachea. Control animals received the same amount of rat IgG (Sigma-Aldrich), following the same protocol.
Determination of Airway Responsiveness
Airway function was assessed as previously described by measuring changes in lung resistance (Rl) in response to increasing doses of inhaled methacholine (MCh) (25). Data are expressed as percentage of changes from baseline Rl obtained after inhalation of saline.
BAL
Immediately after assessment of airway function, lungs were lavaged via the tracheal tube with 1 ml Hank's balanced salt solution at room temperature. Total leukocyte numbers were measured with a Coulter Counter (Coulter Corporation, Hialeah, FL). Cytospin slides were stained with Leukostat (Fisher Diagnostics, Pittsburgh, PA) and differentiated by standard hematologic procedures in a blinded fashion.
Histochemistry
Lungs were fixed in 10% formalin and processed into paraffin. Mucus-containing goblet cells were detected by staining of paraffin sections (5 μm thick) with periodic acid-Schiff (PAS). Histology analysis was done in a blinded manner by light microscopy linked to an image system. Numbers of PAS-positive goblet cells were determined only in cross-sectional areas of the airway wall. Six to 10 different sections were evaluated per animal. The obtained measurements were averaged for each animal, and the mean values and SEs were determined for each group.
Measurement of Cytokine Levels in BALF and Culture Supernates
Levels of cytokines were determined using commercially available ELISAs following the manufacturers' instructions. ELISA kits for the detection of IL-4, IL-5, IL-10, IL-12 (p70), and IFN-γ in supernatants were obtained from BD Pharmingen (San Diego, CA). The IL-13 ELISA kit was purchased from R&D Systems. The limits of detection for each assay were as follows: 4 pg/ml for IL-4 and IL-5; 10 pg/ml for IL-10, IL-12, and IFN-γ; and 1.5 pg/ml for IL-13.
Lung Cell Isolation
Lungs were dissected into small pieces and exposed to an enzymatic digestion solution containing 175 IU/ml collagenase V (Sigma-Aldrich) in Hanks' balanced salt solution for 1 h. After enzymatic digestion, total lung leukocytes were further enriched by 35% Percoll (Sigma-Aldrich) gradient centrifugation. The pellets were resuspended in 5 ml Red Blood Cell Lysing Buffer (Sigma-Aldrich) for 10 min on ice. The cells were washed twice and resuspended in PBS containing 1% BSA.
Intracellular Cytokine Staining
Intracellular cytokine staining was performed as previously described (26). Briefly, lung MNC were stimulated for 6 h with phorbol myristate acetate (10 ng/ml) and ionomycin (500 μg/ml) in the presence of brefeldin A (10 μg/ml). After washing, cells were stained for cell-surface markers with mAbs against CD3 (145–2C11, hamster IgG), CD4 (RM4–5, rat IgG2a), and CD8 (53–6.7, rat IgG2a). All fluorochrome-labeled mAbs and isotype control IgGs were purchased from BD Pharmingen. After fixation and permeabilization, cells were stained with PE- or biotin-conjugated anticytokine Abs or similarly labeled isotype-matched control Abs against IL-4 and IFN-γ (purchased from BD Pharmingen) and biotinylated goat anti-mouse IL-13 and control Ab (purchased from R&D Systems). The cells were washed, and staining was analyzed by flow cytometry on FACS Calibur using CellQuest software (BD Biosciences, Mountain View, CA).
Ex Vivo Culture of Lung T Cells
Lung cell suspensions were prepared, and lung T cells were isolated using mouse T cell immunocolumns (Cedarlane, Hornby, ON, Canada). The purity of T cells after isolation was over 90% as assessed by flow cytometry. Ex vivo culture of purified lung T cells (2 × 105/well) with irradiated splenocytes (3,000 rads, 2 × 105/well) and OVA (10 μg/ml) was performed. After 48 h, the culture supernates were harvested and frozen at –20°C until use.
Statistical Analysis
Mann-Whitney U tests were used to determine the levels of difference between all groups. The data were pooled from three independent experiments with four mice per group in each experiment (n = 12). Comparisons for all pairs were performed by Kruskal-Wallis test. Significance was assumed at P < 0.05. Values for all measurements were expressed as mean ± SEM.
RESULTS
AHR after Modulation of RANTES
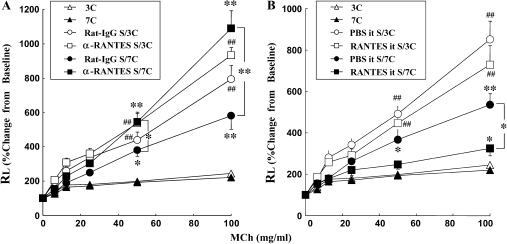
Mice received injections of anti-RANTES antibody (αRANTES Ab) or control rat IgG (control IgG) 30 min before each allergen challenge on Days 28, 29, and 30 in short-term–challenged mice (three challenges) or on Days 44 and 48 in seven repeated-challenged mice (seven challenges). αRANTES Ab administration before each of three daily allergen challenges after OVA sensitization resulted in limited increases in Rl to MCh when compared with control IgG-treated mice (Figure 1). When αRANTES Ab was given to sensitized and repeatedly allergen-challenged mice, significant increases in Rl were observed compared with control IgG-treated mice (Figure 1A).
Figure 1.
Changes of Rl in short-term– and long-term–challenged mice. Increasing concentrations of nebulized MCh were administered through the tracheal cannula 48 h after the last OVA challenge. (A) Groups of mice treated with anti-RANTES antibody or control rat IgG. (B) Groups of mice treated with RANTES or PBS. Data represent mean ± SEM. *P < 0.05 between groups indicated or S/7C (sensitized, seven challenges) versus 7C (seven challenges) mice. **P < 0.01 between groups indicated or S/7C versus 7C mice. ##P < 0.01 compared with S/3C (sensitized, three challenges) with 3C (three challenges) mice. 3C and 7C: nonsensitized mice exposed to three or seven OVA challenges. S/3C and S/7C: sensitized mice exposed to three or seven OVA challenges.
When mice were treated with recombinant mouse RANTES before challenge on Days 44 and 48, a further attenuation of AHR was shown in repeatedly challenged mice compared with the PBS-treated group (Figure 1B). No significant differences were found in airway responsiveness with RANTES treatment of the short-term–challenged mice (data not shown).
Inflammatory Cell Composition in BALF
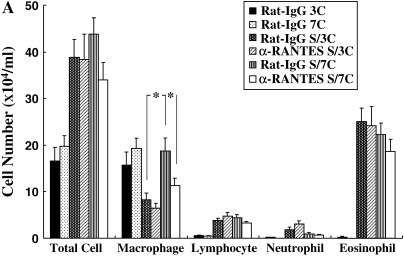
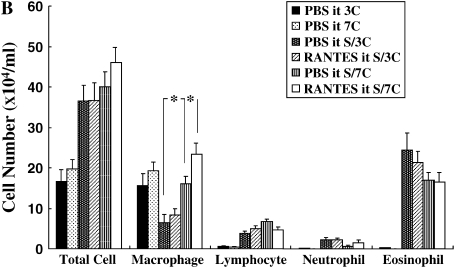
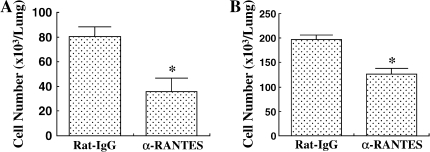
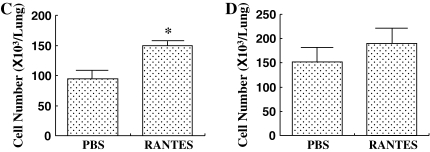
The number of inflammatory cells in the BALF was determined 48 h after the last allergen challenge. In the repeated-allergen–challenged mice, administration of αRANTES Ab significantly reduced the number of macrophages in the BALF (Figure 2A). In contrast, RANTES increased the number of macrophages in the BALF (Figure 2B). Treatment with αRANTES Ab or RANTES did not alter the numbers of lymphocytes or eosinophils in the BALF. In short-term–challenged mice, administration of αRANTES Ab or RANTES showed no effect in cell composition in the BALF (data not shown).
Figure 2.
Cellular composition in BALF from mice sensitized and challenged with OVA. (A) Groups of mice treated with anti-RANTES antibody or control rat IgG. (B) Groups of mice treated with RANTES or PBS. *P < 0.05 between groups indicated.
Lung Tissue Analysis
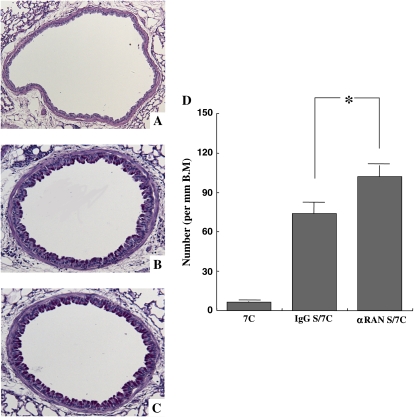
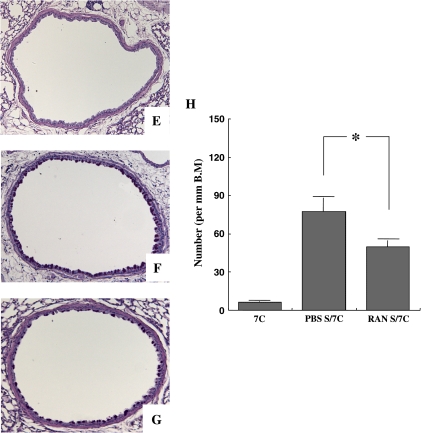
Goblet cell hyperplasia and mucus hyperproduction were evaluated by PAS staining and quantification of positively stained cells. In allergen-sensitized and repeated-allergen–challenged mice, treatment with αRANTES Ab augmented the numbers of PAS-positive cells (Figures 3B–3D), and administration of RANTES suppressed the number of PAS-positive cells (Figures 3F–3H). In short-term–challenged mice, similar numbers of positive cells were detected with αRANTES Ab or RANTES treatment (data not shown).
Figure 3.
Representative photomicrographs (A–C and E–G) and quantitative analysis of PAS-positive cells (D and H) in the lung tissues. The tissues were obtained 48 h after the last challenge. Shown are photomicrographs from groups of nonsensitized and seven challenges (A and E), sensitized/seven challenges with control rat IgG (B), sensitized/seven challenges with anti-RANTES antibody (C), sensitized/seven challenges with PBS (F), and sensitized/seven challenges with RANTES (G). Quantitative analysis on lung tissues was performed in mice with anti-RANTES antibody or PBS (D) and with RANTES or PBS (H). Results for each group are expressed as mean ± SEM. *P < 0.05 between groups indicated.
Cytokine Levels in BALF
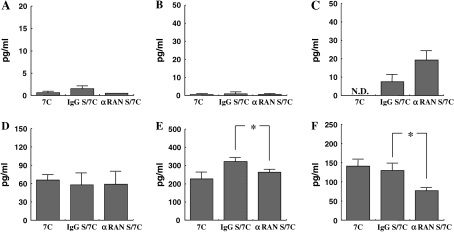
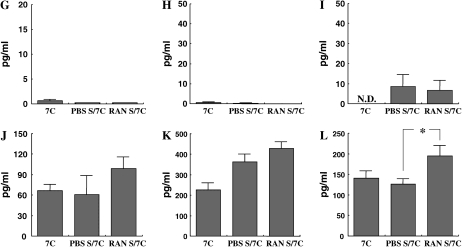
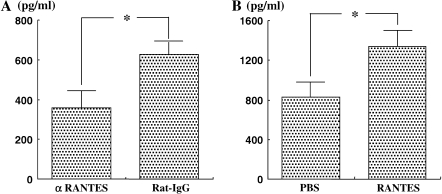
Cytokine levels in the BALF were measured in short-term or repeated allergen challenged mice to determine the effects of αRANTES Ab or RANTES treatment. Short-term challenge of sensitized mice significantly increased the levels of the Th2-type cytokines, IL-4, IL-5, IL-10, and IL-13 in the BALF compared with nonsensitized mice. IL-12 and IFN-γ levels were the same in both groups. None of these cytokine levels was altered by αRANTES Ab or RANTES treatment (data not shown). However, after repeated challenges, IFN-γ levels were sustained and IL-12 levels were increased, and these levels were decreased with αRANTES Ab and increased with RANTES treatment (Figures 4E, 4F, 4K, and 4L).
Figure 4.
Cytokine levels. IL-4 (A, G), IL-5 (B, H), IL-13 (C, I) IL-10 (D, J), IFN-γ (E, K), and IL-12 (F, L) in BALF were determined in long-term–challenged mice by ELISA as described in Materials and Methods. (A–F) Groups of mice treated with anti-RANTES antibody or control rat IgG. (G–L) Groups of mice treated with RANTES or PBS. Results of each group are expressed as mean ± SEM. *P < 0.05 between groups indicated. N.D., not detectable.
Intracellular IFN-γ Staining of Lymphocytes
These data suggested that in sensitized and repeatedly challenged mice, RANTES may play a regulatory role in airway inflammation, AHR, and cytokine production. To address whether RANTES functioned through the upregulation of IFN-γ production from lymphocytes in the lung, we quantified IFN-γ–producing cells by intracellular cytokine staining. Lung MNCs were obtained from each treatment group and stimulated with phorbol myristate acetate and ionomycin, and intracellular staining for IFN-γ was performed. In αRANTES-treated mice, the numbers of IFN-γ–producing CD4+ and CD8+ T cells were significantly decreased compared with control mice (Figures 5A and 5B). In contrast, the number of CD4+ T cells producing IFN-γ was significantly augmented after administration of RANTES (Figures 5C and 5D). IFN-γ–producing CD8+ T cells were also increased but did not achieve statistical significance. There were no differences in the number of IL-4– or IL-13–producing CD4+ and CD8+ T cells in each treatment group (data not shown). There was also no difference in number of CD4+ and CD8+ T cells in the lung in each treatment group (data not shown).
Figure 5.
The numbers of IFN-γ–producing CD4 (A and C) or CD8 T cells (B and D) in the lung were determined by intracellular cytokine staining as described in Materials and Methods. Results are shown as mean ± SEM. *P < 0.05 compared with sensitized seven OVA-challenged mice with control rat IgG (A and B) or PBS (C and D).
IFN-γ Production from Lung T Cells
Ex vivo cultures of lung T cells with OVA showed that IFN-γ production in the mice that received anti-RANTES antibody was significantly decreased compared with mice that received control antibody (Figure 6A). In parallel, lung T cells from mice that received RANTES intratracheally produced more IFN-γ than the mice that received PBS (Figure 6B).
Figure 6.
IFN-γ levels in culture supernates from lung T cells. Cytokine levels after stimulation of purified lung T cells co-cultured with irradiated splenocytes and OVA were measured in supernates by ELISA as described in Materials and Methods. The results for each group are expressed as mean ± SEM (n = 6 in each group). *P < 0.05 between groups indicated.
The Effect of Anti–IFN-γ Antibody
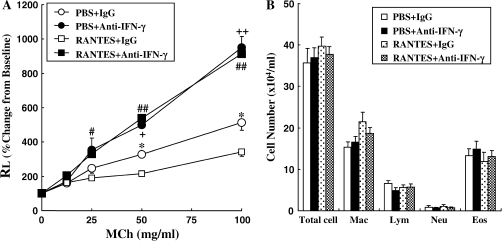
To determine the importance of the increased levels of IFN-γ after RANTES treatment in the regulation of the response to repeated challenge, anti–IFN-γ was administered 3 h after RANTES or PBS administration. Anti–IFN-γ treatment augmented AHR compared with the IgG-treated group (Figure 7A). Anti–IFN-γ plus PBS treatment also increased AHR significantly compared with the control IgG plus PBS treated group. However, the cell composition in BALF was not altered by antibody treatment (Figure 7B).
Figure 7.
Changes in RL (A) to inhaled MCh and BAL cellular composition (B). Anti–IFN-γ antibody or control rat IgG was administered to sensitized and seven OVA-challenged mice with RANTES treatment or PBS through the trachea. Forty-eight hours after the last OVA challenge, airway function measurements and BALF cell analysis were performed. The data are expressed as mean ± SEM. *P < 0.05 compared with RANTES and control rat IgG–treated mice with PBS and control rat IgG–treated mice. #P < 0.05 or ##P < 0.01 between RANTES and anti–IFN-γ–treated mice versus RANTES and control rat IgG–treated mice, respectively. +P < 0.05 or ++P < 0.01 between PBS and anti–IFN-γ–treated mice versus PBS and control rat IgG–treated mice, respectively.
DISCUSSION
Allergic asthma has several characteristic features, including airway eosinophilia, AHR, and mucus metaplasia, and many studies have demonstrated that the production of Th2 cytokines is the cornerstone for the development of these responses. Chemokines produced by immune cells also play a key role in the recruitment of various inflammatory cells (27, 28). Among these proteins, RANTES, eotaxin, MCP-3, and MCP-4 have been shown to contribute to the accumulation of eosinophils in allergic airway inflammation (29). Studies that have attempted to neutralize RANTES or block RANTES receptors have demonstrated a reduction in airway eosinophilia, but these treatments were not sufficient to modulate AHR (20, 30). In animals infected with respiratory syncytial virus, anti-RANTES did suppress the development of AHR (31). All of these studies were only performed early in the response to allergen or respiratory syncytial virus. In the present study, anti-RANTES treatment augmented AHR but only after repeated challenge where AHR was declining as the number of allergen challenges increased. As a corollary, after repeated challenge, RANTES administration further reduced AHR without altering the levels of Th2 cytokines in the BALF or airway eosinophil numbers. The results were not strain dependent because similar results were obtained in C57Bl/6 and BALB/c mice (data not shown). The possible factors associated with the regulation of airway responsiveness by RANTES were IL-12 and IFN-γ because these levels in the BALF were increased with administration of RANTES and decreased after administration of anti-RANTES in repeatedly challenged mice.
The effects of RANTES were restricted to mice that received repeated allergen challenge; after sensitization and only three allergen challenges, neither anti-RANTES nor RANTES had a significant effect on AHR or numbers of eosinophils in the BALF. We previously investigated the kinetics of cytokine and chemokine production after short-term and repeated-allergen challenge (23). In these studies, RANTES levels in the BALF were elevated after short-term challenge but were significantly increased after repeated-allergen challenge. Because increased levels of RANTES paralleled the decline in AHR and decreases in levels of Th2 cytokines and increases in levels of Th1 cytokines in the airways, RANTES may be involved in shifting of the Th1–Th2 balance but is dependent on the stage of the disease. For example, RANTES may be a Th2 response enhancer and chemotactic factor for eosinophils after short-term challenge, but its role shifts toward augmenting Th1 responses in sensitized mice as the number of allergen challenges increases. RANTES is known to be a chemotactic factor for lymphocytes, monocyte/macrophages, neutrophils, dendritic cells, natural killer cells, and eosinophils and has been shown to induce migration of Th1 lymphocytes, monocyte/macrophages, and natural killer cells into inflammatory sites, which is important in the pathogenesis of chronic inflammatory diseases (32). Further, RANTES strongly enhances the production of Th1-type (IL-2 and IFN-γ) cytokines (and that of Th2-type [IL-5 and IL-6] cytokines more modestly) with antigen stimulation in vitro (21).
Anti-RANTES treatment reduced the number of IFN-γ–producing T cells in the lung, whereas RANTES administration increased the number of these cells after repeated allergen challenge. As a result, RANTES may not only play an important role in supporting accumulation of monocyte/macrophages at inflammatory sites but may also enhance IL-12 production from these cells in the presence of IFN-γ (33). Support not only for the association with but the involvement of IFN-γ in RANTES-mediated regulation of AHR has been shown in experiments with anti–IFN-γ treatment, which abolished the effects of RANTES treatment on AHR. Thus, RANTES may upregulate IL-12 production in the lung followed by increased levels of IFN-γ after repeated allergen challenge.
The role of IFN-γ in AHR is somewhat controversial. Some studies have demonstrated that IFN-γ has the potential to inhibit AHR (34–36), whereas others have shown that IFN-γ may play a role in the development of AHR (37, 38). It is well known that IFN-γ inhibits production of Th2-cytokines (IL-4, IL-5, and IL-13) from antigen-specific T cells, in part by skewing toward Th1-type cells (34). However, in the present study, we did not find any significant alteration in Th2 cytokine levels in the BALF after anti-RANTES treatment or RANTES treatment. IFN-γ may have direct effects on AHR by acting on airway smooth muscle. IFN-γ was shown to inhibit airway smooth muscle contraction to carbachol, KCl, and TGF-β with isoproterenol (39, 40) and to inhibit the proliferation of airway smooth muscle (41). Moreover, several studies have reported that intranasal administration of IFN-γ effectively inhibited goblet cell metaplasia and AHR in a mouse model (34, 35, 42). However, no beneficial effects were observed on AHR or symptom scores with IFN-γ in patients with asthma (43, 44).
In summary, this study demonstrates that anti-RANTES treatment enhances AHR, whereas RANTES treatment ameliorates AHR, but only after repeated allergen challenge of sensitized mice. The changes in levels of the Th1 cytokines, IL-12, and IFN-γ paralleled the changes in AHR; anti-RANTES treatment decreased the levels of IL-12 and IFN-γ, and RANTES increased IFN-γ levels in the BALF. It is tempting to speculate that the role of RANTES evolves or changes with increasing allergen challenge of sensitized mice.
Acknowledgments
The authors thank Diana Nabighian for help in preparing the manuscript and Lynn Cunningham for performing the immunolabeling studies.
This work was supported by NIH grants HL-36577 and HL-61005 and EPA grant R825702.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0394OC on March 9, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Peebles RS Jr, Hamilton RG, Lichtenstein LM, Schlsoberg M, Liu MC, Proud D, Togias A. Antigen-specific IgE and IgA antibodies in bronchoalveolar lavage fluid are associated with stronger antigen-induced late phase reactions. Clin Exp Allergy 2001;31:239–248. [DOI] [PubMed] [Google Scholar]
- 2.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol 1994;12:295–335. [DOI] [PubMed] [Google Scholar]
- 3.Kay AB. Asthma and inflammation. J Allergy Clin Immunol 1991;87:893–910. [DOI] [PubMed] [Google Scholar]
- 4.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol 2002;38:881–885. [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000;12:121–127. [DOI] [PubMed] [Google Scholar]
- 6.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol 2001;22:83–87. [DOI] [PubMed] [Google Scholar]
- 7.Schall TJ. Biology of the RANTES/SIS cytokine family. Cytokine 1991;3:165–183. [DOI] [PubMed] [Google Scholar]
- 8.Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med 1992;176:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukacs NW, Standiford TJ, Chensue SW, Kunkel RG, Strieter RM, Kunkel SL. C–C chemokine-induced eosinophil chemotaxis during allergic airway inflammation. J Leukoc Biol 1996;60:573–578. [DOI] [PubMed] [Google Scholar]
- 10.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990;347:669–671. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff SC, Krieger M, Brunner T, Rot A, von Tscharner V, Baggiolini M, Dahinden CA. RANTES and related chemokines activate human basophil granulocytes through different G protein-coupled receptors. Eur J Immunol 1993;23:761–767. [DOI] [PubMed] [Google Scholar]
- 12.Taub DD, Ortaldo JR, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukoc Biol 1996;59:81–89. [DOI] [PubMed] [Google Scholar]
- 13.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol 1998;72:4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsukura S, Kokubu F, Kubo H, Tomita T, Tokunaga H, Kadokura M, Yamamoto T, Kuroiwa Y, Ohno T, Suzaki H, et al. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol 1998;18:255–264. [DOI] [PubMed] [Google Scholar]
- 15.Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, Krensky AM. A human T cell-specific molecule is a member of a new gene family. J Immunol 1988;141:1018–1025. [PubMed] [Google Scholar]
- 16.Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest 1998;102:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkman N, Krishnan VL, Gilbey T, Newton R, O'Connor B, Barnes PJ, Chung KF. Expression of RANTES mRNA and protein in airways of patients with mild asthma. Am J Respir Crit Care Med 1996;154:1804–1811. [DOI] [PubMed] [Google Scholar]
- 18.Folkard SG, Westwick J, Millar AB. Production of interleukin-8, RANTES and MCP-1 in intrinsic and extrinsic asthmatics. Eur Respir J 1997;10:2097–2104. [DOI] [PubMed] [Google Scholar]
- 19.Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med 1996;153:1398–1404. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, Martinez-A C, Dorf M, Bjerke T, Coyle AJ, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med 1998;188:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chensue SW, Warmington KS, Allenspach EJ, Lu B, Gerard C, Kunkel SL, Lukacs NW. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol 1999;163:165–173. [PubMed] [Google Scholar]
- 22.Lillard JW Jr, Boyaka PN, Taub DD, McGhee JR. RANTES potentiates antigen-specific mucosal immune responses. J Immunol 2001;166:162–169. [DOI] [PubMed] [Google Scholar]
- 23.Koya T, Kodama T, Takeda K, Miyahara N, Yang ES, Taube C, Joetham A, Park JW, Dakhama A, Gelfand EW. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen challenge. Am J Respir Crit Care Med 2006;173:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kline JN, Kitagata K, Businga TR, Jain VV. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol 2002;283:170–179. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophillic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med 1997;186:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, Miyahara S, Balhorn A, Dakhama A, Gelfand EW. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol 2004;172:2549–2558. [DOI] [PubMed] [Google Scholar]
- 27.Eum SY, Maghni K, Hamid Q, Eidelman DH, Campbell H, Isogai S, Martin JG. Inhibition of allergic airway inflammation and airway hyperresponsiveness in mice by dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol 2003;111:1049–1061. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol 2003;111:227–242. [DOI] [PubMed] [Google Scholar]
- 29.Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol 2001;1:108–116. [DOI] [PubMed] [Google Scholar]
- 30.Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C–C family chemokines in allergic airway inflammation. J Immunol 1997;158:4398–4404. [PubMed] [Google Scholar]
- 31.Tekkanat KK, Maassab H, Miller A, Berlin AA, Kunkel SL, Lukacs NW. RANTES (CCL5) production during primary respiratory syncytial virus infection exacerbates airway disease. Eur J Immunol 2002;32:3276–3284. [DOI] [PubMed] [Google Scholar]
- 32.Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol 1996;157:3598–3604. [PubMed] [Google Scholar]
- 33.Dorner BG, Scheffold A, Rolph MS, Huser MB, Kaufmann SH, Radbruch A, Flesch IE, Kroczek RA. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci USA 2002;99:6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lack G, Renz H, Saloga J, Bradley KL, Loader J, Leung DY, Larsen G, Gelfand EW. Nebulized but not parenteral IFN-gamma decreases IgE production and normalizes airways function in a murine model of allergen sensitization. J Immunol 1994;152:2546–2554. [PubMed] [Google Scholar]
- 35.Lack G, Bradley KL, Hamelmann E, Renz H, Loader J, Leung DY, Larsen G, Gelfand EW. Nebulized IFN-gamma inhibits the development of secondary allergic responses in mice. J Immunol 1996;157:1432–1439. [PubMed] [Google Scholar]
- 36.Dow SW, Schwarze J, Heath TD, Potter TA, Gelfand EW. Systemic and local interferon gamma gene delivery to the lungs for treatment of allergen-induced airway hyperresponsiveness in mice. Hum Gene Ther 1999;10:1905–1914. [DOI] [PubMed] [Google Scholar]
- 37.Hessel EM, Van Oosterhout AJ, Van Ark I, Van Esch B, Hofman G, Van Loveren H, Savelkoul HF, Nijkamp FP. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am J Respir Cell Mol Biol 1997;16:325–334. [DOI] [PubMed] [Google Scholar]
- 38.Kumar RK, Herbert C, Webb DC, Li L, Foster PS. Effects of anticytokine therapy in a mouse model of chronic asthma. Am J Respir Crit Care Med 2004;170:1043–1048. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Munakata M, Amishima M, Ukita H, Masaki Y, Homma Y, Kawakami Y. Gamma-interferon modifies guinea pig airway functions in vitro. Eur Respir J 1994;7:74–80. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa T, Kume H, Kondo M, Ito Y, Yamaki K, Shimokata K. Inhibitory effects of interferon-gamma on the heterologous desensitization of beta-adrenoceptors by transforming growth factor-beta 1 in tracheal smooth muscle. Clin Exp Allergy 2003;33:808–815. [DOI] [PubMed] [Google Scholar]
- 41.Amrani Y, Tliba O, Choubey D, Huang CD, Krymskaya VP, Eszterhas A, Lazaar AL, Panettieri RA Jr. IFN-gamma inhibits human airway smooth muscle cell proliferation by modulating the E2F–1/Rb pathway. Am J Physiol Lung Cell Mol Physiol 2003;284:L1063–L1071. [DOI] [PubMed] [Google Scholar]
- 42.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VP, Warnock M, Grunig G. Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol 2001;167:1769–1777. [DOI] [PubMed] [Google Scholar]
- 43.Boguniewicz M, Schneider LC, Milgrom H, Newell D, Kelly N, Tam P, Izu AE, Jaffe HS, Bucalo LR, Leung DY. Treatment of steroid-dependent asthma with recombinant interferon-gamma. Clin Exp Allergy 1993;23:785–790. [DOI] [PubMed] [Google Scholar]
- 44.Boguniewicz M, Martin RJ, Martin D, Gibson U, Celniker A, Williams M, Leung DY. The effects of nebulized recombinant interferon-gamma in asthmatic airways. J Allergy Clin Immunol 1995;95:133–135. [DOI] [PubMed] [Google Scholar]