Abstract
We have previously shown that mice that are genetically deficient in the CCR2 gene (CCR2−/− mice) are protected from fluorescein isothiocyanate (FITC)-induced lung fibrosis. Protection from fibrosis correlated with impaired recruitment of fibrocytes (bone marrow–derived cells, which share both leukocyte and mesenchymal markers). There are three ligands for CCR2 in the mouse: CCL2, CCL7, and CCL12. CCL2 and CCL12 are both elevated in the lung after FITC injury, but with different kinetics. CCL2 is maximal at Day 1 and absent by Day 7 after FITC. In contrast, CCL12 peaks at Day 3, but remains elevated through Day 21 after FITC. We now demonstrate that while CCR2−/− mice are protected from FITC-induced fibrosis, CCL2−/− mice are not. CCL2−/− mice are able to recruit fibrocytes to FITC-injured airspaces, unlike CCR2−/− mice. Adoptive transfer of CCR2-expressing fibrocytes augments FITC-induced fibrosis in both wild-type and CCR2−/− mice, suggesting that these cells play a pathogenic role in the disease process. Both CCL2 and CCL12 are chemotactic for fibrocytes. However, neutralization of CCL12 in wild-type mice significantly protects from FITC-induced fibrosis, whereas neutralization of CCL2 was less effective. Thus, CCL12 is likely the CCR2 ligand responsible for driving fibroproliferation in the mouse. As murine CCL12 is homologous to human CCL2, we suggest that the pathobiology of murine CCL12 in fibroproliferation may correlate to human CCL2 biology.
Keywords: chemokines, fibrocytes, fibrosis, lung
Idiopathic pulmonary fibrosis (IPF) is a devastating fibrotic pulmonary disorder. The disease is characterized by alveolar epithelial cell injury, variable inflammation, and progressive deposition of extracellular matrix (ECM) components such as collagen and fibronectin (1–4). Patients diagnosed with the usual interstitial pneumonia (UIP) form of the disease have a particularly poor prognosis, with an average survival of < 2 yr from time of diagnosis (4, 5). Standard therapy offers little improvement in morbidity or mortality in these patients. Thus, a better understanding of the disease process is urgently needed.
Although the disease has generally been thought to involve dysregulation of local lung fibroblasts resulting in the accumulation of myofibroblasts (most notably in the fibrotic foci of UIP), recent studies have highlighted the important contribution played by bone marrow–derived cells in the pathogenesis of pulmonary fibrosis (6–11). Bone marrow transplants using green fluorescent protein (GFP)-expressing mice as donors have demonstrated that the majority of collagen-producing cells in bleomycin-induced fibrotic lungs are derived from bone marrow (8). Further, the use of a GFP-expressing mouse as one partner in parabiotic mice studies have shown that lung injury in the wild-type partner results in the recruitment of mesenchymal cells from the circulation of the GFP-expressing partner (7). More recently, adoptive transfer of human blood fibrocytes into severe combined immune deficiency (SCID) mice has demonstrated that human fibrocytes can be recruited to bleomycin-injured lungs (10).
The recruitment of fibrocytes to the lung may be an important factor in the development of fibrotic responses. Fibrocytes themselves make collagen and can be stimulated to upregulate collagen production (6, 9–13). In addition, fibrocytes make growth factors such as TGF-β1, which may serve to augment fibrotic responses (13). Fibrocytes have been shown to transition into fibroblasts and myofibroblasts in culture (9–11). Thus, these cells may serve as the circulating precursor for the fibroblasts responsible for ECM deposition in fibrotic responses.
Fibrocyte recruitment is likely mediated via chemokine and chemokine receptor interactions. Both human and murine fibrocytes have been shown to express chemokine receptors, and functional studies have indicated that CCR2-, CCR7-, and CXCR4-mediated signals may all serve to recruit fibrocytes to the lung (9–12). We have previously shown that wild-type mice are susceptible to fluorescein isothiocyanate (FITC)-induced pulmonary fibrosis, whereas CCR2−/− mice are protected (14). The protection seen in the CCR2−/− mice is not due to deficiencies in the recruitment of classical inflammatory cells such as monocytes, lymphocytes, neutrophils, or eosinophils (14). However, protection from FITC-induced fibrosis on two separate genetic backgrounds is associated with a failure of CCR2−/− mice to recruit fibrocytes to the injured alveolar spaces (11). Fibrocytes are defined as cells that dually express both leukocyte (CD45, CD13) and mesenchymal (Col 1) markers. Ligands for the CCR2 receptor in the mouse include CCL2 (also known as JE or monocyte chemoattractant protein [MCP]-1), CCL7 (MCP-3) and CCL12 (MCP-5). Of these ligands, only CCL2 and CCL12 mRNA are upregulated in lung tissue at Day 7 after FITC (14). Previous in vitro studies have shown that lung fibrocytes migrate in response to CCL2 and that CCL2 can augment production of ECM proteins by fibrocytes (11). We wanted to determine whether CCL2 was the ligand responsible for the recruitment of fibrocytes to the lung in vivo. To date, murine CCL2 (JE) is widely thought to equate to human CCL2 (MCP-1), however, murine CCL12 (MCP-5, which is only found in the mouse) has a higher homology to human CCL2 (15). Until now the tools were not available to assess the biology of both murine CCL2 and CCL12. We demonstrate that CCL2 is not essential for fibrocyte recruitment to FITC-treated lungs or the development of fibrosis. Rather, CCL12 is likely the CCR2 ligand responsible for fibrocyte recruitment and enhanced fibrotic responses in vivo. This manuscript shows for the first time the importance of murine CCL12 in disease pathogenesis which, we suggest, may correlate to human CCL2 biology.
MATERIALS AND METHODS
Mice
C57Bl/6 and Balb/c mice were from Jackson Laboratories (Bar Harbor, ME). CCL2−/− mice on the Balb/c background (16) were the kind gift of Barrett Rollins (Dana Farber Cancer Institute, Boston, MA) and were bred at the University of Michigan. CCR2−/− mice on the C57Bl/6 or Balb/c background were bred in the University of Michigan Laboratory Animal Medicine facilities under SPF conditions and have been described previously (17). Experiments depicted in Figures 1–3 used Balb/c background mice to allow comparisons with CCL2−/− mice. All other studies used C57Bl/6 background mice. Mice were used at 6–8 wk of age. The University Committee on the Use and Care of Animals (UCUCA) approved these experiments.
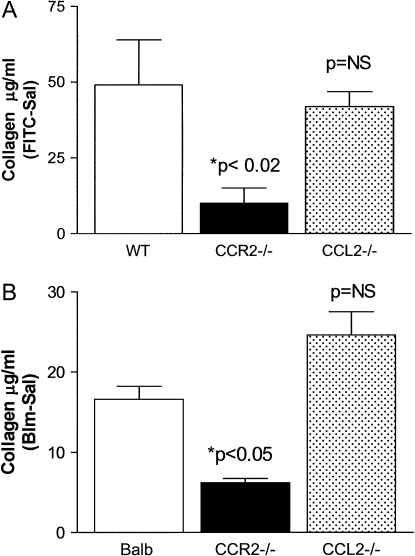
Figure 1.
CCL2 is not required for the development of FITC- or bleomycin-induced fibrosis. (A) WT, CCR2−/−, or CCL2−/− mice were injected with FITC or saline on Day 0. On Day 21, lungs were harvested and total lung collagen content was determined by Sircol assay (n = 6 group). Data are presented as the mean of the value of collagen in the saline-treated samples subtracted from the collagen values in FITC-treated mice. CCR2−/− mice are protected from FITC-induced fibrosis (P < 0.02), whereas CCL2 −/− mice are not. Data represent three separate experiments. (B) The same experiment was repeated using bleomycin as the fibrotic stimulus. CCR2−/− mice are protected from bleomycin-induced pulmonary fibrosis (P < 0.05), but CCL2−/− mice were not.
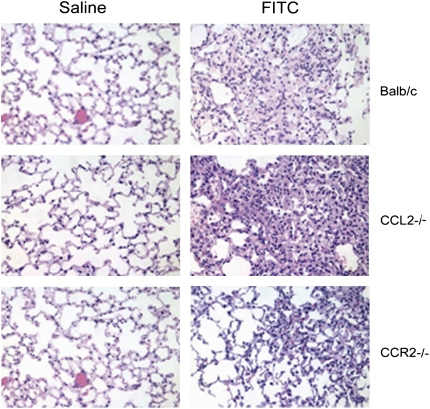
Figure 2.
Histologic analysis confirms the protection of CCR2−/−, but not CCL2−/− mice to FITC-induced fibrosis. WT, CCR2−/−, or CCL2−/− mice were injected with saline or FITC on Day 0. Lungs were harvested for processing on Day 21. Paraffin-embedded sections of lung were stained with hemotoxylin and eosin and examined under ×20 magnification. Results shown are representative of n = 4 mice per group.
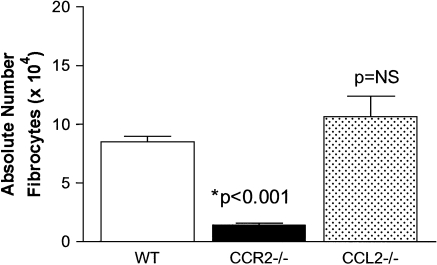
Figure 3.
CCL2−/− mice are able to recruit fibrocytes to FITC-injured airspaces. WT, CCR2−/−, or CCL2−/− mice were injected with FITC on Day 0. On Day 5 after FITC, BAL was collected and cultured for fibrocyte purification. Absolute numbers of fibrocytes were determined post-culture via flow cytometry for the dual expression of CD45 and collagen (n = 5 per group). CCR2−/− mice show a paucity of fibrocyte recruitment to the alveolar space following FITC administration (P < 0.001). Conversely, there is abundant fibrocyte recruitment in CCL2−/− mice. Data were similar in three separate experiments.
FITC or Bleomycin Inoculation
FITC inoculation was performed as previously described (14). Briefly, mice were anesthetized with sodium pentobarbital. The trachea was exposed and entered with a needle under direct visualization. FITC (21 mg, #F-7250; Sigma, St. Louis, MO) was dissolved in 10 ml of sterile PBS, vortexed extensively, and sonicated for 30 s. This slurry was transferred to multi-use vials, and vortexed extensively before each 50-μl aliquot was removed for intratracheal injection using a 23-gauge needle. For bleomycin injections, mice were given 0.025 U bleomycin (Sigma) dissolved in sterile saline in a 30-μl volume.
Lung Collagen Measurements
Total lung collagen levels were determined by harvesting lungs from mice on Day 21 after FITC, bleomycin, or saline administration. Animals were killed and perfused with 3 ml normal saline before all five lung lobes were removed and snap-frozen in liquid nitrogen. Before analysis, lungs were homogenized in 1 ml of normal saline and spun at 2,000 rpm for 10 min. Aliquots of lung homogenate (100 μl) were then assayed for total lung collagen levels and compared with a standard curve prepared from rat tail collagen using the Sircol collagen dye binding assay (Accurate, Westbury, NY) according to the manufacturer's instructions. In Figure 1, the average collagen content of the saline-treated mice of each genotype were subtracted from the values of FITC- or bleomycin-treated mice of each genotype to obtain the mean increase in collagen content.
Bronchoalveolar Lavage for Fibrocyte Purification or Inflammatory Cells
Mice were killed by CO2 asphyxiation, and the tracheae were exposed in a sterile fashion. The tracheae were cannulated with polyethylene tubing (PE50, Intramedic; Clay Adams, Parsippany, NJ) attached to a 25-guage needle on a tuberculin syringe, and the lungs were lavaged three times with 0.75 ml sterile 1× PBS. The lavage fluid from a single mouse was combined, spun at 1,500 rpm, and the supernatant removed. The cell pellet was resuspended in complete media (DMEM + 10% FBS + 1% pen/strep + 1% L-glutamine + 0.1% fungizone) and cultured for 10–14 d to allow mesenchymal cells to expand before analysis. For inflammatory cell composition, cell pellets were counted, cytospins were prepared and stained with Diff-Quick (Baxter, Deerfield, IL), and the percentages of monocyte/macrophages, polymorphonuclear leukocytes (PMNs), eosinosphils, or lymphocytes was determined by differential analysis as in Table 1.
TABLE 1.
PERCENTAGES OF CLASSICAL INFLAMMATORY CELLS FOUND IN THE BRONCHOALVEOLAR LAVAGE OF BALB/C, CCR2−/−, AND CCL2−/− MICE ON DAY 7 AFTER FLUORESCEIN ISOTHIOCYANATE
| Genotype | Total Cells | % Monocyte-Macrophages | % PMNs | % Eos | % Lymphocytes |
|---|---|---|---|---|---|
| Balb/c | 5.7 ± 0.3 × 105 | 68.9 ± 2.4 | 17.8 ± 1.1 | 8.6 ± 1.3 | 3.7 ± 1.8 |
| CCR2−/− | 7.9 ± 0.8 × 105 | 80.1 ± 1.6 | 13 ± 2 | 2.3 ± 0.3* | 4.5 ± 0.3 |
| CCL2−/− | 5.1 ± 0.6 × 105 | 82.1 ± 1 | 7.6 ± 1.4* | 1.4 ± 0.5* | 8.9 ± 1.4 |
Definition of abbreviations: Eos, eosinophils; PMN, polymorphonuclear leukocytes.
Fibrocyte and Fibroblast Isolation from Whole Lungs
Murine lungs were perfused with 5 ml normal saline and removed using aseptic conditions. Lungs were minced with scissors in DMEM complete media containing 10% FCS. Lungs from a single animal were placed in 10 ml of media in 100 cm2 tissue culture plates. Mesenchymal cells were allowed to grow out of the minced tissue, and when cells reached 70% confluence they were passaged using trypsin digestion. Mesenchymal cells were grown for 14 d before being harvested by trypsin digestion. Cells were stained with anti-CD45 Abs coupled to magnetic beads (Miltenyi Biotech, Auburn, CA). Labeled cells were then sorted by binding the cell population to MS- or LS-positive selection columns using a SuperMacs apparatus (Miltenyi Biotech) according to manufacturer's instructions. Cells are then washed extensively. CD45+ cells are retained on the column and can be removed by flushing the column with buffer once it is removed from the magnetic field. CD45− cells are collected in the original flow through. For extra purity, CD45+ cells were sometimes reapplied to a second MS-positive selection column. The absolute number of lung fibrocytes is determined by counting the cells that were retained on the column by a hemocytometer. Immunohistochemical staining or flow cytometry staining on this population confirmed that these cells were CD45+, CD13+, and col 1+. For some experiments, lungs were lavaged on Day 5 after FITC, and recovered cell pellets were stained immediately for flow cytometry as noted below.
Flow Cytometry Analysis
Cells were incubated for 15 min on ice with Fc block (clone 24G2; BD PharMingen, San Diego, CA) before surface staining with CD45-PerCPCy5.5 (BD PharMingen) followed by fixation/permeabilization using the BD PharMingen Cytofix/cytoperm kit according to manufacturer's instructions. Cells were then blocked with goat IgG before staining with col 1 (rabbit anti-mouse; Accurate) followed by a donkey anti-rabbit PE-secondary (Jackson Immunoresearch, West Grove, PA). Cells were analyzed on the flow cytometer (FACScan; BD Biosciences, Mountain View, CA).
Adoptive Transfer
Fibrocytes were purified by magnetic separation from lung mince cultures from untreated wild-type or CCR2−/− mice as described above. A quantity of 5 × 105 fibrocytes were injected via tail vein in a 100-μl volume into mice that had received an FITC inoculation intratracheally 4 d previously. Mice were killed on Day 21 after FITC, and lung collagen contents were determined.
ELISAs
CCL2 and CCL12 were measured in lung homogenates and plasma by specific ELISA. CCL2 was measured using the Opti-EIA kits from PharMingen, and CCL12 was measured using a kit from R&D Systems (Minneapolis, MN).
Chemotaxis
Lung mince cultures were serum-starved for 24 h before magnetic purification of CD45+ and CD45− cells. Chemotaxis on cells at 1 × 106/ml was performed in Boyden chambers to recombinant proteins (fibronectin at 100 μg/ml; Sigma) or CCL12 (50 ng/ml; R&D Systems) through gelatin-coated 5- to 8-μm filters. Checkerboard analysis proved that the migration was directional.
Antibodies
Anti-murine CCL2 (CNTO13, murine IgG2a) and anti-murine CCL12 (CNTO2637, rat IgG2a) were provided by Centocor, Inc. (Radnor, PA). Control sera were mouse IgG or rat IgG at the same concentration (Sigma). Antibodies were administered at a dose of 0.5 mg per injection at weekly intervals.
Statistics
In all graphs where error bars are shown, data represent mean ± SEM. Statistical significance was analyzed using the InStat v. 3 program (GraphPad Software, San Diego, CA) for Windows on a Dell GX260 computer (Dell Inc., Round Rock, TX). Student's t tests were run to determine P values when comparing two groups. For three or more groups, ANOVA analysis was performed with a post hoc Bonferroni test. A value of P < 0.05 was considered significant.
RESULTS
CCR2−/− but Not CCL2−/− Mice Are Protected from FITC-Induced or Bleomycin-Induced Fibrosis
To determine whether CCL2 signaling is required for the development of FITC-induced or bleomycin-induced fibrosis, we injected wild-type (Balb/c), CCR2−/−, or CCL2−/− mice with saline, FITC, or bleomycin on Day 0. On Day 21 after injection, lungs were harvested and collagen content was determined using the Sircol collagen dye binding assay. Results are presented as the average amount of collagen in saline-treated lungs subtracted from the amount of collagen present in FITC-treated lungs (Figure 1A) or from bleomycin-treated lungs (Figure 1B). As we have previously reported for two other genetic backgrounds, mice on the Balb/c background which are genetically deficient in the CCR2 chemokine receptor are protected from the development of FITC-induced pulmonary fibrosis (P < 0.02). Wild-type mice accumulated ∼ 50 μg/ml of collagen in response to FITC, whereas CCR2−/− mice accumulated an average of 10 μg/ml of collagen. In contrast, mice deficient in the CCL2 ligand accumulated an average of 42 μg/ml of collagen. This result was not statistically different from wild-type mice. Thus, CCL2−/− mice are not protected from FITC-induced pulmonary fibrosis.
Table 1 demonstrates the composition of classical inflammatory cells within the bronchoalveolar lavage among the three genotypes. The percentage of eosinophils noted in CCR2−/− and CCL2−/− mice was less than that in wild-type mice, but this did not correlate with fibrotic susceptibility between the strains.
The same pattern of response was seen with bleomycin-induced fibrosis. Note that the overall fibrotic response to bleomycin was less than that noted to FITC. Figure 2 demonstrates the histologic pattern that is observed on Day 21 after injection with saline or FITC in each respective strain. A similar pattern was observed in bleomycin-treated mice (not shown).
CCL2−/− and Wild-Type Mice, but Not CCR2−/− Mice Accumulate Fibrocytes in the Alveolar Space after FITC
We determined the number of fibrocytes that were present in bronchoalveolar lavage cell cultures harvested on Day 5 after FITC from all three mouse genotypes (Figure 3). As reported previously, there were a paucity of fibrocytes present in BAL cultures from FITC-treated CCR2−/− mice compared with wild-type mice (P < 0.001) (11). In contrast, BAL cultures from CCL2−/− mice contained numbers of fibrocytes that were not statistically different from the numbers found in wild-type mice. Thus, CCL2 is not required for fibrocyte accumulation in the alveolar space following FITC administration.
Adoptive Transfer of Fibrocytes Worsens FITC-Induced Fibrosis
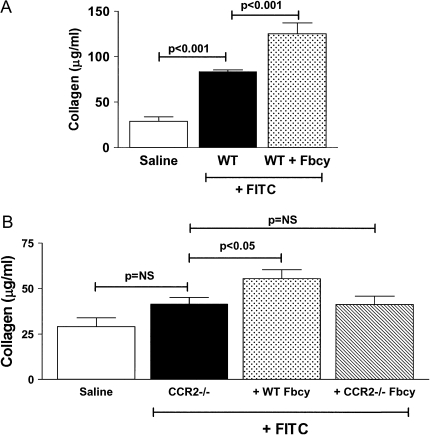
To determine whether fibrocytes could adoptively transfer an enhanced susceptibility to FITC-fibrosis, we compared the fibrotic responses of wild-type mice injected with saline or FITC on Day 0 to the response of wild-type mice injected with FITC on Day 0 and given 5 × 105 fibrocytes purified from lung mince cultures of untreated mice via tail vein infusion on Day 4 after FITC. The adoptive transfer of fibrocytes is able to augment the fibrotic response to FITC challenge in wild-type mice (Figure 4A; P < 0.001). We next wanted to determine whether adoptive transfer of fibrocytes from wild-type mice could create a fibrotic response in the protected CCR2−/− mice. Figure 4B demonstrates that adoptive transfer of wild-type fibrocytes to CCR2−/− mice can induce a level of fibrosis that is statistically significant compared with the CCR2−/− mice treated with FITC alone. In contrast, adoptive transfer of CCR2−/− fibrocytes into CCR2−/− mice had no effect. It should be noted that the overall level of fibrosis achieved with fibrocyte infusion into FITC-treated wild-type mice exceeds the levels achieved with fibrocyte infusion and FITC treatment in CCR2−/− mice.
Figure 4.
Adoptive transfer of fibrocytes augments FITC-induced pulmonary fibrosis. (A) WT mice were injected with FITC on Day 0. On Day 5 after FITC, 5 × 105 fibrocytes purified by magnetic separation from lung mince cultures from untreated WT mice were injected intravenously. Control mice received a saline injection. Lungs were then harvested at Day 21 after FITC, and collagen content was determined by Sircol assay. The adoptive transfer of WT fibrocytes significantly augments FITC-induced lung fibrosis in WT mice (P < 0.001). (B) CCR2−/− mice were injected with saline or FITC on Day 0. On Day 5, FITC-injected mice received 5 × 105 fibrocytes purified by magnetic separation from lung mince cultures from untreated WT mice or from untreated CCR2−/− mice or received a saline injection. Lungs were harvested on Day 21, and collagen content was determined by Sircol assay. WT fibrocytes could significantly augment fibrosis in CCR2−/− mice (P < 0.05); however, adoptive transfer of fibrocytes from CCR2−/− mice did not. Note that the magnitude of fibrosis in CCR2−/− mice injected with FITC and fibrocytes (B) is less than the magnitude of fibrosis seen in WT mice injected with FITC and fibrocytes (A). Each bar represents data from 8–10 mice per group.
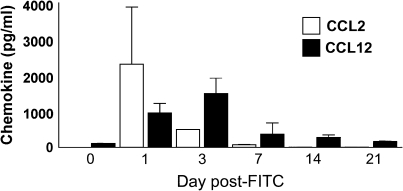
CCL12 Production Shows Sustained Kinetics after FITC
To determine whether CCL2 and CCL12 protein levels were increased during fibrogenesis, we injected wild-type mice with FITC on Day 0. Lungs and plasma were harvested on Days 1, 3, 7, 14, and 21 and tested for the presence of CCL2 or CCL12 by specific ELISA. Figure 5 demonstrates that CCL2 expression peaked at Day 1 after FITC and returned to baseline by Day 7. In contrast, CCL12 expression was increased on Day 1 after FITC, peaked at Day 3 after FITC, and remained elevated until Day 21. Similarly, CCL12 expression is produced at higher levels in the lung and with longer kinetics after bleomcyin as well (data not shown). Plasma levels of CCL2 and CCL12 were not significantly elevated above those at Day 0 at any time point (data not shown). Thus CCL12 expression displays sustained kinetics in the lung after FITC. Furthermore, fibrocytes themselves may be potent producers of CCL12. Purified fibrocytes cultured at 50,000 cells/ml for 24 h produced 396 ± 83 pg/ml of CCL12 (n = 3).
Figure 5.
CCL2 and CCL12 are expressed with different kinetics after FITC administration. Mice were injected with FITC on Day 0. On Days 1, 3, 7, 14, and 21 after FITC, lungs were harvested and lung homogenates were analyzed for presence of CCL2 or CCL12 by specific ELISA. Values represent the mean ± SEM of four mice per time point and are representative of two experiments.
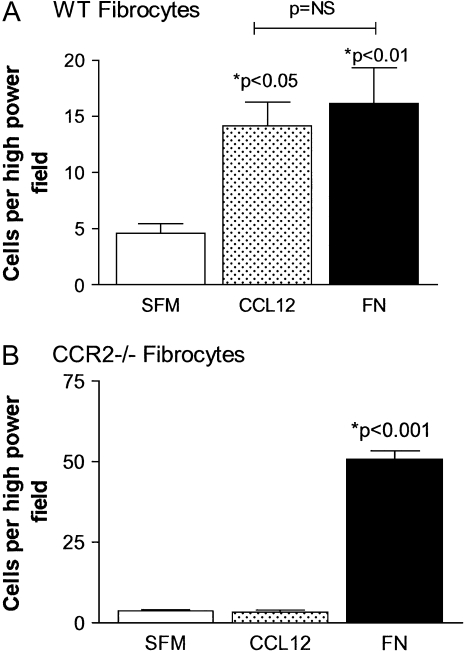
Fibrocytes Migrate in Response to CCL12
We next wanted to determine whether fibrocytes could migrate in response to CCL12. Fibrocytes were purified from wild-type or CCR2−/− lung digest cultures. Migration of wild-type (Figure 6A) or CCR2−/− (Figure 6B) fibrocytes was determined in Boyden chamber chemotaxis assays to either 50 ng/ml CCL12 or 100 mg/ml fibronectin (FN) as a positive control. Serum-free media (SFM) was used as a negative control for chemotaxis. Wild-type fibrocytes are able to migrate in response to both CCL12 and FN stimulation. These responses are significant when compared with migration to SFM (P < 0.05 and P < 0.01, respectively). In contrast, fibrocytes from CCR2−/− mice were unable to migrate to CCL12, but showed active migration to FN (P = 0.001). These results demonstrate that CCL12 is a chemotactic ligand for CCR2-bearing fibrocytes in vitro.
Figure 6.
CCR2-expressing fibrocytes migrate in response to CCL12. Fibrocytes were purified by magnetic separation from lung digest cultures from WT (A) or CCR2−/− mice (B). Fibrocytes were tested for chemotaxis (n = 3) to SFM, CCL12 (50 ng/ml), or fibronectin (FN) (100 μg/ml). Both genotypes were able to show significant migration in response to FN (P < 0.01 and P < 0.001, respectively). Only WT fibrocytes were able to migrate in response to CCL12 (P < 0.05). Data are representative of three similar experiments.
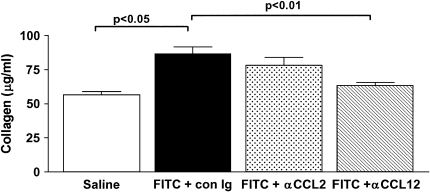
Neutralization of CCL12, but Not of CCL2, Protects against FITC-Induced Pulmonary Fibrosis
To determine whether neutralization of CCL2 or CCL12 could protect against FITC-induced fibrosis in wild-type mice, we injected mice with saline or FITC on Day 0. FITC-treated mice were then given injections of control Ig, anti-CCL2, or anti-CCL12 on Days 0, 7, and 14. Lungs were harvested on Day 21 and collagen content was determined. Figure 7 demonstrates that anti-CCL12 treatment significantly protects wild-type mice from FITC-induced fibrosis, whereas anti-CCL2 treatment does not. In response to FITC + control rat Ig, 3.1 ± 0.58 × 106 total cells were recruited to the BAL on Day 5. In the anti-CCL12–treated mice, the total number of inflammatory cells was reduced ∼ 4-fold to 8.1 ± 2.8 × 105 total cells (n = 3, P = 0.02). Not surprisingly, the total number of fibrocytes recruited to the BAL of FITC + control rat Ig mice was 16.6 ± 0.4 × 104, whereas the number of fibrocytes recruited to the anti-CCL12–treated mice was also reduced ∼ 4-fold to 3.9 ± 1.3 × 104 (n = 3, P = 0.001). Thus neutralization studies confirm our results in CCL2−/− mice and demonstrate that CCL12 is likely the CCR2 ligand mediating FITC-induced fibrotic responses in the mouse. Furthermore, our studies suggest that anti-CCL12 treatment reduces inflammatory cell accumulation (including fibrocytes) in the injured alveolar space.
Figure 7.
Neutralization of CCL12, but not of CCL2, protects against FITC-induced pulmonary fibrosis. WT mice were injected with saline or FITC on Day 0. On Days 0, 7, and 14, FITC-treated mice received a 0.5-mg injection of control rat Ig, anti-CCL2, or anti-CCL12. Lungs were harvested at Day 21 after FITC, and collagen content was determined by Sircol assay. Collagen content in FITC-treated mice receiving mouse Ig as a control sera were not significantly different than the data shown for control rat Ig. Anti-CCL2 treatment reduced FITC fibrosis, but the change did not reach statistical significance, whereas anti-CCL12 treatment significantly reduced FITC-induced fibrosis compared with control Ig (n = 8, P < 0.01).
DISCUSSION
Our data demonstrate that CCR2−/− mice are protected from FITC-induced pulmonary fibrosis. Both CCL2 and CCL12 are produced in the lung in response to FITC deposition, and fibrocytes can chemotax to both ligands in vitro. Genetic deletion of the CCL2 ligand or neutralization of CCL2 does not confer protection from FITC-induced fibrosis. In contrast, neutralization of CCL12 does protect mice from FITC-induced pulmonary fibrosis. Protection in the CCR2−/− mice is associated with a lack of fibrocyte recruitment to the alveolar space, but there is no lack of fibrocyte recruitment in CCL2−/− mice. We show for the first time that adoptive transfer of wild-type fibrocytes can worsen lung fibrosis in response to FITC. Adoptive transfer of wild-type, but not CCR2−/− fibrocytes can augment FITC induced fibrosis in CCR2−/− mice as well, but cannot restore fibrosis to the levels seen in wild-type mice. These results suggest that CCL12 is likely the CCR2 ligand responsible for the augmentation of FITC-induced pulmonary fibrosis in mice.
CCR2 is a molecule that is known to be central to the development of pulmonary fibrosis. CCR2−/− mice are protected from experimental pulmonary fibrosis induced by either bleomycin or FITC on at least three genetic backgrounds that have been tested (11, 14, 18, and the present study). This is the first description of protection in Balb/c mice. The fibrotic response to bleomycin is attenuated when compared with the response to FITC in Balb/c mice. This is consistent with previous work showing that mice on the Balb/c background are relatively resistant to bleomycin-induced fibrosis, likely due to enhanced activity of bleomycin hydrolase in this strain (19).
CCR2 is important for the recruitment of fibrocytes to the alveolar spaces after FITC-induced fibrosis (11), and this is likely one mechanism by which it promotes fibrosis. Fibrocytes have been shown to differentiate into effector fibroblasts, which lose expression of CD45, acquire α-smooth muscle actin expression, and secrete abundant ECM (9–13). In addition, fibrocytes are known to secrete profibrotic cytokines such as TGF-β (13). We have previously demonstrated that CCR2 agonists enhance ECM production by CCR2-expressing fibrocytes (11). In addition, CCR2 is known to play important roles in epithelial cell biology during fibrosis. During homeostasis, alveolar epithelial cells (AECs) are believed to keep fibroproliferation in check at least in part by secreting prostaglandin E2 (PGE2), a potent fibroblast inhibitor (20–22). Alveolar epithelial cells express CCR2, and stimulation of this receptor can inhibit PGE2 production by AECs. AECs co-cultured with CCR2 agonists in the presence of fibroblasts were unable to limit fibroproliferation. Conversely, AECs from CCR2−/− mice were potent inhibitors of fibroblast proliferation (20). Thus, CCR2 is both an important regulator of fibrocyte recruitment and function as well as serving as a modulator of AEC function.
Fibrocytes have been shown to migrate to sites of both upper and lower respiratory tract fibrosis (9–11). Their presence correlates with enhanced fibrotic responses, but until now, there has been no direct evidence that they augment fibrosis. We and others have shown previously that adoptively transferred fibrocytes home to fibrotic lung sites (9–11). In this report, we demonstrate for the first time that as few as 5 × 105 adoptively transferred fibrocytes can significantly augment FITC-induced fibrosis in both wild-type and CCR2−/− mice, although the magnitude of the fibrotic response in CCR2−/− mice was not as great as that seen in wild-type mice. It is important to note that these effects were not seen when 5 × 105 lung monocytes and macrophages were injected intravenously (data not shown) or when fibrocytes purified from CCR2−/− mice were injected.
Therefore, we postulate that the CCR2-mediated recruitment and activation/differentiation of these adoptively transferred fibrocytes worsens disease progression.
CCR2 signaling likely regulates activities of inflammatory cells, fibrocytes, and AECs during lung fibrosis. The fact that CCR2−/− mice do not develop fibrosis to the extent that wild-type mice do even when injected with CCR2+/+ fibrocytes suggests that other protective mechanisms are still intact in these mice. Our previous studies suggest that CCR2−/− mice produce less TNF-α, and more GM-CSF and PGE2 than do wild-type mice (14, 20). Thus these factors likely serve to protect the lung epithelium and limit fibroproliferation even in the face of augmented fibrocyte recruitment/activation. CCR2 has four ligands in human (MCP 1–4) and three ligands in the mouse. In the mouse, the ligands are CCL2 (JE/MCP-1), CCL7 (MCP-3), and CCL12 (MCP-5) (23). Murine CCL2 is the most well-studied ligand, and we have previously shown CCL2 to have potent effects on fibrocyte chemotaxis and ECM production (11). Thus, we were somewhat surprised to discover that the CCL2−/− mice were not protected from FITC-induced fibrosis. Human CCL2 has been shown to be present in both fibroproliferative acute respiratory distress syndrome (ARDS) and also in IPF (24, 25). Interestingly, the murine CCL12 (MCP-5) is the most structurally homologous murine ligand to the human CCL2 (MCP-1) ligand, with 66% identity at the amino acid level (15). Thus, it is likely that murine CCL12 responses will be most similar to human CCL2 responses. Accordingly, it is likely that CCL12 in the mouse is the CCR2 ligand responsible for fibrocyte recruitment. This statement is based on several observations. (1) Only CCL2 and CCL12 are elevated in whole lung mRNA in response to FITC challenge (14). (2) CCL2−/− mice are not protected from FITC-induced fibrosis, but CCR2−/− mice are. (3) CCL12 is a potent chemoattractant for fibrocytes in vitro. (4) CCL12 is the CCR2 ligand that remains elevated throughout fibrotic responses. (5) CCL2 neutralization did not protect against FITC-induced fibrosis, but CCL12 neutralization did. Thus, it seems likely that CCL12 is the relevant ligand for the in vivo recruitment of fibrocytes and the pro-fibrotic effects on multiple cell types. However, since neutralization of CCL12 limited recruitment of all inflammatory cells to the alveolar space after FITC (not just fibrocytes), we cannot exclude the possibility that CCL12 serves to augment fibrosis via effects on other inflammatory cells as well.
Previous studies by other investigators have suggested that CXCR4 and CCR7 may also mediate recruitment of fibrocytes to bleomycin-injured murine lungs (10). This raises the question of which chemokine and chemokine interactions are most important. The differences in the study design and fibrotic stimulus used in the two studies preclude direct comparisons, but several interesting points should be noted. Our previous studies suggest that CCR2 is ubiquitously expressed on lung-derived fibrocytes (11). In contrast, Phillips and coworkers noted that CXCR4 was present on subsets of peripheral blood–derived fibrocytes (10). Neither neutralization of CXCL12 nor CCL12 nor CCR2 deficiency completely blocks development of fibrosis in any of the studies (10, 11, 14, and the present study). Thus, redundant fibrotic mechanisms likely do exist. Our studies have looked at recruitment to the alveolar space (11), whereas the studies by Phillips and colleagues analyzed recruitment to the whole lung, including lung interstitium (10). It is intriguing to hypothesize that different chemokine receptors may be responsible for recruitment to unique anatomic locations within the lung. Further experiments will be needed to fully characterize the contribution each receptor makes to fibrotic development.
In summary, our work has elucidated a key role for the CCR2 receptor in mediating pulmonary fibrotic responses in mice. One important function of CCR2 is to recruit fibrocytes to injured alveolar spaces. We have demonstrated for the first time that adoptive transfer of fibrocytes augments fibrotic responses. Our current work suggests for the first time that murine CCL12 in important for disease pathogenesis. As murine CCL12 is most homologous to human CCL2 (15), we suggest that these findings may correlate to human CCL2 biology. Thus, future therapies aimed at blocking fibrocyte recruitment (possibly via blocking CCR2 or human CCL2) may be beneficial for patients with fibrosis.
Acknowledgments
The authors would like to thank Ping Tsui, Jill Carton, Ted Petley and Hai Sheng for their help in generating the anti-CCL2 and anti-CCL12 antibodies used in this study.
This work was supported by NIH grants HL071586 (B.B.M.) and P50HL56402 (B.B.M. and G.B.T.) as well as by a research grant from the Coalition for Pulmonary Fibrosis and the Martin Edward Galvin Fund for Idiopathic Pulmonary Fibrosis Research. Additional support comes from a Career Investigator award from the American Lung Association of Michigan to B.B.M.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0239OC on March 16, 2006
Conflict of Interest Statement: B.B.M. is a consultant for Centocor. L.M. is an employee of Centocor and has stock options in the company. Centocor provided the anti-CCL2 and anti-CCL12 antibodies used in this study. A.D. is an employee of Centocor and has stock options in the company. Centocor provided the anti-CCL2 and anti-CCL12 antibodies used in this study. C.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.B.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.B.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Thannickal VJ, Toews GB, White E, Lynch JI, Martinez F. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–471. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JI, Toews G. Idiopathic pulmonary fibrosis. In: Fishman A, editor. Pulmonary diseases and disorders. Philadelphia, PA: McGraw-Hill; 1997. pp. 1069–1084.
- 3.Kuhn C. Pathology. In: Phan S, Thrall R, editors. Pulmonary fibrosis. New York: Marcel Dekker, Inc.; 1995. pp. 59–83.
- 4.American Thoracic Society/European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KR, Thwaite EL, Kazerooni EA, Gross BH, Toews GB, Colby TV, Travis WD, Mumford JA, Murray S, Flint A, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore BB, Thannickal VJ, Toews GB. Bone marrow-derived cells in the pathogenesis of lung fibrosis. Current Respiratory Medicine Reviews 2005;1:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med 2004;170:1158–1163. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto N, Jin H, Liu T, Chensue S, Phan S. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Sun G, Stacey M, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;170:380–389. [DOI] [PubMed] [Google Scholar]
- 10.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–7562. [DOI] [PubMed] [Google Scholar]
- 13.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol 1998;160:419–425. [PubMed] [Google Scholar]
- 14.Moore B, Paine R, Christensen P, Moore T, Sitterding S, Ngan R, Wilke C, Kuziel W, Toews G. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001;167:4368–4377. [DOI] [PubMed] [Google Scholar]
- 15.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med 1997;185:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 1998;2:275–281. [DOI] [PubMed] [Google Scholar]
- 17.Kuziel W, Morgan S, Dawson T, Griffin S, Smithies O. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC cheomokine receptor 2. Proc Natl Acad Sci USA 1997;04:12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine 2003;24:266–276. [DOI] [PubMed] [Google Scholar]
- 19.Filderman AE, Lazo JS. Murine strain differences in pulmonary bleomycin metabolism. Biochem Pharmacol 1991;42:195–198. [DOI] [PubMed] [Google Scholar]
- 20.Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R III, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol 2003;284:L342–L349. [DOI] [PubMed] [Google Scholar]
- 21.Lama V, Moore B, Christensen P, Toews G, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2 dependent. Am J Respir Cell Mol Biol 2002;27:752–758. [DOI] [PubMed] [Google Scholar]
- 22.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol 2003;29:537–544. [DOI] [PubMed] [Google Scholar]
- 23.Kunkel S, Strieter R, Lindley I, Westwick J. Chemokines: new ligands, receptors, and activities. Immunol Today 1993;16:559–561. [DOI] [PubMed] [Google Scholar]
- 24.Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 1992;89:5371–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman R, Strieter R, Martin D, Steinberg K, Milberg J, Maunder R, Kunkel S, Wals A, Hudson L, Martin T. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1996;154:602–611. [DOI] [PubMed] [Google Scholar]