Abstract
T-cell activation plays an essential role in the generation of the pulmonary inflammation that is manifest in allergic asthma. Optimal T-cell activation requires not only presentation of antigen with the major histocompatibility complex, but also concurrent signaling through costimulatory molecules. The costimulatory molecule SLAM (Signaling Lymphocytic Activation Molecule, CD150) is a glycoprotein expressed on activated lymphocytes and antigen-presenting cells. Disruption of the SLAM gene demonstrated that SLAM-induced signal transduction pathways regulate cytokine production by T helper (Th)2 cells and macrophages. Here we tested the postulate that the costimulatory molecule SLAM may be critical for allergic inflammation in a murine model. SLAM-deficient mice did not manifest allergen-induced bronchoalveolar lavage eosinophilia, increased serum IgE, or heightened airway responses compared with wild-type mice. Allergen-induced Th2 cytokines and Th1 cytokines were decreased in SLAM-deficient mice. These data support the concept that SLAM plays a crucial role in allergic responses.
Keywords: allergic inflammation, asthma, costimulatory molecules, murine, SLAM
T-cell activation plays a critical role in the initiation and mediation of the airway inflammation observed in allergic asthma. The recognition of allergen in the context of major histocompatibility complex on the surface of an antigen-presenting cell and an antigen-specific T-cell receptor (TCR) is a critical first step toward immune activation. In addition, costimulatory molecules on the T cell and antigen-presenting cell interact for complete T-cell activation. Pathways involving the costimulatory molecules CD28 and CD80/86 are the best-described costimulatory interactions and have been shown to play an important role in the pathogenesis of allergic asthma in murine models (1–4). These costimulatory molecules may be only partially responsible for allergic responses because blocking or deficiency of these molecules does not equally affect all allergic parameters. The recently described costimulatory molecule SLAM (Signaling Lymphocytic Activation Molecule, CD150) has been shown to have modulatory effects during T-cell activation (5). The role of SLAM in allergic inflammation has not been previously analyzed.
SLAM, a type I transmembrane glycoprotein, is a member of a small CD2 subfamily of the Ig superfamily. SLAM is constitutively expressed on CD45ROhigh memory T cells, B cells, dendritic cells, and macrophages; it is rapidly induced on naive T cells after activation (6). A stimulating anti-SLAM antibody evokes antigen-specific T-cell proliferation and IFN-γ production by CD4+ T cells (7). SLAM directly induces proliferation of preactivated T cells in the absence of CD28 involvement or other stimuli. CD4+ T cells from SLAM-deficient mice have a defect in TCR-mediated production of IL-4 (7). SLAM also promotes the proliferation and differentiation of human B cells (8). SLAM-deficient macrophages exhibit abnormal functions, as evidenced by the reduced production of IL-12, TNF-α, and nitric oxide and the inability to clear Leishmania major infection (7, 9).
SLAM has been viewed with importance as a distinct costimulatory molecule. We tested the postulate that the costimulatory molecule SLAM may be critical for allergic inflammation in a murine model. We analyzed allergic parameters including serum IgE, bronchoalveolar lavage (BAL) cytokines, and lung inflammation in a model of pulmonary inflammation with SLAM-deficient mice. Our results indicate that expression of SLAM is essential for allergic pulmonary inflammation and local pulmonary cytokine production.
MATERIALS AND METHODS
Mice
Eight-week-old, specific pathogen–free, SLAM−/− (BALB/c) mice were generated as previously described (6, 9). Eight-week-old wild-type (BALB/c) mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were maintained according to the guidelines of the Committee on Animals of the Harvard Medical School and the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources National Research Council.
Protocol for Allergen Sensitization and Challenge
Mice were sensitized and challenged with allergen ovalbumin (OVA) as previously described (4, 10–15). Briefly, mice were sensitized by intraperitoneal injection with 10 μg chicken OVA and 1 mg Al(OH)3 (alum) on Days 0 and 7. On Days 14–20, mice received aerosolized OVA challenge with 6% OVA for 20 min/d. OVA was dissolved in 0.5× PBS. Control mice received 1 mg alum in 0.5× PBS via intraperitoneal injection on Days 0 and 7 and received aerosolized PBS on Days 14–20. An ultrasonic nebulizer (Model 5000; DeVilbiss, Somerset, PA) was used for nebulizations into a plastic chamber.
Serum IgE
Blood was withdrawn by cardiac puncture and centrifuged to recover serum at 13,000 rpm for 20 min. Total serum IgE levels were determined by ELISA as previously described (12–14). Total serum IgE concentrations were calculated using a standard curve generated with commercial IgE standard (BD Pharmingen, San Diego, CA).
BAL and Histologic Analysis
Twenty-four hours after the final challenge, mice were anesthetized with ketamine/xylazine intraperitoneally and killed by cardiac puncture. Each mouse underwent BAL, as previously described (4, 10–15). Cells were resuspended in RPMI. Slides for differential cell counts were prepared with Cytospin (Shandon Inc., Pittsburgh, PA) and fixed and stained with Diff-Quik (Dade Behring, Newark, DE). For each sample, an investigator blinded to the treatment groups performed two counts of 100 cells. For histopathologic assessment, lungs were removed from the thoracic cavity, placed in formalin, cut, and stained with hematoxylin and eosin. The level of lung inflammation was evaluated by comparison with known positive and negative sections and ranked from mild to severe on a scale of 0–3, based on quantity and uniformity of inflammation (0 = no inflammation; 1 = 1–5, 2 = 5–10, and 3 = > 10 inflammatory cells per high-power field). An investigator blinded to group classification evaluated lung tissue inflammation.
BAL Cytokines
BAL fluid (BALF) cytokine concentrations were measured by ELISA according to the manufacturer's specifications (R&D Systems, Minneapolis, MN). Briefly, BALF samples were aliquoted in duplicate into 96-well plates that were precoated with antibody to specific cytokines and assayed according to the manufacturer's instructions. Optical density was measured at 450–540 nm. Cytokine concentrations were determined by comparison with known standards. The sensitivities of the ELISA kits used in this study are as follows: IL-4, 2 pg/ml; IL-13, 1.5 pg/ml; IL-10, 4 pg/ml; IL-12p70, 2.5 pg/ml, TNF-α, 5.1 pg/ml.
Determination of Airway Measurements
Twenty-four hours after the final aerosol challenge, airway measurements were assessed using whole-body plethysmography. Mice were placed in individual chambers, and 100 mg/ml methacholine was nebulized into the chambers via an inlet for 2 min as previously described (10, 15–17). Readings were averaged over 7 min from the beginning of the nebulization. The whole-body plethysmography system measures changes in box pressure during expiration and inspiration, peak expiratory pressure (PEP), peak inspiratory pressure (PIP), inspiratory time (Ti), expiratory time (Te), and relaxation time (Tr = time of the pressure decay to 36% of total box pressure during expiration) and generates a value called enhanced Pause (Penh) (Penh = PEP/PIP × [{Te − Tr}/Tr]), which is associated with airway resistance (10, 15, 18). We have previously analyzed the identical OVA allergic model with classical measures of lung resistance using tracheostomized and anesthetized mice in Balb/c and C57/BL6 strains (3, 4, 11, 13).
Statistical Analysis
Statistical analysis of data was performed by t test using Sigma Stat software. Nonparametric data were analyzed using a Mann-Whitney test. Data are reported as means ± SEM. Statistical significance was defined by P < 0.05.
RESULTS
SLAM−/− OVA Mice Have Reduced Allergen-Induced Eosinophil Recruitment and Inflammation in the Lung
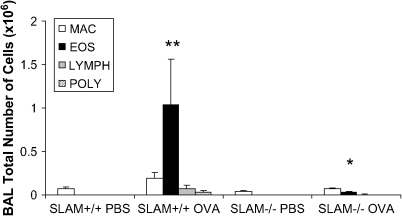
The presence of eosinophils in the BALF and lungs after allergen sensitization and challenge is an important indicator of airway inflammation. Wild-type (SLAM+/+ OVA) mice have increased total cell counts and high levels of eosinophils in the BALF after sensitization and challenge with OVA (**P = 0.01) (Figure 1). In contrast, SLAM−/− OVA sensitized and challenged mice (SLAM−/− OVA mice) have significantly decreased allergen-induced BAL eosinophils when compared with SLAM+/+ OVA mice (*P = 0.029).
Figure 1.
SLAM−/− OVA mice have significantly reduced BAL eosinophils when compared with SLAM+/+ OVA mice. Each mouse underwent BAL after OVA sensitization and challenge as described in Materials and Methods. Cell counts were determined by differential staining of cells isolated from the BALF. Data are shown as percentage of total cells ± SEM (n = 2 experiments; 6–11 mice per group).
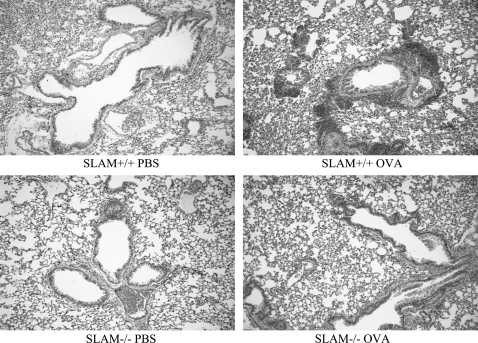
To determine whether allergic lung inflammation was also affected by SLAM deficiency, histologic sections of lung were examined (Figure 2). Lungs from SLAM−/− OVA mice had less pulmonary inflammation than SLAM+/+ OVA mice. Pathology-averaged scores by an investigator blinded to the groups were as follows: SLAM+/+ OVA, 3; SLAM+/+ PBS, 0; SLAM−/− OVA, 1.2; SLAM −/− PBS, 0. The scores were on a scale of 0–3 (n = 4 mice per group).
Figure 2.
SLAM−/−OVA mice show decreased pulmonary inflammation compared with SLAM+/+OVA mice. Mice were sensitized and challenged with allergen OVA or PBS control. Hematoxylin/eosin of right lung was reviewed as described in Materials and Methods. Original magnification: ×20.
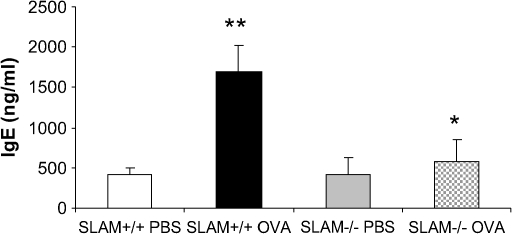
SLAM−/− Mice Have Decreased Levels of Total Serum IgE after OVA Allergen Sensitization and Challenge
OVA sensitization and challenge resulted in an increase in total serum IgE in SLAM+/+ OVA mice (**P < 0.001). SLAM−/− OVA mice had diminished IgE levels compared with SLAM+/+ OVA mice (*P < 0.001) (Figure 3).
Figure 3.
Levels of total serum IgE are significantly reduced in SLAM−/− OVA mice compared with SLAM+/+ OVA control mice. Blood was extracted by cardiac puncture, and total serum IgE was measured by ELISA 24 h after aerosol challenge as described in Materials and Methods. Data are shown as mean IgE concentration in ng/ml ± SEM (n = 2 experiments; 6–11 mice per group).
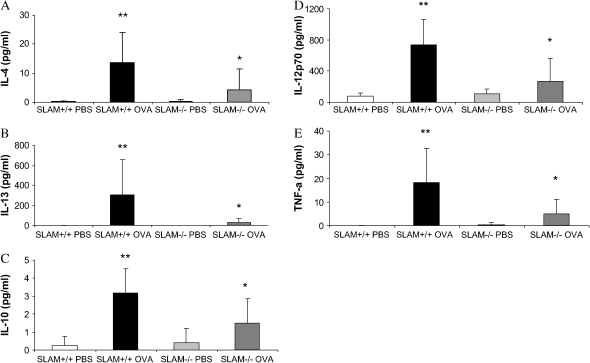
SLAM−/− Mice Do Not Produce Th2 or Th1 Cytokines in Response to OVA Allergen Sensitization and Challenge
Previous data indicate the importance of Th2 cytokine production in the development of allergic inflammation (11, 13, 19). SLAM+/+ OVA mice had increased secretion of Th2 cytokines (IL-4, IL-13, IL-10) in the BALF compared with SLAM+/+ PBS control mice (**P < 0.05) (Figures 4A–4C). This increase in cytokine secretion was absent in SLAM−/− OVA mice compared with SLAM+/+ OVA mice (*P < 0.05) (Figures 4A–4C). Cytokines associated with a Th1 response were also analyzed. Concentrations of TNF-α and IL-12p70 were significantly reduced in the BALF of SLAM−/− OVA compared with SLAM+/+ OVA mice (Figures 4D and 4E). Levels of IL-4, IL-13, IL-10, TNF-α, and IL-12p40 in the BALF of SLAM−/− OVA mice were not significantly increased compared with SLAM−/− PBS control mice.
Figure 4.
(A–C) Secretion of allergen-induced Th2 cytokines IL-4, IL-10, and IL-13 measured in the BALF was significantly decreased in SLAM−/− OVA mice when compared with SLAM+/+ OVA mice. (C and D) Secretion of allergen-induced Th1 cytokines TNF-α and IL-12p70 measured in the BALF was significantly decreased in SLAM−/− OVA mice when compared with SLAM+/+ OVA mice. Cytokine concentration in the BALF was measured by ELISA (R&D systems) according to the manufacturer's instructions. Data are shown as pg/ml ± SEM (n = 2 experiments; 4–12 mice per group).
Allergen-Induced Airway Measurements Are Decreased in SLAM−/− Mice
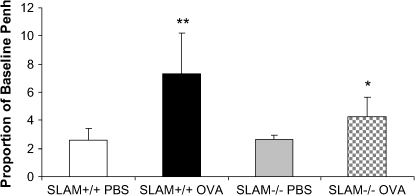
To determine whether SLAM plays a role in the development of allergen-induced airway measurements, responsiveness to methacholine was measured in SLAM+/+ OVA and SLAM−/− OVA mice. SLAM−/− OVA mice had decreased allergen-induced airway measurements (Penh) relative to SLAM+/+ OVA mice (P = 0.04) (Figure 5).
Figure 5.
Airway measurements in SLAM−/− OVA mice are significantly decreased compared with SLAM+/+ OVA mice. Airway measurements (Penh) were assessed using whole-body plethysmography, challenged with methacholine, as described in Materials and Methods. Data are shown as proportion of baseline Penh ± SEM (n = 2 experiments; 8–10 mice per group).
DISCUSSION
Pulmonary allergic inflammation is dependent upon T-cell activation with engagement of the antigen-specific TCR and costimulatory molecules. Previous studies have focused primarily on the costimulatory pathways involving CD28 and the ligands CD80 and CD86 and have found them to be essential for allergic responses (1, 3, 20–22).
We postulated that additional costimulatory pathways that involve SLAM might be crucial for allergic asthma. Using a murine model of allergen OVA–induced inflammation, allergic responses were attenuated in the absence of SLAM. BAL eosinophilia and total serum IgE were significantly reduced in SLAM-deficient mice compared with wild-type control mice. Allergen-induced Th2 cytokine secretion in SLAM-deficient mice was reduced to levels typically seen only in non–allergen-sensitized control mice. Allergic inflammation in the lung (as characterized by eosinophils) and perivascular and peribronchial inflammation were decreased, suggesting that SLAM deficiency impairs tissue inflammation and circulating mediators of inflammation.
Previous analysis of SLAM-deficient mice showed that SLAM interactions are necessary for Th2 and macrophage functions (7). CD4+ cells from SLAM-deficient mice after stimulation with anti-CD3 and anti-CD28 secreted reduced amounts of IL-4 compared with wild-type mice (7). Our data suggest an essential role of SLAM pathways in allergic responses that are classically presumed to be mediated by Th2 cytokines. Modification of the cytokine secretion could be a possible mechanism for the reduction of allergic responses in SLAM-deficient mice. Our data also indicate that Th1 cytokines (TNF-α and IL-12p70) are significantly decreased in the absence of SLAM. Although asthma has been characterized as predominantly a Th2 disease, recent data suggest a role for Th1 responses, consistent with the notion that Th1 and Th2 responses may be critical for the generation of allergic responses (23). Our analysis of SLAM-deficient mice showing reduction of Th1 and Th2 allergen–induced cytokines is consistent with this concept. SLAM is expressed on macrophages and on lymphocytes. Recent data have focused on macrophages and their mediators in the amplification of allergic responses (24–26). Given that macrophage functions in SLAM-deficient mice have previously been shown to be impaired (7), we cannot exclude the possibility that this cell type may contribute to the failure of SLAM-deficient mice to develop allergic responses. A recently described receptor for SLAM, termed SLAM-associated protein, has been identified (27). Whether SLAM-mediated effects in allergic asthma predominate by lymphocyte, macrophage, or SLAM-associated protein signaling–mediated mechanisms warrants further investigation.
We report our analysis of the role of the newly described costimulatory molecule, SLAM, in mediating allergic immune responses. Our results indicate that the expression of SLAM is essential for allergic pulmonary inflammation and the development of local pulmonary cytokine production. These findings suggest that SLAM-mediated pathways involving Th1 and Th2 responses are present in the genesis of allergic responses. These data suggest that multiple costimulatory signaling pathways should be considered in the generation of pulmonary allergic inflammation.
This work was supported by NIH grants HL 5672, HL 67684, and AI 142681 (P.W.F.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0294OC on March 9, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Keane-Myers A, Gause WC, Linsley PS, Chen SJ, Wills-Karp M. B7–CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J Immunol 1997;158:2042–2049. [PubMed] [Google Scholar]
- 2.Mathur M, Herrmann K, Qin Y, Gulmen F, Li X, Krimins R, Weinstock J, Elliott D, Bluestone JA, Padrid P. CD28 interactions with either CD80 or CD86 are sufficient to induce allergic airway inflammation in mice. Am J Respir Cell Mol Biol 1999;21:498–509. [DOI] [PubMed] [Google Scholar]
- 3.Krinzman SJ, De Sanctis GT, Cernadas M, Mark D, Wang Y, Listman J, Kobzik L, Donovan C, Nassr K, Katona I, et al. Inhibition of T cell costimulation abrogates airway hyperresponsiveness in a murine model. J Clin Invest 1996;98:2693–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mark DA, Donovan CE, De Sanctis GT, Krinzman SJ, Kobzik L, Linsley PS, Sayegh MH, Lederer J, Perkins DL, Finn PW. Both CD80 and CD86 co-stimulatory molecules regulate allergic pulmonary inflammation. Int Immunol 1998;10:1647–1655. [DOI] [PubMed] [Google Scholar]
- 5.Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol 2003;3:813–821. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Morra M, Wu C, Gullo C, Howie D, Coyle T, Engel P, Terhorst C. CD150 is a member of a family of genes that encode glycoproteins on the surface of hematopoietic cells. Immunogenetics 2001;53:382–394. [DOI] [PubMed] [Google Scholar]
- 7.Wang N, Satoskar A, Faubion W, Howie D, Okamoto S, Feske S, Gullo C, Clarke K, Sosa MR, Sharpe AH, et al. The cell surface receptor SLAM controls T cell and macrophage functions. J Exp Med 2004;199:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature 2003;421:282–287. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, Sosa MR, Edwards MJ, Borrow P, Satoskar AR, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol 2001;2:410–414. [DOI] [PubMed] [Google Scholar]
- 10.Haley KJ, Ciota A, Contreras JP, Boothby MR, Perkins DL, Finn PW. Alterations in lung collectins in an adaptive allergic immune response. Am J Physiol Lung Cell Mol Physiol 2002;282:L573–L584. [DOI] [PubMed] [Google Scholar]
- 11.Mark DA, Donovan CE, De Sanctis GT, He HZ, Cernadas M, Kobzik L, Perkins DL, Sharpe A, Finn PW. B7–1 (CD80) and B7–2 (CD86) have complementary roles in mediating allergic pulmonary inflammation and airway hyperresponsiveness. Am J Respir Cell Mol Biol 2000;22:265–271. [DOI] [PubMed] [Google Scholar]
- 12.Arestides RS, He H, Westlake RM, Chen AI, Sharpe AH, Perkins DL, Finn PW. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol 2002;32:2874–2880. [DOI] [PubMed] [Google Scholar]
- 13.Donovan CE, Mark DA, He HZ, Liou HC, Kobzik L, Wang Y, De Sanctis GT, Perkins DL, Finn PW. NF-kappa B/Rel transcription factors: c-Rel promotes airway hyperresponsiveness and allergic pulmonary inflammation. J Immunol 1999;163:6827–6833. [PubMed] [Google Scholar]
- 14.Velasco G, Campo M, Manrique OJ, Bellou A, He H, Arestides RS, Schaub B, Perkins DL, Finn PW. Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am J Respir Cell Mol Biol 2005;32:218–224. [DOI] [PubMed] [Google Scholar]
- 15.Fleming CM, He H, Ciota A, Perkins D, Finn PW. Administration of pentoxifylline during allergen sensitization dissociates pulmonary allergic inflammation from airway hyperresponsiveness. J Immunol 2001;167:1703–1711. [DOI] [PubMed] [Google Scholar]
- 16.Hamelmann E, Oshiba A, Schwarze J, Bradley K, Loader J, Larsen GL, Gelfand EW. Allergen-specific IgE and IL-5 are essential for the development of airway hyperresponsiveness. Am J Respir Cell Mol Biol 1997;16:674–682. [DOI] [PubMed] [Google Scholar]
- 17.Hamelmann E, Oshiba A, Loader J, Larsen GL, Gleich G, Lee J, Gelfand EW. Antiinterleukin-5 antibody prevents airway hyperresponsiveness in a murine model of airway sensitization. Am J Respir Crit Care Med 1997;155:819–825. [DOI] [PubMed] [Google Scholar]
- 18.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997;156:766–775. [DOI] [PubMed] [Google Scholar]
- 19.Cernadas M, De Sanctis GT, Krinzman SJ, Mark DA, Donovan CE, Listman JA, Kobzik L, Kikutani H, Christiani DC, Perkins DL, et al. CD23 and allergic pulmonary inflammation: potential role as an inhibitor. Am J Respir Cell Mol Biol 1999;20:1–8. [DOI] [PubMed] [Google Scholar]
- 20.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol 2001;2:203–209. [DOI] [PubMed] [Google Scholar]
- 21.Padrid PA, Mathur M, Li X, Herrmann K, Qin Y, Cattamanchi A, Weinstock J, Elliott D, Sperling AI, Bluestone JA. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of Th1/Th2 subsets in a murine model of asthma. Am J Respir Cell Mol Biol 1998;18:453–462. [DOI] [PubMed] [Google Scholar]
- 22.Greene JL, Leytze GM, Emswiler J, Peach R, Bajorath J, Cosand W, Linsley PS. Covalent dimerization of CD28/CTLA-4 and oligomerization of CD80/CD86 regulate T cell costimulatory interactions. J Biol Chem 1996;271:26762–26771. [DOI] [PubMed] [Google Scholar]
- 23.Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, Dufour JH, Luster AD. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol 2002;168:5278–5286. [DOI] [PubMed] [Google Scholar]
- 24.Stein-Streilein J, Sonoda KH, Faunce D, Zhang-Hoover J. Regulation of adaptive immune responses by innate cells expressing NK markers and antigen-transporting macrophages. J Leukoc Biol 2000;67:488–494. [DOI] [PubMed] [Google Scholar]
- 25.Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol 2005;77:321–327. [DOI] [PubMed] [Google Scholar]
- 26.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol 2002;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howie D, Okamoto S, Rietdijk S, Clarke K, Wang N, Gullo C, Bruggeman JP, Manning S, Coyle AJ, Greenfield E, et al. The role of SAP in murine CD150 (SLAM)-mediated T-cell proliferation and interferon gamma production. Blood 2002;100:2899–2907. [DOI] [PubMed] [Google Scholar]