Abstract
Previous studies have suggested that the proinflammatory cytokine, TNF-α, contributes to airway hyperresponsivness by altering airway smooth muscle (ASM) Ca2+ responses to agonist stimulation. The present study examined the effects of TNF-α on Ca2+ influx pathways in cultured human ASM cells (HASMCs). Proteins encoded by the transient receptor potential (TRP) gene family function as channels through which receptor-operated and store-operated Ca2+ entry (SOCE) occur. In the present study, the presence of TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 mRNA and protein expression was confirmed in cultured HASMCs using RT-PCR and Western blot analysis. TNF-α treatment significantly increased TRPC3 mRNA and protein levels in HASMCs as well as SOCE. TNF-α treatment also increased both the peak and plateau intracellular Ca2+ concentration responses in HASMCs elicited by acetylcholine and bradykinin. The effects of TNF-α treatment on SOCE and agonist-induced intracellular Ca2+ concentration responses were attenuated using small interfering RNA transfection, which knocked down TRPC3 expression. Thus, in inflammatory airway diseases, TNF-α treatment may result in increased myocyte activation due to altered Ca2+ influx pathways. These results suggest that TRPC3 may be an important therapeutic target in inflammatory airway diseases such as asthma and chronic obstructive pulmonary disease.
Keywords: human airway smooth muscle, receptor-operated calcium entry, store-operated calcium entry, TNF-α, transient receptor potential channels
TNF-α is a potent proinflammatory cytokine that is found in bronchoalveolar lavage fluid (1) and sputum (2) from patients with asthma, and has been implicated as a mediator in the pathophysiology of asthma (3–5) and chronic obstructive pulmonary disease (COPD) (6, 7). Recent evidence suggests that TNF-α influences airway smooth muscle (ASM) function in a number of ways, including enhanced Ca2+ mobilization (8, 9), increased Ca2+ sensitivity (10, 11), and increased ASM force generation in response to contractile agonists (12–14).
In ASM, changes in intracellular Ca2+ concentration ([Ca2+]i) controls a number of cellular responses, including contractile responses. Two membrane systems are involved in the regulation of [Ca2+]i in smooth muscle: the plasma membrane and the sarcoplasmic reticulum (SR). Increases in [Ca2+]i occur through Ca2+ influx across the plasma membrane or by SR Ca2+ release. Ca2+ influx can occur through voltage gated (15), receptor-operated (16), and/or store-operated channels (17). In the latter case, store-operated Ca2+ entry (SOCE) is triggered by depletion of SR Ca2+ stores (18, 19). Mammalian homologs of the Drosophila transient receptor potential (TRP) channel family of proteins mediate a large number of signal and sensory signal transduction pathways (20). Members of the TRP subfamily, TRPC, have been recently implicated as the channels through which receptor- and store-operated Ca2+ influx occurs (20–24). Indeed, TRPC1, 3, 4, and 6 have been noted to be expressed at the mRNA level in human airway myocytes (25); however, their functional translation and influence by TNF-α have not been explored.
Acetylcholine (ACh) stimulation of ASM results in a biphasic [Ca2+]i response, characterized by a transient peak increase followed by a decline to a sustained plateau phase during the continued presence of agonist (26). After agonist stimulation, both the initial transient peak increase in [Ca2+]i and the sustained plateau phase are concerted events that are influenced by both Ca2+ release from the SR and repletion of SR Ca2+ by SOCE, as well as by other Ca2+ influx pathways. TNF-α treatment of ASM cells results in augmented agonist-induced Ca2+ responses (8, 9). TNF-α increases both the peak [Ca2+]i responses and the steady-state plateau phase of the responses (27). This suggests that Ca2+ influx mechanisms may be influenced by TNF-α. Recently, Deshpande and colleagues have provided evidence that the CD38/cyclic adenosine diphosphate ribose signaling pathway contributes to the augmented responses observed in ASM upon TNF-α treatment (28). The authors conclude that an increase in cyclic adenosine diphosphate ribose–induced Ca2+ mobilization is a critical component to the augmented [Ca2+]i responses. In these studies, the global Ca2+ responses were observed in the presence of extracellular Ca2+. As such, the relative contribution of Ca2+ influx and/or Ca2+ release from intracellular stores to the augmented responses could not be discerned, as Ca2+ influx via SOCE affects the state of repletion of the SR, and thus could affect both transient (peak) and steady-state responses (17, 29).
In the studies described herein, we investigated the contribution of Ca2+ influx to the TNF-α–induced enhancement of agonist-elicited Ca2+ responses, SOCE, receptor-operated Ca2+ entry (ROCE), and TRPC protein and mRNA expression in cultured human bronchial smooth muscle cells (HBSMCs). We found that: (1) TNF-α enhanced basal and agonist-elicited peak and steady-state [Ca2+]i.; (2) TNF-α significantly increased SOCE; (3) TNF-α specifically increased TRPC3 mRNA and protein expression; and (4) using small interfering RNA (siRNA) technology (30), we were able to knock down TRPC3, which resulted in attenuation of the TNF-α–induced enhancement of SOCE, basal [Ca2+]i, and agonist-elicited Ca2+ responses. These data suggest that, in inflammatory airway diseases such as asthma and COPD, TRPC3 inhibition would likely produce a therapeutic benefit.
MATERIALS AND METHODS
HBSMC Isolation and Culture
Human bronchi were obtained from surgical specimens, as detailed in procedures that were reviewed and approved by the Mayo Foundation institutional review board. Tissues obtained were incidental to the patient surgery, and were discarded by the surgical pathologist. All tissues were immersed in ice-cold Hank's balanced salt solution (HBSS) with 2.25 mM CaCl2, 0.8 mM MgSO4, and 12 mM glucose (pH 7.4). Third- to sixth-generation bronchi were freed from adherent tissue under a dissecting microscope. Epithelium was removed by gently rubbing the rings with a pipe cleaner moistened with HBSS. The remaining tissue was finely minced in ice-cold Ca2+-free HBSS (“0” Ca2+ HBSS), and smooth muscle cells were isolated as previously described (31). Briefly, HBSS was removed, the tissue was incubated for 1 h at 37°C in Earl's balanced salt solution (EBSS) containing 30 mg/ml BSA, 20 U/ml papain, and 0.005% DNase (Worthington Biochemical Corp., Lakewood, NJ), and subsequently with 1 mg/ml type-IV collagenase and 0.4 U/ml elastase for 45 min at 37°C. The tissue was gently triturated with a fire-polished pipette to disperse individual cells. Undissociated tissue debris was allowed to settle from suspended cells, and the cell suspension was transferred to a new tube, centrifuged at 100 × g for 5 min at room temperature, and the resultant cell pellet was dispersed in EBSS containing 1 mg/ml ovomucoid protease inhibitor, 1 mg/ml BSA, and 0.005% DNase. The cell suspension was then carefully layered onto EBSS, containing 10 mg/ml BSA and 10 mg/ml ovomucoid protease inhibitor, and centrifuged at 100 × g for 5 min at room temperature. The cell pellet was resuspended in Dulbecco's modified Eagle's medium (DMEM)/F12 medium, containing antibiotics/antimycotic and 10% FBS (DMEM complete), and centrifuged at 100 × g for 5 min at room temperature. This resuspension/centrifugation was repeated two additional times. The final cell pellet was resuspended in 1 ml DMEM complete and seeded into a T-75 culture flask in an additional 20 ml of DMEM complete. All cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Upon reaching 70–90% confluence, cells were passaged, plated for use in experiments, and/or saved in cryopreservation medium for future use. Cells were used between passages 2 and 5 for all experiments. Presence of smooth muscle cells were confirmed by immunohistochemistry and RT-PCR for α–smooth muscle actin (α-SMA) and immunostaining for sm22, as previously described (32, 33).
Cell Treatments
At time of passage, cells were plated onto 8-well borosilicate coverglass chambers and maintained in DMEM complete, as described above, for use in calcium imaging experiments. Some cells were also seeded into T-75 flasks to make cell lysates for Western blot analysis, or 6-well chambers for RNA extraction. At ∼ 50–60% confluence, DMEM complete medium was replaced with serum-free medium consisting of DMEM/F12, containing 1 mg/ml insulin, 0.67 μg/ml selenium, 0.55 mg/ml transferrin and antibiotic/antimycotic. Durations of this treatment greater than 48 h results in cells that have a previously determined contractile phenotype (32, 33). After 72 h, the medium was removed and replaced with HBSS for imaging experiments, or cells were immediately processed for cell lysates or RNA extracts, as described below. In experiments examining the effects of TNF-α, ∼ 18–22 h before their use, cells were treated with 20 ng/ml TNF-α.
siRNA for TRPC3
To confirm a role for TRPC3 in TNF-α–induced changes in Ca2+ responses, HBSMCs (passages 1–5) were grown to 50–60% confluence and transfected with 100 nM stealth siRNA directed toward TRPC3 (TRPC3 si; Invitrogen, Carlsbad, CA), or transfected with a negative control siRNA with a similar guanosine and cytosine (GC) content as TRPC3 si (siCon; Invitrogen). Stealth TRPC3 si is a 25-bp duplex corresponding to nucleotides 1,405–1,429 of the reported human TRPC3 mRNA sequence. The sense sequence of TRPC3 si is 5′-AUA CCA GAU CGU CAA GAG CUG CUG C-3′. To determine transfection efficiencies, we transfected cells with an FITC-tagged siRNA; 24 h after transfection, FITC-positive cells were counted and compared with the total cell number. Transfection efficiency was determined to be 81 ± 2% (n = 6 experiments). Lipofectamine (Invitrogen) was used as the transfection reagent at a concentration of 2 μl/ml in the final transfection volume. Six hours after initiation of transfection, serum-containing medium was added to the transfection medium. After an additional 48 h, the medium was replaced with serum-free medium to elicit an ACh-responsive phenotype (32, 33). After 24 h of serum deprivation, cells were treated with TNF-α (20 ng/ml), and serum deprivation was continued for an additional 18–24 h.
Measurement of Intracellular Ca2+ Responses
To measure [Ca2+]i, cells plated in 8-well borosilicate coverglass chambers were incubated with 5 mM Fura-2AM in HBSS for 60 min at room temperature. Cells were then washed twice with fresh HBSS, and subsequently maintained in HBSS. Cells were continuously perfused during the acquisition of all Ca2+ measurements. Fluorescence excitation, image acquisition, and Ca2+ data analyses were controlled using a dedicated video fluorescence imaging system (Metafluor; Universal Imaging Corporation, Downingtown, PA). Cells were imaged using an inverted Nikon Diaphot microscope, equipped with a Nikon Fluor 20× objective lens (Nikon Corporation, Tokyo, Japan). Fura-2–loaded cells were alternately excited at 340 and 380 nm using a Lambda 10–2 filter changer (Sutter Instrument Co., Novato, CA). Fluorescence emissions were collected separately for each wavelength using a 510 nm barrier filter. Images were acquired using a Micromax 12-bit camera system (Princeton Instruments, Trenton, NJ). [Ca2+]i was calculated from the ratio of intensities of Fura-2 emissions at 340 nm and 380 nm (calculated approximately every 0.75 s), by extrapolation from an in vitro calibration curve, as previously described (34).
SOCE
These experiments were designed to examine the effects of TNF-α on SOCE in HBSMCs. HBSMCs were loaded with Fura-2AM, as described above, and perfused for 60 s in HBSS, 60 s in “0” Ca HBSS, and 180 s in “0” Ca HBSS, 1 μM nifedipine, and 10 μM cyclopiazonic acid (CPA). This treatment lead to the depletion of Ca2+ from the SR, at which time the cells were perfused with Ca2+-containing HBSS, resulting in SOCE. In some experiments, the effects of lanthanum (1 mM), nickel (1 mM), 2-aminoethoxydiphenyl borate (2-APB; 100 μM), or gadolimium (100 μM) on SOCE were added to the perfusates 60 s before the reintroduction of extracellular Ca2+.
ROCE
These experiments were designed to examine the effects of TNF-α on ROCE in HBSMCs. HBSMCs were loaded with Fura-2AM, as described above. The cells were then perfused as for 60 s in HBSS, 60 s in “0” Ca HBSS, 180 s in “0” Ca HBSS, 1 μM ACh, and 180 s in HBSS, 1 μM ACh. To block ROCE, cells were perfused with 50 μM atropine 60 s before the reintroduction of extracellular Ca2+. Perfusion of HBSMCs with 50 μM atropine 60 s before the addition of ACh was sufficient to completely abolish any ACh-elicited [Ca2+]i responses.
Western Blot Analysis
Cells maintained as described were rinsed with HBSS containing “0” Ca2+ and “0” Mg2+, scraped from the flask surface, and pelleted by centrifugation at 100 × g for 5 min. The resulting cell pellets were resuspended in ice-cold lysis buffer consisting of 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphoshate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 10 μM PMSF, and homogenized. The resulting crude homogenates were centrifuged at 10,000 × g for 10 min to remove cellular debris, and the resultant supernatants were assayed for protein using the DC protein assay (BioRad, Hercules, CA), transferred to new tubes, and stored at −80°C until used. Lysates were diluted 1:2 in Laemmli sample buffer with 5% β-mercaptoethanol and placed in a boiling water bath for 3 min. Equal amounts of proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and immunoblotted using primary antibodies to TRPC1, 3, 4, 5, and 6 (Alomone Labs, Ltd., Jerusalem, Israel), and the protein bands were visualized by enhanced chemiluminescence. In siRNA experiments, transfected or control cells were rinsed with HBSS, scraped from the culture dish surface, and centrifuged as described above, but cell pellets were resuspended directly in 1× Laemmli sample buffer with 5% β-mercaptoethanol and subsequently immunoblotted for TRPC3, as described above. In these experiments, immunoblotting for α-SMA was also carried out to ensure equal loading.
RT-PCR for TRP Channel Expression
TRP channel mRNA expression from TNF-α or control cells was determined by reverse transcription followed by PCR. Total RNA from control and TNF-α–treated cells was isolated using the Trizol method, per the manufacturer's protocol (Invitrogen). RNA was then further purified using an RNeasy Mini kit (Qiagen, Valencia, CA) using the RNA cleanup protocol, including the on-column DNase digestion. RNA was quantified via spectrophotometry, and equimolar amounts from control and TNF-α–treated cells were used for RT-PCR. First-strand cDNAs synthesis was performed using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen), per the manufacturer's protocol. PCR conditions were optimized for each of the products amplified. Primers for TRPC1, 3, 4, 5, and 6, as well as for α-SMA and GAPDH, were designed against known mRNA sequences obtained from GenBank, and were as follows: TRPC1 forward (GCG TGC GAC AAG GG), reverse (ACT GGC CAA ACA TCG ATA); TRPC3 forward (GCA CTG CCA GAA ATA CGA AG), reverse (CCA CGA CCA GCA CAA CGA); TRPC4 forward (CCC GAA GAT TGC AAC T), reverse (GCC AAG GCC TTG TAG ATG); TRPC5 forward (CAG ATC TCT TTG GGA CGC), reverse (CGG CAA TAA GCT GAT AGG); TRPC6 forward (GCC AGA ATG CCC TAC AGT), reverse (CTA GGG CTG GTT GCT AAC); α-SMA forward (GAT CAC GGC CCT AGC ACC), reverse (AGG CCC GGC TTC ATC GTA); and GAPDH forward (AAC AGC GAC ACC CAC TCC TC), reverse (GGA GGG GAG ATT CAG TGT GGT). Primers were also designed for use in real-time PCR reactions, as detailed below for TRPC1, TRPC3, and CD38, as follows: TRPC1 forward (GCC CGG AAT TCT CGT GA), reverse (AGG TGG GCT TGC GTC GGT); TRPC3 forward (CAG GCC TAA GGG AGC AGA CCA TAG), reverse (ACT GTG ATA TTG GGC AGC GTG GTG); and CD38 forward (CTC TGT CTT GGC GTC AGT ATC CTG), reverse (AGC AAG GTA GCC TAG CAG CGT GTC). To verify the specificity of amplification by PCR for the various mRNAs, products from both PCR and real-time PCR were separated in 1% agarose gels containing ethidium bromide and visualized using UV luminescence. A DNA ladder was run in parallel to approximate product sizes. Bands corresponding to the predicted size of each product were excised from the gels, and the products were extracted using a QIAquick gel extraction kit (Qiagen) and subsequently sequenced by Mayo Clinic's core facilities to verify authenticity of all products.
Real-Time Semiquantitative PCR for TRPC3
To determine whether mRNA expression for TRPC3 was increased by TNF-α treatment, real-time PCR, using LightCycler (Roche Applied Science, Indianapolis, IN) and SYBR Green (Molecular Probes, Eugene, OR) technologies, were employed. A LightCycler-human-glucose-6-phosphate dehydrogenase housekeeping gene set (Roche Applied Science) was also used to normalize expression levels of TRPC3, TRPC1, and CD38 to expression levels of hG6PDH in all samples examined. Expression of TRPC1 mRNA was included as a negative control, as the levels of TRPC1 protein did not change with TNF-α treatment. CD38 mRNA was examined as a positive control, because mRNA expression of CD38 is known to increase in response to TNF-α in ASM (28). Products from these real-time RT-PCR reactions were separated on agarose gels, and the products produced were confirmed by sequence analysis, as described above. TRPC3 mRNA levels were also examined in RNA extracts from cells transfected with TRPC3 si or siCon, with or without TNF-α, to confirm the effectiveness of the siRNA transfections at reducing TRPC3 mRNA.
Materials
DMEM and antibiotic/antimycotic mixture were obtained from Invitrogen. ACh chloride, bradykinin, and all fine chemicals were purchased from Sigma-Aldrich (St. Louis, MO). CPA, nifedipine, and Fura-2AM were acquired from Calbiochem (La Jolla, CA).
Statistical Analysis
In all experiment, differences between control and treated groups were analyzed for statistical significance using either a one-way ANOVA or a Student's t test (two-tailed) for unpaired sample. In the case of ANOVA, a multiple comparison test was used to compare all groups. Experiments were performed using cells obtained from the airways of six different patients. No differences were noted in the responsivity of cells obtained from different patients. Thus, each experiment resulted from cells from 1 individual. However, results from individual experiments were grouped, and represent averages from two to four different subjects. A value of P ⩽ 0.05 was accepted as significant. All results are expressed as mean ± SEM.
RESULTS
TNF-α Effects on Agonist-Elicited [Ca2+]i Mobilization
After HBSMC incubation with TNF-α or BSA (control), basal and ACh- (10 μM) or BK (10 nM)-elicited net peak and plateau [Ca2+]i responses were determined in the presence or absence of extracellular Ca2+. TNF-α significantly increased basal [Ca2+]i compared with control cells (111 ± 2 versus 79 ± 1 nM; Figure 1A). However, in the absence of extracellular Ca2+, basal [Ca2+]i was significantly decreased in both groups, and there was no difference in basal [Ca2+]i between cells treated with or without TNFα (72 ± 1 versus 71 ± 1 nM; Figure 1A).
Figure 1.
Intracellular Ca2+ homeostasis is altered in HBSMCs after TNF-α treatment. (A) Basal [Ca2+]i increase after incubation with TNF-α is extracellular Ca2+–dependent. TNF-α (TNF) treatment significantly increases basal [Ca2+]i compared with control (Control). This effect is dependent upon extracellular Ca2+. Results are mean ± SEM (n = 6 experiments, 100 individual cells analyzed per experiment; P < 0.001 for the following comparisons: *compared with control HBSS; ‡compared with control 0 Ca2+; †compared with TNF HBSS). (B) Net peak responses to ACh are increased by incubation with TNFα. ACh-elicited net peak [Ca2+]i increase in control (Control), or TNF-α (TNF)–treated HBSMCs in the presence (HBSS), or absence (0 Ca2+), of extracellular Ca2+. Responses in both groups are significantly decreased in the absence of extracellular Ca2+, and are significantly increased in cells incubated with TNF-α. Results are mean ± SEM (n = 3 experiments; 100 individual cells analyzed per experiment; P < 0.001 for the following comparisons: *compared with control HBSS; ‡compared with control 0 Ca2+; †compared with TNF HBSS). (C) Net plateau responses to ACh are increased by incubation with TNF-α in an extracellular [Ca2+]i–dependent mechanism. In TNF-α (TNF)–incubated HBSMCs, ACh- elicited net plateau [Ca2+]i was increased compared with control (Control) cells. This effect was dependent on the presence of extracellular Ca2+. Results are mean ± SEM (n = 3 experiments; 100 individual cells analyzed per experiment; P < 0.001 for the following comparisons: *compared with control HBSS; ‡compared with control 0 Ca2+; †compared with TNF HBSS).
In HBSS, TNF-α treatment significantly increased ACh-elicited net peak and plateau [Ca2+]i responses (Figures 1B and 1C, respectively). Similarly, in HBSS, BK-elicited net peak and plateau [Ca2+]i. were significantly increased after TNF-α incubation compared with control cells (net peak = 487 ± 5 versus 356 ± 3 nM, respectively; and plateau = 424 ± 5 versus 286 ± 3 nM, respectively; P < 0.001). In both control and TNF-treated cells, ACh- and BK-elicited net peak [Ca2+]i responses were significantly diminished in the absence of extracellular Ca2+.
Finally, in the absence of extracellular Ca2+, the ACh-elicited plateau [Ca2+]i was significantly decreased (Figure 1C), whereas the BK-elicited plateau [Ca2+]i was abolished (data not shown) and TNF-α treatment was not different from control. Taken together, these results suggest that TNF-α increases basal [Ca2+]i, via enhanced constitutive influx, increases agonist-elicited intracellular Ca2+ release, and increases sustained Ca2+ influx.
Effects of TNF-α on SOCE
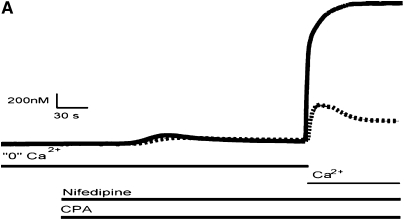
HBSMC SR Ca2+ stores were depleted using 10 μM CPA, which inhibits the sarcoplasmic–endoplasmic reticulum CaATPase, resulting in a “leak” of Ca2+ from the SR in the absence of extracellular Ca2+. Nifedipine (1 μM) was present in all experiments to eliminate any contribution of Ca2+ influx through L-type voltage gated Ca2+ channels. Upon the reintroduction of extracellular Ca2+, a rapid rise in [Ca2+]i was observed. [Ca2+]i traces from a single experiment as well as mean data are shown in Figure 2. In control cells, there was a transient rapid rise in [Ca2+]i, peaking at 523 ± 10 nM, and subsequently declining to a plateau. In HBSMCs that were treated with TNF-α, the magnitude of this influx was significantly higher (1,967 ± 32 nM), and the peak was maintained throughout the duration of the experiment.
Figure 2.
SOCE in HBSMCs. (A) Representative [Ca2+]i traces of control and TNF-α–treated HBSMCs demonstrating SOCE (control, dashed line; TNF-α, solid line). Cells were first exposed to CPA in the absence of extracellular Ca2+ to diminish Ca2+ from the SR, and subsequently re-exposed to extracellular Ca2+, resulting in an influx of Ca2+ into the cells. Notice a significant augmentation of Ca2+ influx in cells treated with TNF-α compared with control. (B) Cumulative data from 8 individual experiments, 100 cells per experiment (mean ± SEM; *P < 0.0001 compared with controls).
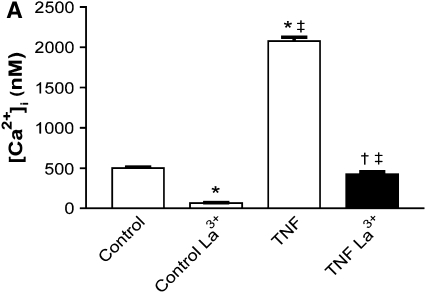
The store-operated Ca2+ influx described above was significantly inhibited by the trivalent cation lanthanum (1 mM lanthanum chloride; Figure 3A), as well as the divalent cation nickel (1 mM nickel chloride; Figure 3B) in both control (501 ± 9 versus 67 ± 5 nM and 424 ± 8 versus 323 ± 11 nM, respectively) and TNF-α–treated cells (2,077 ± 48 versus 424 ± 32 nM and 1,840 ± 52 versus 430 ± 15 nM, respectively). In experiments examining the effects of 2-APB (100 μM) or gadolinium (10 μM) on SOCE, supposed more selective inhibitors of SOCE (35), inhibition of influx was observed with 2-APB in control cells (869 ± 29 versus 602 ± 35 nM), whereas there was no significant effect on influx in TNF-α–treated cells (2,485 ± 85 versus 2,584 ± 60 nM; Figure 3C). Gadolinium had no significant effect on influx in TNF-α–treated cells (1,869 ± 40 versus 1,944 ± 37 nM), whereas it significantly augmented the influx observed in control cells (371 ± 15 versus 497 ± 19 nM; Figure 3D).
Figure 3.
Effects of lanthanum (La3+), nickel (Ni2+), 2-APB, and gadolinium (Gd3+) on SOCE. (A and B) Lanthanum and nickel inhibit SOCE in both control and TNF-α–treated HBSMCs. Cumulative data from 3 individual experiments, 100 cells per experiment (mean ± SEM; P < 0.001 for the following comparisons: *compared with control; ‡compared with control La3+; †compared with TNF; ‡‡P < 0.01 compared with control Ni2+). (C) 2-APB attenuates SOCE in control but has no effect in TNF-α–treated HBSMCs. Results are mean ± SEM (n = 3 experiments, 100 individual cells analyzed per experiment; *P < 0.01 compared with control; **P < 0.001 compared with control; ‡P < 0.001 compared with control 2-APB). (D) Gadolinium has no effect on SOCE in HBSMCs treated with TNF-α, although slightly augmenting SOCE in control cells. Results are mean ± SEM (n = 3 experiments, 100 individual cells analyzed per experiment; *P < 0.05 compared with control; **P < 0.001 compared with control; ‡P < 0.001 compared with control Gd3+).
Effects of TNF-α on ROCE
The protocols in these experiments were designed to differentiate between ROCE and SOCE upon agonist stimulation using ACh (1 μM). TNF-α or control cells were stimulated with ACh in the absence of extracellular Ca2+, and then extracellular Ca2+ was reintroduced, either in the continued presence of ACh or with atropine (50 μM) substituted for ACh, and Ca2+ influx was examined. In control cells, the reintroduction of extracellular Ca2+ elicited an influx of Ca2+ in the presence of ACh (218 ± 9 nM) that was significantly inhibited in the presence of atropine (108 ± 6 nM; Figure 4). In the case of HBSMCs treated with TNF-α, reintroduction of extracellular Ca2+ led to a dramatic rise in [Ca2+]i (993 ± 31 nM), which was not significantly reduced by the replacement of ACh with atropine (952 ± 24 nM; Figure 4). These results suggest that TNF-α treatment of HBSMCs results in the reduction of ROCE, and may trigger a switch to a primarily SOCE mechanism.
Figure 4.
ROCE in HBSMCs. In the absence of extracellular Ca2+, control cells or TNF-α–treated cells were stimulated with 1 μM ACh; ACh was either continued (Control) or replaced with 50 mM atropine (Atropine), and extracellular Ca2+ was subsequently introduced and Ca2+ influx examined. In untreated cells, the presence of atropine significantly attenuated the Ca2+ influx upon reintroduction of Ca2+; this is in contrast to TNF-α–treated cells in which atropine had no significant effect on Ca2+ influx. This suggests that ROCE is present in control cells, although absent in TNF-α–treated cells. Cumulative data from 7 individual experiments, 100 cells per experiment (mean ± SEM; *P < 0.001 compared with control; ‡P < 0.001 compared with control Atropine).
Expression of TRPC Family Members in HBSMCs
Western blot analysis (Figure 5) combined with RT-PCR (Figure 6) confirmed the presence and expression of TRPC1, 3, 4, 5, and 6 in primary cultured HBSMCs, in support of findings by others (25, 36, 37). We consistently observed a significant increase in TRPC3 protein in HBSMCs treated with TNF-α compared with controls (TRPC3, 3,681 ± 1701%; n = 5; P < 0.05, Figure 5). However, TNF-α treatment had no significant effect on the quantity of other members of the TRPC family at the protein level (Figure 5). Specifically, using densitometry of the Western blots, the percent change in relative TRPC protein expression in control cells compared with those receiving TNF-α treatment were: TRPC1, 93 ± 9%; TRPC4, 105 ± 17%; TRPC5, 146 ± 20; and TRPC6, 110 ± 10% (n = 3–5 for each comparison; nonsignificant). We found siRNA technology to be a useful tool (38, 39) to “knock down” the mRNA expressed from the TRPC3 gene and specifically diminish TPRC3 protein. Transfection of HBSMCs with a siRNA specific for TRPC3 (TRPC3 si) resulted in a decrease in TRPC3 protein in TNF-α–treated HBSMCs, as determined by Western blot analysis, when compared with cells transfected with a control siRNA (siCon; Figure 7). In all but one experiment, TRPC3 protein was below detectable levels in control (non–TNF-α) cells; therefore, we could not determine the effects of TRPC3 si transfection on TRPC3 protein expression in these cells.
Figure 5.
Western blot analysis of TRPC1, 3, 4, 5, and 6 in cell lysates from untreated (Control) or TNF-α –treated (TNF) HBSMCs. Notice the increase in TRPC3 protein in HBSMCs treated with TNF-α. There were no significant differences in any of the other TRPC family members examined.
Figure 6.
RT-PCR analysis of total RNA extracted from untreated HBSMCs. Specific primer pairs were designed for TRPC1, 3, 4, 5, and 6, as well as for GAPDH as a positive control. After RT-PCR, products were separated by agarose gel electrophoresis and visualized using ethidium bromide and UV transillumination. Specific products corresponding to each of the TRPC family members were observed, and sequence analysis of excised products confirmed their presence. The number on top corresponds to the specific TRPC homolog or GAPDH, whereas the bottom number indicates the predicted product size. A DNA ladder was also run along side the samples to estimate observed product sizes. Data are representative of a single experiment, which was repeated at least four times.
Figure 7.
Western blot analysis of TRPC3 protein expression in cells treated with a nonspecific siRNA (siCon) or an siRNA directed specifically toward TRPC3 (TRPC3 si), and treated with (TNF) or without (Con) TNF-α. Notice the significant increase in TRPC3 protein in HBSMCs treated with TNF-α, and the decrease in the TNF-α–induced increases in TRPC3 protein in cells transfected with TRPC3 si. Shown is a representative blot from a single experiment that was repeated three times.
Real-time semiquantitative RT-PCR confirmed that mRNA levels of CD38 and TRPC3 were significantly increased in HBSMCs treated with TNF-α, and also that levels of TRPC1 mRNA remained unchanged (Table 1). The relative magnitude of the TNF-α–induced increase in TRPC3 mRNA expression (∼ 1.7-fold) was relatively small when compared with that of CD38 (∼ 59-fold), which suggests that the observed increase in TRPC3 protein may be due to mechanisms other than transcriptional upregulation. In experiments using siRNA, we observed decreases in TRPC3 mRNA levels in cells transfected with TRPC3 si when compared with cells treated with siCon; however, the differences were only statistically significant in the TNF-α–treated cells (Figure 8).
TABLE 1.
REAL-TIME SEMIQUANTITATIVE PCR*
| Primer | Control-Normalized Copy No.† | TNF-α–Normalized Copy No.† |
|---|---|---|
| TRPC1 | 78.0 ± 10.0 | 79.0 ± 10.0 |
| TRPC3 | 2.3 ± 0.4 | 4.1 ± 0.7‡ |
| CD38 | 0.30 ± 0.02 | 16.0 ± 2.0 |
Definition of abbreviation: TRPC, transient receptor potential subfamily C.
PCR was performed on first-strand cDNA generated from total RNA isolated from control or TNF-α–treated HBSMCs using primers specific for TRPC1, TRPC3, and CD38. The amplification rate was monitored using light cycler and SYBR green technologies, and data were analyzed as described in Materials and Methods. Data are expressed as copy number normalized to the house keeping gene glucose-6-phosphate dehydrogenase.
Per 1,000 copies.
P < 0.05 compared to control.
Figure 8.
Effects of TRPC3 si on TRPC3 mRNA expression in HBSMCs. Transfection of HBSMCs with an siRNA directed toward TRPC3 leads to a decrease in TRPC3 mRNA in both control and TNF-α–treated cells; however, these decreases were only statistically significant in the TNF-α–treated cells. Results are mean ± SEM (n = 6 experiments; P < 0.05 for the following comparisons: *compared with siCon Con; ‡compared with TRPC3 si Con; and †compared with TRPC3 si TNF).
Effects of TRPC3 si on ACh-Elicited [Ca2+]i Mobilization and SOCE
In experiments designed to examine the effects of TRPC3 siRNA on agonist-elicited Ca2+ responses, TRPC3 si transfection resulted in the inhibition of ACh-induced peak [Ca2+]i responses when compared with siCon transfection in control cells (170 ± 7 versus 106 ± 8 nM) and in TNF-α–treated cells (537 ± 24 versus 363 ± 18 nM; Figure 9B). Examination of basal [Ca2+]i in these experiments revealed that TRPC3 si transfection of HBSMCs also brought the TNF-α–induced increase in basal [Ca2+]i down to control levels, from 120 ± 4 to 101 ± 4 (Figure 9A). TRPC3 si had no significant effect on basal [Ca2+]i in control HBSMCs (91 ± 3 versus 99 ± 3; Figure 8A). TNF-α–induced increases in steady-state [Ca2+]i responses to ACh were significantly inhibited in TRPC3 si–transfected HBSMCs, from 306 ± 22 to 138 ± 12 (Figure 9C). Steady-state [Ca2+]i responses to ACh were not significantly affected in control HBSMCs (40 ± 3 versus 59 ± 6). In parallel experiments, we also examined the effects of TRPC3 si on SOCE, and observed that influx was significantly attenuated in TNF-α–treated cells (2,705 ± 74 versus 1,326 ± 49), whereas there was no significant effect in control cells (620 ± 22 versus 543 ± 22 nM; Figure 9D).
Figure 9.
Effects of siRNA transfection on basal (A) and ACh-elicited peak (B), and steady-state [Ca2+]i responses (C) and SOCE (D). (A) Transfection of HBSMCs with an siRNA directed toward TRPC3 (TRPC3 si) led to significant attenuation of the TNF-α–elicited increase in basal [Ca2+]i when compared with cells transfected with a control siRNA (siCON). Cumulative data from 4 individual experiments, 100 cells per experiment (mean ± SEM). (B) TRPC3 si attenuated the net peak [Ca2+]i responses to ACh in both control cells and TNF−α–treated cells. Cumulative data from 4 individual experiments, 100 cells per experiment (mean ± SEM). TRPC3 si transfection led to the attenuation of the steady-state [Ca2+]i responses to ACh (C) as well as SOCE (D) in TNF-α–treated cells, but having no significant effect in siCON-transfected cells. Results are mean ± SEM (n = 4 experiments, 100 cells per experiment; P < 0.05 for the following comparisons: *compared with siCon Con; ‡compared with TRPC3 si Con; †compared with siCon TNF).
DISCUSSION
The results of this study confirm that treatment of human ASM cells with the proinflammatory cytokine, TNF-α, results in significant changes in [Ca2+]i regulation. These effects include an increase in basal and agonist-elicted [Ca2+]i, which are influenced by extracellular [Ca2+]. Moreover, TNF-α treatment elicits a significant increase in SOCE. The latter effect is not diminished during antagonism of receptor-operated Ca2+ influx. In addition, TNF-α treatment leads to a significant increase in TRPC3 mRNA and protein expression. Finally, we were able to significantly reduce TRPC3 protein levels using siRNA techniques, leading to a reduction in TNF-α–induced increases in agonist-elicited Ca2+ responses, as well as in SOCE, supporting the hypothesis that increases in TRPC3 contribute to these TNF-α–elicited changes in Ca2+ regulation.
Several observations lead to the hypothesis that TNF-α may play a pivotal role in airway inflammatory diseases. Previous studies have shown that TNF-α is elevated in the airways of patients with symptomatic asthma (1), and that instillation of TNF-α into the lungs of humans (4) or other animal species (40) results in airway hyperresponsiveness (AHR). Although evidence exists that, in patients with severe asthma, treatment with soluble TNF-α receptor IgG1 Fc fusion protein is associated with improvement in asthma symptoms, lung function, and AHR (41), the specific cells affected by TNF-α and the mechanisms of altered AHR underlying this improvement are not known.
Previous studies by Amrani and colleagues have demonstrated that exposure of ASM cells to TNF-α leads to potentiation of [Ca2+]i responses to various contractile agonists, including ACh and BK (9, 27). The results from the current studies confirm these previous results, and also provide evidence that Ca2+ influx pathways are clearly altered in human ASM cells treated with TNF-α. In cells treated with TNF-α, basal [Ca2+]i levels were increased when compared with controls. Omission of extracellular Ca2+ lead to a significant decrease in basal [Ca2+]i in both control and TNF-α–treated cells; however, there is no difference in basal [Ca2+]i between control and TNF-α–treated cells when extracellular Ca2+ is omitted (Figure 1A). This would suggest that a constitutive Ca2+ influx occurs in unstimulated HBSMCs cells that is increased after incubation with TNF-α. In TNF-α–treated ASM cells, peak [Ca2+]i responses to ACh (Figure 1B) and BK (data not shown) were significantly augmented when compared with untreated cells. This is in agreement with previous studies by Amrani and colleagues (9, 27). We also confirmed their findings that the plateau phase of the ACh-induced [Ca2+]i responses are also increased in cells treated with TNF-α (Figure 1C). We also determined that the increase in the plateau phase of the responses to ACh (Figure 1C) and BK (data not shown) are due to enhanced Ca2+ influx, as the omission of extracellular Ca2+ significantly diminished this effect.
SOCE or capacitative Ca2+ entry was initially described by Putney in 1986 (18). Since then, many investigators have described SOCE in many cell types. The presence of store-operated Ca2+ influx pathways has been demonstrated previously in guinea pig, rat, human, and porcine ASM (25, 37, 42–44). Receptor-operated Ca2+ influx has also been detailed in ASM (16, 45). Sweeney and coworkers have demonstrated a role for SOCE in bronchial contraction and proliferation, and therefore propose that SOCE may play an important role in the airway constriction and remodeling observed in inflammatory diseases such as asthma (43). In the present study, we confirm the presence of both receptor- and store-operated Ca2+ influx mechanisms in primary cultured human bronchial myocytes. We also report that treatment of HBSMCs with TNF-α leads to a significant increase in SOCE (Figure 2), which is not diminished by antagonism of receptor-operated Ca2+ influx (Figure 4). It is possible that these changes in Ca2+ influx pathways could contribute to the altered AHR observed in bronchial asthma.
Western blot analysis revealed the presence of TRPC1, 3, 4, 5, and 6 in whole cell lysates of HBSMCs (Figure 5). Expression of TRPC1, 3, 4, 5, and 6 were confirmed by RT-PCR of whole cell RNA extracts (Figure 6). The level of TRPC3 protein in control cells was minimal, and was dramatically increased upon treatment of HBSMCs with TNF-α (Figure 5). We also examined whether changes in TRPC1, TRPC3, and CD38 mRNA expression occurred with TNF-α treatment using real-time semiquantitative RT-PCR. As we predicted, no change in TRPC1 mRNA expression was observed, and TRPC3 mRNA expression was increased by TNF-α treatment of HBSMCs (Table 1). CD38 expression is known to increase in HBSMCs upon TNF-α treatment (28), and was used as a positive control; as expected, CD38 expression was increased in HBSMCs treated with TNF-α (Table 1). The relative increase in TRPC3 mRNA expression (∼ 1.7-fold) was relatively small in comparison with the increase in CD38 mRNA expression (∼ 59-fold). We therefore cannot be certain that the increase in TRPC3 protein observed in HBSMCs treated with TNF-α is exclusively due to the upregulation of TRPC3 mRNA expression, as studies on translational regulation and TRPC3 degradation pathways were beyond the scope of this study.
Recently, TNF-α treatment of vascular endothelial muscle cells has been shown to induce an increase in TRPC1 expression (46, 47). These investigators demonstrated that the increase in TRPC1 protein was also accompanied by an increase in TRPC1 mRNA through an NF-κB–dependent mechanism. The authors also demonstrated an increase in Ca2+ influx upon Ca2+ store depletion corresponding to the increase in TRPC1 upon TNF-α treatment of these cells. In HBSMCs, TNF-α treatment has no effect on TRPC1 expression, as demonstrated by real-time PCR analyses (Table 1). We did, however, observe a TNF-α–induced increase in TRPC3 mRNA (Table 1) and protein expression (Figure 5), with a corresponding increase in Ca2+ influx upon intracellular Ca2+ store depletion. Thus, it would appear that there are different expression patterns of TRPCs induced by TNF-α in different cell types.
In the present study, we demonstrate that HBSMCs treated with TNF-α have altered regulation of [Ca2+]i. In control cells, Ca2+ influx upon the reintroduction of extracellular Ca2+ after ACh stimulation, in the absence of extracellular Ca2+, is attenuated by the addition of the muscarinic antagonist, atropine. However, TNF-α treatment results in influx of Ca2+, which is not affected by receptor blockade with atropine. This important difference suggests that there is a change in the mode of regulation of Ca2+ influx in HBSMCs determined by levels of expression of TRPC3. This finding is consistent with the findings of Vasquez and colleagues (23) that indicate that TRPC3 can form or contribute to the formation of ion channels regulated in two very distinct ways (i.e., either by a receptor-coupled phospholipase C–derived messenger or Ca2+ store-depletion), and that the mechanism of regulation of the channels depends upon the level of TRPC3 expression.
Using siRNA technology, we were able to reduce the TNF-α–induced increases in TRPC3 protein (Figure 7) and mRNA (Figure 8) with a siRNA directed specifically toward TRPC3. TRPC3 mRNA “knock down” significantly attenuated the TNF-α–induced increases in basal [Ca2+]i, SOCE, and agonist-elicited [Ca2+]i responses (Figure 9). In control cells, TRPC3 si had no significant effect on SOCE; however, agonist-elicited [Ca2+]i responses were significantly inhibited. This would support our hypothesis that the changes in [Ca2+]i regulation that we observe upon TNF-α treatment of HBSMCs are due, at least in part, to an increase in TRPC3 levels.
In summary, we have further confirmed the presence of TRPC1, 3, 4, 5, and 6 in primary cultured HBSMCs. We have demonstrated that TNF-α treatment of these cells leads to altered [Ca2+]i regulation, including significantly enhanced basal [Ca2+]i, agonist-elicited [Ca2+]i responses, and SOCE. These changes in Ca2+ regulation coincide with significant increases in TRPC3 expression. Using siRNA directed specifically at TRPC3, we confirmed that these TNF-α–induced changes in HBSMC Ca2+ regulation are due, at least in part, to changes in TPRC3 expression levels. The TRPC3 siRNA data would further support the notion that, under inflammatory conditions, TRPC3 plays an exaggerated role in HBSMC [Ca2+]i regulation. Taken together, these findings suggest that, in inflammatory airway diseases such as asthma and COPD, TNF-α results in increased airway myocyte activation due to increased SOCE as a result of increased TRPC3 protein. We therefore suggest that TRPC3 might be a relevant therapeutic target in inflammatory diseases of the airway, such as asthma and COPD.
Acknowledgments
The authors thank Jeff Bailey for his molecular biology advice.
This work was supported by National Institutes of Health grants AI050494, AI07047, GM56686, GM74309, and HL074309.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0003OC on March 30, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol 1992;89:958–967. [DOI] [PubMed] [Google Scholar]
- 2.Taki F, Kondoh Y, Matsumoto K, Takagi K, Satake T, Taniguchi H, Matsuzaki M. Tumor necrosis factor in sputa of patients with bronchial asthma on exacerbation [article in Japanese]. Arerugi 1991;40:643–646. [PubMed] [Google Scholar]
- 3.Shah A, Church MK, Holgate ST. Tumour necrosis factor α: a potential mediator of asthma. Clin Exp Allergy 1995;25:1038–1044. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-α increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 1995;152:76–80. [DOI] [PubMed] [Google Scholar]
- 5.Thomas PS. Tumour necrosis factor-α: the role of this multifunctional cytokine in asthma. Immunol Cell Biol 2001;79:132–140. [DOI] [PubMed] [Google Scholar]
- 6.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes PJ. Cytokine-directed therapies for the treatment of chronic airway diseases. Cytokine Growth Factor Rev 2003;14:511–522. [DOI] [PubMed] [Google Scholar]
- 8.Amrani Y, Bronner C. Tumor necrosis factor α potentiates the increase in cytosolic free calcium induced by bradykinin in guinea-pig tracheal smooth muscle cells. C R Acad Sci III 1993;316:1489–1494. [PubMed] [Google Scholar]
- 9.Amrani Y, Martinet N, Bronner C. Potentiation by tumour necrosis factor-α of calcium signals induced by bradykinin and carbachol in human tracheal smooth muscle cells. Br J Pharmacol 1995;114:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter I, Cobban HJ, Vandenabeele P, MacEwan DJ, Nixon GF. Tumor necrosis factor-α-induced activation of Rhoa in airway smooth muscle cells: role in the Ca2+ sensitization of myosin light chain20 phosphorylation. Mol Pharmacol 2003;63:714–721. [DOI] [PubMed] [Google Scholar]
- 11.Parris JR, Cobban HJ, Littlejohn AF, MacEwan DJ, Nixon GF. Tumour necrosis factor-α activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle. J Physiol 1999;518:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukkar MB, Hughes JM, Armour CL, Johnson PR. Tumour necrosis factor-α potentiates contraction of human bronchus in vitro. Respirology 2001;6:199–203. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds AM, Holmes MD, Scicchitano R. Cytokines enhance airway smooth muscle contractility in response to acetylcholine and neurokinin A. Respirology 2000;5:153–160. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Tliba O, Van Besien CR, Panettieri RA, Jr, Amrani Y. TNF-α modulates murine tracheal rings responsiveness to G-protein–coupled receptor agonists and kcl. J Appl Physiol 2003;95:864–872. (discussion 863). [DOI] [PubMed] [Google Scholar]
- 15.Worley JF III, Kotlikoff MI. Dihydropyridine-sensitive single calcium channels in airway smooth muscle cells. Am J Physiol 1990;259:L468–L480. [DOI] [PubMed] [Google Scholar]
- 16.Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol 1991;435:123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L909–L917. (see comment). [DOI] [PubMed] [Google Scholar]
- 18.Putney JW, Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986;7:1–12. [DOI] [PubMed] [Google Scholar]
- 19.Takemura H, Putney JW, Jr. Capacitative calcium entry in parotid acinar cells. Biochem J 1989;258:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci 2001;2:387–396. [DOI] [PubMed] [Google Scholar]
- 21.Putney JW Jr, McKay RR. Capacitative calcium entry channels. Bioessays 1999;21:38–46. [DOI] [PubMed] [Google Scholar]
- 22.Venkatachalam K, Ma HT, Ford DL, Gill DL. Expression of functional receptor-coupled TRPC3 channels in DT40 triple receptor InsP3 knockout cells. J Biol Chem 2001;276:33980–33985. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez G, Wedel BJ, Trebak M, St John Bird G, Putney JW Jr. Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J Biol Chem 2003;278:21649–21654. [DOI] [PubMed] [Google Scholar]
- 24.Qian F, Huang P, Ma L, Kuznetsov A, Tamarina N, Philipson LH. TRP genes: candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes 2002;51:S183–S189. [DOI] [PubMed] [Google Scholar]
- 25.Corteling RL, Li S, Giddings J, Westwick J, Poll C, Hall IP. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am J Respir Cell Mol Biol 2004;30:145–154. [DOI] [PubMed] [Google Scholar]
- 26.Hyvelin JM, Martin C, Roux E, Marthan R, Savineau JP. Human isolated bronchial smooth muscle contains functional ryanodine/caffeine- sensitive Ca-release channels. Am J Respir Crit Care Med 2000;162:687–694. [DOI] [PubMed] [Google Scholar]
- 27.Amrani Y, Krymskaya V, Maki C, Panettieri RA Jr. Mechanisms underlying TNF-α effects on agonist-mediated calcium homeostasis in human airway smooth muscle cells. Am J Physiol 1997;273:L1020–L1028. [DOI] [PubMed] [Google Scholar]
- 28.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose–mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J 2003;17:452–454. [DOI] [PubMed] [Google Scholar]
- 29.Pabelick CM, Ay B, Prakash YS, Sieck GC. Effects of volatile anesthetics on store-operated Ca(2+) influx in airway smooth muscle. Anesthesiology 2004;101:373–380. [DOI] [PubMed] [Google Scholar]
- 30.Trian T, Girodet PO, Ousova O, Marthan R, Tunon-de-Lara JM, Berger P. RNA interference decreases PAR-2 expression and function in human airway smooth muscle cells. Am J Respir Cell Mol Biol 2006;34:49–55. [DOI] [PubMed] [Google Scholar]
- 31.Kannan MS, Prakash YS, Johnson DE, Sieck GC. Nitric oxide inhibits calcium release from sarcoplasmic reticulum of porcine tracheal smooth muscle cells. Am J Physiol 1997;272:L1–L7. [DOI] [PubMed] [Google Scholar]
- 32.Halayko AJ, Camoretti-Mercado B, Forsythe SM, Vieira JE, Mitchell RW, Wylam ME, Hershenson MB, Solway J. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. Am J Physiol 1999;276:L197–L206. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell RW, Halayko AJ, Kahraman S, Solway J, Wylam ME. Selective restoration of calcium coupling to muscarinic M(3) receptors in contractile cultured airway myocytes. Am J Physiol Lung Cell Mol Physiol 2000;278:L1091–L1100. [DOI] [PubMed] [Google Scholar]
- 34.White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. FASEB J 2003;17:482–484. [DOI] [PubMed] [Google Scholar]
- 35.Trebak M, Bird GS, McKay RR, Putney JW Jr. Comparison of human TRPC3 channels in receptor-activated and store-operated modes: differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem 2002;277:21617–21623. [DOI] [PubMed] [Google Scholar]
- 36.Ong HL, Chen J, Chataway T, Brereton H, Zhang L, Downs T, Tsiokas L, Barritt G. Specific detection of the endogenous transient receptor potential (TRP)-1 protein in liver and airway smooth muscle cells using immunoprecipitation and Western-blot analysis. Biochem J 2002;364:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corteling RL, Li S, Giddings J, Westwick J, Poll C, Hall IP. Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am J Respir Cell Mol Biol 2003;30:145–154. [DOI] [PubMed] [Google Scholar]
- 38.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998;391:806–811. [DOI] [PubMed] [Google Scholar]
- 39.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 2002;3:737–747. [DOI] [PubMed] [Google Scholar]
- 40.Kips JC, Tavernier J, Pauwels RA. Tumor necrosis factor causes bronchial hyperresponsiveness in rats. Am Rev Respir Dis 1992;145:332–336. [DOI] [PubMed] [Google Scholar]
- 41.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, Beckett P, Al Ali M, Chauhan A, Wilson SJ, et al. Tumour necrosis factor (TNFα) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax 2005;60:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong HL, Brereton HM, Harland ML, Barritt GJ. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology 2003;8:23–32. [DOI] [PubMed] [Google Scholar]
- 43.Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol 2002;92:1594–1602. [DOI] [PubMed] [Google Scholar]
- 44.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L909–L917. [DOI] [PubMed] [Google Scholar]
- 45.Murray RK, Fleischmann BK, Kotlikoff MI. Receptor-activated Ca influx in human airway smooth muscle: use of Ca imaging and perforated patch-clamp techniques. Am J Physiol 1993;264:C485–C490. [DOI] [PubMed] [Google Scholar]
- 46.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-α–induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol 2004;287:L1303–L1313. [DOI] [PubMed] [Google Scholar]
- 47.Paria BC, Malik AB, Kwiatek AM, Rahman A, May MJ, Ghosh S, Tiruppathi C. Tumor necrosis factor-α induces nuclear factor-κB–dependent TRPC1 expression in endothelial cells. J Biol Chem 2003;278:37195–37203. [DOI] [PubMed] [Google Scholar]