Abstract
Transforming growth factor (TGF)-β family members regulate multiple aspects of wound repair through effects on cell proliferation, matrix production, and tissue inflammation, but the effects of TGF-β on wound closure itself have been controversial. We found that blocking antibodies to TGF-β enhanced the degree of closure of scratch wounds in primary airway epithelial monolayers, while addition of exogenous TGF-β1 inhibited the degree of closure, suggesting that endogenous activation of TGF-β normally serves as a brake on the degree of wound closure. Although these cells secreted large amounts of TGF-β2 and small amounts of TGF-β1, blockade of TGF-β1 enhanced the degree of wound closure, whereas blockade of TGF-β2 had no effect. TGF-β1 (but not TGF-β2) can be activated by two members of the integrin family, αvβ6 and αvβ8, which are both expressed on airway epithelial cells. Wounding induced activation of TGF-β through effects of both integrins, but antibodies against αvβ8 enhanced the degree of wound closure, whereas antibodies against αvβ6 did not.
Keywords: airway epithelium, integrin αvβ6, integrin αvβ8, TGF-β, wound closure
In response to injury to surface epithelium, transforming growth factor (TGF)-β isoforms are secreted and activated. TGF-β isoforms are thought to play important roles in inducing fibroblasts to produce extracellular matrix proteins to cover the wound and in limiting the local inflammatory response. However, the effects of TGF-β on the behavior of the cells involved in primary closure of epithelial wounds have not been completely elucidated.
Injury to the airway epithelium has been shown to be an early, prominent feature of several respiratory diseases. For example, chronic asthma has been suggested to involve impaired ability of the epithelium to restitute itself after initial injury, which could contribute to the airway remodeling that characterizes this disease (1). A growing body of evidence suggests that idiopathic pulmonary fibrosis (IPF) also involves abnormal epithelial wound healing (2). Furthermore, in obliterative bronchiolitis (OB), the leading cause of limited long-term survival after lung transplantation, airway epithelial injury may be an early, crucial event (3). In each of these diseases, TGF-β has been shown to contribute to disease pathology, suggesting that TGF-β is likely to be activated in the presence of airway epithelial injury. TGF-β family members play important roles at multiple steps in wound healing (4–6). These proteins regulate a broad array of biologic functions, including cellular proliferation, migration, apoptosis, differentiation, developmental specification, adhesion, immunoregulation, and extracellular matrix production (7, 8). Each of the TGF-β isoforms is made as a large precursor that is cleaved in the endoplasmic reticulum to form a short carboxy-terminal fragment (the active cytokine) and a longer amino-terminal fragment (termed the latency-associated peptide [LAP]). These two fragments each form disulfide linked homodimers that noncovalently associate with one another to form what is called the small latent complex, since in this form the associated LAP prevents the mature cytokine from binding to its receptors and inducing TGF-β's known effects. In virtually all cells, this complex is further linked to members of another protein family, the latent TGF-β–binding proteins (LTBP), forming what is called the large latent complex. A number of mechanisms have been described by which these latent complexes can be activated, including direct interaction with members of the integrin family of cell surface adhesion receptors. A subset of integrins can bind to TGF-β1 LAP and TGF-β3 LAP, through an arginine-gylcine-aspartic acid (RGD) recognition sequence. This RGD site is not present in TGF-β2 LAP, and TGF-β2 has thus far not been shown to be activated by interaction with integrins (9).
Integrins are heterodimeric transmembrane receptors that themselves contribute to a variety of cellular responses, including survival, proliferation, and migration (10). At least eight members of the integrin family have been shown to be capable of binding to RGD sites (e.g., αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, α5β1, α8β1, αIIbβ3) (11). At least three of these, αvβ6, αvβ8, and αvβ5, have been reported to be capable of activating TGF-β (12–14). Whereas previous data published by our laboratory suggested that the integrin αvβ6 might not be expressed in normal subjects, we have been able to detect αvβ6 on airway epithelial cells of normal human subjects in vivo by using newer, more sensitive antibodies (15; D. Sheppard and coworkers, unpublished observation). Moreover, the integrins αvβ5 and αvβ8 have been demonstrated to be expressed in airway epithelial cells in normal subjects in vivo (16, 17). However, the significance of each of these integrins in modulating effects of endogenous TGF-β in injured conducting airways has not been evaluated.
Several previous studies have examined the effects of exogenous TGF-β on migration of epithelial cells and have suggested that TGF-β can enhance epithelial cell migration (18, 19). However, most of these have been done with either epithelial cell lines derived from carcinomas or cells immortalized by expression of transforming oncogenes. In contrast, mice that exhibit a defect in TGF-β signaling (as a result of a null mutation of the TGF-β signaling protein, SMAD3) have been reported to have acceleration of the rate of cutaneous wound closure, suggesting that in vivo endogenous activation of TGF-β might actually suppress the rate of wound closure (20). Furthermore, at least one study of the effects of exogenous TGF-β on the rate of closure of primary airway epithelial cell wounds found that TGF-β slowed the rate of wound closure (21).
In the current study, we sought to determine the effects of epithelial wounding on the activation of endogenously produced TGF-β and to determine how such activation might affect the degree of wound closure. We also sought to determine what role, if any, specific epithelial integrins play in this process.
MATERIALS AND METHODS
Airway Epithelial Cell Culture and Reagents
Primary normal human bronchial epithelial cells (NHBE), bronchial epithelial basal medium, and cell culture supplements were purchased from Cambrex Bioscience (Walkersville, MD). Cells were expanded in 100-mm dishes (Corning Inc., Corning, NY) using complete bronchial epithelial cell growth medium (BEGM) according to the protocol provided by Cambrex (East Rutherford, NJ). At confluence, passage 2 NHBE cells were seeded onto Transwell culture inserts (6.5 mm diameter; Costar 3470; Costar, Cambridge, MA) coated with collagen I at a density of 300,000 cells/well. Cells were further cultivated using Dulbecco's minimal essential medium (DMEM)/BEGM (1:1) containing 10% FCS. Cells were maintained in culture for 10–14 d, and medium was replaced every other day. Differentiated cultures showed a typical “cobblestone” appearance and were selected for experiments by measuring the transepithelial resistance (Rt) using an ohmmeter (EVOM; World Precision Instruments, Sarasota, FL). Cultures were considered confluent and differentiated if the Rt was stable and > 500 Ωcm2. Cells were grown in supplement- and serum-free DMEM/BEGM (1:1) medium for 24 h before and throughout the experiments.
Scratch Wound Assay and Determination of Degree of Wound Closure
The cell monolayer was wounded with a sterile 0.1- to 10-μl pipet tip (TipOne; USA Scientific, Ocala, FL) by one perpendicular linear scratch, creating a wound of ∼ 500 μm width across the diameter of the well. The wells were washed twice with PBS to remove detached cells or cell debris followed by incubation with the different stimuli. Human recombinant active TGF-β1, TGF-β2, and TGF-β3, monoclonal anti-pan–TGF-β (1D11), and affinity-purified anti–TGF-β1, -β2, and -β3 antibodies were purchased from R&D Systems (Minneapolis, MN). Polyclonal TGF-β2 antibody was purchased from BioVision (Mountain View, CA). The concentrations used were based on the neutralization doses and ED50 provided by the manufacturers. Mouse monoclonal antibody against human αvβ6 (6.3G9, 10D5) and αvβ8 (37E1) were generated as previously described (13, 22). A mouse monoclonal antibody against αvβ5 (ALULA) was generated in our laboratory by immunizing β5 knockout mice with murine L cells, which express αvβ5, and screening by flow cytometry, immunoprecipation, and inhibition of cell adhesion to vitronectin. As negative control, wounded cultures were exposed to an isotype mouse monoclonal IgG1 control antibody with no observed reaction with human cell surface (Chemicon, Temecula, CA) in the same concentrations as the neutralizing antibodies, or to PBS.
Wound closure was monitored immediately after initial wounding with time-lapse video phase contrast microscopy (×50 magnification) at 37°C and 5% CO2 in a humidified and climatized chamber using a Leica DMI 6000 microscope (Wetzlar, Germany) over 24 h. Images of the wounds were taken with a digital camera (Leica DFC350) at various time points. Wound areas were calculated using the publicly available ImageJ (1.34 s) software from the NIH (http://rsb.info.nih.gov/ij/; Bethesda, MD). The degree of wound closure was quantified by calculating the difference between the initial wound area and the remaining wound area expressed as percentage of their respective controls, 10 h after wounding. For each condition, three wounded areas were analyzed in parallel, and all experiments were repeated at least three times.
TGF-β Bioassay
To determine TGF activation, TMLC (mink lung epithelial reporter cells that stably express a portion of the plasminogen activator inhibitor 1 promoter) were co-cultured with NHBE as previously described (12). TMLC were suspended at 5 × 105 cells/ml in DMEM/BEGM with or without additions (e.g., antibodies). NHBE cell medium in the upper compartment was removed and immediately after wounding replaced with 100 μl of TMLC suspension. NHBE and TMLC were co-cultured for 16–20 h. Lysates were assayed for luciferase activity as described (23). Relative luciferase units were defined as activity minus the background activity of the TMLC reporter cells.
TGF-β ELISA
The secretion of TGF-β1, TGF-β2, and TGF-β3 was measured by determining their concentration in the culture medium in the apical and basal compartments of the filter system using commercial, sandwich ELISA systems (R&D Systems). The supernatants were removed, acid activated, and directly assayed using visualization with tetramethylbenzidine on an ELISA plate reader at 450 nm according to the manufacturer's instructions. All values were corrected using a seven-point standard curve with 2-fold serial dilutions and a high standard of 2,000 pg/ml.
Flow Cytometry
Cells were blocked with normal goat serum (10 mg/ml; Jackson ImmunoResearch Laboratories, West Grove, PA) for 10 min, washed with PBS, and incubated with primary antibody (10 μg/ml) for 20 min and then with phycoerythrin-conjugated goat anti-mouse secondary antibody (Boehringer Mannheim, Mannheim, Germany) for 20 min at 4°C. Cells were re-suspended with PBS and analyzed by FACScan (Becton Dickinson, Rutherford, NJ).
Immunocytochemistry
For immunohistochemistry studies, cells were grown on filters and wounded as described above. Primary antibodies used were mouse monoclonal anti-αvβ6 antibody, 10D5, and rabbit polyclonal antibodies against TGF-β1 (Promega, Madison, WI) or TGF-β2 (Santa Cruz Biotech, Santa Cruz, CA). The medium was removed and cells were washed with PBS. Cells were then fixed for 10 min with PBS containing 2% paraformaldehyde. Cells were washed with staining buffer PBS (supplemented with 4 mM MgCl2 and 0.5% Tween 20 [Bio Rad, Hercules, CA] for 10D5 staining only), blocked for 1 h with staining buffer containing 10% Normal Goat Serum (NGS), and incubated for 1 h with primary antibody in staining buffer + 10% NGS at 37°C. Samples were washed and incubated for 45 min with FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) or goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) diluted to 3 μg/ml in staining buffer + 10% NGS. Cells were then washed, and Transwell culture insert membranes were mounted with coverslips using VECTASHIELD mounting medium (Vector Laboratories).
5-Bromo-2′-Doxyuridine Staining and Mitomycin C Treatment
Mitomycin C (10 μg/ml) was dissolved in the experimental medium and added to the lower and upper compartments of the inserts at the time of wounding. 5-Bromo-2′-doxyuridine (BrdU) labeling was performed to measure new DNA synthesis as an indication of cell proliferation. For BrdU studies, cells were grown on filters and wounded as described above. Nine hours after wounding, BrdU (10 μM; Amersham, Piscataway, NJ) was added for 60 min. The cells were washed with PBS, fixed with 4% paraformaldehyde for 15 min and permeabilized with Triton-X-100 0.5% for 10 min. The denaturation step with 4 N HCl at 37°C lasted for 30 min followed by three washing steps with PBS. The cells were subsequently incubated with monoclonal antibody against BrdU diluted in PBS (1:1,000, Clone Bu20a; DakoCytomation, Glostrup, Denmark) at 4°C overnight. The reaction was visualized by fluorescein-conjugated anti-mouse antibody (1:200; Jackson Immunoresearch Laboratories) with a Leica DM 5000B microscope. The number of stained cells was determined by counting at least four high-power fields (×200 magnification) per culture insert.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Software (GraphPad Software Inc., San Diego, CA). Comparison between groups was made using one-way ANOVA followed by the Tukey test. Data are reported as mean value ± SE of at least three independent experiments done in triplicate. A P value of < 0.05 was considered significant.
RESULTS
Effects of TGF-β on Epithelial Wound Closure
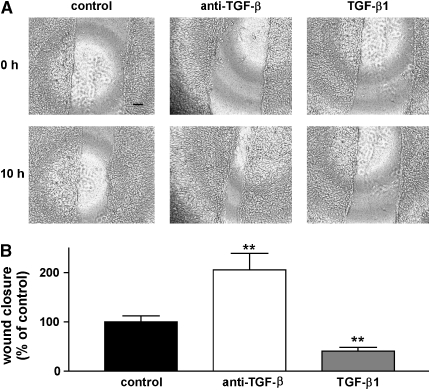
Epithelial cell scratch wounds usually close as a consequence of collective cell (sheet) migration (24). Wound closure in our assay was the result of a continuous, multicellular sheet movement over 15–20 h (Figure 1A). To evaluate the role of endogenously secreted and activated TGF-β in this process, wounded monolayers were incubated with either a control antibody (100 μg/ml) or an antibody that blocks all TGF-β isoforms (1D11, 100 μg/ml) or with recombinant active TGFβ1 (Figures 1A and 1B). Addition of pan–TGF-β–blocking antibody induced a significant increase in the degree of wound closure, whereas treatment of scratch wounds with recombinant, active TGF-β1 (10 ng/ml) significantly reduced the degree of wound closure.
Figure 1.
Effects of TGF-β1 and anti–TGF-β antibody on wound closure. (A) Phase contrast microscopy (×50 magnification) of NHBE cell monolayers on transwell culture inserts immediately after wounding (upper panels, 0 h) and after 10 h (lower panels). Incubation with either control antibody (100 μg/ml), anti-pan–TGF-β (1D11, 100 μg/ml) or TGF-β1 (10 ng/ml). Scale bar: 100 μm. (B). Degree of wound closure expressed as percent of control, 10 h after wounding. The data are shown as means ± SE. **P < 0.001 versus control.
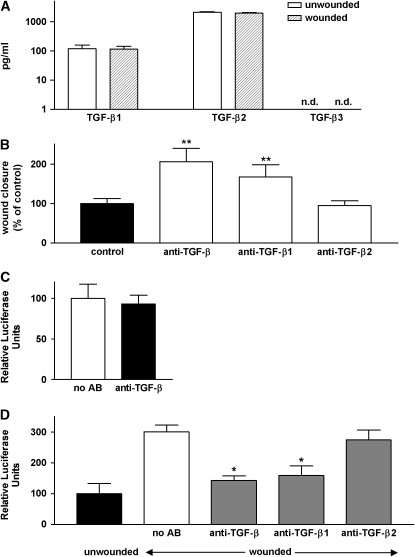
The effect of TGF-β–blocking antibody suggested that one or more TGF-β isoforms was present in our culture system and was being endogenously activated by airway epithelial cells. To determine which TGF-β isoforms were being secreted by these cells, we measured the concentration of each of the three mammalian TGF-β isoforms (TGF-β1, -β2, and -β3) in the culture medium in the apical and basal compartments of our filter culture system. Because of the large volume of basal medium, we could not detect any TGF-β in the basal compartments of wounded or unwounded cultures. Both TGF-β1 and TGF-β2 were detectable in the apical compartment, but, as previously reported by others, these cells made ∼ 20 times more TGF-β2 than TGF-β1 (Figure 2A). TGF-β3, if present, was at a concentration below the limit of detection of our assay (30 pg/ml). Over the time course studied (18 h), wounding had no effect on the amount of either TGF-β isoform secreted by these cells.
Figure 2.
Role of specific TGF-β iosforms in slowing epithelial wound closure. (A) Culture supernatants (apical compartment) were analyzed by ELISA for TGF-β1, TGF-β2, and TGF-β3 (logarithmic scale). TGF-β3 was not detectable (n.d.). (B) Degree of wound closure expressed as percent of control, 10 h after wounding in the absence of antibodies (control) or in the presence of blocking antibodies against all TGF-β isoforms or against TGF-β1 or -β2. **P < 0.001 versus control. (C) Unwounded NHBE (test cells) and TMLC (reporter) cells were co-cultured for 16–20 h and lysed for measurement of luciferase activity. Measurements for unwounded cells in the absence of blocking antibody were adjusted to 100%. (D) Unwounded and wounded NHBE (test cells) and TMLC (reporter) cells were co-cultured for 16–20 h in the presence or absence of blocking antibodies against all TGF-β isoforms or against TGF-β1 or -β2 and lysed for measurement of luciferase activity. Measurements for unwounded cells in the absence of blocking antibody were adjusted to 100%. The data are shown as means ± SE. *P < 0.01 versus control.
Since the airway epithelial cells we studied made both TGF-β1 and -β2, we next evaluated the contributions of each isoform to inhibition of the degree of wound closure. Blocking antibodies against TGF-β1 substantially enhanced the degree of closure, whereas two different blocking antibodies to TGF-β2 had no effect at concentrations up to 100 μg/ml (Figure 2B and data not shown).
Taken together, the above results suggested that airway epithelial wounding, at least over the time course we studied, does not alter the protein concentration of any TGF-β isoforms, but does induce an effect on wound closure that is preferentially mediated by TGF-β1. One possible explanation for these findings would be that wounding specifically activates TGF-β1, but not TGF-β2. To address this possibility we performed a co-culture bioassay in which we incubated either wounded or unwounded airway epithelial cells together with mink lung epithelial cells stably transfected with a TGF-β–inducible luciferase reporter. As shown in Figure 2C, reporter cell activity in unwounded monolayers was not inhibited by TGF-β–blocking antibody, meaning that there was no detectable TGF-β activity in the absence of wounding. However, monolayer wounding induced a substantial increase in reporter cell activity that could be inhibited down to the levels seen for unwounded monolayer by anti-pan–TGF-β. To determine which TGF-β isoform was being activated, we also performed co-culture assays with wounded monolayers in the presence of isoform-specific blocking antibodies to TGF-β1 or -β2. TGF-β1 antibody significantly inhibited reporter cell activity in this assay, whereas TGF-β2 antibody had no effect (Figure 2D) suggesting that wounding induced activation of TGF-β1 but not TGF-β2.
Roles of αv Integrins in Wound-Induced Activation of TGF-β
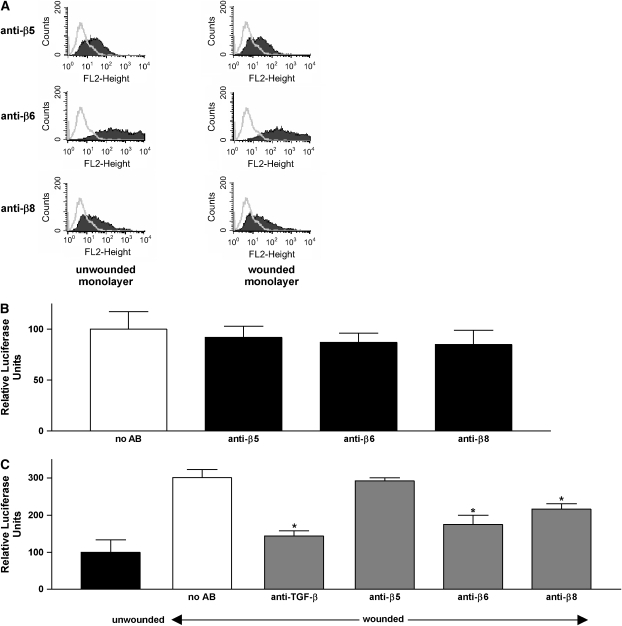
Having found that endogenous active TGF-β plays a critical role in our wound repair model, we sought to determine whether endogenous activity could be explained by TGF-β activation by epithelial integrins (25). Thus far, three integrins have been suggested to be capable of activating latent TGF-β, αvβ5 (14), αvβ6 (12), and αvβ8 (13). First, expression of integrins αvβ5, αvβ6, and αvβ8 was assessed by flow cytometry. As shown in Figure 3A, NHBE cells in our culture model expressed all three integrins on their cell surface. Creation of the wound did not induce a significant shift in the expression pattern of these integrins over the 15-h time period used for our assays (Figure 3A). Moreover, as assessed by immunocytochemistry, wounding did not affect the spatial distribution of αvβ6, TGF-β1, or TGF-β2 throughout the monolayer (data not shown). We could not assess the spatial distribution of αvβ8 because no antibodies are available that are suitable for immunocytochemistry.
Figure 3.
Role of specific integrins in TGF-β activation after epithelial wounding. (A) Flow cytometry of NHBE cells with and without wounding. Cells were stained with anti-αvβ5, anti-αvβ6, and anti-αvβ8 antibodies. Open peaks represent control (i.e., secondary antibody alone), shaded peaks represent NHBE cells stained with primary antibodies indicated. (B) Unwounded NHBE (test cells) and TMLC (reporter) cells were co-cultured for 16–20 h in the presence or absence of antibodies to αvβ5 (ALULA, 10 μg/ml), αvβ6 (6.3G9, 10 μg/ml), or αvβ8 (37E1, 100 μg/ml) integrins and lysed for measurement of luciferase activity. Measurements for unwounded cells in the absence of blocking antibody were adjusted to 100%. (C) Unwounded and wounded NHBE (test cells) and TMLC (reporter) cells were co-cultured for 16–20 h in the presence or absence of anti-integrin antibodies and lysed for measurement of luciferase activity. Measurements for unwounded cells in the absence of blocking antibody were adjusted to 100%. The data are shown as means ± SE. *P < 0.01 versus control.
To evaluate the potential contribution of these integrins to TGF-β activation, co-culture bioassays were performed for wounded or unwounded monolayers incubated with monoclonal antibodies against human αvβ5 (ALULA, 10 μg/ml), αvβ6 (6.3G9, 10 μg/ml), or αvβ8 (37E1, 100 μg/ml). None of these antibodies had any effect on the basal reporter cell activity in unwounded monolayers (Figure 3B). In wounded monolayers, both anti-ανβ6 and anti-αvβ8 significantly inhibited reporter cell activity, suggesting that both integrins contribute to the activation of TGF-β1 in response to wounding (Figure 3C). Antibody to αvβ5 had no effect.
Roles of αv Integrins in Epithelial Wound Repair
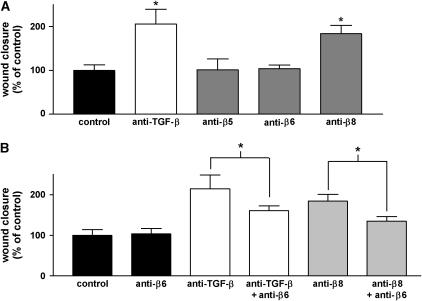
We next sought to determine whether TGF-β activation by αvβ6, αvβ8, or both was responsible for the delay in wound closure caused by endogenously activated TGF-β. As expected, antibody against αvβ5 had no effect on the degree of wound closure. αvβ8-blocking antibody significantly increased the degree of wound closure, but, surprisingly, antibody against αvβ6 had no significant effect (Figure 4A).
Figure 4.
Role of specific integrins in regulating the degree of epithelial wound closure. (A) Degree of wound closure in the presence or absence of anti-pan–TGF-β (1D11, 100 μg/ml) and antibodies to αvβ5 (ALULA, 10 μg/ml), αvβ6 (6.3G9, 10 μg/ml), or αvβ8 (37E1, 100 μg/ml) integrins, 10 h after wounding. *P < 0.01 versus control. (B) Effects of anti-αvβ6 antibody on the degree of wound closure in the presence or absence of combined treatment with antibodies against TGF-β or αvβ8, 10 h after wounding. The data are shown as means ± SE. *P < 0.01 versus treatment with either anti–TGF-β or anti-αvβ8 alone.
Since both αvβ6 and αvβ8 apparently contribute to activation of TGF-β after airway epithelial wounding, we were initially puzzled by the failure of blocking antibody against αvβ6 to accelerate the degree of wound closure. However, αvβ6 has been previously shown to accelerate epithelial cell migration. We therefore tested the possibility that αvβ6 might exert competing effects on wound closure—inhibition mediated by activation of TGF-β and simultaneous acceleration through TGF-β–independent effects on cell migration. To test this possibility, we evaluated the effects of blocking αvβ6 on closure of wounds in which TGF-β or αvβ8 had already been inhibited. Although anti-αvβ6 antibody again had no effect on its own, addition of anti-αvβ6 antibody to either anti-αvβ8 or anti–TGF-β antibody significantly inhibited wound closure compared with treatment with anti-αvβ8 or anti–TGF-β alone (Figure 4B).
Contribution of Proliferation to Epithelial Wound Healing
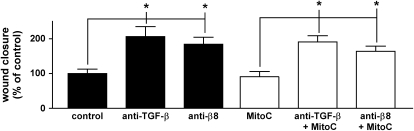
TGF-β is a well-established inhibitor of epithelial cell proliferation, an effect that could contribute to the degree of wound closure over the time course of our assay. To evaluate the contribution of proliferation in our scratch wound assay, we used the DNA-crosslinking inhibitor Mitomycin C. We verified that Mitomycin blocked DNA synthesis by demonstrating negligible BrdU incorporation in Mitomycin-treated cells. Inhibition of cell proliferation had little if any effect on the degree of wound closure and did not abrogate the effects of pan–TGF-β– or αvβ8-blocking antibodies on the degree of wound closure (Figure 5).
Figure 5.
Effects of mitomycin C on the degree of wound closure. Degree of wound closure expressed as percent of control, 10 h after wounding and incubation with the indicated stimuli in the absence or presence of mitomycin C (MitoC, 10 μg/ml). The data are shown as means ± SE. *P < 0.01.
DISCUSSION
The results of the present study show that confluent airway epithelial cells, despite secreting two TGF-β isoforms and expressing two members of the integrin family that can activate TGF-β, do not activate either isoform under resting conditions. In response to mechanical wounding, however, there is rapid induction of integrin-dependent activation of TGF-β, which slows the degree of wound closure. TGF-β activation following wounding appears to be mediated by both the αvβ6 and αvβ8 integrins, but αvβ8-mediated activation is principally responsible for the reduction in the degree of wound closure, since blocking antibodies to αvβ8 substantially accelerated wound closure, whereas antibodies to αvβ6 had no effect on their own. This difference is explained in part by a TGF-β–independent contribution of αvβ6 to the degree of wound closure, as demonstrated by inhibition of the degree of closure by antibodies to αvβ6 after TGF-β activity was inhibited by antibodies to either TGF-β or αvβ8. Airway epithelial cells make substantial amounts of TGF-β2 and smaller amounts of TGF-β1, but the concentrations of each TGF-β isoform are not affected by wounding, at least during the time course of our experiments. The αvβ8-mediated decrease in the degree of wound closure is mediated by activation of TGF-β1, but TGF-β2 does not appear to contribute, consistent with the identification of the arginine-glycine–aspartic acid site in TGF-β1 LAP as the site of binding of this integrin. The RGD site is present in TGF-β1 and -β3 LAP, but absent from TGF-β2 LAP (25).
Numerous previous reports have examined effects of TGF-β on individual migration of a variety of cell types. Based on these studies, it is clear that TGF-β can exert dramatically different effects on different cell types and under different circumstances. TGF-β isoforms have been shown to be chemotactic for macrophages, mast cells, and fibroblasts, enhancing the migration of each cell type toward gradients of diffusible TGF-β (26–28). TGF-β has also been reported to enhance the migration of epithelial tumor cells and immortalized epithelial cells (18, 29, 30). Finally, TGF-β is a potent inducer of epithelial-to-mesenchymal transformation (EMT), inducing primary epithelial cells to take on mesenchymal cell characteristics, including enhanced detachment from epithelial sheets and enhanced speed of migration (31, 32). However, EMT occurs over a time course of several days, much longer than the time course of primary epithelial wound closure (33, 34).
Primary epithelial wounds, like the scratch wounds studied here, close by a process of continuous sheet migration, which appears to be distinct from migration of isolated individual cells. The one previous study that looked at the effects of exogenous TGF-β on the rate of closure of airway epithelial wounds found significant slowing of the rate of closure, just as we did in the current study (21). Furthermore, this effect is consistent with results reported for the rate of cutaneous wound closure in vivo in mice with impaired responsiveness to TGF-β as a consequence of a null mutation of the TGF-β signaling molecule, SMAD3. These mice have dramatically accelerated wound closure, as expected if endogenously activated TGF-β normally slows the rate of wound closure (20).
Many of the previous studies that examined the potential roles of TGF-β on migration of epithelial cells focused primarily on exogenously administered TGF-β. In the current study, we demonstrated that TGF-β is activated by airway epithelial cells themselves in response to mechanical wounding, and that locally activated TGF-β significantly slows the degree of wound closure. Based on studies with blocking antibodies, it is clear that TGF-β1 is endogenously activated and contributes to this response. In response to mechanical wounding, TGF-β1 is activated by the actions of at least two members of the integrin family, αvβ6 and αvβ8. Airway epithelial cells express one other integrin, αvβ5, that has also recently been reported to be capable of activating TGF-β on the surface of fibroblasts from patients with sceleroderma (14, 35). Blockade of αvβ5 had no effect on either TGF-β activity or on the degree of wound closure in our assay. These results are consistent with our own inability to demonstrate αvβ5-mediated TGF-β activation by any primary cells or cell lines we have examined (data not shown). We are uncertain why our results in this regard are different from those reported by Asano and coworkers (14, 35), but perhaps this function of αvβ5 uniquely occurs on fibroblasts from patients with scleroderma. An unanswered question from our experiments is how wounding stimulates αvβ6- and αvβ8-mediated TGF-β activation. This effect does not appear to be due to either a change in the level of expression of either integrin or a change in expression of any TGF-β isoforms, since the levels of each of these proteins was unaffected by wounding over the time course of our experiments.
Our finding that airway epithelial cells make large amounts of TGF-β2, but do not appear to activate it in response to injury raises the questions of how TGF-β2 becomes activated and whether or not it might contribute to the slowing of wound closure or other TGF-β-dependent effects in wounded airways in vivo. In addition to integrins, TGF-β isoforms can be activated by a variety of proteases and by interaction with the secreted protein, thrombospondin and it is certainly possible that other cells that are present in or recruited to injured airways could secrete these other activators and lead to an important contribution of TGF-β2 to in vivo responses (36, 37, 38).
Interestingly, although we found that both αvβ6 and αvβ8 contributed to TGF-β activation after wounding, and the contribution of αvβ6 was at least as large as that of αvβ8, we could not demonstrate any contribution of αvβ6 to the decrease in the degree of wound closure we observed. As noted above, one possible explanation for this confusing result might be that, in addition to activating TGF-β (an effect that would be expected to slow the degree of wound closure), αvβ6 itself enhances the degree of wound closure through a TGF-β–independent mechanism. This possibility is supported by our finding that blockade of αvβ6 actually slowed the degree of wound closure under conditions in which the effects of endogenously activated TGF-β were prevented by addition of TGF-β–blocking antibodies. However, there may be additional explanations for these results. Although αvβ6 and αvβ8 both bind to the same RGD sites in TGF-β1 and -β3, the mechanisms of TGF-β activation by these two integrins are actually quite different. αvβ6 induces activation of latent complexes through a mechanism that depends on actin polymerization and appears to induce a conformational change in latent TGF-β but does not release any free TGF-β from the latent complex. Rather, this pathway is completely dependent of direct contact between the cell expressing the activated integrin and an adjacent target cell expressing TGF-β receptors (12). In contrast, αvβ8-mediated TGF-β activation involves presentation of the latent complex to transmembrane proteases, with resultant proteolytic cleavage of the LAP and release of freely diffusible active TGF-β from the cell surface (13). It is thus conceivable that TGF-β locally activated by wounding of the epithelial cells at the wound edge might not be sufficient to globally affect the migration of a continuous sheet of epithelial cells, whereas the diffusible TGF-β released by αvβ8-mediated activation could have more long-range effects on the entire epithelial sheet. However, we have not been able to demonstrate any difference in TGF-β activity between the wound edge and the rest of the wounded epithelial sheet, so we have no data to support this possibility.
In summary, we have found that airway epithelial cells respond to injuring by inducing TGF-β activation mediated by two different integrins, αvβ6 and αvβ8. Although these cells make large amounts of TGF-β2 and small amounts of TGF-β1, only TGF-β1 is activated by this pathway. At least in the case of αvβ8, activation of endogenous TGF-β1 slows the degree of wound closure, an effect that can be prevented by blocking either TGF-β or the αvβ8 integrin. These observations could have important implications for understanding the mechanisms underlying a variety of lung and airway diseases that are characterized by abnormal airway repair.
Acknowledgments
The authors thank Mark Travis and Greg deHart for valuable scientific discussion and technical assistance.
This work was funded by Deutsche Forschungsgemeinschaft (DFG) grant NE 1168/1–1 (to C.N.) and by National Heart, Lung and Blood Institute grants HL56385, HL64353, HL66600, and HL53949 (to D.S.)
Originally Published in Press as DOI: 10.1165/rcmb.2006-0013OC on March 30, 2006
Conflict of Interest Statement: C.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.L.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S. is PI on a sponsored research agreement with Biogen Idec ($99,000/year since 2003) and is a co-owner of a patent that covers antibody 3G9.
References
- 1.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 2004;1:93–98. [DOI] [PubMed] [Google Scholar]
- 2.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 3.Vilchez RA, Dauber J, Kusne S. Infectious etiology of bronchiolitis obliterans: the respiratory viruses connection—myth or reality? Am J Transplant 2003;3:245–249. [DOI] [PubMed] [Google Scholar]
- 4.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816–827. [DOI] [PubMed] [Google Scholar]
- 5.Frank S, Madlener M, Werner S. Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem 1996;2717:10188–10193. [DOI] [PubMed] [Google Scholar]
- 6.McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-beta3. Am J Physiol 1999;276:G1087–G1093. [DOI] [PubMed] [Google Scholar]
- 7.Whitman M. Smads and early developmental signaling by the TGFβ superfamily. Genes Dev 1998;12:2445–2462. [DOI] [PubMed] [Google Scholar]
- 8.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors 1993;8:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci 2003;116:217–224. [DOI] [PubMed] [Google Scholar]
- 10.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 1995;268:233–239. [DOI] [PubMed] [Google Scholar]
- 11.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev 2005;24:395–402. [DOI] [PubMed] [Google Scholar]
- 12.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–329. [DOI] [PubMed] [Google Scholar]
- 13.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 2002;157:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum 2005;52:2897–2905. [DOI] [PubMed] [Google Scholar]
- 15.Weinacker A, Ferrando R, Elliott M, Hogg J, Balmes J, Sheppard D. Distribution of integrins αvβ6 and α9β1 and their known ligands, fibronectin and tenascin, in human airways. Am J Respir Cell Mol Biol 1995;12:547–556. [DOI] [PubMed] [Google Scholar]
- 16.Pilewski JM, Latoche JD, Arcasoy SM, Albelda SM. Expression of integrin cell adhesion receptors during human airway epithelial repair in vivo. Am J Physiol 1997;273:L256–L263. [DOI] [PubMed] [Google Scholar]
- 17.Cambier S, Mu DZ, O'Connell D, Boylen K, Travis W, Liu WH, Broaddus VC, Nishimura SL. A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth. Cancer Res 2000;60:7084–7093. [PubMed] [Google Scholar]
- 18.Howat WJ, Holgate ST, Lackie PM. TGF-β isoform release and activation during in vitro bronchial epithelial wound repair. Am J Physiol Lung Cell Mol Physiol 2002;282:L115–L123. [DOI] [PubMed] [Google Scholar]
- 19.Boland S, Boisvieux-Ulrich E, Houcine O, Baeza-Squiban A, Pouchelet M, Schoevaert D, Marano F. TGF beta 1 promotes actin cytoskeleton reorganization and migratory phenotype in epithelial tracheal cells in primary culture. J Cell Sci 1996;109:2207–2219. [DOI] [PubMed] [Google Scholar]
- 20.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1999;1:260–266. [DOI] [PubMed] [Google Scholar]
- 21.Spurzem JR, Sacco O, Rickard KA, Rennard SI. Transforming growth factor-beta increases adhesion but not migration of bovine bronchial epithelial cells to matrix proteins. J Lab Clin Med 1993;122:92–102. [PubMed] [Google Scholar]
- 22.Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, et al. Function-blocking integrin αvβ6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem 2004;279:17875–17887. [DOI] [PubMed] [Google Scholar]
- 23.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 1994;216:276–284. [DOI] [PubMed] [Google Scholar]
- 24.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol 2004;16:14–23. [DOI] [PubMed] [Google Scholar]
- 25.Sheppard D. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol 2004;16:552–557. [DOI] [PubMed] [Google Scholar]
- 26.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA 1987;84:5788–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med 1987;165:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson N, Piek E, Sundstrom M, ten Dijke P, Nilsson G. Transforming growth factor-beta-mediated mast cell migration depends on mitogen-activated protein kinase activity. Cell Signal 2001;13:483–490. [DOI] [PubMed] [Google Scholar]
- 29.Kim ES, Kim MS, Moon A. Transforming growth factor (TGF)-beta in conjunction with H-ras activation promotes malignant progression of MCF10A breast epithelial cells. Cytokine 2005;29:84–91. [DOI] [PubMed] [Google Scholar]
- 30.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 2002;115:3193–3206. [DOI] [PubMed] [Google Scholar]
- 31.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 2005;16:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 2004;23:1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;165:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, Yano H, Schaefer E, Sabe H. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 during epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene 2001;20:2626–2635. [DOI] [PubMed] [Google Scholar]
- 35.Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Increased expression levels of integrin alphavbeta5 on scleroderma fibroblasts. Am J Pathol 2004;164:1275–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol 1993;122:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol 1988;106:1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol 1990;111:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]