Abstract
When the fungus Stachybotrys chartarum is inhaled, its mycotoxins may cause lung injury and inflammation. The severity of human responses to S. chartarum in both occupational and home settings varies widely. To explore these differences, we intratracheally instilled C3H/HeJ, BALB/c, and C57BL/6J mice with S. chartarum spores suspended in saline. One day later, the mice were humanely killed, bronchoalveolar lavage (BAL) was performed, and biochemical and cellular indicators of lung injury and inflammation were measured. BALB/c mice showed the highest myeloperoxidase activity, albumin and hemoglobin levels, and neutrophil numbers in their BAL among the three strains. BALB/c was the only strain to show significant increases in keratinocyte-derived cytokine (KC), monocyte chemotactic protein (MCP)-1, MCP-3, macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-1γ, MIP-2, RANTES, IL-1α, IL-1β, IL-3, IL-6, IL-18, leukemia inhibitory factor, macrophage colony-stimulating factor, and TNF-α. A model of allergen-induced airway inflammation was examined to assess whether underlying allergic inflammation might contribute to increased susceptibility to S. chartarum–induced pulmonary inflammation and injury. Surprisingly, in BALB/c mice, ovalbumin-induced airway inflammation produced a protective effect against some S. chartarum–induced pulmonary responses. This is the first report of mammalian strain differences affecting responses to S. chartarum. These responses differ from those reported for LPS and other fungi. Analogous underlying genetic differences may contribute to the wide range of sensitivity to Stachybotrys among humans.
Keywords: BALB/c; C57BL/6; cytokines; lung diseases, fungal; mold
Inhalation of fungal particles can cause inflammatory and allergic responses as well as pulmonary infections (1–3). Exposure to mycotoxin-producing fungi may cause other severe effects (4–6). In particular, Stachybotrys chartarum (previously called Stachybotrys atra) has been associated with diverse symptoms such as muscle aches, headaches, cough, pulmonary hemorrhage, dermatitis, and interstitial lung disease after inhalation exposures (7–10). Acute idiopathic pulmonary hemorrhage leading to the deaths of infants has been attributed to this fungus (11, 12), although there may have been other contributing causes such as environmental tobacco smoke which may alter host defenses (12, 13). There are no data addressing whether some babies were more sensitive to S. chartarum due to their genetic background. Interestingly, African-American race has been correlated with the likelihood of babies experiencing acute idiopathic pulmonary hemorrhage (11, 12). Current data do not allow us to distinguish whether race is associated with the likelihood of living in mold-contaminated housing, or if race is a marker of genetic differences that might alter sensitivity to S. chartarum. This context informs our use of multiple mouse strains to explore whether genetic differences may contribute to mammalian mold responses.
Higher levels of household mold are associated with an increase in respiratory symptoms in the first year of life in a cohort at risk for asthma (14, 15). In addition, 63% of children with asthma show sensitization to mold, but there is no clear-cut relationship between sensitization and asthma symptoms (15). It is possible that people with a history of asthma are more likely than those without asthma to report respiratory symptoms from exposure to molds. Alternatively, the presence of asthma after a similar environmental exposure may be the result of a genetic predisposition. In this study, we used BALB/c mice that are sensitized to ovalbumin (OVA) as a model for underlying asthma before S. chartarum exposure to begin to address the hypothesis that a prior history of asthma may exacerbate the subsequent pulmonary response to S. chartarum. We recognize that neither the mouse OVA-sensitization model, nor any other mouse model, fully replicates every symptom of human asthma.
S. chartarum is a fungus that uses cellulose (e.g., paper, ceiling tiles, cardboard) as a carbon source. It grows best at 15–25°C under wet conditions. With appropriate substrate, temperature, light, and relative humidity, it produces mycotoxins, especially macrocyclic trichothecenes (16–19). Trichothecenes are potent inhibitors of protein and DNA synthesis, resulting in disruption of cellular function as well as cellular injury (20). Some strains also produce a hemolysin called stachylysin that may lead to pulmonary hemorrhage (21, 22).
Most studies on Stachybotrys chartarum or its specific mycotoxins used ingestion, injection, or dermal application of purified toxins (23–29). Mouse exposures to S. chartarum spores given intranasally were examined for histologic changes in lung tissue (30, 31). Rats were used to examine the time course of these responses (32). Severe inflammatory changes and hemorrhaging of mucosal surfaces have been consistently reported as consequences of intense exposures to S. chartarum (9, 31) and an extract of S. chartarum can induce allergic asthma-like inflammation in BALB/c mice using repeated pulmonary exposures (33). The effects of inhaled spores in humans have primarily been reported as case studies (9, 34, 35).
The purpose of this study was to explore whether mouse strain differences influence pulmonary responses to acute exposures. In particular, we wanted to test the hypothesis that TLR4 status, which is involved in innate recognition of both LPS and Aspergillus (36, 37), would influence the pulmonary responses to S. chartarum. We also wanted to see whether underlying OVA-induced airway hyperresponsiveness would exacerbate susceptibility to pulmonary injury and inflammation from pulmonary exposure to S. chartarum.
MATERIALS AND METHODS
Experimental Design
We examined three strains of 8.5-wk-old (± 1 wk) male mice: C57BL/6J, C3H/HeJ (both from The Jackson Laboratory, Bar Harbor, ME), and BALB/c (Taconic, Germantown, NY). All three are independently developed inbred strains that differ from each other at over 50% of genetic loci (38). All are TLR2+ and only C3H/HeJ is TLR4− (37, 39). Both TLR2 and TLR4 have been shown to mediate responses to the fungi Aspergillus fumigatus and A. niger (36). BALB/c mice are generally Th2-dominant (40), while C57BL/6 mice are generally Th1-dominant. These designations, however, are model-dependent: in a fungal model using Paracoccidioides brasiliensis, BALB/c are Th1-dominant (41), and in another fungal model using Coccidioides posadasii there is no clear Th1/Th2 polarization (42). Alveolar macrophages from C57BL/6 mice have a significantly higher phagocytic capacity than alveolar macrophages from BALB/c mice (43).
All mice were housed at 25°C for a minimum 1-wk acclimation period and fed Purina Mouse Chow and water ad libitum. In an additional group of BALB/c mice, airway inflammation was induced with OVA to test whether or not underlying allergic inflammation exacerbated pulmonary injury and inflammation in response to S. chartarum. All mice were intratracheally instilled (2.5 ml/kg) with S. chartarum spores suspended in saline (105–107 spores/ml), for final doses of 6.25 × 103 to 6.25 × 105 spores (62.5 μl) per 25-g mouse. Each mouse was individually weighed and the actual dose was adjusted according to the weight of the mouse on the morning of instillation. Weights ranged from 20.7–30.1 g. The mean weight was 24.7 ± 1.7 g. One day after instillation (with the exception of cytokine and chemokine experiments, which employed a time course described below), the mice were humanely killed and bronchoalveolar lavage (BAL) was performed. The data in this report represent a combination of separate and combined results from a total of seven experiments that included two to four animals per strain and dose in each experiment.
Fungal Strains and Spore Suspensions
A toxin-producing strain of S. chartarum was obtained from Professor Harriet Burge, Harvard School of Public Health (32), and grown on potato dextrose agar (PDA) plates at room temperature. Spores were vacuumed from the surface of 14- to 21-d agar cultures using a modified filter cassette with a 37 mm, 0.4 μm polycarbonate membrane filter (Poretics Corp., Livermore, CA), and suspended in 0.9% saline to concentrations of 105–107 spores per milliliter. Minor hyphal fragment content and negligible spore clumping were observed; most spores were ovoid in shape with an average size of 6 × 9 μm.
Intratracheal Instillation
Delivery of spore suspensions into the lungs was achieved by intratracheal instillation as described (44) and detailed online. Although intratracheal instillation does not simulate human exposure, the method allows delivery of a relatively precise dose directly into the lungs (45). Comparisons to control treated with sterile pyrogen-free saline assessed the possible effects of the carrier and the instillation protocol. Between 6 and 12 mice per day were instilled, randomly alternating the strain of mouse. We used the same spore suspensions for all three strains. After instillation, the animals were kept under pre-instillation housing conditions. At least five mice for each combination of strain and dose of spores were studied (0, 1 million, 3 million, and 10 million spores/ml). In addition, five BALB/c mice were instilled at doses of 100,000 and 300,000 spores/ml to evaluate the effects of lower doses on BALB/c mice.
Six BALB/c and five C57BL/6J mice were each instilled with 5 × 105 spores to verify that the two strains received comparable spore doses and to evaluate effectiveness of intratracheal instillation to deliver the spores to mouse lungs of these two strains. Immediately after each spore instillation, we removed the lungs and homogenized them in 4 ml distilled water by pushing them through a sieve with a wire mesh spacing of 100 μm. A measured aliquot was then evaluated on a hemocytomer under a light microscope at 20× magnification. Spore numbers were calculated based on counts per unit volume of sample and total volume of lung homogenate.
Histopathology
BALB/c and C57BL/6J mice were instilled with the 1 million spores S. chartarum/ml dose which also contained carbon black to identify areas of the lung where spores were retained. One day later, the lungs were excised and then fixed by intratracheal instillation of 10% formalin at 23 cm H2O pressure. Tissues were embedded in paraffin, and 8-μm sections were stained with hematoxylin and eosin and examined under light microscopy.
Bronchoalveolar Lavage
Analysis of bronchoalveolar lavage (BAL) constituents provides estimates of relative toxicity of various agents, including poorly characterized toxins and complex mixtures (46–48). BAL is a sensitive method for measuring airway and alveolar injury and inflammation (49, 50), and thus can yield quantitative estimates of pulmonary inflammatory responses to mycotoxin-containing fungal particles. The severity of inflammation and injury is measured by the release of cellular and serum constituents into alveolar spaces after lung injury and the recruitment of circulating red and white cells. One day after intratracheal instillation, mice from each group were humanely killed by intraperitoneal lethal dose (10 mg/mouse) of pentobarbital sodium (Anthony Products Co., Arcadia, CA). The abdomen was then opened, the mouse exsanguinated by cutting the abdominal aorta, and the hemidiaphragm punctured to create a bilateral pneumothorax. BAL was performed and analyzed as described previously (48) and as detailed in the online supplement. Hemoglobin, albumin, lactate dehydrogenase (LDH), myeloperoxidase (MPO), and white cell count values represent pooled data from two separate experiments.
Cytokine and Chemokine Analysis
To explore mechanisms, studies of cytokine and chemokine release were performed on two of the strains described above, C57BL/6J and BALB/c. They were selected because they had the greatest differences in pulmonary responses to S. chartarum. Both strains were instilled with the 10 million S. chartarum spores/ml dose, as described above. The mice were lavaged as described earlier and in the online supplement, and the first two washes of the lavage fluid were pooled, centrifuged, stored at −80°C, and analyzed as described in the next two paragraphs.
Enzyme-linked immunosorbent assay analysis.
To establish a time course of cytokine release after S. chartarum exposure, at 0, 6, and 24 h, the cytokines TNF-α, keratinocyte-derived cytokine (KC), and macrophage inflammatory protein (MIP)-2 were measured by enzyme-linked immunosorbent assay (ELISA) using 50 μl of the thawed BAL fluid (R&D Systems, Minneapolis, MN). Two to three animals of each strain and time were studied in two separate experiments and the data were pooled for a total of at least five mice per dose and time.
Multi-analyte profile analysis.
A Luminex multi-analyte profile (MAP) analysis (Charles River Laboratories, Wilmington, MA) was used to analyze the panel of proteins in mouse BAL fluid 6 h after instillation of either saline (negative control) or S. chartarum spores. The MAP analysis used a Luminex bead-based technology sorted by fluorescence-activated cell sorter to quantitate the measured protein levels of 57 cytokines, chemokines, and other proteins in 10 μl of the thawed BAL fluid.
OVA-Induced Airway Inflammation Model
OVA-induced airway inflammation was induced as previously described (51). BALB/c mice were sensitized with three intraperitoneal injections of OVA at 4, 5, and 6 wk of age. At 7 wk, they received OVA aerosol challenge for 3 d for 10 min/d. At 8 wk they were intratracheally instilled with multiple doses of S. chartarum as described above. After 24 h, lungs were lavaged and analyzed for the same cellular and biochemical parameters described above, and the results compared with controls not sensitized to OVA.
Statistical Analyses
Statistical analyses used SAS statistical software (Version 8.2; SAS Institute, Cary, NC). The spore dose to response variable (hemoglobin, albumin, etc.) slopes were compared among the three mouse strains using a multivariable ANOVA (MANOVA). A type III analysis (for different sized data sets) was used. The critical value was set at P < 0.05. The pairwise comparisons are based on a least squares mean post hoc test. We ran an additional MANOVA analysis leaving out the highest dose to test the persistence of significance at lower spore doses. The cytokine ELISA and OVA-induced airway inflammation BAL data from experiments using two mouse strains were analyzed using an independent t test. A Bonferroni's adjusted MANOVA was used to determine significance of the MAP analysis.
RESULTS
Strain Differences Influence Pulmonary Responses to S. chartarum
There is a significantly greater response as seen in the BAL parameters to the same S. chartarum doses in the BALB/c mice when compared with the C57BL/6J and C3H/HeJ mice (P < 0.0001, MANOVA). We did observe significant responses to instilled mold in all strains as shown in Figures 1 and 2 (P < 0.0001). TNF-α, KC, and MIP-2 all show significantly higher levels in both BALB/c mice and C57BL/6J mice at 6 h when compared with baseline. The magnitude of this increase is significantly higher in the BALB/c mice than in the C57BL/6J mice. These differences were not due to differences in deposited spore dose between the most and least responsive strains. Comparable spore numbers were recovered from the lungs of BALB/c and C57BL/6J mice immediately after intratracheal instillation. In C57BL/6J mice, 96.3 ± 11.1% (mean ± SEM) were found, and in BALB/c mice 81.4 ± 9.9% were recovered.
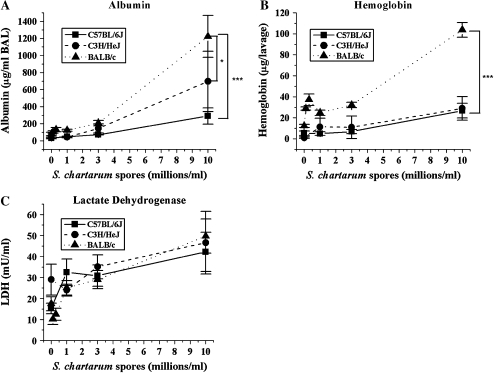
Figure 1.
Dose response of albumin, hemoglobin, and LDH. (A) Mean concentrations of albumin in BAL fluid ± SEM versus instilled dose S. chartarum. (MANOVA, spore dose to albumin slope different at P = 0.0007 for C57BL/6J versus BALB/c, P = 0.03 for C3H/HeJ versus BALB/c, and P = 0.3 for C57BL/6J versus C3H/HeJ). (B) Mean concentrations of hemoglobin in BAL cell pellet ± SEM versus instilled dose S. chartarum. (MANOVA, spore dose to hemoglobin slope different at P < 0.0001 for C57BL/6J versus BALB/c, P < 0.0001 for C3H/HeJ versus BALB/c, and P = 0.8 for C57BL/6J versus C3H/HeJ). (C) Mean concentrations of LDH in BAL fluid ± SEM versus instilled dose S. chartarum (MANOVA, spore dose to LDH slope different at P = 0.5 for C57BL/6J versus BALB/c, P = 0.2 for C3H/HeJ versus BALB/c, and P = 0.5 for C57BL/6J versus C3H/HeJ). * Slopes of lines significantly different at P < 0.05; *** slopes of lines significantly different at P < 0.005.
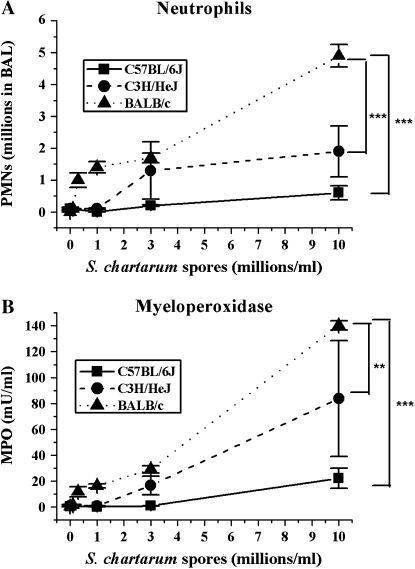
Figure 2.
Dose response of PMNs and MPO. (A) Mean number of PMNs in BAL ± SEM versus instilled dose S. chartarum (MANOVA, spore dose to PMN slope different at P < 0.0001 for C57BL/6J versus BALB/c, P = 0.0008 for C3H/HeJ versus BALB/c, and P = 0.09 for C57BL/6J versus C3H/HeJ). (B) Mean concentrations of MPO in BAL fluid ± SEM versus instilled dose S. chartarum (MANOVA, spore dose to MPO slope different at P < 0.0001 for C57BL/6J versus BALB/c, P = 0.01 for C3H/HeJ versus BALB/c, and P = 0.06 for C57BL/6J versus C3H/HeJ). ** Slopes of lines significantly different at P < 0.01; *** slopes of lines significantly different at P < 0.005.
The P values represented in Figures 1–3 reflect significant strain differences in the slopes of the curves over multiple doses. We wanted to be sure that this significance was not unduly driven by the highest dose. Significant differences in responses are maintained when the statistical analysis is done without the highest spore dose. The significance of the pairwise comparisons in the least square means analysis is also maintained.
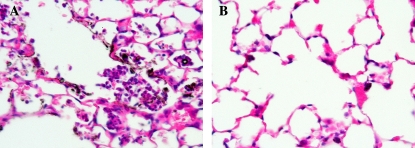
Figure 3.
Lung tissue section. Representative histological images from C57BL/6J and BALB/c mice 24 h after intratracheal instillation of 1 million spores S. chartarum/ml (2.5 ml/kg body weight), ×25 magnification. (A) Focal inflammation of the lungs in BALB/c mice. (B) Minor inflammation of the lungs in C57BL/6J mice.
BAL Proteins
BAL fluid albumin levels (Figure 1A), a measure of capillary permeability in the lungs, show significant strain differences in their dose response (P < 0.0005). All three strains show a significant dose response in the albumin levels in response to S. chartarum (P < 0.0001) (Figure 1A). But BALB/c mice show the greatest response with increasing dose, with a 20-fold increase in albumin levels from baseline to the highest dose. C57BL/6J mice show the smallest responses with only a 4-fold increase over baseline. C3H/HeJ shows an intermediate responsiveness, with a 12-fold increase over baseline. The BALB/c mice show a significantly greater increase in albumin with increasing dose with respect to both other strains (spore dose to albumin slope significantly different at P = 0.0007 for C57BL/6J versus BALB/c, P = 0.033 for C3H/HeJ versus BALB/c, MANOVA). The difference between the albumin levels in C57BL/6J and C3H/HeJ is not significant (P = 0.25 for C57BL/6J versus C3H/HeJ, MANOVA).
The level of hemoglobin in BAL reflects the degree of alveolar hemorrhage. Hemoglobin levels in the BAL fluid (Figure 1B) again show significant strain differences in their dose response (P < 0.0004). While all three strains show a significant dose response in hemoglobin levels (P < 0.0001), BALB/c mice show the largest increase, with 5-fold higher levels than in C57BL/6J and C3H/H3J mice at the highest S. chartarum dose. The spore dose to hemoglobin slope was significantly different at P < 0.0001 for C57BL/6J versus BALB/c, P < 0.0001 for C3H/HeJ versus BALB/c (MANOVA). C57BL/6J and C3H/HeJ mice show very similar hemoglobin levels at every dose (P = 0.75, MANOVA). LDH, a measure of cell death or damage, did not demonstrate the same strain dependency. All three strains have a significant dose response in the levels of LDH (P < 0.0001), but none has a significantly different response in LDH levels (P = 0.27) (Figure 1C).
Lung Inflammation
Numbers of neutrophils (polymorphonuclear leukocytes [PMNs]) also showed the same strain dependence. PMNs enter alveoli in response to inflammatory stimuli, after being recruited from the systemic circulation by chemokines secreted by alveolar macrophages and other pulmonary cells. BALB/c mice show more infiltration and degranulation of neutrophils at higher doses than did C57BL/6J and C3H/HeJ mice. One to five percent of the white blood cells recovered in the BAL of all three strains after saline instillation were neutrophils. At the highest S. chartarum dose of 10 million spores/ml, 21% of the C57BL/6J white cells, 48% of the C3H/HeJ white cells, and 83% of the BALB/c white cells are neutrophils. The numbers of PMNs in the BAL fluid show significant strain differences in their dose response (P < 0.0001) (Figure 2A). Importantly, BALB/c mice show the largest increase in the number of PMNs present 24 h after instillation of the highest dose of S. chartarum, and C57BL/6J mice show a modest increase (spores to PMNs slopes significantly different at P < 0.0001 for C57BL/6J versus BALB/c, MANOVA). The PMN increase in C3H/HeJ mice is significantly less than in BALB/c, but borderline significantly greater than in C57BL/6J mice (spores to PMNs slope significantly different at P = 0.0008 for C3H/HeJ versus BALB/c, and spore dose to PMN slope trends toward difference at P = 0.0873 for C57BL/6J versus C3H/HeJ, MANOVA). In contrast, the total number of macrophages in the BAL fluid did not change significantly among the doses in any of the mouse strains (data not shown).
MPO is a mediator of oxidant production, and an indicator of PMN degranulation when measured in BAL supernatant. All three strains show a significant dose response in MPO (P < 0.0001) (Figure 2B), consistent with the PMN data. MPO levels show significant strain differences in their dose response (P < 0.0001). BALB/c mice show the largest increase in MPO, with levels 14-fold higher than the C57BL/6J mice and 2-fold higher than the C3H/HeJ mice at the highest dose of S. chartarum (spore dose to MPO slope significantly different at P < 0.0001 for C57BL/6J versus BALB/c, P = 0.014 for C3H/HeJ versus BALB/c, MANOVA). The C57BL/6J mice and the C3H/HeJ mice do not show significantly different dose responses in their MPO levels (spore dose to MPO slope trends toward difference at P = 0.057 for C57BL/6J versus C3H/HeJ, MANOVA).
These BAL results are consistent with histopathology (Figure 3). The lungs of BALB/c mice showed more pulmonary pathologic changes after instillation with the 1 million spores/ml dose of S. chartarum (Figure 3A). They had focal inflammation surrounding spores and large numbers of neutrophils in the alveolar spaces. These neutrophils sometimes occluded affected alveoli. In contrast, the C57BL/6J mice had a small number of isolated sites of minor inflammatory responses near areas of carbon black accumulation, but very few spores were visible (Figure 3B).
Cytokines and Chemokines
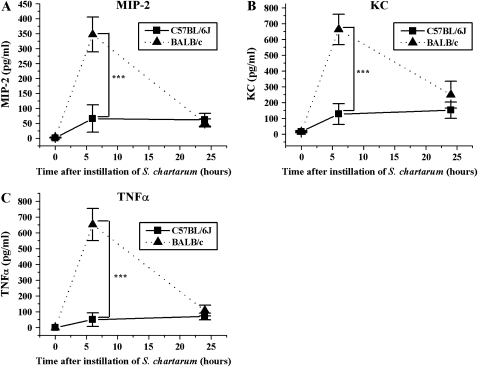
ELISA assays revealed that alterations in chemoattractants for PMNs were consistent with the PMN increases we observed. Mouse MIP-2 and mouse KC are homologs of human IL-8 and exhibit potent neutrophil chemotactic activity. Moreover, they are mediators of neutrophil recruitment in response to tissue injury and infection (52–55). We found that MIP-2 levels peak 6 h after instillation for both C57BL/6J and BALB/c mice, with the BALB/c mice producing 5.2 times more MIP-2 than the C57BL/6J mice in response to S. chartarum. (P = 0.005, t test) (Figure 4A). KC levels peak at 6 h in both strains. The KC levels are 5.2 times higher in the BALB/c mice than in the C57BL/6J mice at 6 h after instillation (P = 0.002, t test) (Figure 4B).
Figure 4.
Time course of MIP-2, KC, and TNF-α. (A) MIP-2 in BAL ± SEM versus time after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight) (t test, P = 0.005). (B) KC in BAL ± SEM versus time after instillation of 5 × 105 spores S. chartarum/20-g mouse (t test, P = 0.002). (C) TNF-α in BAL ± SEM versus time after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight) (t test, P = 0.0007). ***P = 0.005.
TNF-α, a mediator of permeability and inflammation, shows strain-specific changes. We measured TNF-α (Figure 4C) levels by ELISA in both C57BL/6J and BALB/c mice, the two mouse strains showing the greatest difference in albumin levels. TNF-α levels peak 6 h after instillation for both C57BL/6J and BALB/c mice. BALB/c mice produce 13 times more TNF-α than the C57BL/6J mice at the 6-h time point (P = 0.0007, t test).
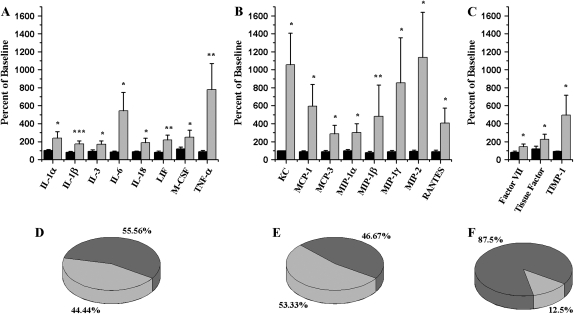
To better clarify the mechanistic differences in the inflammatory responses between BALB/c and C57BL/6J mice, we performed a more thorough investigation of BAL proteins with Luminex technology. The results support the conclusion that profound strain differences exist. In BALB/c mice, 8 of the 15 chemokines (Figure 5E) we measured increased (P < 0.05) after S. chartarum instillation, particularly the monocyte chemotactic protein (MCP) (increased 3- to 6-fold) and MIP (increased 2- to 11-fold) families (Figure 5B). Eight of eighteen cytokines (Figure 5D), especially TNF-α (increased 8-fold) and IL-6 (increased 6-fold) (Figure 5A), also increased. We saw a significant increase in KC, MCP-1, MCP-3, MIP-1α, MIP-1β, MIP-1γ, MIP-2, RANTES, IL-1α, IL-1β, IL-3, IL-6, IL-18, leukemia inhibitory factor (LIF), macrophage colony-stimulating factor (M-CSF), and TNF-α in the BALB/c mice but not in the C57BL/6J mice exposed to the same dose of S. chartarum. Three of twenty-four of the other proteins measured increase significantly too—factor VII, tissue factor, and tissue inhibitor of metalloproteinase-1 (Figure 5F). Cytokine and chemokine levels were negligible in both strains after saline instillation and in C57BL/6J after S. chartarum instillation. The full list of proteins measured with their values and standard errors are available in the online supplement.
Figure 5.
Multi-analyte profile (MAP) analysis. (A) Cytokine level changes in BAL as measured by a MAP analysis after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight) described as percentage of cytokine levels after saline instillation in the same strain (Bonferroni's adjusted MANOVA). (B) Chemokine level changes in BAL as measured by an MAP analysis after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight) described as percentage of chemokine levels after saline instillation in the same strain (Bonferroni's adjusted MANOVA). (C) Other protein level changes in BAL as measured by a MAP analysis after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight) described as percentage of protein levels after saline instillation in the same strain (Bonferroni's adjusted MANOVA). Solid bars, C57BL/6J mice; shaded bars, BALB/c mice. (D) Cytokines (18). Percent of cytokines in a MAP analysis with a significant increase in either C57BL/6J or BALB/c mice after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight). (E) Chemokines (15). Percent of chemokines in a MAP analysis with a significant increase in either C57BL/6J or BALB/c mice after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight). (F) Other proteins (24). Percent of other proteins in a MAP analysis with a significant increase in either C57BL/6J or BALB/c mice after instillation of 10 million spores S. chartarum/ml (2.5 ml/kg body weight). Light shading indicates increased levels in BALB/c only; dark shading indicates no response in either strain. *P < 0.05; **P < 0.01; ***P < 0.005.
OVA-Induced Airway Inflammation Decreases Some of the Pulmonary Responses to S. chartarum
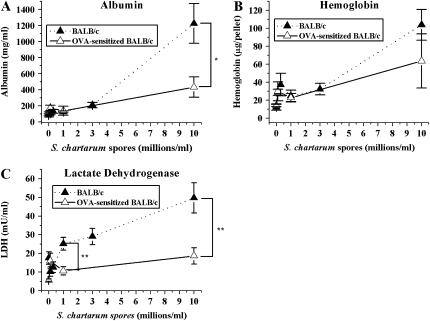
OVA-induced airway inflammation in BALB/c mice significantly increases both mucus production and methacholine airway responsiveness, and is generally characterized by inflammation (51). However, we show in this study that this OVA-induced airway inflammation phenotype reduces the magnitude of some of the murine dose responses to inhaled mold, although the converse, that S. chartarum decreases already existing inflammation from the OVA aerosol, is not true. Albumin and LDH (Figures 6A and 6C) are both significantly lower (t test, P = 0.05 and P = 0.01, respectively) in the sensitized mice than in the nonsensitized mice after intratracheal instillation with S. chartarum (5 × 105 spores/20-g mouse). In addition, even at the middle dose of 5 × 104 spores/20-g mouse, OVA-induced airway inflammation appears protective (t test, P = 0.01) against S. chartarum–induced cell death, as measured by LDH levels. Less than 0.5% of the white cells in BALB/c mice are eosinophils at the highest dose of S. chartarum (10 million spores/ml). In OVA-sensitized mice, 35% of the white cells are eosinophils, and this does not change with increasing S. chartarum dose. The allergic mice have significantly more eosinophils in the BAL at all doses (P = 0.0001, independent t test; data not shown). Other parameters measured including hemoglobin, macrophages, neutrophils, and MPO show no significant difference between the S. chartarum dose responses in sensitized animals and the dose responses in nonsensitized BALB/c mice (Figure 6B and data not shown), although macrophages show higher numbers at all spore doses in sensitized mice (P = 0.002, independent t test; data not shown), which is consistent with the data that macrophage numbers do not increase 1 d after spore exposure.
Figure 6.
Allergen-induced airway inflammation phenotype. (A) Mean concentrations of albumin in BAL fluid ± SEM versus instilled dose S. chartarum in normal BALB/c mice and BALB/c mice with OVA-sensitized airways (t test, P = 0.05). (B) Mean concentrations of hemoglobin in BAL fluid ± SEM versus instilled dose S. chartarum in normal BALB/c mice and OVA-sensitized BALB/c mice (t test, P = 0.3). (C) Mean concentrations of LDH in BAL fluid ± SEM versus instilled dose S. chartarum in normal BALB/c mice and OVA-sensitized BALB/c mice (t test, P = 0.01). *P < 0.05; **P < 0.01.
DISCUSSION
Not all mice respond the same way to Stachybotrys fungal spores. BALB/c mice are more sensitive to pulmonary exposure to S. chartarum than are C57BL/6J and C3H/HeJ mice. We observed more vigorous cytokine and chemokine release, increased capillary permeability and BAL leukocyte numbers, as well as increased release of white cell constituents in the lungs of BALB/c mice (56). Interestingly, these strain differences are distinct from the strain differences seen in response to LPS or to other fungi. We have data showing that C3H/HeJ is the least responsive strain to LPS as measured by LDH, MPO, albumin, and hemoglobin levels and by infiltration of macrophages and neutrophils (data not shown). In this study we show that C57BL/6 is the least responsive strain to S. chartarum. There are no published data comparing the strain differences of all three of these strains to other fungi, but several earlier studies explored pairwise comparisons (57–63). Pulmonary exposure to Coccidioides immitis, Cryptococcus neoformans, and A. fumigatus shows that C57BL/6 mice are more sensitive to infection and inflammation and that BALB/c are more resistant (58, 59, 63); in contrast, we show the opposite. In the case of vaginal Candida and pulmonary C. immitis, BALB/c and C57BL/6 are both sensitive (57, 60– 62). S. chartarum is the only fungus that we know of to which BALB/c mice are sensitive and C57BL/6J are resistant.
Cellular Recruitment and Cell Death
Because the MIP-2 and KC levels are significantly different between the two strains, KC and MIP-2 are strong candidates for causing the recruitment of such large numbers of neutrophils in the BALB/c mice. The levels of TNF-α seen in the lungs of the BALB/c mice six hours after instillation are high, and over a longer timeframe, the increased pulmonary inflammation may help kill and/or clear the spores from the lungs. In addition, sustained high levels of TNF-α have been reported to lead to pulmonary hemorrhage in rats (64), so a similar increase in TNF-α levels in susceptible infants might contribute to pulmonary hemorrhage.
IL-18 causes increases in vascular permeability and in BAL content of neutrophils and proinflammatory cytokines including TNF-α, IL-1β, as well as neutrophil chemoattractants, and therefore may be initiating the cytokine and chemokine increases seen in the BALB/c mice (65–69). TNF-α can also upregulate the IL-18 receptor on epithelial cells (70), thus further sensitizing the tissue to any increase in IL-18. IL-18 can polarize toward a Th1 response by signaling for increased IFN-γ production, leading to a Th1-mediated atopic allergy (66). In this model, though, we cannot say definitively that the BALB/c mice are mounting a Th1 response because we do not see increased levels of IL-12 or IFN-γ. IL-6, LIF, TNF-α, IL-1α, and IL-1β are all key components of the inflammatory pathway in response to tissue damage and all increased significantly in the BALB/c mice, but not C57BL/6J mice. They may be partly responsible for the inflammation we see in response to S. chartarum. IL-3, IL-6, LIF, and M-CSF are members of the differentiation pathway for myeloid progenitor cells, which give rise to monocytes, including alveolar macrophages (71). The increase in these cytokines in BALB/c mice suggests that macrophage numbers may remain constant by a balance between increased macrophage cell death, as is suggested by increasing LDH levels at higher doses, and increased production of new macrophages, which would be required to maintain alveolar macrophage levels at a constant level as we observe.
Allergen-Induced Airway Inflammation Phenotype
In our study, salient features of allergen-induced airway inflammation such as underlying chronic inflammation, especially mucus hypersecretion (72), may cause the apparent higher tolerance for S. chartarum in the sensitized mice. Thicker airway mucus could constitute a more effective barrier for fungal mycotoxins. In addition, the Th2-mediated airway inflammation induced by OVA may be counterbalancing a more exuberant Th1 inflammatory response, which is mediated by IL-18 and elevated in the BALB/c mice in response to S. chartarum, thus leading to the decrease in albumin and LDH levels seen in the OVA-sensitized mice (73, 74).
An alternate explanation for our results is that we see blunted increases in albumin and LDH because the airway was already injured by the OVA-induced allergic inflammatory response. For example, there may have been denudation of airway epithelium in this model, resulting in less LDH to be released in response to the mold. This explanation is unlikely because control animals that were sensitized to OVA but received saline instead of spores showed identical LDH levels to nonsensitized animals, but it is possible that LDH, albumin, and hemoglobin levels rose significantly and then returned to normal in the 4 d between the final OVA aerosol challenge and the intratracheal instillation of S. chartarum spores.
The pathways used by S. chartarum spores to elicit LDH, albumin, and hemoglobin release are unknown. One explanation for the similar levels of hemoglobin release after S. chartarum exposure in OVA-sensitized and unsensitized animals, in contrast to decreases in LDH and albumin after OVA sensitization, may be that hemoglobin in BAL is possibly due to hemorrhage caused by the stachylysin mycotoxin. Stachylysin may be acting through a sensitization-independent pathway. LDH release (a marker of cell death or damage) and albumin levels (a marker for permeability of the air–blood barrier) may have a different signal transduction pathway. However, we have no direct evidence supporting this hypothesis.
Genetic Differences among Strains
The genetic differences and resulting phenotypic differences among the three mouse strains are likely contributing to the observed differences in their inflammatory responses. The results of this study suggest that the pulmonary response to S. chartarum is largely independent of TLR4, because the C3H/HeJ mice show intermediate sensitivity to S. chartarum even though they are the only TLR4− strain we studied. This is consistent with other data suggesting that most innate immune responses to fungi are mediated in part by TLR2 and TLR6 (75). However, responses to both A. fumigatus and A. niger involve TLR4 (36).
BALB/c mice have been reported to be Th2-dominant (40), while C57BL/6 mice are Th1-dominant, although these designations are model-dependent. In fungal models using P. brasiliensis and C. neoformans, BALB/c are Th1-dominant (41, 59, 76, 77), and in another fungal model using C. posadasii there is no clear Th1/Th2 polarization (42). Mills and colleagues (78) have shown that this phenotype is related to innate macrophage function. M-1 and M-2 phenotypes of macrophages from prototypical Th1 and Th2 strains of inbred mice show different immunologic responses and different phagocytic capacities (43, 78). Alveolar macrophages from C57BL/6 mice have been shown to have a significantly higher phagocytic capacity than the alveolar macrophages from BALB/c mice (43), which may allow the C57BL/6 mice to clear spores from the lungs more quickly and more efficiently, leading to diminished inflammation. In contrast, perhaps the BALB/c mice clear the spores more slowly, leading to infiltration of PMNs and a larger inflammatory response. More quantitative measurements of spore clearance are underway.
Dectin-1 is expressed on phagocytic cells, including macrophages and neutrophils, and mediates both the internalization and cellular responses to the β-glucan cell wall of fungi (79). C57BL/6J mice have been reported to splice out exon 3 of dectin-1, which may change the nature of their inflammatory response to S. chartarum spores (80). In particular, the dectin-1 splice variant has been shown to reduce innate recognition of the fungi C. immitis and C. posadasii (80, 81). A similar deficiency in innate recognition of β-glucan cell wall of S. chartarum spores could lead to the reduced inflammatory response seen in the lungs of C57BL/6J mice compared with BALB/c and C3H/HeJ mice.
Human Health Effects
The strain differences we observed in mice may be relevant to humans. The C57BL/6J dectin-1 splice variant is homologous to a splice variant found in humans. If dectin-1 is a primary receptor involved in innate recognition of the β-glucan cell wall of fungi, as has been suggested (80), then the presence of this splice variant in human populations suggests the possibility that these populations may share the decreased recognition of and decreased inflammation in response to S. chartarum exposures. In addition, variations in Th1- and Th2-dominant immune responses among people, whether from genetic predisposition or past environmental exposures, might help explain the variety of responses seen among people exposed to the same moldy environments. In particular, if some infants have a tendency to clear spores more slowly, as may be associated with a Th2-dominant immune response, they will have a higher chronic exposure; this greater exposure, coupled with an exaggerated inflammatory response, could lead to acute idiopathic pulmonary hemorrhage and in some cases death, before the immature immune system could kill or clear the spores. This could be a feature of the spore-related deaths reported by the CDC and others (82–84).
CONCLUSION
This is the first evidence for strain differences in, and thus possible genetic determinants of, susceptibility to S. chartarum. Our data show that BALB/c mice are more sensitive to pulmonary exposure to S. chartarum than are C57BL/6J and C3H/HeJ mice. BALB/c mice show the most hemorrhage, the highest permeability of the air–blood barrier, the most white blood cell recruitment, and the highest inflammatory cytokine and chemokine levels in response to intratracheally instilled S. chartarum. Experiments with other fungi have either shown that BALB/c mice are more resistant than C57BL/6 mice to infection and inflammation or that both strains are similarly sensitive. OVA-induced airway inflammation in the BALB/c mice reduces changes in permeability and cell death induced by exposure to S. chartarum.
Similar underlying genetic variability in human populations may account for some of the wide variation among individuals responding to similar mold exposure environments in contaminated occupational and home settings. Future directions include evaluation of the pulmonary pharmacokinetics of S. chartarum spores, and determination of the genetic loci responsible for differential responses to S. chartarum.
Supplementary Material
Acknowledgments
The authors thank Harriet Burge, Barbara Burleigh, Joseph Mizgerd, Lester Kobzik, and Brett Coull for their helpful comments about this work.
This work was funded by: NIH ES-000002 NIH HL-07118.
Requests for reprints should be addressed to Joseph D. Brain, Harvard School of Public Health, Molecular and Integrative Physiological Sciences, Department of Environmental Health, 665 Huntington Ave., Building I Room 1411, Boston, MA 02115. E-mail: brain@hsph.harvard.edu
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2005-0483OC on May 11, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Burge HA. Airborne allergenic fungi: classification, nomenclature, and distribution. Immunol Allergy Clin North Am 1989;9:307–319. [Google Scholar]
- 2.Larsen FO, Christensen LH, Clementsen P, Gravesen S, Stahl Skov P, Norn S. Microfungi in indoor air are able to trigger histamine release by non-IgE-mediated mechanisms. Inflamm Res 1996;45:S23–S24. [DOI] [PubMed] [Google Scholar]
- 3.Rylander R, Persson K, Goto H, Yuasa K, Tanaka S. Airborne beta-1,3 glucan may be related to symptoms in sick buildings. Indoor Environ 1992;1:263–267. [Google Scholar]
- 4.Flannigan B. Mycotoxins in the air. Int Biodeterior 1987;23:73–78. [Google Scholar]
- 5.Hendry KM, Cole EC. A review of mycotoxins in indoor air. J Toxicol Environ Health 1993;38:183–198. [DOI] [PubMed] [Google Scholar]
- 6.Sorenson W. Mycotoxins as potential occupational hazards. Dev Ind Microbiol 1990;31:205–211. [Google Scholar]
- 7.Cooley JD, Wong WC, Jumper CA, Straus DC. Correlation between the prevalence of certain fungi and sick building syndrome. Occup Environ Med 1998;55:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croft WA, Jarvis BB, Yatawara CS. Airborne outbreak of trichothecene toxicosis. Atmos Environ 1986;20:549–552. [Google Scholar]
- 9.Hintikka EL. Human stachybotryotoxicosis. In: Wyllie T, Morehouse L, editors. Mycotoxic fungi, mycotoxins and mycotoxicosis. New York: Marcel Dekker; 1978. pp. 87–89.
- 10.Sudakin DL. Toxigenic fungi in a water-damaged building: an intervention study. Am J Ind Med 1998;34:183–190. [DOI] [PubMed] [Google Scholar]
- 11.Etzel RA, Montana E, Sorenson WG, Kullman GJ, Allan TM, Dearborn DG, Olson DR, Jarvis BB, Miller JD. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch Pediatr Adolesc Med 1998;152:757–762. [DOI] [PubMed] [Google Scholar]
- 12.Dearborn DG, Yike I, Sorenson WG, Miller MJ, Etzel RA. Overview of investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ Health Perspect 1999;107:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stampfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2004;170:1164–1171. [DOI] [PubMed] [Google Scholar]
- 14.Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, Leaderer BP. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect 2002;110:A781–A786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dill I, Niggemann B. Domestic fungal viable propagules and sensitization in children with IgE mediated allergic diseases. Pediatr Allergy Immunol 1996;7:151–155. [DOI] [PubMed] [Google Scholar]
- 16.Bata A, Harrach B, Vanyi A, Lepom P. Macrocyclic trichothecene toxins produced by Stachybotrys atra. Acta Vet Hung 1988;36:221–227. [PubMed] [Google Scholar]
- 17.Jarvis BB, Lee YW, Comezoglu SN, Yatawara CS. Trichothecenes produced by Stachybotrys atra from Eastern Europe. Appl Environ Microbiol 1986;51:915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasanen A, Nikulin J, Tuimainen M, Berg S, Parikka P, Hinnikka EL. Laboratory experiements on membrane filter sampline of airborne mycotoxins produced by Stachybotrys atra Corda. Atmos Environ 1993;27A:9–13. [Google Scholar]
- 19.Sorenson WG, Frazer DG, Jarvis BB, Simpson J, Robinson VA. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl Environ Microbiol 1987;53:1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno Y. Mode of action of trichothecenes. Ann Nutr Aliment 1977;31: 885–900. [DOI] [PubMed] [Google Scholar]
- 21.Vesper SJ, Magnuson ML, Dearborn DG, Yike I, Haugland RA. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect Immun 2001;69:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesper SJ, Vesper MJ. Stachylysin may be a cause of hemorrhaging in humans exposed to Stachybotrys chartarum. Infect Immun 2002;70: 2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergmann F, Yagen B, Jarvis BB. The toxicity of macrocyclic trichothecenes administered directly into the rat brain. Toxicon 1992;30:1291–1294. [DOI] [PubMed] [Google Scholar]
- 24.Bubien JK, Woods WT Jr. Direct and reflex cardiovascular effects of trichothecene mycotoxins. Toxicon 1987;25:325–331. [DOI] [PubMed] [Google Scholar]
- 25.Glavits R, Vanyi A. Effect of trichothecene mycotoxins (satratoxin H and T-2 toxin) on the lymphoid organs of mice. Acta Vet Hung 1988;36: 37–41. [PubMed] [Google Scholar]
- 26.Hughes BJ, Hsieh GC, Jarvis BB, Sharma RP. Effects of macrocyclic trichothecene mycotoxins on the murine immune system. Arch Environ Contam Toxicol 1989;18:388–395. [DOI] [PubMed] [Google Scholar]
- 27.Ohff R, Kwella M, Booth W. Studies into Stachybotrys atra: use of skin test on rats for verification of toxicity. Monatsh Veterinaermed 1985;40:774–776. [Google Scholar]
- 28.Schiefer HB, Hancock DS, Bhatti AR. Systemic effects of topically applied trichothecenes. I. Comparative study of various trichothecenes in mice. Zentralbl Veterinarmed A 1986;33:373–383. [DOI] [PubMed] [Google Scholar]
- 29.Yoshizawa T, Ohtsubo K, Sasaki T, Nakamura K. Acute toxicities of satratoxins G and H in mice - a histopathological observation with special reference to the live injury caused by satratoxin G. Proc Jap Assoc Mycotoxicol 1986;23:53–57. [Google Scholar]
- 30.Nikulin M, Reijula K, Jarvis BB, Hintikka EL. Experimental lung mycotoxicosis in mice induced by Stachybotrys atra. Int J Exp Pathol 1996; 77:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikulin M, Reijula K, Jarvis BB, Veijalainen P, Hintikka EL. Effects of intranasal exposure to spores of Stachybotrys atra in mice. Fundam Appl Toxicol 1997;35:182–188. [PubMed] [Google Scholar]
- 32.Rao CY, Burge HA, Brain JD. The time course of responses to intratracheally instilled toxic Stachybotrys chartarum spores in rats. Mycopathologia 2000;149:27–34. [DOI] [PubMed] [Google Scholar]
- 33.Viana ME, Coates NH, Gavett SH, Selgrade MK, Vesper SJ, Ward MD. An extract of Stachybotrys chartarum causes allergic asthma-like responses in a BALB/c mouse model. Toxicol Sci 2002;70:98–109. [DOI] [PubMed] [Google Scholar]
- 34.Le Bars J, Le Bars P. Etude du nuage de spores de Stachybotrys atra contaminant de pailles: risques d'inhalation. Bull Soc Fr Mycol Med 1985;14:321–324. [Google Scholar]
- 35.Le Bars J, Le Bars P. Toxinogenesis and development conditions of Stachybotrys atra in France. Acta Vet Scand Suppl 1991;87:349–351. [Google Scholar]
- 36.Meier A, Kirschning CJ, Nikolaus T, Wagner H, Heesemann J, Ebel F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol 2003;5:561–570. [DOI] [PubMed] [Google Scholar]
- 37.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 38.Taconic Guide to Products and Services. Germantown, NY: Taconic; 2003. pp. 23–25.
- 39.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol 2003; 10:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2002;2:845–858. [DOI] [PubMed] [Google Scholar]
- 41.Taborda CP, Juliano MA, Puccia R, Franco M, Travassos LR. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun 1998;66:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magee DM, Friedberg RL, Woitaske MD, Johnston SA, Cox RA. Role of B cells in vaccine-induced immunity against coccidioidomycosis. Infect Immun 2005;73:7011–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su SH, Chen H, Jen CJ. C57BL/6 and BALB/c bronchoalveolar macrophages respond differently to exercise. J Immunol 2001;167:5084–5091. [DOI] [PubMed] [Google Scholar]
- 44.Brain JD, Knudson DE, Sorokin SP, Davis MA. Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ Res 1976;11:13–33. [DOI] [PubMed] [Google Scholar]
- 45.Leong BK, Coombs JK, Sabaitis CP, Rop DA, Aaron CS. Quantitative morphometric analysis of pulmonary deposition of aerosol particles inhaled via intratracheal nebulization, intratracheal instillation or nose-only inhalation in rats. J Appl Toxicol 1998;18:149–160. [DOI] [PubMed] [Google Scholar]
- 46.Henderson RF. Commentary on 'Cellular and biochemical indices of bronchoalveolar lavage for detection of lung injury following insult by airborne toxicants' by M. Firoze Khan and G.S.D. Gupta. Toxicol Lett 1991;58:235–238. [DOI] [PubMed] [Google Scholar]
- 47.Khan MF, Gupta GS. Cellular and biochemical indices of bronchoalveolar lavage for detection of lung injury following insult by airborne toxicants. Toxicol Lett 1991;58:239–255. [DOI] [PubMed] [Google Scholar]
- 48.Beck BD, Brain JD, Bohannon DE. An in vivo hamster bioassay to assess the toxicity of particulates for the lungs. Toxicol Appl Pharmacol 1982;66:9–29. [DOI] [PubMed] [Google Scholar]
- 49.Blythe S, England D, Esser B, Junk P, Lemanske RF Jr. IgE antibody mediated inflammation of rat lung: histologic and bronchoalveolar lavage assessment. Am Rev Respir Dis 1986;134:1246–1251. [DOI] [PubMed] [Google Scholar]
- 50.Lecours R, Laviolette M, Cormier Y. Bronchoalveolar lavage in pulmonary mycotoxicosis (organic dust toxic syndrome). Thorax 1986;41: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krinzman SJ, De Sanctis GT, Cernadas M, Kobzik L, Listman JA, Christiani DC, Perkins DL, Finn PW. T cell activation in a murine model of asthma. Am J Physiol 1996;271:L476–L483. [DOI] [PubMed] [Google Scholar]
- 52.Schall T. Chapter 22: The chemokines. In: Thomson A, editor. The cytokine handbook, 2nd ed. New York: Academic Press; 1994. pp. 419–460.
- 53.Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res 1994;20:473–490. [DOI] [PubMed] [Google Scholar]
- 54.Heinrich JN, Bravo R. The orphan mouse receptor interleukin (IL)-8R beta binds N51. Structure-function analysis using N51/IL-8 chimeric molecules. J Biol Chem 1995;270:4987–4989. [DOI] [PubMed] [Google Scholar]
- 55.Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol 1995;155:2158–2164. [PubMed] [Google Scholar]
- 56.Grossblatt N, Paulson LR, editor. Biologic markers in pulmonary toxicology. Washington, DC: National Academy Press; 1989.
- 57.Calderon L, Williams R, Martinez M, Clemons KV, Stevens DA. Genetic susceptibility to vaginal candidiasis. Med Mycol 2003;41:143–147. [DOI] [PubMed] [Google Scholar]
- 58.Cox RA, Kennell W, Boncyk L, Murphy JW. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun 1988;56:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol 1997;17:733–739. [DOI] [PubMed] [Google Scholar]
- 60.Magee DM, Cox RA. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect Immun 1996;64:3609–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mullick A, Elias M, Picard S, Bourget L, Jovcevski O, Gauthier S, Tuite A, Harakidas P, Bihun C, Massie B, et al. Dysregulated inflammatory response to Candida albicans in a C5-deficient mouse strain. Infect Immun 2004;72:5868–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitarch A, Diez-Orejas R, Molero G, Pardo M, Sanchez M, Gil C, Nombela C. Analysis of the serologic response to systemic Candida albicans infection in a murine model. Proteomics 2001;1:550–559. [DOI] [PubMed] [Google Scholar]
- 63.Stephens-Romero SD, Mednick AJ, Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect Immun 2005;73:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Debs RJ, Fuchs HJ, Philip R, Montgomery AB, Brunette EN, Liggitt D, Patton JS, Shellito JE. Lung-specific delivery of cytokines induces sustained pulmonary and systemic immunomodulation in rats. J Immunol 1988;140:3482–3488. [PubMed] [Google Scholar]
- 65.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol 2001;167:7060–7068. [DOI] [PubMed] [Google Scholar]
- 66.Tsutsui H, Yoshimoto T, Hayashi N, Mizutani H, Nakanishi K. Induction of allergic inflammation by interleukin-18 in experimental animal models. Immunol Rev 2004;202:115–138. [DOI] [PubMed] [Google Scholar]
- 67.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol 2005;32:311–318. [DOI] [PubMed] [Google Scholar]
- 68.Randolph AG, Lange C, Silverman EK, Lazarus R, Weiss ST. Extended haplotype in the tumor necrosis factor gene cluster is associated with asthma and asthma-related phenotypes. Am J Respir Crit Care Med 2005;172:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 2005;171:1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krasna E, Kolesar L, Slavcev A, Valhova S, Kronosova B, Jaresova M, Striz I. IL-18 receptor expression on epithelial cells is upregulated by TNF alpha. Inflammation 2005;29:33–37. [DOI] [PubMed] [Google Scholar]
- 71.Knight D. Leukaemia inhibitory factor (LIF): a cytokine of emerging importance in chronic airway inflammation. Pulm Pharmacol Ther 2001;14:169–176. [DOI] [PubMed] [Google Scholar]
- 72.Walter MJ, Holtzman MJ. A centennial history of research on asthma pathogenesis. Am J Respir Cell Mol Biol 2005;32:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol 2001;1:69–75. [DOI] [PubMed] [Google Scholar]
- 74.Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 2004;31:3–7. [DOI] [PubMed] [Google Scholar]
- 75.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity 2003;19:311–315. [DOI] [PubMed] [Google Scholar]
- 76.Hoag KA, Street NE, Huffnagle GB, Lipscomb MF. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol 1995;13:487–495. [DOI] [PubMed] [Google Scholar]
- 77.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol 1994;55:35–42. [DOI] [PubMed] [Google Scholar]
- 78.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:6166–6173. [DOI] [PubMed] [Google Scholar]
- 79.Herre J, Willment JA, Gordon S, Brown GD. The role of Dectin-1 in antifungal immunity. Crit Rev Immunol 2004;24:193–203. [DOI] [PubMed] [Google Scholar]
- 80.Jimenez-Alzate Md WL, Viriyakosol S, Fierer J. Inherited susceptibility to Coccidioides immitis in C57BL/6 mice is associated with a truncated splice variant of dectin-1. FASEB J 2005;19:A935. [Google Scholar]
- 81.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun 2005;73:1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention. Update: pulmonary hemorrhage/hemosiderosis among infants–Cleveland, Ohio, 1993–1996. MMWR Morb Mortal Wkly Rep 1997;46:33–35. [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention. Update: Pulmonary hemorrhage/hemosiderosis among infants–Cleveland, Ohio, 1993–1996. MMWR Morb Mortal Wkly Rep 2000;49:180–184. [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention. Update: pulmonary hemorrhage/hemosiderosis among infants–Cleveland, Ohio, 1993–1996. JAMA 2000;283:1951–1953. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.