Abstract
The class A macrophage scavenger receptor SR-AI/II is implicated as a pattern recognition receptor for innate immunity, but its functional role in lung defense has not been studied. We used mice genetically deficient in SR-AI/II and their wild-type C57BL/6 counterparts to investigate the contribution of this receptor to defense against pneumococcal infection and inhaled particles. SR-AI/II deficiency caused impaired phagocytosis of fluorescent bacteria in vivo, diminished clearance of live bacteria from the lungs, and substantially increased pneumonic inflammation. Survival studies also showed increased mortality in SR-AI/II–deficient mice with pneumococcal lung infection. Similarly, after challenge of the airways with TiO2 particles, SR-AI/II–deficient mice showed increased proinflammatory cytokine levels in lung lavage fluid and a more pronounced neutrophilic inflammation. The data indicate that the lung macrophage class A scavenger receptor SR-AI/II contributes to innate defense against bacteria and inhaled particles.
Keywords: environmental particles, lung, macrophages, scavenger receptors
Macrophages recognize pathogens through a rich repertoire of receptors expressed on their surface. These receptors constitute a large family of pattern recognition receptors, which can be subdivided based on their structure and function. The class A scavenger receptors (SR-As) are a small group of pattern recognition receptors composed of four members: SR-AI/II, MARCO (MAcrophage Receptor with COllagenous structure) (1, 2), and the recently identified SRCL (scavenger receptor with C-type lectin) (3) and SCARA5 receptor (4). SR-AI/II is expressed primarily by macrophages and has been extensively studied in the context of atherosclerosis (5–8), where it was initially identified as the macrophage receptor for oxidatively modified lipoproteins (9, 10).
SR-AI/II has been reported to participate in other important biologic processes (11, 12), such as macrophage–host cell interactions (9, 13), endocytosis of modified proteins and ligands (9), phagocytosis of apoptotic cells (14) and microbes (15), and clearance of microbial products, such as LPS (16) and lipoteichoic acid (17). In addition, SR-AI/II engagement by its ligands may be involved in intracellular signaling events in the macrophage (18–22).
SR-AI/II has also been implicated in innate immune responses to systemic infections (6, 15–17, 23–26), as has been demonstrated in mouse models using intravenous or intraperitoneal challenges. For example, SR-AI/II–deficient mice display impaired protection against intravenous infection by Listeria monocytogenes and HSV-1 virus (6) and increased sensitivity to intraperitoneally administered Staphylococcus aureus (23). These observations, and our previous finding that the class A receptor MARCO contributes significantly to lung host defense (27), and the expression of SR-AI/II on lung macrophages (28) suggest a potential function for SR-AI/II in lung host defense.
The studies reported in this article tested this postulate using mice genetically deficient in SR-AI/II and receiving pulmonary challenges with pneumococcal bacteria and environmental particles.
MATERIALS AND METHODS
Animals
Male or female mice genetically deficient in SR-AI/II (SR-AI/II−/−) were used in all experiments. SR-AI/II−/− mice were generated by disrupting exon 4 of the SR-A gene, which is essential for the formation of functional trimeric receptors (6). Age- and sex-matched wild-type (C57BL/6 SR-AI/II+/+) mice purchased from Charles River Laboratories (Wilmington, MA) were used as controls. All animals were housed in sterile microisolator cages and had no evidence of spontaneous infection. All experimentation involving animals was approved by the governing institutional review panel.
Reagents and Particles
Titanium dioxide (TiO2) particles (median diameter ∼ 1 μm) were generously provided by Dr. J. Brain (Harvard School of Public Health, Boston, MA). The particles were baked at 200°C for 4 h to destroy any contaminating endotoxins. All particles were suspended in BSS− (124 mM NaCl, 5.8 mN KCl, 10 mM dextrose, and 20 mM HEPES) as stock solution and sonicated before use. All reagents not otherwise specified were obtained from Sigma Chemical Co. (St. Louis, MO).
Bacteria
Streptococcus pneumoniae serotype 3 strain ATCC 6303 (American Type Culture Collection, Rockville, MD) was cultured overnight on 5% sheep blood–supplemented agar Petri dishes (VWR, West Chester, PA). A stock suspension was prepared, aliquoted in small volumes, and kept at −80°C. For each experiment, an aliquot was grown overnight on a blood agar plate and resuspended in sterile isotonic saline. Bacterial concentration of the obtained suspension was estimated by OD600 measurements, and the appropriate dilution was prepared in saline to be administered intranasally to mice. The true concentration was determined by overnight culture.
Mouse Model of Pneumococcal Pneumonia
A well characterized mouse model of pneumococcal pneumonia (27) was used in this study. Pneumonia was induced by intranasal instillation of 25 μl of a bacterial suspension containing ∼ 105 CFU of S. pneumoniae type 3 of mice under short-term anesthesia with halothane. Four or 24 h postinfection, mice were killed with an intraperitoneal injection of a lethal dose of sodium pentobarbital (FatalPlus; Vortech Pharmaceuticals, Dearborn, MI). Separate experiments were performed for bronchoalveolar lavage (BAL) and lung harvest for histology or total lung bacteria counts (CFU). At 4 or 24 h after infection, whole lungs were harvested and homogenized in 1 ml sterile isotonic saline with a tissue homogenizer (Omni International, Warrenton, VA). Serial 10-fold dilutions in sterile saline were made from these homogenates, and 100 μl volumes were plated onto sheep-blood agar plates and incubated at 37°C. CFUs were counted after 18–20 h. In some experiments, to assess survival, mice were instilled intranasally with a single 25-μl suspension of S. pneumoniae (∼ 4.5 × 105 CFU). Animal survival was monitored twice a day over a period of 13 d.
BAL of Infected Mice
Animals were killed 4 or 24 h after bacterial challenge. BAL was performed in situ with a 20-gauge catheter inserted into the proximal trachea, flushing the lower airways five times with 0.8 ml of PBS. The fluid retrieved from the first lavage was used for ELISA assays. The BAL cells were separated from the BAL fluid (BALF) by centrifugation, resuspended in PBS, and counted, and a fraction was cytospun on microscopic slides for staining with Diff-Quick (Baxter Scientific Products, McGaw Park, IL) and for subsequent differential counts.
Preparation of FITC-Labeled Pneumococci and Phagocytosis Assay
S. pneumoniae were labeled with FITC (Molecular Probes, Eugene, OR). Bacteria were grown overnight on sheep blood agar plates, washed twice, and resuspended in PBS. Bacterial concentration was set to ∼ 109 CFU/ml as determined by OD600. Bacteria were heat killed at 95°C for 10 min, centrifuged, and resuspended in 1 ml of labeling buffer (0.1 M NaHCO3, pH 9.2). Newly prepared FITC in DMSO (0.25 mg/ml of suspension) was added, and bacteria were incubated under constant stirring in the dark at room temperature for 1 h. Bacteria were washed three times and dialyzed overnight against PBS. Cell number was determined using a hemocytometer under a fluorescence microscope. The final concentration was set to 109 bacteria/ml.
Mice received intranasally 107 FITC–S. pneumoniae under light halothane anesthesia. Lungs were lavaged with PBS 3 h later, and the amounts of bacteria associated with the alveolar macrophages (AMs) in the BALF were quantified by flow cytometry.
Particle Effects In Vivo
Mice were lightly anaesthetized with halothane, and solutions of particles in pyrogen-free saline were administered via intranasal insufflation (50 μl). Pilot experiments in normal wild-type mice showed minimal response to 1 mg/ml and 5 mg/ml of the inert particle TiO2. These concentrations were used in subsequent experiments comparing responses of SR-AI/II–deficient mice. Mice were killed 4 h after instillation, and BAL was performed. Total cell yield was determined by hemocytometer and differential by microscopic evaluation of cytocentrifuge preparations stained with modified Wright-Giemsa. Aliquots of cell-free BALF were stored at −70°C for subsequent ELISA assay of cytokines. In some experiments, whole lung tissue was harvested without lavage for RNA extractions using the Trizol reagent (GibcoBRL, Gaithersburg, MD) according to manufacturer's protocol. Expression of a panel of cytokine mRNAs (RANTES, Eotaxin, MIP-1a, MIP-1b, MIP-2, IP-10, MCP-1, and TCA-3) was evaluated using the RNase protection assay kit (mCK5; BD Biosciences, San Jose, CA) following the manufacturer's protocol. Blots were visualized using an InstantImager Phosphoimager (Packard, Downers Grove, IL), and densitometric analysis was performed using the dedicated Imager software package.
Quantitation of BALF Cytokine Content by ELISA
MIP-2 and TNF-α were quantitated in BALF of infected/TiO2-treated SR-AI/II−/− and control mice using commercially available ELISA kits (R&D Systems Inc., Minneapolis, MN) following the manufacturer's instructions.
Statistical Analysis
Data were analyzed using the StatView software (Abacus Concepts, Berkeley, CA). Differences in survival were determined by χ2 analysis. Kaplan-Meier curves were used to show survival over time, and differences between curves were analyzed using a log-rank test. For other measurements, differences between groups were examined by Student's t test or ANOVA with correction for multiple groups. Data are presented as mean ± SEM. Results were considered significant at P ⩽ 0.05.
RESULTS
Increased Susceptibility to Lethal Pneumococcal Infection in SR-AI/II−/− Mice
We first tested whether the lack of SR-AI/II would affect survival after lung infection with S. pneumoniae. We inoculated SR-AI/II−/− and SR-AI/II+/+ mice with 4.5 × 105 CFU of S. pneumoniae and monitored their survival over 2 wk. In the wild-type mice group, we observed 40% mortality, with deaths occurring between Days 4 and 6 after infection. SR-AI/II−/− mice showed a much greater mortality at about this same time point, with 50% mortality at Day 5 postinfection and an additional 40% at Day 6, resulting in a total mortality of 90%. No subsequent deaths were recorded in either group. The difference in survival between the two groups of mice was statistically significant (Figure 1).
Figure 1.
Absence of SR-AI/II increases mortality in S. pneumoniae–infected mice. SR-AI/II+/+ and SR-AI/II−/− mice were intranasally inoculated with 4.5 × 105 CFU of S. pneumoniae serotype 3, and survival was monitored over 13 d. Data are expressed as the percentage of mice surviving at each time point. Kaplan-Meier survival plots are shown for 10 animals per group. Mantel-Cox logrank test showed a P value of 0.0416.
Impaired Bacterial Clearance in the Lungs of Infected SR-AI/II−/− Mice
We investigated whether the increased vulnerability of the SR-AI/II−/− mice to pneumococcal infection derives from defects in bacterial clearance from the lungs of infected mice. We inoculated the animals with live S. pneumoniae and determined total CFU in lung homogenates after 4 and 24 h. The viable bacteria recovered from the lungs of infected SR-AI/II+/+ male mice 4 h after infection represented only 8.31 ± 3.37% of the inoculum, whereas 47.8 ± 10.12% of the inoculated bacteria were recovered from the lungs of SR-AI/II−/− mice (Figure 2A). We observed differences in bacterial clearance in male and female mice; therefore, the data are presented separately. In female mice, recovered viable bacteria represented 4.9 ± 1.2% and 23.54 ± 6.06% of the input in SR-AI/II+/+ and SR-AI/II−/−, respectively (Figure 2B). Similar results showing decreased bacterial clearance in the lungs of SR-AI/II−/− mice were seen at 24 h postinfection (Figure 2C).
Figure 2.
Diminished bacterial clearance from the airways of SR-AI/II−/− after infection with S. pneumoniae. SR-AI/II+/+ and SR-AI/II−/− (n > 6) were intranasally infected with ∼ 105 CFU of S. pneumoniae. Lung bacterial load was determined at 4 h (A) and 24 h (B) postinfection. Data shown are representative of at least three separate experiments and are presented as mean ± SEM.
SR-AI/II−/− Mice Show Increased Pulmonary Inflammation in Response to Pneumococci
Streptococcal pneumonia is characterized by an intense production of proinflammatory cytokines and chemokines by activated AMs and other lung cells. Consequently, large numbers of neutrophils are recruited from the circulation into the alveoli (29, 30). We examined these early features of host defense to determine the consequences of SR-AI/II deficiency on the inflammatory response to pneumococcal infection.
Four hours postinoculum, SR-AI/II−/− mice showed markedly increased BAL cellularity compared with wild-type control mice (e.g., total cells ×104: 262 ± 28.5 versus 145 × 6.45, respectively, in one representative experiment). Similarly, deficient mice showed more neutrophils (14.93 ± 3.23% of total BAL leukocytes) than did the wild-type animals (2.45 ± 0.46% of total BAL leukocytes) (Figure 3A).
Figure 3.
SR-AI/II−/− mice with pneumococcal pneumonia develop a stronger inflammatory response in the airways. BALF was harvested from wild-type (n = 10) and deficient mice (n = 8) 4 h postinfection, and the total number of leukocytes and the percentage of PMNs were determined (A). MIP-2 and TNF-α contents in the BALF were determined using ELISA (B). Data shown are representative of at least three separate experiments and are presented as mean ± SEM.
Consistent with the elevated neutrophilic inflammation, BALF from infected SR-AI/II−/− mice showed higher amounts of the cytokines TNF-α and MIP-2 (0.75 ± 0.1 ng/ml and 6.46 ± 0.18 ng/ml, respectively) than did SR-AI/II+/+ mice (0.13 ± 0.01 ng/ml and 4.48 ± 0.39 ng/ml, respectively) (Figure 3B).
Diminished Phagocytosis of S. pneumoniae in SR-AI/II−/− AMs
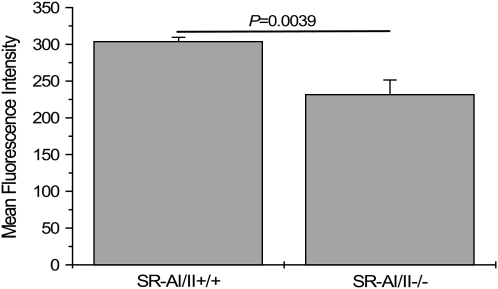
To directly evaluate the contribution of SR-AI/II to the phagocytic activity of macrophages within the alveolar space, we tested AM uptake of FITC-labeled S. pneumoniae in situ. After intranasal administration of bacteria into wild-type and SR-AI/II–deficient mice, AMs were harvested by lavage 3 h later and analyzed by flow cytometry to measure uptake of fluorescent bacteria. The total fluorescence intensity, reflecting the total number of bacteria, associated with AMs is significantly higher in the wild-type mice than in the deficient mice (304 ± 5 and 232 ± 20 MFI units, respectively) (Figure 4).
Figure 4.
Decreased phagocytic capacity in SR-AI/II−/− AMs. Mice were intranasally given 107 FITC-S. pneumoniae under slight anesthesia. Lungs were lavaged with PBS 3 h later, and the amount of bacteria associated with AMs was quantified by flow cytometry. Data shown are pooled from two separate experiments representing a total of seven mice per group.
SR-AI/II Modulates TiO2-Induced Inflammation in the Lungs
AMs are also responsible for ingestion and clearance of inhaled environmental particles (31, 32), as can be modeled with the inert particulate TiO2 (27, 33–37). We sought to test the potential role of SR-AI/II in this process in vivo by administrating TiO2 particles intranasally to mice and assessing the inflammatory response in the lower airways. All mice showed similar total leukocyte counts in the BALF after exposure to PBS and TiO2 (data not shown). However, SR-AI/II–deficient mice exhibited a higher influx of PMNs into the airways in response to 50 μg TiO2 (157 ± 51 × 103 PMNs/ml) and 250 μg TiO2 (400 ± 108 × 103 PMNs/ml) when compared with wild type-mice (30 ± 21 × 103 PMNs/ml and 191 ± 83 × 103 PMNs/ml in response to 50 μg and 250 μg TiO2, respectively) (Figure 5A).
Figure 5.
Elevated lung inflammation in SR-AI/II–deficient mice in response to inhaled TiO2 particles. (A) PMN recruitment into BALF of PBS and TiO2–treated mice (n = 3–35 mice/group). (B) BALF TNF-α after instillation with PBS or two different doses of TiO2, as measured by ELISA (n = 3–9 mice/group). (C) Analysis of lung gene expression in the lungs of PBS or TiO2-treated mice by RNase protection assay shows increased MIP-2 response in SRA-I/II–deficient mice. (D) Fold-increase of MIP-2 mRNA expression in TiO2-treated mice over PBS-treated mice in SR-AI/II+/+ (n = 3) and SR-AI/II−/− mice (n = 4).
The levels of TNF-α secreted into the BALF from SR-AI/II+/+ mice in response to 50 μg TiO2 (0.314 ± 0.177 ng/ml) and 250 μg TiO2 (0.081 ± 0.084 ng/ml) were not significantly different from the amount found in mice exposed to PBS (0.127 ± 0.022 ng/ml). In contrast, BALF from SR-AI/II−/− contained higher amounts of TNF-α in response to 50 μg TiO2 and 250 μg TiO2 (0.828 ± 0.348 and 1.613 ± 0.349 ng/ml, respectively) than in response to PBS (0.097 ± 0.032 ng/ml) (Figure 5B). SR-AI/II−/− mice were more responsive to both doses of TiO2 than were their respective control mice.
Similarly, exposure to TiO2 induced a higher rate of MIP-2 mRNA expression in the lung tissue from SR-AI/II−/− than from SR-AI/II+/+ mice as assessed by RPA (Figure 5C). Overall, TiO2 treatment induced a 3.45 ± 0.7-fold increase in MIP-2 expression over PBS treatment in SR-AI/II−/−, compared with a 0.56 ± 0.05-fold increase in wild-type mice (Figure 5D).
DISCUSSION
Our data support a role of the class A scavenger receptor SR-AI/II on AMs in the innate immune response to bacterial pneumonia and inhaled environmental particles. SR-AI/II−/− mice show defective bacterial clearance, with increased neutrophil and cytokine responses and diminished survival. Similarly, SR-AI/II−/− mice show increased lung inflammatory responses to an “inert” particle, TiO2. These findings indicate that this class A scavenger receptor contributes substantially to innate defense of the lung in the murine models studied.
Certain aspects of the infection model we used merit discussion. We chose to study infection by S. pneumoniae in large part because it is the most common causative microorganism in community-acquired pneumonia (38, 39). We also used a relatively low-dose challenge, aiming to model the frequent low-dose challenge to the lungs of bacteria within aspirated nasopharyngeal secretions. The pathogenesis of pneumococcal pneumonia in most cases involves initial colonization of the upper airways. Pneumococcal infection is relatively common but is far less common than might be predicted from high rates of nasopharyngeal colonization in normal adults and children (40) and from the frequency of normal nocturnal aspiration of upper airway secretions (41). These data imply that lung macrophage uptake and clearance can provide effective innate defense against these low-dose inocula, and our experimental design sought to analyze the role of macrophage SR-AI/II in this process.
SR-AI/II−/− mice showed only partial impairment of in vivo uptake of fluorescent S. pneumoniae. The deficiencies in clearance observed using live bacteria were more profound, and one possible explanation is that the smaller effect observed with fluorescent bacteria may reflect changes induced by the heat-killing process used to prepare the bacteria for labeling. Nevertheless, these data are qualitatively consistent with diminished in vivo uptake of bacteria by lung macrophages in SR-AI/II–deficient mice.
Prior studies have found that SR-AI/II protects against systemic infections by gram-positive and gram-negative bacteria. Our data expand the role of SR-AI/II to protection of the lungs against pneumococcal infection, a major cause of community-acquired pneumonia (42). Our present findings, together with previously published work (27), suggest that SR-AI/II and another class scavenger receptor, MARCO, may act together to prevent the onset of pneumococcal pneumonia after aspiration of S. pneumoniae from the nasopharynx, providing an explanation for the low percentage of pneumococcal infections when compared with the total number of people carrying pneumococcus (41, 43). SR-AI/II–deficient mice show increased basal expression of MARCO on their normal AMs (A. Holian, personal communication). The questions of whether and how much this increased expression contributes to the residual clearance capacity seen in our experiments remain unanswered. The use of mice lacking MARCO and SR-AI/II may further inform assessment of the relative contribution to innate defense by these two receptors.
Another challenge to innate defenses of the lung is air pollution. Chronic exposure to particulate air pollution has been associated with various adverse health effects in humans (44, 45). Absence of SR-AI/II was associated with markedly increased neutrophilic inflammation after TiO2 challenge, likely mediated by the increased expression of MIP-2, a potent neutrophil chemoattractant cytokine. These findings prompt the speculation that the enhanced effects of TiO2 in SR-AI/II–deficient mice may result from prolonged contact of particles with other cell types of the lungs, such as the epithelial cells, leading to MIP-2 synthesis and subsequent recruitment of neutrophils (27).
A final question to consider is the relevance of these data using mice to human biology. Although SR-AI/II is expressed on normal human AMs, we have recently observed a dominant role for MARCO in human AM scavenger receptor functions tested in vitro. Blocking antibodies to human SR-AI/II did not affect human AM binding of fluorescent bacteria or TiO2 (33). This raises the possibility that the observations reported here using murine models may not apply to human biology. However, the in vitro studies of normal human AMs have not addressed the potential requirement for surfactant components to optimize SR-AI/II function in vivo (46) or the potential for increased function after modulation of scavenger receptor expression by cytokines or other components of inflammation (47, 48). This remains a question to be addressed in future studies.
This study was supported by NIH grants ES0002 and ES11008 and by the Jere Mead Fellowship (M.S.A.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0128OC on May 4, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Platt N, Haworth R, Darley L, Gordon S. The many roles of the class A macrophage scavenger receptor. Int Rev Cytol 2002;212:1–40. [DOI] [PubMed] [Google Scholar]
- 2.Arredouani M, Kobzik L. The structure and function of Marco, a macrophage class a scavenger receptor. Cell Mol Biol 2004;OL657–OL665. [PubMed]
- 3.Nakamura K, Funakoshi H, Tokunaga F, Nakamura T. Molecular cloning of a mouse scavenger receptor with C-type lectin (SRCL)(1), a novel member of the scavenger receptor family. Biochim Biophys Acta 2001;1522:53–58. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Oliver P, Davies KE, Platt N. Identification and characterisation of murine SCARA5, a novel class A scavenger receptor that is expressed by populations of epithelial cells. J Biol Chem 2006;281: 11834–11845. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Sakaguchi H, Kruijt JK, Higashi T, Suzuki T, et al. The multiple roles of macrophage scavenger receptors (MSR) in vivo: resistance to atherosclerosis and susceptibility to infection in MSR knockout mice. J Atheroscler Thromb 1997;4:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 1997;386:292–296. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi H, Takeya M, Suzuki H, Hakamata H, Kodama T, Horiuchi S, Gordon S, van der Laan LJ, Kraal G, Ishibashi S, et al. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest 1998;78:423–434. [PubMed] [Google Scholar]
- 8.de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: a multifunctional receptor in atherosclerosis. Arterioscler Thromb Vasc Biol 2000;20:290–297. [DOI] [PubMed] [Google Scholar]
- 9.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem 1994;63:601–637. [DOI] [PubMed] [Google Scholar]
- 10.Ling W, Lougheed M, Suzuki H, Buchan A, Kodama T, Steinbrecher UP. Oxidized or acetylated low density lipoproteins are rapidly cleared by the liver in mice with disruption of the scavenger receptor class A type I/II gene. J Clin Invest 1997;100:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell 2002;111:927–930. [DOI] [PubMed] [Google Scholar]
- 12.Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? The mouse's tale. J Clin Invest 2001;108:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature 1993;364:343–346. [DOI] [PubMed] [Google Scholar]
- 14.Platt N, da Silva RP, Gordon S. Class A scavenger receptors and the phagocytosis of apoptotic cells. Immunol Lett 1999;65:15–19. [DOI] [PubMed] [Google Scholar]
- 15.Pearson AM. Scavenger receptors in innate immunity. Curr Opin Immunol 1996;8:20–28. [DOI] [PubMed] [Google Scholar]
- 16.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature 1991;352:342–344. [DOI] [PubMed] [Google Scholar]
- 17.Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA 1994;91:1863–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu HY, Chiu SL, Wen MH, Chen KY, Hua KF. Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. J Biol Chem 2001;276:28719–28730. [DOI] [PubMed] [Google Scholar]
- 19.Hsu HY, Hajjar DP, Khan KM, Falcone DJ. Ligand binding to macrophage scavenger receptor-A induces urokinase-type plasminogen activator expression by a protein kinase-dependent signaling pathway. J Biol Chem 1998;273:1240–1246. [DOI] [PubMed] [Google Scholar]
- 20.Pollaud-Cherion C, Vandaele J, Quartulli F, Seguelas MH, Decerprit J, Pipy B. Involvement of calcium and arachidonate metabolism in acetylated-low-density-lipoprotein-stimulated tumor-necrosis-factor-alpha production by rat peritoneal macrophages. Eur J Biochem 1998; 253:345–353. [DOI] [PubMed] [Google Scholar]
- 21.Palkama T. Induction of interleukin-1 production by ligands binding to the scavenger receptor in human monocytes and the THP-1 cell line. Immunology 1991;74:432–438. [PMC free article] [PubMed] [Google Scholar]
- 22.Jozefowski S, Kobzik L. Scavenger receptor A mediates H2O2 production and suppression of IL-12 release in murine macrophages. J Leukoc Biol 2004;76:1066–1074. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, El Khoury J. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med 2000;191:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peiser L, De Winther MP, Makepeace K, Hollinshead M, Coull P, Plested J, Kodama T, Moxon ER, Gordon S. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect Immun 2002;70:5346–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med 1997;186:1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi Y, Miyaji C, Watanabe H, Umezu H, Hasegawa G, Abo T, Arakawa M, Kamata N, Suzuki H, Kodama T, et al. Role of macrophage scavenger receptor in endotoxin shock. J Pathol 2000;192:263–272. [DOI] [PubMed] [Google Scholar]
- 27.Arredouani M, Yang Z, Ning YY, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for normal lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 2004;200:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naito M, Kodama T, Matsumoto A, Doi T, Takahashi K. Tissue distribution, intracellular localization, and in vitro expression of bovine macrophage scavenger receptors. Am J Pathol 1991;139:1411–1423. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev 2000;173:39–51. [DOI] [PubMed] [Google Scholar]
- 30.Moore TA, Standiford TJ. Cytokine immunotherapy during bacterial pneumonia: from benchtop to bedside. Semin Respir Infect 2001;16: 27–37. [DOI] [PubMed] [Google Scholar]
- 31.Brain JD. Macrophages in the respiratory tract. In: Fishman AP, Fisher AB, editors. Handbook of physiology. Bethesda, MD: American Physiological Society; 1984. pp. 447–471.
- 32.Bowden DH. Macrophages, dust, and pulmonary diseases. Exp Lung Res 1987;12:89–107. [DOI] [PubMed] [Google Scholar]
- 33.Arredouani MS, Palecanda A, Koziel H, Huang YC, Imrich A, Sulahian TH, Ning YY, Yang Z, Pikkarainen T, Sankala M, et al. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol 2005;175:6058–6064. [DOI] [PubMed] [Google Scholar]
- 34.Kobzik L. Lung macrophage uptake of unopsonized environmental particulates: role of scavenger-type receptors. J Immunol 1995;155:367–376. [PubMed] [Google Scholar]
- 35.Palecanda A, Paulauskis J, Al-Mutairi E, Imrich A, Qin G, Suzuki H, Kodama T, Tryggvason K, Koziel H, Kobzik L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med 1999;189:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stringer B, Imrich A, Kobzik L. Flow cytometric assay of lung macrophage uptake of environmental particulates. Cytometry 1995;20:23–32. [DOI] [PubMed] [Google Scholar]
- 37.Stringer B, Kobzik L. Alveolar macrophage uptake of the environmental particulate titanium dioxide: role of surfactant components. Am J Respir Cell Mol Biol 1996;14:155–160. [DOI] [PubMed] [Google Scholar]
- 38.Brown PD, Lerner SA. Community-acquired pneumonia. Lancet 1998; 352:1295–1302. [DOI] [PubMed] [Google Scholar]
- 39.Marrie TJ. Community-acquired pneumonia. Clin Infect Dis 1994;18:501–513 (quiz: 514–515). [DOI] [PubMed] [Google Scholar]
- 40.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004;4:144–154. [DOI] [PubMed] [Google Scholar]
- 41.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest 1997;111:1266–1272. [DOI] [PubMed] [Google Scholar]
- 42.File TM. The epidemiology of respiratory tract infections. Semin Respir Infect 2000;15:184–194. [DOI] [PubMed] [Google Scholar]
- 43.McCullers JA, Tuomanen EI. Molecular pathogenesis of pneumococcal pneumonia. Front Biosci 2001;6:D877–D889. [DOI] [PubMed] [Google Scholar]
- 44.Pope CA III. Air pollution and health: good news and bad. N Engl J Med 2004;351:1132–1134. [DOI] [PubMed] [Google Scholar]
- 45.Handzel ZT. Effects of environmental pollutants on airways, allergic inflammation, and the immune response. Rev Environ Health 2000;15: 325–336. [DOI] [PubMed] [Google Scholar]
- 46.Kuronuma K, Sano H, Kato K, Kudo K, Hyakushima N, Yokota S, Takahashi H, Fujii N, Suzuki H, Kodama T, et al. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J Biol Chem 2004;279:21421–21430. [DOI] [PubMed] [Google Scholar]
- 47.Jozefowski J, Arredouani M, Sulahian T, Kobzik L. Disparate regulation and function of the class a scavenger receptors SR-AI/II and MARCO. J Immunol 2005;175:8032–8041. [DOI] [PubMed] [Google Scholar]
- 48.de Villiers WJ, Fraser IP, Hughes DA, Doyle AG, Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J Exp Med 1994;180: 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]