Abstract
Inorganic phosphate (Pi) plays a critical role in diverse cellular functions. Among three classes of sodium/phosphate co-transporters (NPTs), two types have been identified in mammalian lung. The potential importance of Pi as a novel signaling molecule and pulmonary expression of NPTs with poor prognosis of diverse lung diseases including cancer have prompted us to begin to define the pathways by which Pi regulates nontumorigenic human bronchial epithelial cells. Pi activates Akt phosphorylation on Thr308 specifically, and activated signal transmits on the Raf/MEK/ERK signaling. Here, we report that Pi controls cell growth by activating ERK cascades and by facilitating the translocation of Mnk1 from cytosol into nucleus through an Akt-mediated MEK pathway. Sequentially, translocated Mnk1 increases eIF4E-BP1 phosphorylation. As a result, Pi stimulates cap-dependent protein translation. Such Akt-mediated signaling of inorganic phosphate may provide critical clues for treatment as well as prevention of diverse lung diseases.
Keywords: Akt, inorganic phosphate, Mnk1, nontumorigenic human bronchial epithelial cells
Inorganic phosphate (Pi) is present in bacteria, fungus, plant, and animal cells. Pi plays a critical role in mineral metabolism, and diverse cellular functions involving intermediary metabolism and energy transfer. It is a vital component of membrane phospholipids, nucleotides that provide energy, DNA and RNA, and is necessary for phosphorylated intermediates in cellular signaling (1).
Bronchial epithelial cells, the progenitor cells for bronchogenic carcinomas, are often exposed to diverse xenobiotics, including toxicants. Many investigators elucidated the precise mechanisms of pulmonary toxicant–induced normal bronchial epithelial cell damage. The response of bronchial epithelium to chemical and physical injury has been associated with the induction of hyperplasia and the subsequent development of squamous metaplasia (2). The modified response of nontumorigenic bronchial epithelial cells to external/internal stimuli causes a disturbance in the homeostasis between normal cell survival and growth. Therefore, the elucidation of such disturbed homeostatic process will provide a critical clue to cope with pulmonary diseases such as cancer.
The serine/threonine protein kinase Akt, also termed PKB (protein kinase B), controls key cellular processes such as glucose metabolism (3), cell cycle progression (4), and apoptosis (5). Akt has been identified as a key effector of the phosphatidylinositol 3-kinase (PI3K) signaling pathway and functions to promote cell survival by inhibiting apoptosis through its ability to phosphorylate and inactivate several targets. The binding of PI3K-generated phospholipids to Akt results in the translocation of Akt from the cytoplasm to the inner surface of the membrane, where Akt is phosphorylated (6).
Surveys from various countries indicate that Pi intake is increasing steadily such that Pi additives have increased by ∼ 17% for the decade until 1993, and the use of Pi as a food additive is predicted to continue to increase (7). So far, most studies for Pi have been focused mainly on bone because the bone is the major organ affected by Pi. However, mechanistic studies for the lung's homeostatic maintenance and adaptation to abnormal Pi have not yet been investigated. Therefore, this study was undertaken to elucidate the potential effects of Pi on homeostasis in nontumorigenic human bronchial epithelial (NHBE) cells. Here, we demonstrate that Pi affects cell growth and facilitates Mnk1 translocation from cytosol into nucleus through activating PI3K/Akt as well as Raf/MEK/ERK pathways. These results support the hypothesis that the Pi works as a stimulus capable of increasing or decreasing several pivotal genes for cell survival, and suggest that regulation on Pi consumption is important in maintaining a high quality of life.
MATERIALS AND METHODS
Reagents, Plasmids, and Antibodies
Gentamicin was purchased from GibcoBRL (Grand Island, NY). Sodium phosphate, sodium sulfate, phosphonoformic acid (Foscarnet), and 4′,6′–diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO). Kinase inhibitors LY294002 and PD98059 were purchased from CalBiochem (San Diego, CA) and Tocris (Ellisville, MO), respectively. Fluorescein isothiocyanate (FITC)-conjugated AffiniPure goat anti-rabbit IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). Akt with normal sequence and Akt with a K179M mutation cloned into pCMV5 were kind gifts from Dr. Hemmings (Friedrich Miescher Institute, Basel, Switzerland) and referred to as Akt-WT (wild-type) and Akt-KD (kinase dead), respectively. The bicistronic construct, pcDNA-fLUC-polIRES-rLUC, was from Dr. Gram (Novartis Pharma AG, Basel, Switzerland). Anti–phospho-Akt, anti-Akt1, anti-Raf, anti–phospho-MEK1/2, anti-MEK1, anti-MEK2, anti–phospho-ERK1/2, anti-ERK1, anti-Mnk1, anti-eIF4E-BP1, anti-Histone H1, and anti-GAPDH antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–phospho-Raf (Ser259), anti–phospho-Mnk1 (Thr 197/202), anti–phospho-4E-BP1 (Ser65), and anti–phospho-GSK-3 α/β (Ser21/9) antibodies were from Cell Signaling (Beverly, MA). Antibody against Na/Pi co-transporter (NPT-IIa) was purchased from Alpha Diagnostic International (San Antonio, TX).
Cell Culture and Transfection
NHBE (ATCC no. CRL-2503) cells were incubated in RPMI 1640 medium supplemented with 10% FBS and gentamicin (50 μg/ml) and subsequently cultured with or without Pi. Pi was used in the form of 10 mM NaPO4, pH 7.4, and in all experiments described, 10 mM sodium sulfate was used as a control to clarify the potential salt effects on current study. Cells were transiently transfected using FuGENE 6 Transfection Reagent (Roche, Basel, Switzerland) according to the manufacturer's instructions.
Cell Viability and Luciferase Assay
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays were performed 48 h after the treatment with or without Pi, and the values were described as percentages of the control. For luciferase assay, cells were grown on 6-well plates and were co-transfected with pCMV5 control vector, Akt1-WT, Akt1-KD plasmid, and bicistronic reporter gene. After 24 h incubation, cells were treated with or without Pi for 24 h. The cells were washed twice in ice-cold PBS, extracted in passive lysis buffer (Promega, Madison, WI), and assayed for firefly and renilla luciferase activities, according to the manufacturer's instructions.
Western Blot Analysis
NHBE cells were cultured in the presence or absence of 10 mM Pi for indicated time points. Twenty-five micrograms of cell lysate was separated using 10–15% SDS-PAGE and transferred to nitrocellulose membranes. After membranes were blocked in TTBS containing 5% skim milk for 1 h, immunoblotting was performed by incubating overnight at 4°C with the corresponding primary antibodies in 5% skim milk and then with second antibodies conjugated to horseradish peroxidase (HRP) for 1 h. After washing, the bands-of-interest were visualized by luminescent image analyzer LAS-3000 (Fuji Photo Film, Tokyo, Japan). Results were quantified using a measure program of LAS-3000.
siRNA Preparation
Akt1 protein level was decreased by using the plasmids containing siRNA from siXpress Human U6 PCR Vector System by Mirus Bio Coporation (Madison, WI). The three siRNA sequences targeting human Akt1 (GenBank no.: NM_005163) were as follows: (1) 5′-CGA GUUUGAGUACCUGAAAUU-3′; (2) 5′-CCUCAUGCUGGAC AAGGACUU-3′; (3) 5′-GAUGACAGCAUGGAGUGUGUU-3′. Scrambled sequence 5′-AAUCGCAUAGCGUAUGCCGUU-3′ was used as a control. The siRNA constructs were produced according to manufacturer's instructions. Cells grown to a confluence of 70–80% in 6-well plates were transfected with 1 μg of plasmid using TransITR-LT1 reagent. After 48 h incubation, cells were treated with or without Pi for 8 h and harvested for future experiments.
Akt Kinase Assay
The kinase activity of Akt was measured by using the Akt kinase assay kit according to manufacturer's instructions (Cell Signaling). Briefly, cells were lysed in ice-cold lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/μl leupeptin) plus 1 mM phenylmethylsulfonyl fluoride. Cell lysates were centrifuged, and 200 μl of supernatants were immunoprecipitated with 20 μl of immobilized antibody bead for overnight at 4°C. One microliter of 10 mM ATP and 1 μg of glycogen synthase kinase-3 (GSK-3) fusion protein, an Akt substrate, was added to the suspended pellet with 50 μl of kinase buffer (25 mM Tris [pH 7.5], 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4, 10 mM MgCl2), and incubated for 30 min at 30°C. The reactions were terminated with the addition of 25 μl of 3× SDS sample buffer and boiling at 100°C for 5 min. Reaction samples were resolved by SDS-PAGE and performed by Western blot.
Separation of Nucleus and Cytosol
Nucleus and cytosol were separated by Nuclear Extract Kit from Active Motif (Carlsbad, CA). Cells were grown to confluence in a 100-mm tissue culture plate and washed with 5 μl ice-cold PBS/phosphatase inhibitors. Collected cells were gently resuspended in 500 μl hypotonic buffer and incubated on ice for 15 min. After incubation, 25 μl detergent was added in the tube. By centrifugation, cytoplasmic fraction and nuclear pellet were separated. Nuclear pellet was resuspended with 50 μl complete lysis buffer and incubated for 30 min on ice on a rocking platform at 150 rpm. After centrifuge, supernatant containing nuclear proteins was collected for further analysis.
DAPI and Immunostaining
Cells were grown on chamber slides (Nalge Nunc, Naperville, IL) and treated with 10 mM Pi for indicated time points. Slides were washed with PBS and fixed in 4% paraformaldehyde for 10 min. Cells were washed and fixed again with methanol:acetone (1:1, vol/vol). After being blocked with 3% BSA in TTBS for 1 h, cells were incubated in 1:50 anti-phospho-Mnk1 antibody for overnight at 4°C, washed, and incubated in 1:500 FITC-conjugated secondary antibody for 1 h at room temperature. Nuclei were stained with DAPI for 30 min in incubation at dark room. After washing, coverslips were mounted using poly-L-lysine (Sigma-Aldrich), and the slides were visualized using a fluorescent microscope (Carl Zeiss, Thornwood, NY).
Statistical Analysis
All results are given as means ± SE. Results were analyzed by unpaired Student's t test (GraphPad Software, San Diego, CA). *P < 0.05 was considered significant and **P < 0.01 and ***P < 0.001 highly significant compared with corresponding control.
RESULTS
Pi Increases Cell Growth and Selective Akt Phosphorylation at Thr308
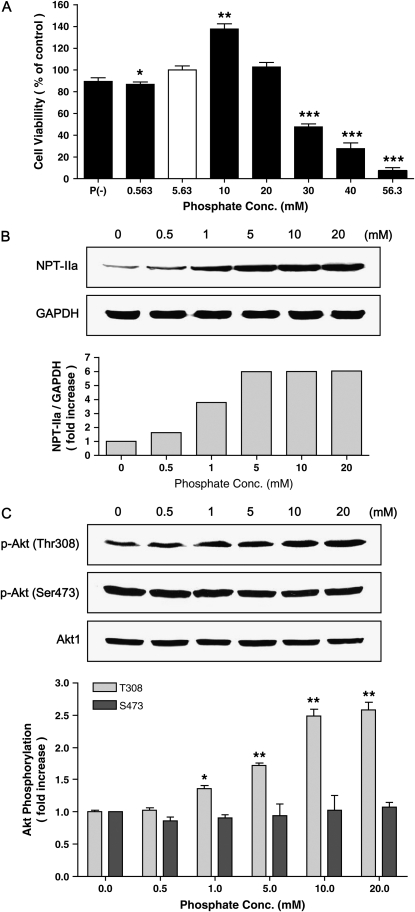
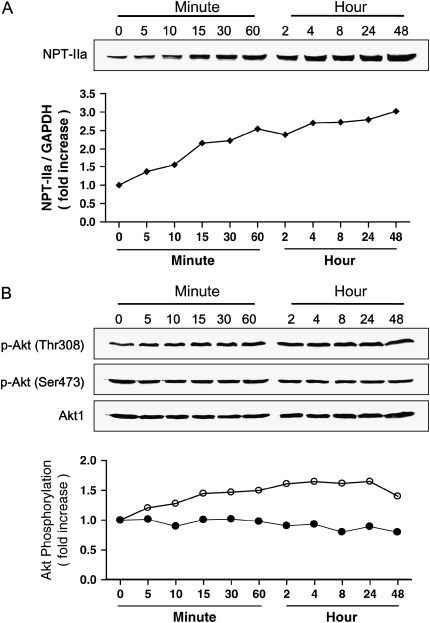
The potential effects of Pi on NHBE cell viability were measured through MTT assay. As shown in Figure 1A, 10 mM Pi increased viable cells significantly; however, above 30 mM Pi was cytotoxic. Deficient and low Pi (0.563 mM) caused slightly less viability than normal Pi condition (5.63 mM). A quantity of 10 mM Pi increased ∼ 38% of cell viability compared with normal Pi group. Pi treatment increased the sodium/phosphate co-transporter IIa (NPT-IIa) protein expression as a function of concentration and time, suggesting a role for this transporter in the Pi response of the cell (Figures 1B and 2A). Pi also increased Akt phosphorylation at Thr308 but not at Ser473 in a concentration- and time-dependent manner; however, total Akt level remained unchanged (Figures 1C and 2B). Such concentration-dependent increasing pattern of selective Akt phosphorylation at Thr308 was clearly demonstrated by densitometric analysis (Figures 1C and 2B).
Figure 1.
Concentration-dependent effects of inorganic phosphate (Pi) on NHBE cells. (A) NHBE cells were treated with various concentrations of Pi and incubated for 48 h, and cell viability was measured using MTT assay. The open bar represents the control/normal media containing 5.63 mM Pi, whereas filled bars represent different Pi concentrations. (B) Concentration-dependent NPT-IIa protein expression. NHBE cells were treated with increasing concentrations of Pi (0–20 mM) for 24 h and 25 μg of protein lysate was used for Western blot. Upper panels, Western blot; lower panel, densitometric analysis. (C) Concentration-dependent Akt phosphorylation on Thr308 and Ser473. NHBE cells were treated with increasing concentrations of Pi (0–20 mM) for 24 h and 25 μg of protein was used for Western blot. Upper panels, Western blot; lower panel, densitometric analysis. Open and filled bars represent degrees of phosphorylation of Akt at Thr308 and S473, respectively. Values are the means ± SE of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2.
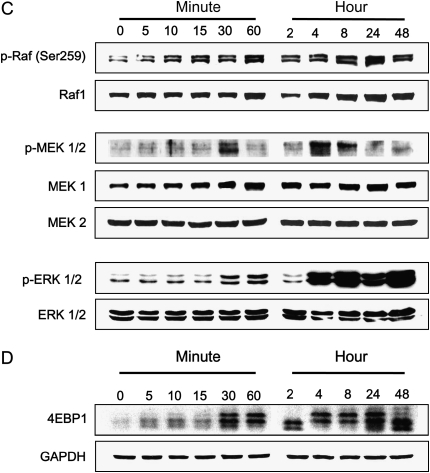
Time-dependent effects of Pi on NPT-IIa, Akt phosphorylation, and Raf/MEK/ERK signals. Cells were treated with 10 mM Pi for various time points, then lysed, and 25 μg of protein was used for Western blot. (A) Time-dependent NPT-IIa protein expression. Upper panel, Western blot; lower panel, densitometric analysis. (B) Time-dependent Akt phosphorylation on Thr308 and Ser473. Upper panels, Western blot; lower panel, Densitometric analysis. Open and filled circles represent degrees of phosphorylation of Akt at Thr308 and S473, respectively. (C) Time-dependent expressions of Raf/MEK/ERK signal proteins. (D) Time-dependent expression of eIF4E-BP1 protein in NHBE cells.
Pi-Induced Biphasic Stimulation of Raf/MEK/ERK and eIF4E-BP1 Signals
Our initial analysis of Akt-related pathways revealed that Pi did not affect either mTOR or p70S6K expressions (data not shown). It is possible that Pi may activate other signaling molecules, such as MAP kinases. To determine whether these signaling molecules were activated by Pi, NHBE cells were treated with 10 mM Pi for indicated times. Western blot analysis using specific antibodies to various MAP kinases revealed that the response of NHBE cells to Pi was selective and specific because phosphorylations of Raf-1, MEK1/2, and ERK1/2 were clearly observed. Interestingly, however, the specific phosphorylation of Raf-1, MEK1/2, and ERK1/2 showed a biphasic pattern with initial activation at early time points (10 min for Raf-1, 30 min for MEK1/2, 30–60 min for ERK1/2, respectively) and the second activation (after 4 h) (Figure 2C). Interestingly, the band-shift pattern of eIF4E-BP1 protein in response to Pi was almost identical to those of Raf-1, MEK1/2, and ERK1/2 phosphorylation (Figure 2D). These results suggest that Pi specifically stimulates Raf-1, MEK/12, ERK1/2, and eIF4E-BP1 and that the activation is biphasic.
Pi-Augmented Cell Growth Is Blocked by a Phosphate Transport Inhibitor
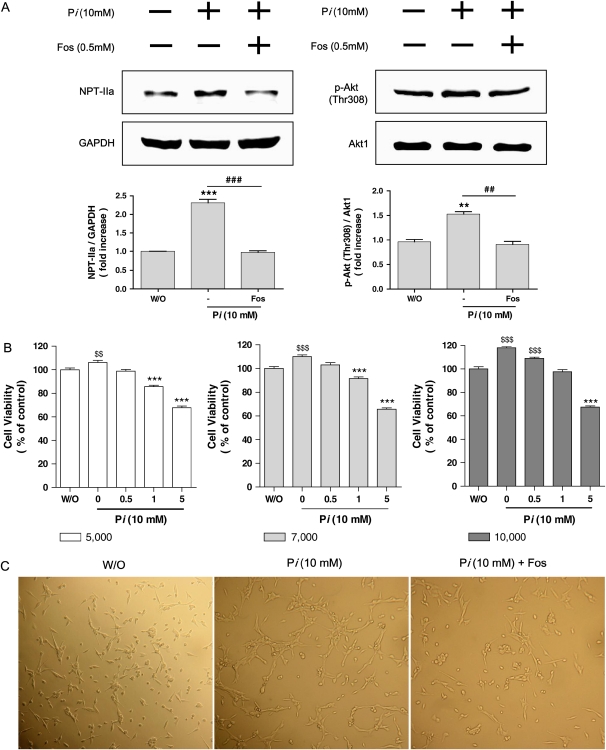
Because Pi treatment increased the cell viability with selective Akt phosphorylation at Thr308, we were interested in defining the role of phosphate transport. The compound Foscarnet (phosphonoformic acid or PFA) has previously been demonstrated to inhibit phosphate transport (8). Pretreatment with 0.5 mM Foscarnet suppressed Pi-induced NPT-IIa protein expression significantly with similar pattern of decreased Akt phosphorylation at Thr308 (Figure 3A). With the unique effects of Foscarnet on Akt phosphorylation, our next interests were in the relationship between cell viability and phosphate transport inhibitor. To maximize the effects of Foscarnet on cell viability, cell viability assay was performed with three different concentrations of Foscarnet and cell numbers. As shown in Figure 3B, 7,000 cells per well was the most appropriate cell density for the 10 mM Pi study. Pi increased the cell size significantly (middle column of Figure 3C) compared with control (left column of Figure 3C); however, Foscarnet treatment suppressed Pi-induced increase of cell size (right column of Figure 3C). As mentioned earlier, control cells were treated with 10 mM Na2SO4, and thus such observed cell size change was not due to salt.
Figure 3.
Effects of phosphate transport inhibitor on NPT-IIa, Akt phosphorylation, and cell growth. Cells were pretreated with Foscarnet phosphate transport inhibitor for 30 min, and then 10 mM of Pi was added for an additional 24 h. After 24 h, cells were lysed and 25 μg of protein was used for Western blot. (A) Changes of NPT-IIa protein (left panels) and Akt phosphorylation at Thr308 (right panels) were detected by Western blot. GAPDH and Akt1 were used as each corresponding internal standard. Upper panels, Western blot; lower panels, densitometric analysis. Values are the means ± SE of three independent experiments. **P < 0.01, ***P < 0.001 versus without Pi, ##P < 0.01, ###P < 0.001 versus without inhibitors. (B) Effects of various concentrations of Foscarnet on viability of cells treated with/without Pi measured by MTT assay. Three different numbers of cells grown in 96 wells (left: 5,000 cells/well; middle: 7,000 cells/well; right: 10,000 cells/well) were treated with increasing concentrations of Foscarnet (0–5 mM) in 10 mM of Pi. Based on above study, optimal concentration of Foscarnet (0.5 mM) as well as appropriate cell number (7,000 cells/well) were selected for further studies shown in panel C. $$P < 0.01, $$$P < 0.001 significantly increased compared with control, and ***P < 0.001 significantly decreased compared with control. (C) Effects of Pi on cell growth. Cells (7,000 cells/well) were grown in wells and treated with Pi in the presence or absence of 0.5 mM Foscarnet and observed under ×200 magnification. Pi caused the increase of cell size (middle panel) compared with control (left panel); however, Foscarnet treatment suppressed the cell growth significantly (right panel). The photograph is representative of three independent experiments. For all above studies, control cells were treated with 10 mM Na2SO4 to clarify the potential effect of salts. Note that the pattern of negative control was almost identical to that of control cells (data not shown).
Pi Downregulated Akt-Related Signals
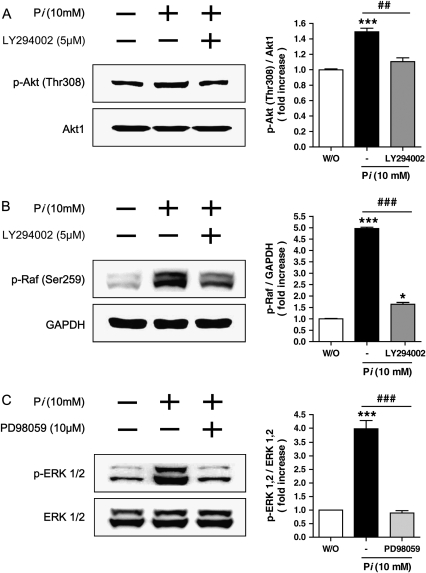
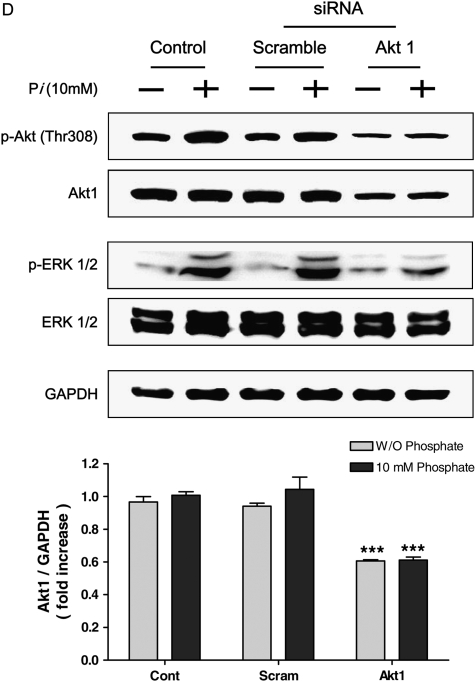
To confirm the precise roles of Pi on PI3K/Akt pathways, the cells were pretreated with appropriate inhibitors for 30 min, then 10 mM Pi was added into media for 24 h. Two different inhibitors, LY294002 for PI3K pathway and PD98059 for MAPK pathway, were used. Our data indicated that selective Akt phosphorylation at Thr308 in response to Pi was inhibited by LY294002 pretreatment (Figure 4A). Pretreatment of LY294002 also suppressed Pi-induced Raf-1 phosphorylation at Ser259 significantly (Figure 4B). Because PD98059 inhibits MEK1/2, the potential effects of such inhibitor on Pi-treated cells were tested. Our results showed that inhibition of the MEK pathway with PD98059 suppressed the ERK phosphorylation (Figure 4C). Since we hypothesized that Pi-induced alteration of Akt activity could be one of critical factors for cell growth, siRNA Akt study was performed. Pi-induced expression of total Akt and Akt phosphorylation at Thr308 was significantly suppressed by siAkt. Such selective suppression of Akt also decreased ERK phosphorylation, whereas total ERK remained unchanged (Figure 4D). The results clearly demonstrated that Akt played a key role for regulating the cell growth through Akt-mediated ERK signaling.
Figure 4.
Continued
Pi Facilitated Cap-Dependent Protein Translation in an Akt-Dependent Manner
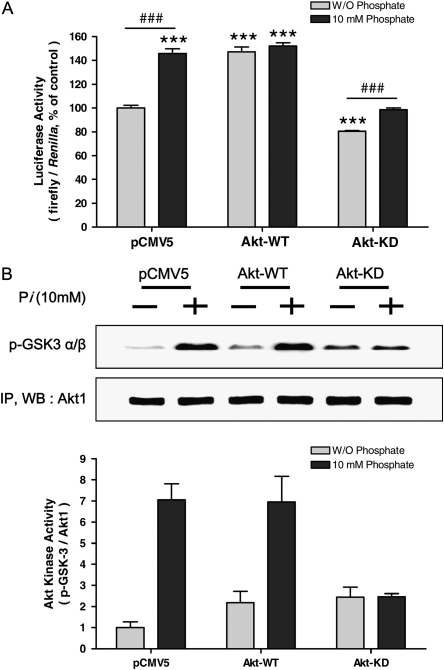
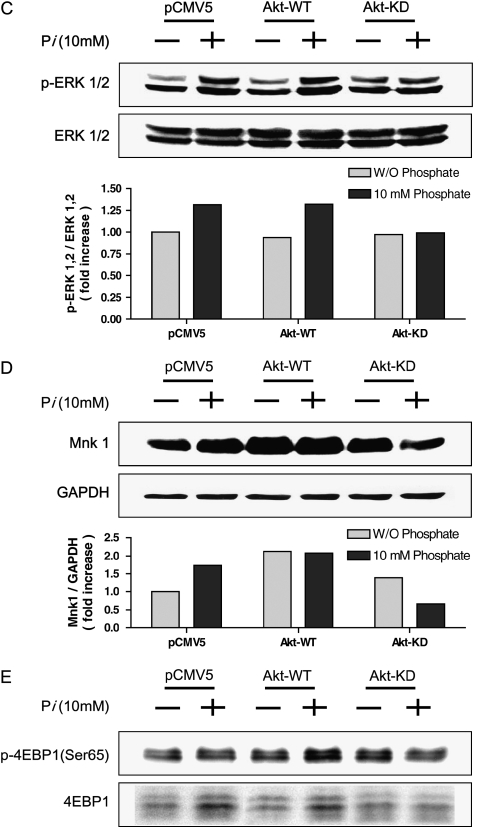
To confirm the precise role of Pi on Akt-mediated protein translation, co-transfection of Akt-WT or Akt-KD with bicistronic reporter gene into the cells was performed. As mentioned earlier, firefly luciferase represents cap-dependent while renilla luciferase activity represents cap-independent protein translation. When Akt-WT was overexpressed, cap-dependent translation was increased ∼ 30% compared with control; however, Akt-KD transfection decreased cap-dependent (and thus increased cap-independent) protein translation ∼ 22% compared with control, significantly. When the cells were co-treated with Pi, cap-dependent protein translation was still suppressed significantly in Akt-KD–treated cells (Figure 5A). Such Akt-mediated protein translation was further confirmed by the Akt kinase assay. The kinase assay with Akt-WT and Akt-KD clearly demonstrated that GSK-3 α/β phosphorylation was well correlated with the pattern of cap-dependent/-independent protein translation. The Pi-induced Akt kinase activation is clearly shown in densitometric analysis, suggesting that Pi exerts its activity through Akt (Figure 5B). Again, close correlation between Pi-induced ERK phosphorylation through Akt signal was clearly observed, as Akt-KD did not induce the ERK phosphorylation significantly (Figure 5C). Pi also affected the Mnk1 protein expression in an Akt-dependent manner, because Pi with Akt-KD transfection suppressed the Mnk1 protein expression. There was no such pattern observed in wild-type Akt (Figure 5D). Finally, Akt played an important role for Pi-induced eIF4E-BP1 activation because Akt-KD suppressed eIF4E-BP1 protein expression as well as phosphorylation (Figure 5D). Taken together with the dual luciferase assay shown in Figure 5A, Pi facilitated cap-dependent protein translation. Such cap-dependent protein translation could occur by controlling 4E-BP1 phosphorylation in an Akt-dependent manner regardless of eIF4E, because there was no change in eIF4E protein (data not shown).
Figure 5.
Continued
Pi Facilitates the Translocation of Mnk1 from Cytosol into Nucleus
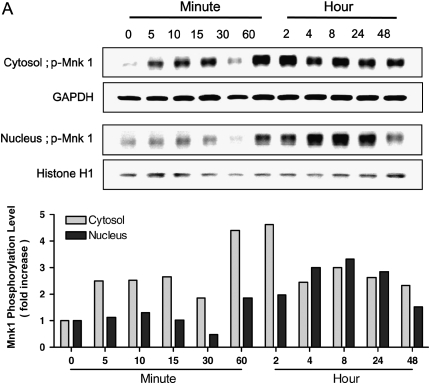
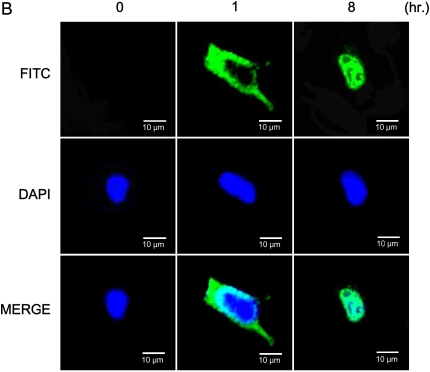
Signal pathways have been elucidated in which the activation of MAP kinases ERK1/2 is upstream of the activation of Mnk1. To test the potential effects of Pi on Mnk1 translocation inside the cell, NHBE cells were treated with Pi, after which cytosol and nucleus were separated. Mnk1 protein was phosphorylated mainly in the cytosol at early time points; however, subsequently the protein expression in the nucleus was increased as a function of time. After 8 h, marked increase of Mnk1 phosphorylation in the nucleus was clearly observed (Figure 6A). Immunofluorescence analysis supports our findings by showing that FITC representing phosphor-Mnk1 expression in the cytosol is decreased, whereas Mnk1 phosphorylation in nucleus is increased in a time-dependent manner (Figure 6B).
Figure 6.
Time-dependent effects of Pi on Mnk1 translocation between cytosol and nucleus. (A) Western blot analysis of phospho-Mnk1 between cytosol and nucleus. Cells were treated with 10 mM Pi for various time points, collected, then, separated into cytosol and nucleic fractions by Nuclear Extract Kit. Twenty-five micrograms of protein was used for Western blot of phospho-Mnk1. GAPDH and Histone H1 were used as controls for cytosol and nucleus, respectively. Upper panels, Western blot analysis; lower panel, densitometric analysis. (B) Fluorescence imaging of the distribution of phospho-Mnk1 between cytosol and nucleus. At different time points, cells were collected, fixed, and assayed by immunostaining for phospho-Mnk1 (green via FITC), and nuclei (blue via DAPI). Two representative time points are shown for better explanation. Scale bars = 10 μm.
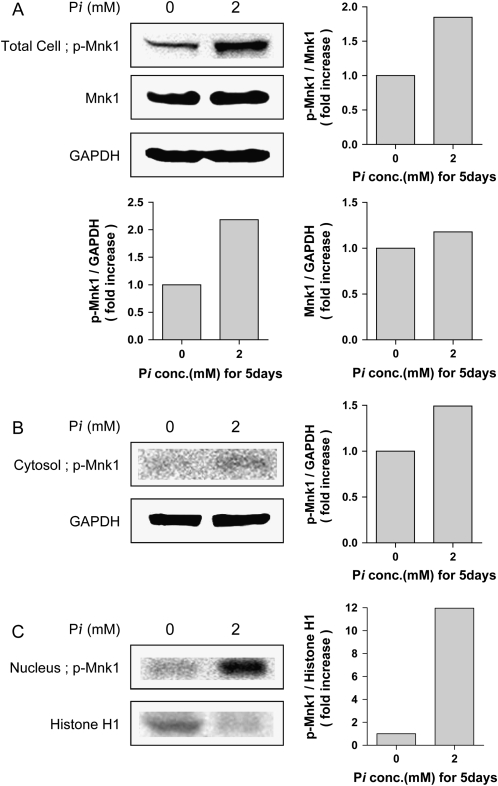
Concentration of Pi Required for Translocation of Mnk1 Is Inversely Proportional to Time
Previous results suggested that the amount of added Pi required to result in changes in intracellular signaling is inversely proportional to time (9–11). To determine if this was the case in the NHBE model, cells were treated with 2 mM inorganic phosphate for 5 d. The resulting total cell (Figure 7A), cytoplasmic (Figure 7B), or nuclear lysates (Figure 7C) were analyzed by Western blot and probed as indicated. The results indicate that, in fact, the addition of 2 mM Pi for 5 d caused an increase in phosphorylated Mnk1 in the nucleus (Figures 7A–7C). In addition, the antibody used specifically detects the phosphorylated form of Mnk1 and suggests that exposure of NHBE cells to elevated inorganic phosphate causes an increase in the phosphorylated form of Mnk1.
Figure 7.
Increase in nuclear phosphorylated Mnk1 in response to small changes in extracellular phosphate. NHBE cells were treated with 2 mM Pi for 5 d, then collected, and separated into total (A), cytosol (B), and nucleic (C) fractions by Nuclear Extract Kit. Twenty-five micrograms of protein was used for Western blot of phospho-Mnk1. GAPDH and Histone H1 were used as controls for cytosol and nucleus, respectively. Left panels, Western blot analysis; right panels, densitometric analysis.
DISCUSSION
Nononcogenic as well as neoplastic lung tissues often display alterations of gene expression in signal transduction pathways responsible for homeostasis; however, the exact mechanisms by which the gene modulates abnormal cell growth/differentiation have yet to be determined. Such alterations are likely associated with cellular changes that involve an imbalance between cell proliferation, DNA repair, and cell death. In addition, these alterations may result from cellular aging and/or insults from endogenous or exogenous chemical exposure.
Pi is abundant in the diet, and intestinal absorption of Pi is efficient and well regulated. The kidney is a major regulator of Pi homeostasis and can increase or decrease its Pi-reabsorptive capacity to accommodate Pi need. The bulk of filtered Pi is reabsorbed in the proximal tubule, where sodium-dependent Pi transport system in the brush-border membrane mediates the rate-limiting step in the overall Pi-reabsorptive process (12). As mentioned earlier, Pi plays a key role in diverse physiologic functions. Among three classes of NPTs (Type I, II, III), two types (Type II and III) have been identified in mammalian lung, and considerable progress has been made in our understanding of their function and regulation. Pi transport into the lung cells is regulated mainly by dietary and serum Pi value (1). Several lines of research indicate that Pi works as a stimulus capable of increasing or decreasing several pivotal genes such as transcriptional regulator, signal transduction, and cell cycle regulator (9). Recently, Wang and coworkers (13) have found that inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Taken together, the potential importance of Pi as a novel signaling molecule and pulmonary expression of NPTs with poor prognosis of diverse lung diseases including cancer have prompted us to begin to define the pathways by which Pi regulates lung cell growth.
Akt is a serine/threonine kinase that is a crucial mediator in signaling pathways, leading to cell survival and cell proliferation (14). Akt requires phosphorylation of both Thr308 and Ser473 for full activity (15). Our data showed that Pi did not affect total Akt protein expression; however, it increased the Akt phosphorylation at Thr308 in a concentration- and a time-dependent manner, while phosphorylation at Ser473 remained unchanged (Figures 1C and 2B). The data suggest that Pi may play a key role in cell proliferation as well as cell differentiation through controlling Akt activity. Inhibition by the known phosphate transport inhibitor Foscarnet suggests that NPT-dependent Pi uptake (Figures 1B and 2A) stimulated NHBE cell growth through activating the Akt phosphorylation at Thr308 because high level of Pi increased cell viability significantly (Figure 1A). Recent research that the level of Akt expression and kinase activity are often associated with the degree of cell proliferation/differentiation, and aggressive behavior of cancer cells, confirms our finding (16). In fact, such Pi-induced cell growth is clearly observed in our results (Figure 3C). Therefore, increased Akt phosphorylation at Thr308 may be closely associated with Pi-stimulated NBHE cell growth.
The ERK pathway is regulated downstream of a number of transmembrane receptors and couples extracellular signals to intracellular events controlling the activity of transcription factors that mediate cell proliferation (17). ERK itself is a component of three kinase cascades at the membrane consisting of the sequential activation of a MAP kinase kinase kinase (Raf-1), a MAP kinase kinase (MEK), and family ERK/MAP kinase itself. In our study, Pi seems to affect the lung cell growth through Raf/MEK/ERK signaling cascade because Pi-induced phosphorylation pattern of Raf-1, MEK, and ERK is almost identical (Figure 2C). In fact, Pi activates Akt by selective phosphorylation at Thr308, then activated Akt may cause serial activation of downstream of Akt. Therefore, Raf-1 is activated, and such activated Raf-1 can further activate related downstream such as MEK in our study. In fact, phosphorylation by MEK is known to be an essential step in ERK activation (18). Such MEK-mediated ERK phosphorylation seems to be Pi-dependent, because inhibitors of PI3K and MEK suppressed Pi-induced augmented phosphorylation of Akt, Raf-1, and ERK (Figures 4A–4C). Together, Pi activates Akt directly; such direct Akt activation may be responsible for Raf/MEK-dependent ERK activation in our study. Our results are further confirmed by the evidence that Akt siRNA treatment suppresses ERK phosphorylation, not total ERK (Figure 4D).
Mnk1 is protein kinase that is directly phosphorylated and activated by ERK and implicated in the regulation of protein synthesis. Recent lines of research have demonstrated that Mnk1 is essential for constitutive and inducible phosphorylation of eIF4E, but not cell growth or development, through phosphorylation of eIF4E at Ser209 (19). However, this is not the case in our study. Inorganic phosphate may exert its activity differently because Pi does not affect eIF4E phosphorylation. Instead, Pi has induced phosphorylation of eIF4E-BP1 at Ser65 (Figure 5E). Such phosphorylated Mnk1 is translocated from the cytosol into nucleus as a function of time (Figures 6A and 6B). Taken together, Pi may affect the cell growth by controlling protein translation through Akt-mediated Raf/MEK/ERK activation and facilitating the translocation of Mnk1 from cytosol to nucleus.
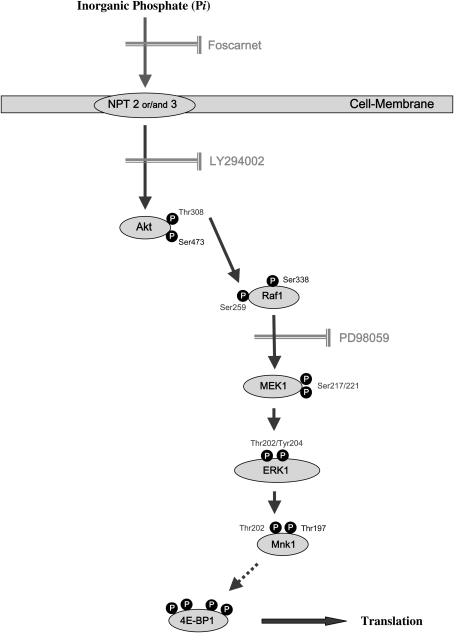
In summary, we report that elevated extracellular Pi controls cell growth by activating the ERK cascades and the translocation of Mnk1 into nucleus through manipulating the Akt-mediated Raf/MEK pathways. Sequentially, activated Mnk1 on Thr202 by ERK signal could increase the eIF4E-BP1 phosphorylation. As a result, inorganic phosphate stimulates cap-dependent protein translation (Figure 8). Such Akt-mediated signaling of inorganic phosphate may provide critical clues for treatment as well as prevention of diverse lung diseases including cancer.
Figure 8.
Schematic signal pathway by which Pi affects in NHBE cells. Based on our studies with the transport inhibitor, Foscarnet, Pi is transported into NHBE cells through a sodium-dependent phosphate transporter (NPT) and results in the activation of Akt by phosphorylation of Thr308. Such activated Akt phosphorylates Raf, MEK, ERK, then facilitates Mnk1 translocation from cytosol into nucleus resulting increased cap-dependent protein translation.
This work was supported by NANO Systems Institute at National Core Research Center program of the KOSEF at SNU. K.H.L. was supported by 21C Frontier Functional Human Genome Project (FG03–0601–003–1-0-0) and National Nuclear R&D Program from Ministry of Science and Technology and a National Cancer Institute Grant CA84573 (G.R.B. Jr.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0477OC on June 8, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Takeda E, Yamamoto H, Nashiki K, Sato T, Arai H, Taketani Y. Inorganic phosphate homeostasis and the role of dietary phosphorus. J Cell Mol Med 2004;8:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fields WR, Desiderio JG, Leonard RM, Burger EE, Brown BG, Doolittle DJ. Differential c-myc expression profiles in normal human bronchial epithelial cells following treatment with benzo[a]pyrene, benzo[a]pyrene-4,5 epoxide, and benzo[a]pyrene-7,8–9,10 diol epoxide. Mol Carcinog 2004;40:79–89. [DOI] [PubMed] [Google Scholar]
- 3.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785–789. [DOI] [PubMed] [Google Scholar]
- 4.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 2001;114:2903–2910. [DOI] [PubMed] [Google Scholar]
- 5.Hovelmann S, Beckers TL, Schmidt M. Molecular alterations in apoptotic pathways after PKB/Akt-mediated chemoresistance in NCI H460 cells. Br J Cancer 2004;90:2370–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription. Mol Cell Biol 2002;22:5962–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo MS. Dietary phosphorous, calcium metabolism, and bone. J Nutr 1993;123:F1627–F1633. [DOI] [PubMed] [Google Scholar]
- 8.Szczepanska-Konkel M, Yusufi AN, VanScoy M, Webster SK, Dousa TP. Phosphonocarboxylic acids as specific inhibitors of Na+-dependent transport of phosphate across renal brush border membrane. J Biol Chem 1986;261:6375–6383. [PubMed] [Google Scholar]
- 9.Beck GR Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res 2003;288:288–300. [DOI] [PubMed] [Google Scholar]
- 10.Beck GR Jr, Knecht N. Osteopontin regulation by inorganic phosphate is ERK1/2-, protein kinase C-, and proteasome-dependent. J Biol Chem 2003;278:41921–41929. [DOI] [PubMed] [Google Scholar]
- 11.Beck GR Jr. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem 2003;90:234–243. [DOI] [PubMed] [Google Scholar]
- 12.Takeda E, Taketani Y, Morita K, Tatsumi S, Kanako K, Nii T, Yamamoto H, Miyamoto K. Molecular mechanisms of mammalian inorganic phosphate homeostasis. Adv Enzyme Regul 2000;40:285–302. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Fraley CD, Faridi J, Kornberg A, Roth RA. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc Natl Acad Sci USA 2003;100:11249–11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 2001;61:3986–3997. [PubMed] [Google Scholar]
- 15.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 2003;111:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill MH, Hemmings BA. Inhibition of protein kinase B/Akt implications for cancer therapy. Pharmacol Ther 2002;93:243–251. [DOI] [PubMed] [Google Scholar]
- 17.Raman M, Cobb MH. MAP kinase module: many roads home. Curr Biol 2003;13:R886–R888. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis JM. Making the connection: coupling of stress-activated ERK/MAPK (extracellular-signal-regulated kinase/mitogen-activated protein kinase) core signaling modules to extracellular stimuli and biological responses. Biochem Soc Symp 1993;64:29–48. [PubMed] [Google Scholar]
- 19.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constituitive and inducible phosphorylation of eukaryotic initiation factor 4E but for cell growth or development. Mol Cell Biol 2004;24:6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]