Abstract
Mucin hypersecretion is a prominent feature of obstructive airway diseases such as asthma. Clara cells conditionally produce mucin in response to inflammatory signals in a process termed mucous metaplasia. This can be followed by mucin secretion stimulated by various signaling molecules. The cellular and molecular mechanisms that regulate mucin production and secretion are not well understood. Adenosine is a signaling nucleoside that has been implicated in airway diseases in which mucus obstruction is prominent. Furthermore, the A3 adenosine receptor (A3AR) is upregulated in mucin-producing goblet cells of the airway, thereby implicating it in processes involved in mucous cell biology. Here we use genetic approaches to investigate the contribution of A3AR signaling to mucus production and secretion in a mouse model of allergen-induced pulmonary disease. We found that the degree of mucin production in response to allergen is similar in wild-type and A3AR-deficient mice, and that overexpression of this receptor in Clara cells neither induces mucin production itself, nor enhances mucin production in response to allergen challenge. Collectively, these experiments demonstrate that the A3AR is neither necessary nor sufficient for mucous cell metaplasia. In contrast to the lack of effect on mucin production, agonist-induced mucin secretion was increased in goblet cells overexpressing the A3AR, and was absent in A3AR-deficient mice. Thus, the A3AR contributes to mucin secretion in allergen-induced metaplasia. Signaling through this receptor may contribute to mucus airway obstruction seen in pulmonary disorders in which adenosine levels are elevated.
Keywords: mucin, mucous cell metaplasia, secretion, adenosine receptors, allergic lung disease
Overproduction and hypersecretion of mucin are prominent features of chronic lung diseases such as asthma, cystic fibrosis, and chronic obstructive pulmonary disease (COPD) (1–3). Obstruction of the airways by mucus is a major contributor to morbidity in patients with these pulmonary conditions. Mucus obstruction is the result of two distinct processes: mucous cell metaplasia, and the increased secretion of mucin (4). Mucous metaplasia and mucin secretion are regulated by distinct extracellular signaling molecules, but detailed information into the concerted regulation of these processes in the diseased airway is lacking. Thus, investigation into basic cellular mechanisms involved in these processes is needed to clarify how coordinate regulation of mucin production and secretion lead to airway obstruction.
Adenosine is a nucleoside that is generated at high levels during situations of cellular stress or injury (5). Consistent with this, adenosine levels are elevated in the airways of individuals with asthma (6), in whom the degree of adenosine elevation correlates with the magnitude of pulmonary inflammation (7). Adenosine has been implicated in chronic lung diseases such as asthma and COPD (8, 9), in part by promoting mast cell degranulation (10), cytokine and chemokine expression (11), and airway hyperresponsiveness (12). Furthermore, a recent study by McNamara and colleagues demonstrates that adenosine within tracheal aspirates from subjects with asthma stimulates the transcription of the gel forming mucin genes MUC5AC and MUC2 in airway epithelial cells (13). These findings have important implications for the ability of endogenously generated adenosine to impact mucin production in the asthmatic airway. Moreover, studies in experimental models of chronic lung disease demonstrate that elevations of adenosine are closely associated with increased mucin production and secretion (14, 15). Collectively, these studies suggest that adenosine produced in the inflamed lung contributes to mucus obstruction in the airways; however, the signaling pathways involved are not yet known.
Adenosine elicits its cellular responses by engaging cell surface receptors (16). There are four such adenosine receptors (A1AR, A2AAR, A2BAR, and A3AR), all of which are seven membrane–spanning G protein–coupled receptors. Adenosine receptors are widely distributed in tissues and have unique affinities for adenosine and its analogs. Engagement of adenosine receptors serves to regulate homeostasis in many physiologic systems, and can impact inflammation and tissue injury in many disease states (5, 16, 17). The A3AR receptor has received attention in asthma due in part to findings that the levels of this receptor are elevated in the airways of patients with asthma (18). In addition, recent studies have shown that levels of the A3AR are substantially elevated in the lungs of mice in experimental models of Th2 and adenosine-dependent lung injury (15, 19). Cellular localization of the A3AR in these models revealed that the A3AR is not expressed in epithelial cells of normal bronchial airways; however, it is upregulated in mucin-producing Clara cells within the airways of diseased mice. The function of the A3AR in mucin-producing epithelial cells is not known; however, its upregulation suggests that it is involved in aspects of mucous cell biology.
The purpose of the current study is to use genetic approaches to investigate the contribution of A3AR signaling to mucin production and secretion in a mouse model of allergen-induced pulmonary disease. Results demonstrate that the degree of mucin production in response to allergen is not altered by the genetic removal or the overexpression of the A3AR in Clara cells. Further, overexpression of the A3AR in Clara cells did not lead to mucous cell metaplasia, even in the presence of an A3AR agonist. These experiments demonstrate that the A3AR is neither necessary nor sufficient for mucous cell metaplasia. In contrast, agonist-induced mucin secretion was increased in Clara cells expressing endogenous or transgenically elevated levels of the A3AR, while there was a lack of agonist-induced mucin secretion in A3AR-deficient (A3AR−/−) mice. Thus, the A3AR is involved in mucin secretion in response to allergen-induced metaplasia, and may thus contribute to mucus airway obstruction seen in various pulmonary disorders.
MATERIALS AND METHODS
Mice
A3AR-deficient (A3AR−/−) mice were generated as previously described (20) and were obtained from Merck Research Laboratories (West Point, PA). Mice were congenic on the C57BL/6J background, and wild-type (A3AR+/+) C57BL/6J mice were purchased from Taconic Laboratories (Germantown, NJ). Genotypes were determined by PCR analysis of genomic DNA obtained from tails at weaning using the following primers: A3AR+/+ (sense: 5′-ATGGAAGCCGACAACACCAC-3′, antisense: 5′-CTACAGCAATGGCCAGCAAG-3′), A3AR−/− (sense: 5′-CTGAG GTTGATGGTCCTGTA-3′, antisense: 5′-GCCTTCTTGACGAGTT CTTC-3′). For the generation of A3AR-overexpressing mice, a 7.5-kb ClaI restriction fragment containing the entire A3AR gene was cloned into the ClaI site of the plasmid containing a 2.3-kb fragment containing the promoter region of the rat Clara cell secretory protein CC10 (gift from Dr. Jamie Lee, Mayo Clinic, Scottsdale, AZ). This transgenic construct (CC10-A3AR) was excised from plasmid sequences by NotI digestion and injected into C57BL/6J one-cell embryos. Transgenic founder mice were identified from genomic PCR analysis of tail DNA using the following primers: CC10-A3AR transgene (sense: 5′-TGCT CGAGCTCTAGATATCG-3′, antisense: 5′-ATCTTGACTTGCAG GCTGAC-3′). All mice were maintained in microisolator cages housed under specific pathogen–free conditions. Sentinel mice within the colony surveyed negative for viral antibodies and the presence of known mouse pathogens. All experiments in mice were approved by the University of Texas Health Science Center at Houston Animal Welfare Committee.
Ovalbumin Sensitization and Challenge and IB-MECA Exposures
Six- to eight-week-old A3AR+/+, A3AR−/−, or CC10-A3AR mice were sensitized by an intraperitenial injection of 500 μl ovalbumin(OVA)/AlOH3 (8 μg/ml OVA and 8 mg/ml AlOH3; Sigma-Aldrich, St. Louis, MO) on Days 0, 7, and 14. Mice were challenged by intranasal installation of 30 μl of either saline or OVA (8 mg/ml) on Days 14, 21, 22, and 23. For IB-MECA exposures, 6-wk-old naïve or OVA-sensitized and -challenged mice were exposed to aerosolized saline or 1 mM IB-MECA for 5 min on Day 24. IB-MECA is a selective A3AR agonist (21), and the exposure protocol described above has been used to engage A3ARs in the mouse airway (22).
RNA Isolation and Quantitative RT-PCR Analysis
RNA was isolated from whole lung tissue using Trizol reagent (Invitrogen, Grand Island, NY). RNase-free DNase was used to eliminate genomic DNA contamination (Invitrogen), and RNA quality was assessed by electrophoresis through formaldehyde agarose gels. Transcript levels were quantified using real-time quantitative RT-PCR. Adenosine receptor and β-actin transcripts were analyzed by using Taqman probes or SYBER green on a Smart Cycler (Cepheid, Sunnyvale, CA), with primer sequences and conditions as previously described (19, 23, 24). Data were analyzed using Smart Cycler (Cepheid) analysis software. To generate a standard curve, PCR amplification was performed with template dilutions for each transcript with the assistance of the Smart Cycler program (Cepheid) to determine concentration by the cycle number where it crosses the threshold (Ct). Final data were normalized to β-actin.
In Situ Hybridization
The cDNA clones used to generate riboprobes for the mouse A3AR were obtained from Marlene Jacobson (Merck Research Laboratories). Plasmids were linearized and either T3 or T7 RNA polymerases were used to generate antisense or sense riboprobes labeled with digoxigenin. Formalin-fixed, paraffin-embedded lungs were sectioned (5 μm) and collected on Superfrost/plus positively charged microscope slides (Fischer Scientific, Houston, TX). The sections were deparaffinized and rehydrated according to a standard protocol and fixed in 4% paraformaldehyde for 15 min at room temperature. Sections were treated with 0.1% Triton X-100 for 10 min at room temperature, then washed in 200 mM HCl for 5 min. Sections were digested with 5–10 μg/ml of proteinase K in 20 μM Tris and 2 mM CaCl2 at 37°C for 20 min. Sections were postfixed in 4% paraformaldehyde for 5 min at room temperature, washed in 0.5% acetic anhydride in Tris (pH 8.0) for 10 min, and hybridized overnight at 60°C in 100 μl of hybridization buffer (2× standard saline citrate, 50% formamide, 10% dextran sulfate, and 0.02% sodium dodecyl sulfate) and 1.5 μl of antisense riboprobe per section. Hybridization using sense riboprobes was performed under identical conditions. Sections were washed three times in 50% formamide in saline citrate at 60°C for 20 min each, and then washed in saline citrate two times for 15 min. Detection was performed using anti-digoxigenin alkaline phosphatase (Roche, Indianapolis, IN) and 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium chloride (BCIP/NBT) as substrate (Invitrogen). Sections were counterstained in nuclear red, coverslipped, and viewed and photographed using an Olympus BX60 microscope equipped with a SPOT digital camera (Diagnostics Instrument, Sterling Heights, MI).
Membrane Preparations and Receptor Binding Assays
Crude membrane preparations were made from whole lungs collected from 6-wk-old mice. Frozen lung tissue was minced with scissors in an ice-cold buffer containing 0.32 M sucrose and 25 mM Tris (pH 7.4 at 37°C), and homogenized using a polytron (PT2100, Brinkmann) with two 30-s bursts at a low setting. The homogenate was centrifuged at 1,000 × g for 10 min at 4°C (Sorvall SS-34, New Town, CT). The filtered supernatant was centrifuged at 40,000 × g for 20 min at 4°C. The pellet was resuspended in ice-cold 25 mM Tris-HCl buffer (pH 7.4) and centrifuged at 40,000 × g for 20 min at 4°C. The final pellet was resuspended in 200 μl 25 mM Tris-HCl (pH 7.4), and membrane protein concentration was determined by BCA protein assay kit (Pierce, Rockford, IL). Next, 20 μg of the membrane suspension was used for binding assays conducted in 96-well plates. For saturation binding, a range of [125I] AB-MECA was added to each well, and 25 mM Tris was added to a final volume of 100 μl. The nonselective AR agonist NECA (100 μM) was used to determine nonspecific binding. After incubation for 1 h at 37°C, cell membranes were retained and washed on a 96-well filter plate. Radioactivity was counted using a γ counter.
Immunohistochemical Analysis
Paraffin-embedded lungs were sectioned (5 μm) and collected on Superfrost/plus positively charged microscope slides. Deparaffinized sections were rehydrated in a series of graded alcohols to water. Endogenous peroxidase activity was blocked by incubation in 0.5% H2O2 in methanol for 20 min. Sections were then incubated for 1 h at room temperature with a 1:20,000 dilution of rabbit anti-mouse CC10 kD protein (gift from Franco DeMayo, Baylor College of Medicine, Houston, TX) followed by development with a rabbit IgG Vectastain Elite ABC Kit (Vector Laboratories Inc., Burlingame, CA) and counterstaining with methyl green.
Mucin and Secretion Index
Sections (5 μm) were stained with PAFS reagent as described (25). Briefly, sections were placed in 1% periodic acid for 10 min at room temperature. Sections were then washed in water and placed in fluorescent Schiff reagent (0.5% acriflavin, 1% potassium metabisulphite, and 0.1 M HCl) for 20 min at room temperature. Sections were then washed in tap water and placed in acid alcohol (0.1 M HCl in 70% EtOH) for two changes of 5 min each. Sections were then dehydrated and cover slipped. A mucin index was calculated by dividing the area of PAFS staining by the total area of the airway epithelium as described (14). In this analysis, multiple airways in the lung, with and without mucin, were analyzed to provide an overall assessment of mucin production in the lungs. A mucin secretion index was determined by examining only mucin containing airway epithelium at ×40 magnification. The area of PAFS staining was divided by the total area of the airway epithelium in regions that contained mucin. This index was used to monitor the amount of mucin within individual cells, with decreases in the degree of PAFS staining being indicative of mucin secretion into the airways (25).
RESULTS
The A3AR Is Not Required for Mucous Metaplasia
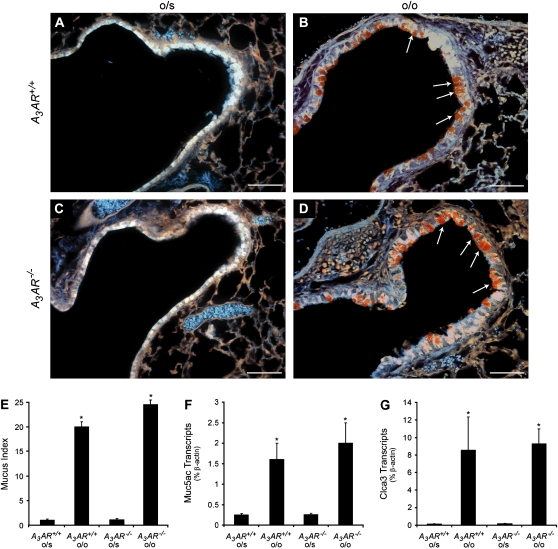
Elevations in adenosine, as well as increased expression of the A3AR, are seen in the airways of animal models exhibiting mucous metaplasia (14, 15, 19). To examine the necessity of the A3AR in mucin production, changes in PAFS staining were assessed in A3AR wild-type (A3AR+/+) and A3AR−/− mice subjected to OVA sensitization and challenge. A3AR+/+ and A3AR−/− mice sensitized with OVA and challenged with saline had no mucin production in their airways (Figures 1A and 1C). In contrast, OVA-sensitized and -challenged A3AR+/+ and A3AR−/− mice showed significant mucin production in their airways (Figures 1B and 1D, orange staining). Quantification of the PAFS staining revealed no significant differences in the amount of mucin produced in the airways of A3AR+/+ and A3AR−/− mice (Figure 1E). Similarly, there was no difference in degree of expression of the major mucin, Muc5ac (Figure 1F), and the mucous metaplasia marker calcium-activated chloride channel 3 (Clca3) (Figure 1G). These results demonstrate that the A3AR is not necessary for the development of the mucous phenotype after allergen challenge in the mouse.
Figure 1.
The A3AR is not required for mucin production after allergen challenge. Mucin production was determined by PAFS staining of lung sections. (A) Airway from an A3AR+/+ mouse sensitized with OVA and challenged with saline. (B) Airway from an A3AR+/+ mouse sensitized and challenged with OVA. (C) Airway from an A3AR−/− mouse sensitized with OVA and challenged with saline. (D) Airway from an A3AR−/− mouse sensitized and challenged with OVA. Arrows denote mucin-producing cells; scale bars = 100 μm. (E) Mucin production was quantified by measuring PAFS staining as described in Materials and Methods. Values are presented as mean mucin index score ± SEM (n = 4). Whole lung RNA extracts were analyzed using quantitative RT-PCR assays for Muc5ac (F) and Clca3 (G). o/s, OVA sensitization and saline challenge; o/o, OVA sensitization and challenge. Values are presented as transcript level (% β-actin) ± SEM; n = 4 (*P < 0.05 A3AR+/+ o/o and A3AR−/− o/o versus A3AR+/+ o/s using Student's t test).
Generation and Characterization of Mice Overexpressing the A3AR in the Airways
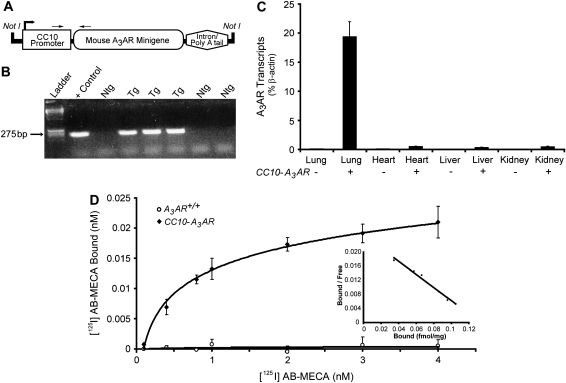
To determine if A3AR signaling is sufficient to induce mucous metaplasia and mucin production, a line of transgenic mice overexpressing the A3AR in the airways was generated. The rat Clara cell 10-kD protein (CC10) promoter was used to target expression of an A3AR minigene to the airways (Figure 2A). A transgenic line containing nine copies of the CC10-A3AR transgene (data not shown) was identified (Figure 2B) and characterized for increased expression of the A3AR in Clara cells. CC10-A3AR transcript levels were elevated 200-fold in lungs of naïve transgenic mice (Figure 2C). Furthermore, A3AR overexpression from this transgene was predominantly lung specific, with only slight expression seen in other tissues (Figure 2C). Increased expression was further verified by conducting A3AR saturation binding assays on cell membranes isolated from the lungs of CC10-A3AR transgenic mice (Figure 2D). Lungs from naive A3AR+/+ mice exhibited no specific binding even at the highest concentration of the A3AR-selective analog [125I] AB-MECA (Figure 2D). In contrast, lungs from naïve CC10-A3AR transgenic mice demonstrated a substantial increase in A3AR binding with a saturation binding curve that gave a Kd of 1.14 nM. Scatchard plot analysis revealed the Bmax to be 0.133 fmol/mg. These findings confirm that A3AR binding is elevated in the lungs of CC10-A3AR transgenic mice.
Figure 2.
Generation, genotyping, and characterization of CC10-A3AR transgenic mice. (A) The transgene was generated by the addition of the A3AR gene into a plasmid that contained the rat CC10 promoter and a β-globin intron and poly A sequences. The transgene was excised from the plasmid by Not1 digestion. (B) Genotypes were determined by PCR analysis using forward and reverse primers specific for the CC10-A3AR construct. Positive control is the plasmid used to create the transgenic mice. (C) Isolated RNA from lung, liver, heart, and kidney from A3AR+/+ and CC10-A3AR mice were analyzed using a Taqman-based quantitative RT-PCR assay for the A3AR transcript. Values are presented as transcript level (% β-actin) ± SEM; n = 5. (D) Binding assays were performed on whole lung crude membrane preparations from A3AR+/+ and CC10-A3AR mice. Saturation binding was determined from the addition of [125I] AB-MECA (0.1–4 nM). Specific binding was determined by the addition of NECA (not shown). Values are presented as mean bound ± SEM; n = 3 in triplicate.
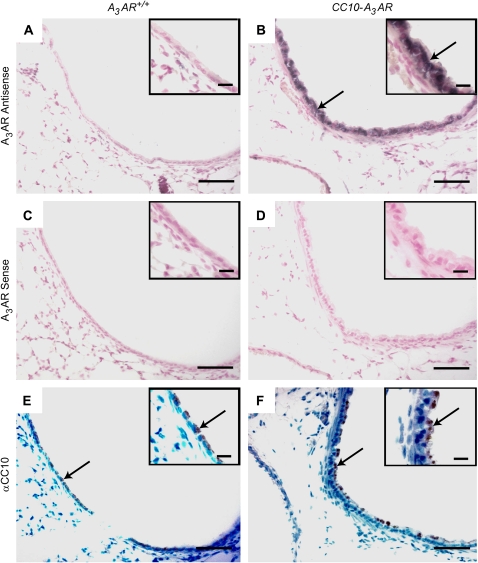
Localization of A3AR in the airways of transgenic mice was analyzed using in situ hybridization. Whereas bronchial epithelium from naive A3AR+/+ mice showed no A3AR signal (Figure 3A), the bronchial epithelium of naïve CC10-A3AR transgenic mice exhibited strong signal for the A3AR (Figure 3B). The majority of the cells in the airways of A3AR+/+ and CC10-A3AR transgenic mice were shown to be Clara cells by immunohistochemical analysis with a CC10 antibody (Figures 3E and 3F). These data demonstrate that the A3AR is elevated in Clara cells of CC10-A3AR transgenic mice.
Figure 3.
Localization of the A3AR in CC10-A3AR transgene in unchallenged mice. Lung sections from CC10-A3AR transgenic (B, D, and F) and A3AR+/+ (A, C, and E) mice were hybridized with A3AR antisense (A and B) and sense (C, and D) digoxigenin-labeled RNA probes. A3AR+/+ mice exhibit no specific A3AR signal (purple) in bronchial epithelial cells (A) that are shown to be Clara cells by positive immunohistochemical staining for CC10 (αCC10) in a neighboring section (E). By contrast, A3AR transcript was abundant in the airway epithelial cells from CC10-A3AR mice (B), which also show positive staining for the CC10 protein (F). Scale bar in figures = 100 μm; scale bar in insets = 30 μm.
Effects of A3AR Overexpression on Mucin Production
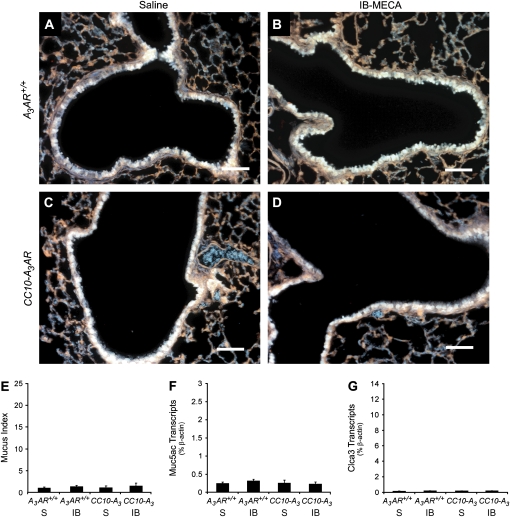
To assess the impact of increased A3AR expression on mucin production, lungs were isolated from CC10-A3AR transgenic mice and stained histochemically for mucin using PAFS. PAFS-positive staining was not detected in the airways of naïve A3AR+/+ (Figure 4A) or CC10-A3AR (Figure 4C) mice. These findings indicate that the increased expression of the A3AR in Clara cells is not sufficient for the induction of mucin production. Since endogenous adenosine levels are low in uninjured lungs (14), the absence of mucin in the lungs of naïve CC10-A3AR mice could likely reflect the absence of sufficient adenosine levels to engage these receptors. To address this, mice were exposed to aerosolized IB-MECA, a selective A3AR agonist (21). However, PAFS-positive staining was not detected in the airways of naïve A3AR+/+ (Figure 4B) or CC10-A3AR (Figure 4D) mice exposed to IB-MECA. Similarly, there was not an increase in mucus scores or the expression of Muc5ac and Clca3 in these mice (Figures 4E–4G). Together, these results indicate that the overexpression of the A3AR is not sufficient to induce Clara cells to produce mucin, even in the presence of an agonist.
Figure 4.
Mucin production in the airways of CC10-A3AR transgenic mice. Mucin production was determined by PAFS staining lung sections. (A) Airway from an A3AR+/+ mouse challenged with aerosolized saline. (B) Airway from an A3AR+/+ mouse challenged with aerosolized IB-MECA. (C) Airway from a CC10-A3AR mouse challenged with aerosolized saline. (D) Airway from a CC10-A3AR mouse challenged with aerosolized IB-MECA. Scale bars = 100 μm. (E) Mucin production was quantified by measuring PAFS staining as described in Materials and Methods. Values are presented as mean mucin index score ± SEM; n = 3. Whole lung RNA extracts were analyzed using quantitative-RT-PCR assays for Muc5ac (F) and Clca3 (G). S, saline; IB, IB-MECA. Values are presented as transcript level (% β-actin) ± SEM; n = 3.
Effects of A3AR Overexpression on Mucin Secretion
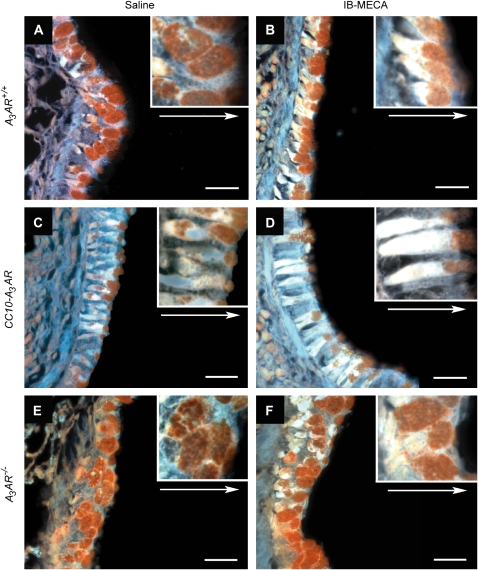
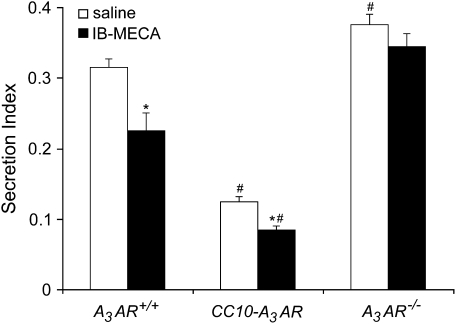
To examine the impact of A3AR signaling on mucin secretion, mice were sensitized and challenged with OVA and basal to apical distributions of mucin staining were monitored. Airway mucous cells from OVA-sensitized and -challenged A3AR+/+ mice appeared oval and laden with PAFS-positive granules (Figure 5A) distributed evenly through the cytoplasm. Upon treatment with IB-MECA, total PAFS staining was reduced and the distribution of residual mucin was shifted to a more apical distribution in OVA-sensitized and -challenged A3AR+/+ mice (Figure 5B), implying that IB-MECA induces secretion. Similarly, compared with OVA-sensitized and -challenged A3AR+/+ mice, OVA-sensitized and -challenged CC10-A3AR transgenic mice showed reduced PAFS staining. This was characterized by a loss of perinuclear mucin localization, with the remainder of the mucin showing localization at the epithelial apices (Figure 5C). Likewise, secretion was enhanced further in A3AR-CC10 transgenic mice after IB-MECA exposure (Figure 5D). These findings suggest there is enhanced steady state and evoked mucin secretion in mice overexpressing the A3AR. Furthermore, mucous cells in the airways of OVA-sensitized and -challenged A3AR−/− mice showed no evidence of altered cellular mucin morphology, even after IB-MECA exposure (Figures 5E and 5F), thus confirming that selective A3AR stimulation results in secretion of mucin from airway mucous cells. Morphometric analysis of these histologic observations was conducted to establish a quantitative measure of mucin secretion. This analysis confirmed that there was an acute reduction in intracellular mucin in A3AR+/+ mice after IB-MECA treatment (Figure 6), indicating evoked secretion. Furthermore, mucous cells in the airways of CC10-A3AR transgenic mice had lower levels of steady-state mucin than A3AR+/+ mice, and activation with IB-MECA enhanced secretion (Figure 6). Finally, A3AR−/− mice exhibited a higher level of intracellular mucin than A3AR+/+ mice, and showed no secretion after IB-MECA exposure. Together, these data suggest that engagement of the A3AR promotes mucin secretion in vivo.
Figure 5.
Morphologic changes in mucin-producing cells after IB-MECA exposure. Mucin distribution was determined by PAFS staining of lung sections. (A) Airway of an OVA-sensitized and -challenged A3AR+/+ mouse exposed to saline. (B) Airway of an OVA-sensitized and -challenged A3AR+/+ mouse exposed to IB-MECA. (C) Airway of an OVA-sensitized and -challenged CC10-A3AR mouse exposed to saline. (D) Airway of an OVA-sensitized and -challenged CC10-A3AR mouse exposed to IB-MECA. (E) Airway of an OVA-sensitized and -challenged A3AR−/− mouse exposed to saline. (F) Airway of an OVA-sensitized and -challenged A3AR−/− mouse exposed to IB-MECA. Images are representative of those found in four animals per each condition. Arrows depict basolateral to apical direction of the airway epithelium. Scale bar in figures = 30 μm. Enlarged insets were taken at ×100 magnification.
Figure 6.
Mucin secretion after IB-MECA exposure. Mucin secretion was quantified by counting 10 random fields per slide that contained PAFS-positive airway epithelial cells. Secretion index was determined by measuring total area of PAFS staining divided by the total area of the airway epithelium in regions that contained mucin. Values are presented as mean secretion index score ± SEM; n = 4 (*P < 0.05 IB-MECA versus saline, #P < 0.05 CC10-A3AR or A3AR−/− versus A3AR+/+ using a Student's t test).
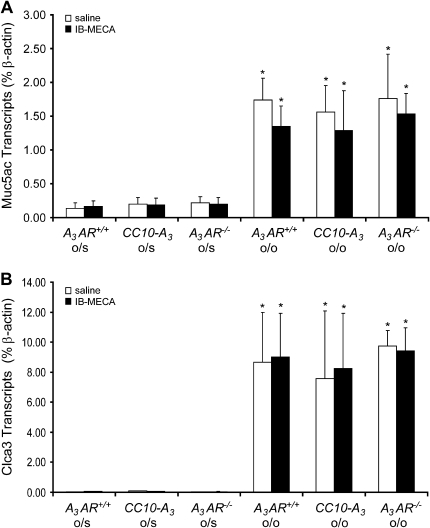
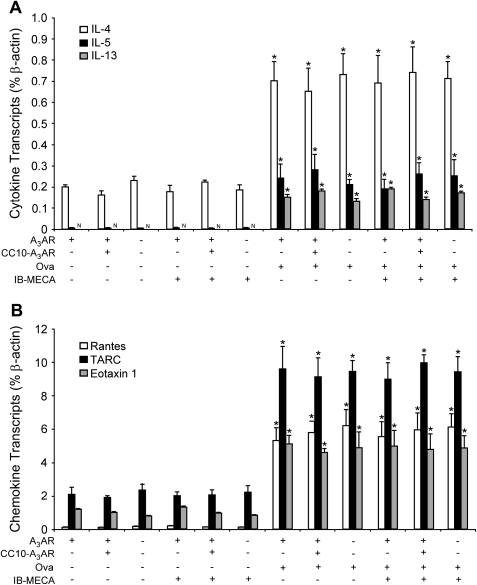
To confirm that the above alterations in mucous cell morphology were not due to alterations in mucin production, the expression of genes associated with the mucous phenotype were examined. Analysis of RNA extracts from whole lungs revealed that there were similar increases in Muc5ac (Figure 7A) and Clca3 (Figure 7B) in the lungs of A3AR+/+, CC10-A3AR transgenic, and A3AR−/− mice sensitized and challenged with OVA. These findings indicate that the increased mucin secretion seen was not related to changes in mucin expression phenotype. Similarly, there was not a significant difference in the degree of inflammatory cell infiltration (data not shown), or cytokine (Figure 8A) and chemokine (Figure 8B) levels in the lungs of A3AR+/+, CC10-A3AR transgenic, or A3AR−/− mice sensitized and challenged with OVA. These data indicate that altered inflammation and mediator production is not the cause of altered IB-MECA–stimulated mucin secretion in these genetic models.
Figure 7.
Muc5ac and Clca3 expression in the lungs of mice with various amounts of A3AR expression. Whole lung RNA extracts were analyzed using quantitative RT-PCR assays for Muc5ac (A) and Clca3 (B). Values are presented as mean transcript level (% β-actin) ± SEM; n = 3 for each condition (*P < 0.05 o/o + IB-MECA and o/o + saline versus o/s + saline using Student's t test).
Figure 8.
Cytokine and chemokine production in the lungs of mice with various amounts of A3AR expression. Mice were sensitized with OVA and then challenged with saline (−) or OVA. In addition, mice were exposed to aerosolized saline (−) or IB-MECA before harvesting whole lungs for RNA extraction. (A) Cytokine and (B) chemokine production was measured in whole lung extracts using quantitative RT-PCR. Values are presented as mean transcript level (% β-actin) ± SEM; n = 3 for each condition (*P < 0.05 o/o + IB-MECA and o/o + saline versus o/s + saline using Student's t test).
DISCUSSION
The cellular mechanisms involved in increased airway mucin production and secretion are not well understood. This study was designed to use genetic approaches to examine the involvement of the A3AR in these processes using a standard mouse model of allergen-induced lung disease. Our findings demonstrate that the A3AR is not involved in the production of mucin by airway mucous cells of allergen-challenged mice, but does contribute to mucin secretion in the airways. Thus, adenosine signaling through the A3AR may contribute to airway mucus obstruction by contributing to the hypersecretion of mucin.
Adenosine levels are controlled in tissues and cells by the enzyme adenosine deaminase (ADA) (26). Mice that lack this enzyme develop severe pulmonary inflammation and airway remodeling in association with elevations in lung adenosine content. Mucin overproduction and hypersecretion are features of this model, and the use of ADA replacement therapy to lower adenosine levels can reverse the degree of mucin production seen (14). Similar associations between adenosine elevations and mucin overproduction are seen in the airways of mice that overexpress the Th2 cytokine IL-13 (15). In addition, adenosine levels are elevated in the lungs of individuals with asthma (6), where mucin overproduction is prominent (1). Hence, there is a close relationship between elevations in lung adenosine concentrations and increased mucin production. In pursuit of the mechanisms by which adenosine might mediate mucin production, we turned our attention to the A3AR for several reasons. The A3AR is upregulated in biopsies taken from the airways of individuals with asthma (18), and levels of this receptor are elevated in the lungs of ADA-deficient and IL-13–overexpressing mice (15, 19). Localization of A3AR expression in the airways demonstrates that it is not found in non–mucin-producing airway epithelial cells, whereas it is upregulated in airway epithelial cells that stain for mucus (15, 19). Lastly, genetic removal of the A3AR from ADA-deficient mice was associated with decreased mucin production (19). Collectively, these data led to the hypothesis that the A3AR mediates adenosine-dependent mucin production in the airways. Results from the current study suggest that this hypothesis is null in mice where mucin production is stimulated in response to allergen exposure. A3AR+/+ and A3AR−/− mice sensitized and challenged with OVA exhibit the same degree of airway mucin production and mucin-associated gene expression in the lungs. Furthermore, overexpression of the A3AR in mucin-producing cells was neither sufficient for inducing mucin production in the naïve airway nor sufficient for increasing levels of mucin production in OVA-challenged mice, suggesting that this receptor is likely not involved in the development of mucous cell metaplasia in allergen-challenged mice. Thus, it appears that the A3AR is neither necessary nor sufficient for the production of mucin in response to allergen.
What mechanisms then, if any, might regulate adenosine's effects on mucin production? It is likely that adenosine may influence mucin production through interaction with adenosine receptors other than the A3AR. A recent study has shown that adenosine within tracheal aspirates of individuals with asthma can lead to the upregulation of mucin gene expression in human airway epithelial cells in vitro (13). In that study, pharmacologic agents were used to implicate the involvement of the A1AR in stimulating mucin gene expression through a mechanism that involved activation of the calcium2+-activated chloride- channel CLCA1. Thus, the A1AR may govern aspects of adenosine-mediated mucus production through engagement of the A1AR. However, recent studies demonstrating enhanced mucin production in the airways of ADA-deficient mice lacking the A1AR (24) would argue against such a mechanism. The A2BAR is a low-affinity receptor that is also expressed in the airway epithelium of mice overproducing mucin (15), making it a possible candidate for mediating the direct effects of adenosine on mucin production. It is also possible that adenosine may contribute to increased mucin production indirectly by promoting aspects of pulmonary inflammation. Consistent with this, adenosine has been shown to promote mast cell degranulation (10, 27) and airway eosinophilia (14, 19), both of which can impinge on mucin production and obstruction in the airways (28, 29). Moreover, adenosine can stimulate production of IL-13 (15, 30), a potent cytokine known to directly influence the production of mucin in the airways (31, 32). The decreased mucin production seen in ADA/A3AR double knockout mice (19) likely results from attenuation of one of these aforementioned pathways. Individual adenosine receptors will have to be examined in the context of specific disease and inflammatory conditions to fully appreciate their relative involvement in mucin production. Doing so will help to clarify the involvement of this signaling nucleoside in the production of mucin.
In contrast to effects on mucin production, results from the current study demonstrate that the A3AR plays an important role in the secretion of mucus from goblet cells in the airways of allergen-challenged mice. Exposure to the A3AR agonist IB-MECA induced mucin secretion as determined by decreased intracellular mucin content, and apical localization of remaining mucin containing vesicles within goblet cells. This effect was increased after the overexpression of the A3AR in Clara cells, and IB-MECA–stimulated secretion was not detected in mucin-producing goblet cells in the airways of allergen-challenged A3AR−/− mice. The levels of Muc5Ac and Clca3, two major mucous metaplasia–associated genes, did not change amongst groups, indicating that mucous metaplasia was not affected whereas secretion was. Thus, upregulation of the A3AR on mucin-producing goblet cells (15, 19) may represent a mechanism for increasing mucin secretion in chronic lung diseases in which adenosine levels are elevated, such as asthma (6).
Interestingly, there was increased mucus secretion in allergen-challenged mice overexpressing the A3AR, even in the absence of agonist administration (Figures 5 and 6), suggesting that overexpression of the A3AR led to increased sensitivity to endogenous adenosine levels. The excessive release of mucin in response to endogenous adenosine levels may account for the relatively modest additional release seen after agonist stimulation. Similarly, the observation that there was persistent mucus present even after agonist stimulation in the lungs of wild-type mice suggests that there may be a pool of mucus that is not accessible to A3AR stimulation. It is likely that there are a number of secretagogues that work in concert in the inflamed lung to propagate mucus obstruction. For example, ATP and UTP are potent stimulators of mucus secretion (25, 33). Direct comparisons between adenosine, ATP, and UTP in vivo would help to clarify this issue.
Consistent with our findings in mice in vivo, studies in the canine trachea have shown that adenosine can have a potent effect on mucin secretion (34). However, other studies using isolated epithelial cells in culture have shown that adenosine has little effect on mucin secretion (33, 35). Discrepancies among these findings may stem from differences in the origin of the cell types examined and the environment in which they were surveyed, since A3AR expression depends on inflammatory signaling (19). To the best of our knowledge, the current study is the first to demonstrate the ability of an adenosine agonist to promote airway mucus secretion in vivo under allergic conditions. Future studies examining the ability of adenosine and A3AR activation to promote mucin secretion in additional in vivo models will help to clarify how universal this pathway is in the secretion of mucin.
The mechanism(s) by which A3AR engagement on goblet cells leads to increased mucin secretion is not known. Most evidence suggests that the A3AR is coupled to the inhibitory G protein Gαi, which downregulates cAMP levels by inactivating adenylate cyclases (16). However, there is evidence to suggest that activation of the A3AR plays important roles in aspects of cellular secretion, which is likely mediated by the ability of this G protein–coupled receptor to access effector pathways that promote this process. For example, engagement of the A3AR on mouse mast cells can lead to degranulation through pathways that involve the βγ subunits of the receptor, activation of PI3 kinase, and subsequently elevation in intracellular Ca2+ levels (22, 36). The mobilization of intracellular Ca2+ is critical for regulated secretion of many substances (37) including mucin from airway mucous cells (38, 39). Therefore, engagement of the A3AR may contribute to increased mucin secretion by leading to increases in Ca2+ levels within mucin-producing Clara cells. Activation of protein kinase C (PKC) isoforms is another proposed mechanism for regulated mucin secretion (39). PKC is known to interact and activate myristoylated-alanine-rich C-kinase substrate (MARCKS) (40), which plays an important role in the regulated secretion of mucin in the airways (41). A3AR engagement can activate PKC (42). Thus, A3AR activation of PKC may activate MARCKS and contribute to mucin secretion. Defining the involvement of downstream A3AR signaling pathways in mucin secretion could provide novel targets for the development of therapies to circumvent mucus obstruction in the lung.
There is substantial evidence that inflammatory mediators such as cytokines can lead to mucin overproduction in the airways of experimental models of asthma (31, 32). Once mucin is produced, subsequent mucin secretion into the airways can lead to fatal airway obstruction (1). Adenosine levels are increased in the lung as result of inflammation and damage (5). Our current findings suggest that such elevations might access A3AR-dependent pathways that selectively influence mucin secretion without affecting mucin production. Therefore, the upregulation of the A3AR expression in the bronchial airways during inflammatory metaplasia (15, 19) may provide a mechanism for promoting the secretion of mucin in injured environments where adenosine levels are elevated. Interestingly, ATP, a metabolic precursor of adenosine, is rapidly released from damaged airway epithelial cells (43), and this adenine nucleotide can stimulate mucin secretion in vitro (33, 35, 44) and in vivo (25), but not mucin production (33), through engagement of P2 purinergic receptors. Thus, the concerted upregulation of ATP and adenosine levels in the lung may represent an important mechanism of regulated mucin hypersecretion that may contribute to mucus occlusion of the airways.
Acknowledgments
The authors thank Dr. Richard Clark and Jackie Freedman for their assistance in conducting the receptor binding assays in this study.
This work was supported by National Institutes of Health Grants AI43572 and HL70952 (to M.R.B.) and HL72984 (B.F.D). In addition, M.R.B. was supported by a young investigator award from The Sandler Program for Asthma Research, and C.M.E. was supported by the American Heart Association, Texas Affiliate Beginning Grant-in-Aid (0506030Y).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0060OC on June 8, 2006
Conflict of Interest Statement: H.W.J.Y., C.-X.S., C.M.E., and B.F.D. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R.B. is a paid consultant with CV Therapeutics for the investigations of adenosine receptor analogs and lung disease.
References
- 1.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest 2002;122:320S–326S. [DOI] [PubMed] [Google Scholar]
- 2.Bedrossian CW, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol 1976; 7:195–204. [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278:1355–1360. [DOI] [PubMed] [Google Scholar]
- 4.Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol 2001;25:533–537. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn MR. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci 2003;24:66–70. [DOI] [PubMed] [Google Scholar]
- 6.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 1993;148:91–97. [DOI] [PubMed] [Google Scholar]
- 7.Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar VG, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J 2002;20:1393–1398. [DOI] [PubMed] [Google Scholar]
- 8.Fozard JR. The case for a role for adenosine in asthma: almost convincing? Curr Opin Pharmacol 2003;3:264–269. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson MA, Bai TR. The role of adenosine in asthma. In: Jacobson KA, Jarvis MF, editors. Purinergic approaches in experimental therapeutics. Danvers, MA: Wiley-Liss; 1997. pp. 315–331.
- 10.Marquardt DL, Parker CW, Sullivan TJ. Potentiation of mast cell mediator release by adenosine. J Immunol 1978;120:871–878. [PubMed] [Google Scholar]
- 11.Banerjee SK, Young HW, Volmer JB, Blackburn MR. Gene expression profiling in inflammatory airway disease associated with elevated adenosine. Am J Physiol Lung Cell Mol Physiol 2002;282:L169–L182. [DOI] [PubMed] [Google Scholar]
- 12.Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol 1983;15:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara N, Gallup M, Khong A, Sucher A, Maltseva I, Fahy J, Basbaum C. Adenosine up-regulation of the mucin gene, MUC2, in asthma. FASEB J 2004;18:1770–1772. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med 2000;192:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn MR, Lee CG, Young HWJ, Chunn JL, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung: evidence for an IL-13-adenosine amplification pathway. J Clin Invest 2003;112:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredholm BB, Jzerma IJP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 17.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 2004;25:33–39. [DOI] [PubMed] [Google Scholar]
- 18.Walker BA, Jacobson MA, Knight DA, Salvatore CA, Weir T, Zhou D, Bai TR. Adenosine A3 receptor expression and function in eosinophils. Am J Respir Cell Mol Biol 1997;16:531–537. [DOI] [PubMed] [Google Scholar]
- 19.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LNL, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol 2004;173:1380–1389. [DOI] [PubMed] [Google Scholar]
- 20.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 2000;275:4429–4434. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci 1998;19:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong H, Sergiy S, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol 2003;170:338–345. [DOI] [PubMed] [Google Scholar]
- 23.Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J Immunol 2001;167:4676–4685. [DOI] [PubMed] [Google Scholar]
- 24.Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the A(1) adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest 2005;115:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackburn MR, Kellems RE. Regulation and function of adenosine deaminase in mice. Prog Nucleic Acid Res Mol Biol 1996;55:195–226. [DOI] [PubMed] [Google Scholar]
- 27.Tilley SL, Tsai M, Williams CM, Wang ZS, Erikson CJ, Galli SJ, Koller BH. Identification of A3 receptor- and mast cell-dependent and -independent components of adenosine-mediated airway responsiveness in mice. J Immunol 2003;171:331–337. [DOI] [PubMed] [Google Scholar]
- 28.Carroll NG, Mutavdzic S, James AL. Increased mast cells and neutrophils in submucosal mucous glands and mucus plugging in patients with asthma. Thorax 2002;57:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776. [DOI] [PubMed] [Google Scholar]
- 30.Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol 2004;172:7726–7733. [DOI] [PubMed] [Google Scholar]
- 31.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RN, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Zhao YH, Wu R. Differential regulation of airway mucin gene expression and mucin secretion by extracellular nucleotide triphosphates. Am J Respir Cell Mol Biol 2001;25:409–417. [DOI] [PubMed] [Google Scholar]
- 34.Johnson HG, McNee ML. Adenosine-induced secretion in the canine trachea: modification by methylxanthines and adenosine derivatives. Br J Pharmacol 1985;86:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KC, Lee BC. P2 purinoceptor regulation of mucin release by airway goblet cells in primary culture. Br J Pharmacol 1991;103:1053–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity 2002;16:441–451. [DOI] [PubMed] [Google Scholar]
- 37.Chapman ER. Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell Biol 2002;3:498–508. [DOI] [PubMed] [Google Scholar]
- 38.Rossi AH, Sears PR, Davis CW. Ca2+ dependency of ‘Ca2+-independent’ exocytosis in SPOC1 airway goblet cells. J Physiol 2004; 559:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol 1997;273:L201–L210. [DOI] [PubMed] [Google Scholar]
- 40.Fujise A, Mizuno K, Ueda Y, Osada S, Hirai S, Takayanagi A, Shimizu N, Owada MK, Nakajima H, Ohno S. Specificity of the high affinity interaction of protein kinase C with a physiological substrate, myristoylated alanine-rich protein kinase C substrate. J Biol Chem 1994;269:31642–31648. [PubMed] [Google Scholar]
- 41.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med 2004;10:193–196. [DOI] [PubMed] [Google Scholar]
- 42.Zhao TC, Kukreja RC. Protein kinase C-delta mediates adenosine A3 receptor-induced delayed cardioprotection in mouse. Am J Physiol Heart Circ Physiol 2003;285:H434–H441. [DOI] [PubMed] [Google Scholar]
- 43.Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol 2001;280:L221–L228. [DOI] [PubMed] [Google Scholar]
- 44.Lethem MI, Dowell ML, Van Scott M, Yankaskas JR, Egan T, Boucher RC, Davis CW. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am J Respir Cell Mol Biol 1993;9:315–322. [DOI] [PubMed] [Google Scholar]