Abstract
Although respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract illness in infants, the effect of RSV on human airway smooth muscle (HASM) has not been studied. We hypothesized that RSV has direct effects on cAMP formation and β2-adrenergic receptor (ADRB2) density and that ADRB2 haplotype influences this response. A recombinant green-fluorescent protein (rg) expressing RSV was used to determine whether RSV could infect cultured HASM. Influence of RSV infection on β2-adrenergic responsiveness was determined by measuring differences in isoproterenol (ISO)-induced cyclic AMP (cAMP) formation, ADRB2 density, and Gi expression in HASM cells challenged with RSV, with ultraviolet-inactivated RSV, and with mock infection. The rgRSV efficiently infected cultured HASM cells. ISO-induced cAMP formation was significantly reduced in cells infected with RSV, compared with mock-infected and ultraviolet-inactivated RSV, in a time- and concentration-dependent manner. Forskolin-induced cAMP formation and Gi expression were not altered in cells infected with RSV, suggesting that the influence of RSV on β2-adrenergic relaxation was upstream of cAMP formation. ADRB2 density was reduced in cells infected with RSV, compared with mock infection, and the Arg16Gln27 ADRB2 haplotype was associated with decreased ISO-induced cAMP formation (P < 0.05) and with decreased ADRB2 density at baseline (P < 0.05). The implications of these results are that limitations of β2-agonists in the treatment of any airway obstruction associated with RSV infection may be related to direct effects of RSV on HASM, and ADRB2 genotype may predict β2-adrenergic responses.
Keywords: β2-adrenergic receptor, haplotype, human airway smooth muscle, isoproterenol, respiratory syncytial virus
Respiratory syncytial virus (RSV) is the most common infectious cause of wheezing in children less than two years of age (1). The mechanisms by which RSV results in airway obstruction that causes wheezing are only partially understood. The contributors to RSV-induced airway obstruction in humans have been hypothesized to include luminal collections of desquamated airway epithelial and inflammatory cells, increased vascular permeability leading to airway edema, mucus secretion, and constriction of the smooth muscle that surrounds the airway (2, 3). A direct test of the importance of smooth muscle constriction in RSV-induced wheezing is to measure responsiveness to β-agonist medications during infection. However, results from these studies in children with RSV infection have not been conclusive due to variability in physiologic response to these pharmacologic agents (4). In vitro data suggest that β2-adrenergic receptor (ADRB2) genotype influences ARDB2 responsiveness after repeated agonist exposures in human airway smooth muscle (HASM) (5, 6). In addition, a number of clinical studies have suggested that ADRB2 genotype influences the severity of clinical symptoms. Although two recent studies from the Asthma Clinical Research Network have suggested that presence of the Arg16 may impact one parameter of clinical disease (AM Peak Expiratory Flow) (7, 8), a recent meta-analysis suggests that the relationship between ADRB2 genotype and desensitization is complex and exceeds the SNP at the 16 position (9).
Studies of how RSV infection regulates smooth muscle constriction have focused primarily on how RSV infection alters neural regulation of the airway in animal models. To date, there have been no studies examining how human smooth muscle function is modulated directly by RSV infection. We hypothesized that: (1) HASM is susceptible to RSV infection; (2) that such RSV infection decreases airway smooth muscle cyclic AMP (cAMP) production; and (3) that ADRB2 genotype influences RSV-infection induced changes in smooth muscle function.
MATERIALS AND METHODS
Cell Culture
Human tracheas were obtained from lung transplant donors in accordance with procedures approved by the Vanderbilt University Institutional Review Board. Tracheal smooth-muscle cells were harvested from the tracheas as previously described (10, 11). The cells were then grown in plastic flasks in Ham's F12 medium with 10% fetal calf serum (FCS) that was supplemented with penicillin (100 U/ml), streptomycin (0.1 mg/ml), NaOH (12 mM), amphotericin-B (2.5 μg/ml), CaCl2 (1.6 mM), and l-glutamine (2 mM). Medium was replaced every 3–4 d, and cells were passaged with 0.25% trypsin and 1 mM ethylene diamine tetraacetic acid (EDTA) every 10–14 d. Cells were studied in Passages 4–7.
Maintenance of RSV Stock
The A2 strain of RSV was provided by Dr. Robert Chanock, National Institutes of Health. Master stocks and working stocks of RSV were prepared as previously described (12).
Infection of HASM
We used rgRSV expressing the enhanced GFP gene (13) to determine if RSV could infect cultured HASM. HASM cells were grown in a T-150 flask to 65–75% confluence (the equivalent of ∼ 650,000 cells) and infected with RSV at a multiplicity of infection (MOI) of 15. Photomicrographs of GFP-expressing cells were acquired using an inverted fluorescence microscope equipped with a charge-coupled device digital camera (Olympus BX51-TRF; Olympus, Center Valley, PA) and with a paired brightfield image.
Experimental Protocol
For each set of experiments, 6-well plates (Sigma, St. Louis, MO) of HASM cells at 65–75% confluence (the equivalent of ∼ 65,000 cells/well) were serum-deprived and supplemented with insulin at 5.7 μg/ml and transferrin at 5 μg/ml at 24–36 h before use, since these conditions maximize expression of smooth muscle–specific contractile proteins (10, 14). We examined the effect of RSV challenge on HASM cells from four different donors by adding live RSV at an MOI of 0.0015, 0.15, or 15; RSV (MOI 15) which had been inactivated with ultraviolet light (UV-RSV); or culture media that did not contain RSV (mock) for periods of 2, 6, and 24 h. Outcome indicators included agonist-induced cAMP formation, ADRB2 density, and Gi expression. For each donor, the protocol was performed on three experimental days in duplicate.
cAMP Measurements
For cAMP measurements, cells were lysed and intracellular cAMP concentration was measured. Specifically, cells were studied at the designated time after challenge, at which time the medium was replaced with 0.5 ml of PBS containing 0.1 mM 3-isobutyl-1-methylxanthine (IBMX) and 300 μM ascorbic acid. Thirty minutes later, the cells either were treated with isoproterenol (ISO) (10−6 M) or Forskolin (10−4 M) or were left untreated (for measurement of basal cAMP formation). The cell supernatant containing cAMP was collected 10 min later, and cAMP was assayed with a Rainen [125I] cAMP radioimmunoassay kit (Amersham, Boston, MA) as previously described (11, 15).
ADRB2 Density
On Day 1, two T-150 flasks of cells were exposed to RSV challenge. On Day 2 after 24 h of incubation, flasks were removed from the incubator and placed on ice and washed with 10 ml of ice cold PBS. Cells were harvested in a harvest buffer containing HEPES (15 mM), EDTA (5 mM), EGTA (5 mM) at pH 7.6, and PMSF (10−5 M) by scraping cells from the flask. The cells were then placed in a 50-ml conical tube. Flasks were washed with an additional 5 ml of the harvest buffer without PMSF and transferred to the same 50-ml conical tube. Cells were homogenized by passing them through a 20-gauge syringe 10 times. The cells then were centrifuged at 6,000 × g for 15 min at 4°C. The supernatant was decanted and 3 ml of the harvest buffer was added. The cells were again homogenized by passing them through a 20-gauge needle and centrifuged again. This homogenization and centrifugation step was again repeated. The supernatant was decanted and the pellet was resuspended in binding buffer (75 mM Tris, 12.5 mM MgCl2, 1.5 mM EDTA, pH to 7.6 with NMDG). The pellet was homogenized with a 20-gauge needle 10 times, and cells were further dispersed with a 25-gauge needle an additional 10 times. A saturation curve was set up in triplicate on ice using I-125 cyanopindolol, 2,000 ci/mmole (Amersham) as the radioligand. Membrane preparations were incubated 3 h at room temperature in a total volume of 100 μl in the absence or presence of propranolol (5 μM), and reactions were terminated via filtration on a Whatman GF-C filter (Whatman Inc., Florham Park, NJ) using a cell harvester apparatus. Counts were performed using a γ counter.
ADBR2 Genotyping
HASM cells from two donors homozygous for the Arg16Gln27 ADBR2 haplotype and from two donors homozygous for the Gly16Glu27 ADBR2 haplotype were used in these experiments. ADBR2 genotyping was performed through standard methods as previously described (15). Briefly, a 241-bp peptide containing the ADRB2 SNPs encoding for amino acid changes at position 16 and 27 were amplified with the primers 5′-CTGAATGAGGCTTCCAGGC-3′ and 5′-GCCAGGACGATGAGAGACAT'3′. Conditions of PCR were 94°C for 3 min, followed by 35 cycles of 45 s at 94°C, of 45 s at 58°C, of 60 s at 72°C, with a final extension time of 7 min at 72°C, and PCR product identified by direct sequencing (Applied Biosystems, Foster City, CA).
Gi Expression
HASM cells were grown in 6-well plates and treated identically as described above for cAMP assays. Cell lysates were harvested by scraping, and nuclei and large particulates were removed by centrifugation. The supernatant was then centrifuged, the membrane pellet resuspended, and the protein concentration was measured. Equivalent amounts (50 μg) of membrane protein were fractionated in 12% SDS-polyacrylamide gels; this was followed by transfer to nitrocellulose membranes. The primary antibody used was rabbit polyclonal Giα3 (Santa Cruz Biotechnology, Santa Cruz, CA) used at 1:5,000 dilution, with goat anti-rabbit IgG used as the secondary antibody.
Reagents
Tissue culture reagents and drugs used in the study were obtained from Sigma, with the exception of amphotericin-B and trypsin-EDTA solution, which were purchased from GIBCO (Grand Island, NY). ISO was dissolved at 10−1 M in distilled water on each experimental day, and because ISO is rapidly oxidized, dilutions of ISO in medium were made immediately before treating cells with ISO.
Data Analysis and Statistics
ANOVA was used to determine statistical significance among the treatment groups before the data were stratified by genotype, with P < 0.05 to reject the null hypothesis. Data are presented as mean ± SE.
RESULTS
RSV Infects Cultured HASM Cells
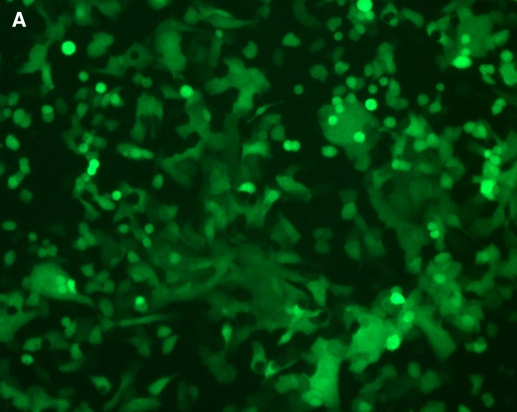
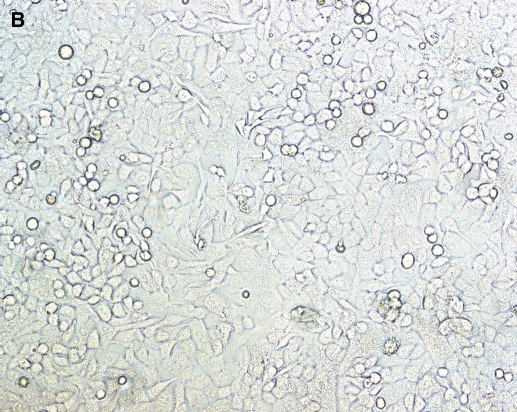
To determine if HASM are susceptible to RSV infection, we inoculated cultured HASM with live rgRSV (MOI 15). At 8 h after inoculation, nearly all cultured cells were green under fluorescence (Figure 1A), indicating that the virus efficiently infected these cells. A paired bright light image (Figure 1B) shows that infection efficiency approaches 100%.
Figure 1.
RSV infects cultured HASM cells. Cultured HASM cells in a T-150 flask at 70% confluence were infected with a recombinant green-fluorescent protein–expressing RSV and visualized under immunofluorescence after 8 h (A) and under paired brightfield analysis (B).
RSV Attenuates ISO-Induced cAMP Formation
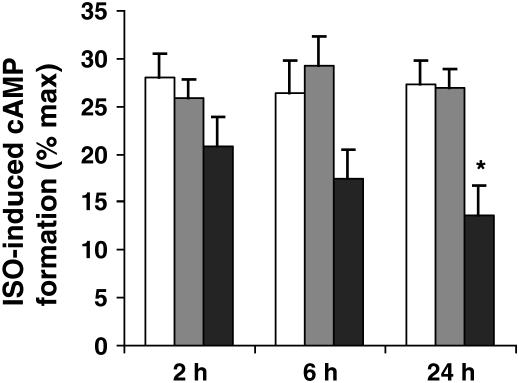
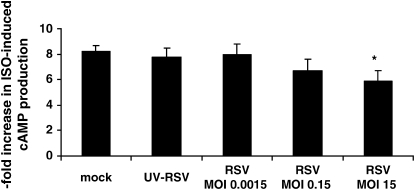
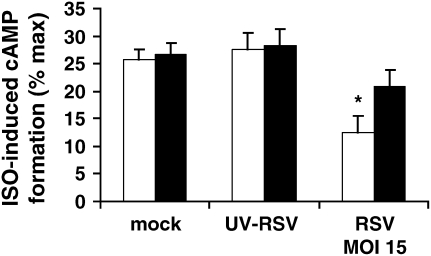
We then questioned whether RSV itself had direct, functional effects on HASM. We first performed a time-course experiment to determine whether RSV altered ISO-induced cAMP formation. We infected cultured HASM cells with RSV (MOI 15), UV-RSV, and mock over three different time periods (2 h, 6 h, and 24 h). In our experiments, ISO caused an ∼ 8-fold increase in cAMP formation, consistent with previous reports (11, 15, 16). We found that RSV had no effects on ISO-induced cAMP formation at 2 h, but that there was a trend for a decrease at 6 h that reached statistical significance at 24 h (P < 0.05; Figure 2), compared with baseline, as well as compared with the 24-h mock and UV-RSV groups. Forskolin (10−4 M) caused an ∼ 30-fold increase in cAMP formation in mock conditions (data not shown), consistent with previous reports (11, 15), and neither RSV nor UV-RSV altered forskolin-induced cAMP formation, suggesting that RSV had no effects on forskolin expression or activity or any downstream signaling events. As Forskolin-induced cAMP formation did not differ among treatment groups, ISO-induced cAMP formation is expressed as % forskolin-induced cAMP formation in Figure 2. We then performed a dose–response experiment to determine the effect of RSV titer (MOI 0.0015, 0.15, and 15) for 24 h on ISO-induced cAMP formation. We found that RSV had no effects on ISO-induced cAMP formation at an MOI of 0.0015, but that there was a trend for a decrease at an MOI of 0.15, which reached statistical significance at an MOI of 15 (P < 0.05; Figure 3), compared with the mock and UV-RSV groups.
Figure 2.
RSV attenuates ISO-induced cAMP formation (time course). Cultured HASM cells on each experimental day were infected with RSV (MOI 15; dark grey bars), UV-RSV (light grey bars), or mock infection (open bars) over three different time periods (2 h, 6 h, and 24 h), and then supernatant was harvested after ISO (10−6 M) stimulation for 10 min. Measurements were performed in duplicate in two separate experiments for each of four donors. Data are expressed as % maximal stimulation (Forskolin 10−4 M–induced cAMP formation). *P < 0.05 compared with baseline and compared with UV-RSV and with mock.
Figure 3.
RSV attenuates ISO-induced cAMP formation (dose response). Cultured HASM cells on each experimental day were infected with increasing RSV titers (MOI 0.0015, 0.15, and 15), UV-RSV, or mock infection for 24 h, and then supernatant was harvested after ISO (10−6 M) stimulation for 10 min. Measurements were performed in duplicate in two separate experiments for each of four donors. *P < 0.05 compared with UV-RSV and with mock.
RSV Does Not Alter Giα-3 Expression
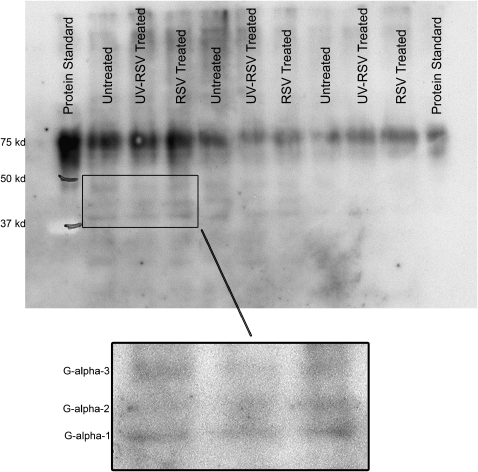
To determine whether RSV influenced Gi signaling in cultured HASM, we performed Western blots on cell lysates from cultured HASM cells exposed to RSV (MOI 15), UV-RSV, and mock. As shown in Figure 4, RSV does not appear to alter Giα-3 expression, compared with the mock and UV-RSV groups. This antibody also detects Gαi-1 and Gαi-2. In the insert in Figure 4, bands corresponding to the molecular weight of all three isoforms of Gαi are shown.
Figure 4.
RSV does not alter Gi expression. Cultured HASM cells on each experimental day were infected with RSV (MOI 15), UV-inactivated RSV, or mock infection for 24 h, after which protein was harvested and Gi protein expression assayed by Western blot. The inset demonstrates the three isoforms of Gi detected by this antibody. In addition, the antibody detects a strong band corresponding to the 75-kD marker, which represents cross detection with albumin, present in the Biorad protein standard and in the cell lanes. A Western blot was performed on cell lysates from three donors.
ADRB2 Density Is Reduced in HASM Cells Exposed to RSV
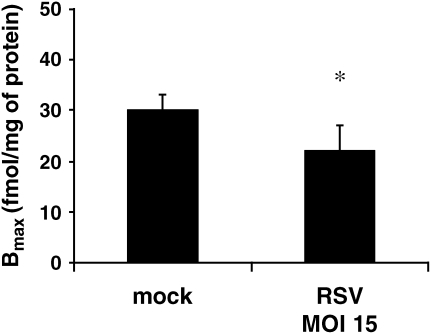
To determine whether RSV had direct effects on ADRB2 density, we measured receptor density using radioligand binding in cultured HASM cells challenged with RSV (MOI 15) or mock for 24 h. Under mock challenge conditions, ADRB2 density (Bmax) was 30 pmol/mg of protein, similar to previously reported levels of ADRB2 expression in HASM (6). By contrast, we found that RSV reduced ADBR2 expression in cultured HASM by 32% (P < 0.05; Figure 5).
Figure 5.
ADRB2 density is reduced in HASM cells exposed to RSV. Cultured HASM cells on each experimental day were infected with RSV (MOI 15) or mock infection for 24 h, and cell membranes were isolated for ADRB2 density measurements using radioligand binding. Measurements were performed in triplicate in two separate experiments from each of four donors. *P < 0.05 compared with mock.
ADRB2 Haplotype Influences RSV Effects on ISO-Induced cAMP Formation and ADRB2 Density
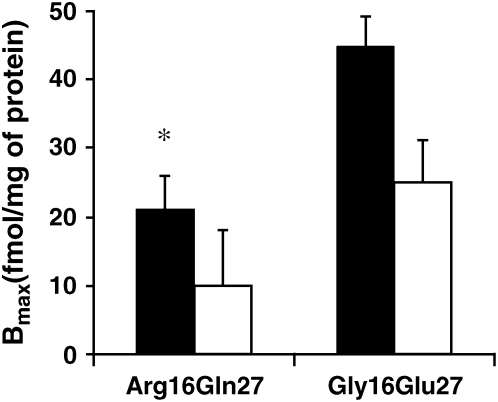
To determine whether ADRB2 haplotype influenced the ability of RSV to modulate ISO-induced cAMP formation, we stratified data from Figure 2 by ADRB2 haplotype. As shown in Figure 6, there was no change in ISO-induced cAMP formation with RSV infection (MOI 15) in cells from individuals homozygous for the Gly16Glu27 haplotype, compared with UV-RSV or mock. However, there was a significant decrease in ISO-induced cAMP formation in cells from individuals homozygous for the Arg16Gln27 haplotype, compared with UV-RSV or mock (P < 0.05). We also stratified data from Figure 5 by ADRB2 genotype. As shown in Figure 7, ADRB2 haplotype influences ADRB2 density in mock-treated cells (P < 0.05). Challenge with RSV led to a trend in decreased ADRB2 density in both groups, which was not statistically significant (P = 0.09).
Figure 6.
ADRB2 haplotype influences RSV effects on ISO-induced cAMP formation. Cultured HASM on each experimental day were infected with RSV (MOI 15), UV-RSV, or mock infection for 24 h. Measurements were performed in duplicate in two separate experiments from each of two donors homozygous at the Arg16Gln27 ADRB2 haplotype (open bars), and also in duplicate in two separate experiments from each of two donors homozygous at the Gly16Glu27 haplotype (filled bars). Data are expressed as % maximal stimulation (Forskolin 10−4 M–induced cAMP formation). *P < 0.05 compared with RSV-exposed cells from Gly16Glu27 donors and compared with UV-RSV and mock from Arg16Gln27 donors.
Figure 7.
ADRB2 haplotype influences RSV effects on ADRB2 density. Cultured HASM cells on each experimental day were infected with RSV (MOI 15; open bars) or mock infection (filled bars) for 24 h, and cell membranes were isolated for ADRB2 density measurements using radioligand binding. Measurements were performed in triplicate in two separate experiments from each of two donors homozygous at the Arg16Gln27 ADRB2 haplotype, and in triplicate in two separate experiments from each of two donors homozygous at the Gly16Glu27 haplotype. *P < 0.05 compared with mock exposure in cells from Gly16Glu27 donors.
DISCUSSION
Our results indicate that RSV can infect HASM (Figure 1), and that it also has direct functional effects on ASM by inhibiting ISO-induced cAMP production in a time- and concentration-dependent fashion (Figures 2 and 3). RSV does not appear to influence HASM via Gi expression (Figure 4), but rather through effects on ADRB2 density (Figure 5). In fact, we demonstrated significant genotypic differences in the ability of RSV to influence ISO-induced cAMP expression (Figure 6), with a trend toward reducing ADRB2 density (Figure 7).
The influence of viral infection on airway smooth muscle has been the focus of a number of recent studies. D'Aprile and coworkers demonstrated that parainfluenza-3 virus infection reduces endothelin receptor density and function in guinea pig airways (17), and Billington and colleagues showed that rhinovirus influences cAMP production in HASM (18). Although epithelial cells may be the primary target of RSV infection as manifest by histopathologic characteristics of massive epithelial sloughing, the release of cytokines and chemokines in the setting of RSV infection may have paracrine effects on HASM. To our knowledge, this is the first study to suggest that RSV has direct effects on HASM. There are no definitive data showing that RSV infects HASM in vivo; however, sloughing of the bronchial epithelium could facilitate access of the virus to these cells.
The mechanistic basis by which RSV alters the ASM relaxation pathway is not known, but may involve the production of cytokines which exert an autocrine effect on ASM. In a murine model, challenge with RSV resulted in IL-4 and IL-13 production (19), and in the only previous in vitro report of RSV effects in HASM, RSV was shown to stimulate IL-11 production (20). A number of cytokines, including IL-1β, TNF-α, IL-13, IL-4, and IL-6, have been associated with decreased β-agonist responses in HASM (21). Hakonarson and coworkers reported that decreased agonist responsiveness elicited in rhinovirus-exposed ASM is largely attributed to the induced autologous expression and autocrine action of IL-1β in the virus-infected ASM (22). We saw that RSV had no impact (Figure 4) on Gi expression in our system, suggesting that the primary effect of RSV is through Gs signaling via cAMP production, rather than through Gi signaling.
The ability of ADRB2 genotype to influence desensitization in HASM led us to question in this study whether ADRB2 genotype could also influence the desensitization to β-agonist responses associated with RSV exposure. Despite a number of clinical studies in patients with asthma, there has been no consensus on which ADRB2 genotype influences desensitization. Screening from four ethnically distinct populations suggests that there are only a few common haplotypes spanning the coding block and the immediate 5′-untranslated region of the ADRB2 gene (7, 8, 23). For this reason, HASM cells were chosen for this study only from individuals homozygous at either the Arg16Gln27 or the Gly16Glu27 allele. The observation that Arg16Gln27 haplotypes had lower basal ADRB2 expression levels, yet did not differ from Gly16Glu27 haplotypes in their ISO-induced cAMP responses, may possibly be explained by receptor expression not being the limiting factor in cAMP formation and therefore spare receptors possibly being present in the Gly16Glu27 haplotype (24, 25).
Although SNPs in the ADRB2 gene have received the greatest scrutiny, we recognize that genetic variants in other targets in the β-adrenergic relaxation pathway may alter the ASM relaxation phenotype. Genetic variants in the AC type 9 have been identified that confer reduced β-adrenergic stimulation (26). Although we did not see differences in AC expression or activity, Billington reported that chronic incubation with rhinovirus in HASM caused a significant increase in Forskolin-induced cAMP formation, suggesting sensitization of AC (18). Thus, it is possible that genetic variation in AC and other downstream genes could influence responses in the setting of RSV infection.
There are several clinical implications of our in vitro observations. Approximately half of the infants with RSV-induced bronchospasm will respond to inhaled β-agonists; however, there is no reliable method for predicting which infants will respond. Perhaps the variation observed in clinical responses to bronchodilators during RSV infection is secondary to differences in ADRB2 haplotype. Genetic screening of patients with RSV may predict patients with a more favorable response to certain therapies, including β-agonists and corticosteroids. In addition, lower respiratory tract infection by RSV is a major risk factor for the development of persistent wheezing and asthma during childhood. Although the mechanism by which RSV alters airway reactivity is not understood, the more long-term sequelae of RSV infection may be related to ADRB2 haplotype. Further in vitro and clinical studies are needed to clarify the role of ADRB2 genotype in RSV infection.
This work was supported by NIH grants HL04395 (P.E.M.), HL 66949 (R.S.P.), and AI 54660 (R.S.P.)
Originally Published in Press as DOI: 10.1165/rcmb.2005-0282OC on June 8, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shay D, Holman R, Newman R. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 1999;282:1440–1446. [DOI] [PubMed] [Google Scholar]
- 2.Tripp R. Pathogenesis of respiratory syncytical virus infection. Viral Immunol 2004;17:165–181. [DOI] [PubMed] [Google Scholar]
- 3.Psarras S, Papadopoulos NG, Johnston SL. Pathogenesis of respiratory syncytial virus bronchiolitis-related wheezing. Paediatr Respir Rev 2004;5:S179–S184. [DOI] [PubMed] [Google Scholar]
- 4.Wainwright C, Altamirano L, Cheney M, Cheney J, Barber S, Price D, Moloney S, Kimberley A, Woolfield N, Cadzow S, et al. A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med 2003;349:27–35. [DOI] [PubMed] [Google Scholar]
- 5.Green S, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry 1994;33:9414–9419. [DOI] [PubMed] [Google Scholar]
- 6.Green S, Turki J, Bejarno P, Hall I, Liggett S. Influence of Beta2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol 1995;13:25–33. [DOI] [PubMed] [Google Scholar]
- 7.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, et al. for the National Heart, and Blood Institute's Asthma Clinical Research Network. The effect of polymorphisms of the beta-2-adrenergic receptor on the response to the regular use of albuterol in asthma. Am J Respir Care Crit Care Med 2000;162:75–84. [DOI] [PubMed] [Google Scholar]
- 8.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack RM, Craig TJ, Deykin A, Fagan JK, Fahy JV, Fish JE, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled, cross-over trial. Lancet 2004;364:1505–1512. [DOI] [PubMed] [Google Scholar]
- 9.Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, Duffy D, Thompson J, Hall I, Kaurfman J, Leung TF, et al. Systematic review and meta-analysis of the association between beta2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol 2005;162:201–211. [DOI] [PubMed] [Google Scholar]
- 10.Panettieri R, Murray R, DePalo L, Yadvish P, Kotlikoff M. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol 1989;256:C329–C335. [DOI] [PubMed] [Google Scholar]
- 11.Shore S, Laporte J, Hall I, Hardy E, Panetteiri R. Effect of IL-1b on responses of cultured human airway smooth muscle cells to bronchodilator agonists. Am J Respir Cell Mol Biol 1997;16:702–712. [DOI] [PubMed] [Google Scholar]
- 12.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol 1988;26:153–162. [DOI] [PubMed] [Google Scholar]
- 13.Hallak L, Spillmann D, Collins P, Peeples M. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 2000;74:10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall I, Kotlikoff M. Use of cultured human airway myocytes for study of airway smooth muscle. Am J Physiol 1995;268:L1–L11. [DOI] [PubMed] [Google Scholar]
- 15.Moore P, Laporte J, Abraham J, Schwartzman I, Yandava C, Silverman E, Drazen J, Wand M, Panettieiri JRA, Shore S. Polymorphism of the beta(2)-adrenergic receptor gene and desensitization in human airway smooth muscle. Am J Respir Crit Care Med 2000;162:2117–2124. [DOI] [PubMed] [Google Scholar]
- 16.Hall I, Widdop S, Townsend P, Daykin K. Control of cyclic AMP levels in primary cultures of human tracheal smooth muscle cells. Br J Pharmacol 1992;107:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Aprile AC, Fernandes LB, Rigby PJ, Goldie RG. Impact of parainfluenza-3 virus infection on endothelin receptor desnity and function in guinea pig airways. Clin Sci 2002;103:3455–3485. [DOI] [PubMed] [Google Scholar]
- 18.Billington CK, Pascual RM, Hawkins ML, Penn RB, Hall I. Interleukin-1b and rhinovirus sensitize adenylyl cyclase in human airway smooth muscle cells. Am J Respir Crit Care Med 2001;24:633–639. [DOI] [PubMed] [Google Scholar]
- 19.Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff H, Lincoln P, Maassab H. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol 2001;167:1060–1065. [DOI] [PubMed] [Google Scholar]
- 20.Elias JA, Wu Y, Zheng T, Panetteiri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6 type cytokines. Am J Physiol 1997;273:L648–L655. [DOI] [PubMed] [Google Scholar]
- 21.Shore S, Moore P. Effects of cytokines on contractile and dilator response of airway smooth muscle. Clin Exp Pharmacol Physiol 2002;29:859–866. [DOI] [PubMed] [Google Scholar]
- 22.Hakonarson H, Carter C, Maskeri N, Hodinka R, Grunstein MM. Rhinovirus-mediated changes in airway smooth muscle responsiveness: induced autocrine role of interleukin-1b. Am J Physiol 1999;277:L13–L21. [DOI] [PubMed] [Google Scholar]
- 23.Drysdale C, McGraw D, Stack C, Stephens J, Judson R, Nandabalan K, Arnold K, Ruano G, Liggett S. Complex promoter and coding region Beta2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci USA 2000;97:10483–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whaley BS, Yan N, Birnbaumer L, Clark RB, Barber R. Differential expression of the beta-adrenergic receptor modifies agonist stimulation of adenylyl cyclase: a quantitative evaluation. Mol Pharmacol 1994; 45:481–489. [PubMed] [Google Scholar]
- 25.George ST, Berrios M, Hadcock JR, Wang H, Malbon CC. Receptor density and cAMP accumulation: analysis in CHO cells exhibiting stable expression of a cDNA that encodes the beta2-adrenergic receptor. Biochem Biophys Res Commun 1988;150:665–672. [DOI] [PubMed] [Google Scholar]
- 26.Small K, Brown K, Theiss C, Seman C, Weiss S, Liggett S. An Ile to Met polymorphism in the catalytic domain of adenylyl cyclase type 9 confers reduced beta2-adrenergic receptor stimulation. Pharmacogenomics 2003;13:535–541. [DOI] [PubMed] [Google Scholar]