Abstract
The human MUC7 gene encodes a low-molecular-mass mucin glycoprotein that functions in modulation of microbial flora in the oral cavity and respiratory tracts. MUC7 gene expression is tissue- and cell-specific, with dominant expression in salivary gland acinar cells. To begin to understand the molecular mechanisms responsible for controlling MUC7 gene expression, we analyzed the promoter activity of MUC7 5′-flanking region in a human lung epithelial cell line A549. We demonstrated that MUC7 gene is expressed constitutively in this cell line and is upregulated by TNF-α stimulation. The promoter activities of a 2,762-bp fragment of the human genomic DNA (−2,732/+30 bp) and its deletion series, subcloned into a luciferase reporter vector, were characterized at the basal level and under stimulation by TNF-α. The results indicated that the minimal functional MUC7 promoter is in the region of −138/+30 bp. This region also revealed the greatest increase in the promoter activity upon TNF-α stimulation. Two putative AP1-binding elements and one NF-κB–binding element were identified within the proximal promoter. Further analyses demonstrated that mutations of these elements dramatically reduced specific DNA-protein binding ability and reporter gene expression. AP1 elements played an essential role in the constitutive expression, while the NF-κB element was crucially important in the response to TNF-α stimulation, demonstrating that TNF-α activates MUC7 transcription via NF-κB signaling pathway.
Keywords: activator protein-1, human lung epithelial cell line, human TNF-α, MUC7 mucin gene promoter, NF-κB
Mucin glycoproteins constitute the major macromolecular components of mucus that covers the epithelial surfaces of mucosal tissues to provide protection and lubrication to underlying cells. To date, at least seventeen human mucin genes, MUC1–4, MUC5AC, MUC5B, MUC6–9, MUC11–13, MUC15–17, and MUC19, have been characterized (http://www.ncbi.nlm.nih.gov/LocusLink/). Most of these mucin genes are expressed in a wide range of tissues with overlapping tissue- and cell-specific expression patterns (1–4). MUC7 gene encodes a protein core of 39 kD of a relatively small mucin glycoprotein (∼ 125 kD) (5). MUC7 expression was originally detected in the human salivary glands and more recently in respiratory tracts, urinary tract and lacrimal glands (6–10). MUC7 mucin is mainly secreted by human sublingual and submandibular glands, and its functions in oral cavity have been extensively studied (11, 12). As a glycoprotein of saliva, MUC7 is involved in mastication, speech, swallowing, and lubrication of the oral cavity. More importantly, MUC7 functions as an antimicrobial agent involved in promoting clearance of various bacteria because it can inhibit bacterial colonization by masking their surface adhesins (11, 13). It is interesting to note that MUC7 mucin can interact, in vitro, with many oral as well as airway microorganisms, including Streptococcus mutans and Pseudomonas aeruginosa, and also with Herpes simplex virus (HSV) and human immunodeficiency virus (HIV) (11, 12). Recent studies in our laboratory have shown that the N-terminal region of MUC7 mucin exhibits potent fungicidal and bactericidal activities (14–16).
Normal mucin expression is necessary to maintain healthy epithelium, but the normal pattern is often changed in response to environmental insults. The expression levels and patterns of mucin genes are often altered during cell differentiation, inflammatory process, and tumor development. For example, abnormal expression of gel-forming mucins, MUC2, MUC5AC, and MUC5B, were observed in cystic fibrosis (CF) airways (17), and aberrant expression of MUC5B was found in gastric carcinoma (18). Two comprehensive review articles, published very recently, summarize up-to-date information on the respiratory tract mucin genes and mucin glycoproteins in health and disease (19), and mucin gene expression and regulation in the normal lung and in chronic inflammatory airway diseases (20).
The biological manifestation of MUC7 expression has been recognized for its involvement in several diseases and carcinogenesis. MUC7 mucin concentration has been examined in clinical patients suffering from Actinobacillus actinomycetemcomitans–associated periodontal disease, and found to be significantly decreased compared with periodontal healthy subjects (21). The study of the relationship between the levels of MUC7 mucin and S. mutans titers in human saliva has demonstrated that elevated level of the etiologic bacterium of dental caries is significantly associated with diminished amount of MUC7 mucin (22–24). These findings indicate that a decline in the salivary defense component might increase the risk for oral bacterial infection. The differential expression of MUC7 gene has also been found during bladder tissue carcinogenesis, and considered as a new diagnostic urinary marker for bladder cancer (9, 25).
A major prerequisite for understanding the regulatory mechanisms of mucin gene expression under normal and pathologic situations is the knowledge of the gene promoter structure and function. The molecular mechanisms responsible for controlling gel-forming mucin (MUC2, MUC5AC, and MUC5B) gene expression have been increasingly defined as their promoters and regulatory regions are characterized in suitable cell model systems (19, 26–29). The MUC7 gene promoter, however, has not been characterized until the present study even though its 5′-flanking region (∼ 3 kb) has been cloned and partially sequenced by our group several years ago (13). This is because MUC7 gene is mainly expressed by acinar cells of human sublingual and submandibular glands, and thus far, there is no appropriate salivary gland cell model available for MUC7 promoter characterization. However, as mentioned above, MUC7 gene is also expressed in human airways, and likely contributes to the protection of airway epithelium.
Currently, human primary airway epithelial cells and cell lines are being used in many laboratories to study various epithelial functions, including mucin gene expression. We have recently studied the transcriptional regulation of MUC7 gene expression in primary normal human tracheobronchial epithelial (NHTBE) cells and MUC7 transgenic mice airways (30). We found that MUC7 gene expression was induced by culturing the NHTBE cells at the air–liquid interface (ALI), in which the cells were well differentiated. Further, we determined that MUC7 transcription was upregulated by a panel of cytokines (IL-1β, IL-4, IL-13, TNF-α), a growth factor (EGF), and a bacterial product (P. aeruginosa LPS). We have also observed (but not reported in that study) that MUC7 gene expression could be modulated by inflammatory cytokines in a human lung epithelial cell line A549, suggesting that this cell line may be an appropriate model system for studying MUC7 promoter. In the present study we characterized MUC7 gene promoter using the A549 cell line in an attempt to identify the cis-acting elements that are responsible for MUC7 transcription and regulation. Bacterial products and inflammatory cytokines have been linked to mucin gene regulation. Particularly, P. aeruginosa LPS and human TNF-α have been shown to upregulate transcription of MUC2 and MUC5AC in certain airway epithelial cells (31–34). We intended to examine whether these agents also regulate MUC7 transcription. However, A549 cells lack Toll-like receptor 4 (TLR4), through which bacterial LPS activates the cells (35), whereas the A549 cells can be activated by TNF-α (36, 37). Thus, the promoter activities were analyzed at the constitutive level and under the TNF-α stimulation. This study for the first time aimed at the regulatory mechanisms of MUC7 gene transcription by characterizing its promoter structure and function.
MATERIALS AND METHODS
Cell Culture and Stimulation
A549 cell line was obtained from ATCC (Rockville, MD) and cultured in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (50 mg/ml). The cells were maintained at 37°C in a 5% CO2 incubator. To stimulate the cells, P. aeruginosa LPS (10 μg/ml) or human TNF-α (20 ng/ml) were added into the cells at ∼ 80% confluence and maintained for 12 h. The LPS and TNF-α were purchased from Sigma Chemical Co. (St. Louis, MO) and dissolved in PBS, pH 7.4. The cells treated with PBS alone served as a control.
Northern Analysis and Real-Time PCR
RNA samples were prepared from the control, LPS-, and TNF-α–treated A549 cells using TRIzol reagent (Life Technologies, Rockville, MD) following the manufacturer's instructions.
Northern blot analysis was performed according to the standard procedures. Briefly, total RNA (15 μg) samples were resolved on a 2% agarose-formaldehyde gel, and transblotted onto a Nytran membrane (Schleicher and Schuell, Keene, NH) by capillary blotting. The blot was hybridized with 32P-labeled MUC7 cDNA probe using Rediprime II random primer labeling kit (Amersham Biosciences, Piscataway, NJ). MUC7 cDNA probe was 403 bp in size (nt: 13–415) (6). Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe (542 bp) was used as an internal control. After hybridization, the blot was washed and visualized by autoradiography. Human salivary gland (HSG) RNA (1 μg) was used as a positive control.
In real-time RT-PCR analysis, the first-strand cDNA was synthesized using the M-MLV reverse transcriptase (Promega, Madison, WI). In brief, a 25-μl reaction containing RNA (2 μg), random hexamer primers (1 μg), dNTP (0.5 mmol), rRNasin (25 U), M-MLV reverse transcriptase (200 U), and 5× first strand buffer (5 μl) was incubated at 37°C for 60 min. Quantitative real-time PCR was performed using Applied Biosystems 7,500 (Foster City, CA) following “TaqMan Gene Expression Assay” protocol. TaqMan primers and probes for human MUC7 and GAPDH genes were pre-developed by Applied Biosystems. PCR amplification was performed under the control of SDS software (Sequence Detection System; Applied Biosystems). To optimize the reaction, serially diluted cDNA templates were used to plot the log of the abundance of target gene product against Ct (threshold cycle). The data were analyzed with Relative Quantification Study Document software (Applied Biosystems). The relative abundance of MUC7 mRNA was normalized with the endogenous control, GAPDH mRNA abundance. Template contamination was monitored by including a negative control reaction that did not contain RNA.
Generation of MUC7 Promoter/Reporter Constructs
A fragment of MUC7 genomic DNA corresponding to −2,732 to +30 (in relation to the transcription start site) was amplified by PCR from a genomic clone (13) with an upstream primer containing a MluI site and a downstream primer containing an XhoI site (see Table 1). The PCR product was restriction digested and cloned into the MluI/XhoI site of the pGL3 basic vector (Promega). This construct is referred to as −2,732/+30. The inserted fragment (2,762 bp) was fully sequenced, and the nucleotide sequence was submitted to GenBank (accession no. DQ157434). This fragment was also screened for putative transcriptional factor–binding sites by the following software: http://www.gene-regulation.com/cgi-bin/pub/programs/alibaba2/; http://www.genomatix.de/cgi-bin/matinspector_prof/). A series of 5′-end deletion constructs were generated from the –2,732/+30 construct by PCR using appropriate upstream primers and the downstream primer that is common for all constructs as listed in Table 1. Restriction enzyme digestion and double-strand DNA sequencing were performed to confirm orientations and sequences of all constructs, respectively.
TABLE 1.
PCR PRIMERS USED FOR GENERATING MUC7 PROMOTER/REPORTER CONSTRUCTS
| Construct | Upstream Primer |
|---|---|
| −2,732/+30 | 5′-CGACGCGTGAACAGGCAGC-3′ |
| −2,014/+30 | 5′-CGACGCGTTCTCACTCACAAGT-3′ |
| −1,449/+30 | 5′-CGACGCGTAAATGCATATCAC-3′ |
| −1,167/+30 | 5′-CGACGCGTGGAGAAGCAAGGAA-3′ |
| −1,064/+30 | 5′-CGACGCGTATTCCAGATTAACG-3′ |
| −478/+30 | 5′-CGACGCGTGTTGATTTTTTGGC-3′ |
| −334/+30 | 5′-CGACGCGTCTGCTTACTTTGAGA-3′ |
| −138/+30 | 5′-CGACGCGTCAATCTCAGCAAAC-3′ |
| −60/+30 | 5′-CGACGCGTAGATTTCCTATTTCC-3′ |
| −37/+30 | 5′-CGACGCGTCGACTGGAGTGTTA-3′ |
| All Constructs | Downstream Primer |
| 5′-CCCTCGAGGATGGTAACTAGAAG-3′ |
All the upstream primers contain MluI site and downstream primer contains XhoI site as underlined.
Cell Transfection and Luciferase Assay
A549 cells were plated onto 12-well plates at 8 × 104 cells/well and cultured in l ml of RPMI 1640 medium. The cells were grown to ∼ 60% confluence before transfection. Transient cell transfection was performed using FuGENE 6 reagent (Roche, Indianapolis, IN). Briefly, 100 μl of transfection mixture was prepared with serum-free medium containing 1 μg of the MUC7 promoter/reporter plasmid, 5 ng of Renilla luciferase plasmid (which served as internal control), and 3 μl of FuGENE 6. After incubation for 15 min at room temperature, the DNA mixture was added drop-wise to each well. The transfection was maintained for 48 h. The control cells were transfected with the basic pGL3 plasmid, a promoterless-vector. For luciferase assays, the transfected cells were washed twice with PBS (pH 7.4) and lysed in 100 μl of Passive Lysis Buffer (Promega). The cell lysates were centrifuged at 10,000 × g for 10 min to remove cell debris. The supernatants were assayed for reporter gene expression, which was shown as firefly luciferase activity, and internal control, which was shown as Renilla luciferase activity. They were measured using 15 μl of cell lysate with the dual luciferase assay system (Promega) on an Orion MPL2 luminometer (Berthol Detection System, Oak Ridge, TN). In each transfection, reporter gene expression was normalized to the internal control. The values were expressed as fold of induction by comparing the activity of the promoter construct with that of the promoterless-vector alone. The results were averaged from three separate transfections.
In the stimulation study, human TNF-α (20 ng/ml) was added into the A549 cells after 24 h of transfection, and maintained for another 24 h before preparation of cell lysates.
Nuclear Extracts and Gel Mobility Shift Assays
Nuclear extracts were prepared from A549 cells using BD TransFactor Extraction Kit (BD Biosciences, Palo Alto, CA) following the manufacturer's instructions. The nuclear protein concentrations were determined using the Bradford assay (Bio-Rad, Hercules, CA) and the extracts stored at −70°C. In the stimulation study, human TNF-α (20 ng/ml) was added to ∼ 80% confluent cells for 30 min, after which nuclear extracts were prepared.
Double-stranded oligonucleotides were custom-made by BIO.Synthesis (Lewisville, TX). Sequences of the oligonucleotides are listed in Table 2. The oligonucleotides were end-labeled with digoxigenin (Dig), using DIG Gel Shift Kit (Roche), following the manufacturer's instructions. Protein–DNA binding reactions were performed under optimized conditions. Briefly, nuclear extracts (10 μg) were mixed with Dig-labeled probe (60 fM) in 20 μl of binding buffer containing 20 mM Hepes (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM DTT, 1% Tween-20 (wt/vol), 30 mM KCI, and 1 μg poly[d(I-C)]. The reaction mixture was maintained at room temperature for 15 min. In competition analysis, a 100-fold excess of unlabeled oligonucleotide was added to the binding reaction. NF-κB and AP1 consensus oligonucleotides (Promega) were used in binding competition for demonstrating the specificity of the observed protein-DNA interactions. Antibodies (0.5 μg) specific to NF-κB p65 or c-Jun (Santa Cruz Biochemicals, Santa Cruz, CA) were used for immunocompetition, and were preincubated with nuclear extracts for 30 min on ice before addition of the oligos. Protein–DNA complexes were resolved on a 6% polyacrylamide gel for 3 h at 200 V in 0.5× TBE buffer. After electrophoresis, the gel was transferred to a positively charged membrane by electroblotting, followed by cross-linking. The blot was then subjected to blocking, washing, and incubating with anti-Dig conjugated to alkaline phosphatase, and application of CSPD solution, as per manufacturer's instructions. The results were visualized by chemiluminescent signal detection on X-ray film.
TABLE 2.
PROBES FOR EMSA AND PCR PRIMERS FOR MAKING MUC7 PROMOTER MUTANTS
| Probes for EMSA | Primers for Mutation | |
|---|---|---|
| NF-κB | wt: 5′-TTAAAAGTAAATTCCTTGAC-3′ | Upper: 5′-TTAAAAGTAAATGAGTTGAC-3′ |
| mut: 5′-TTAAAAGTAAATGAGTTGAC-3′ | Lower: 5′-GTCAACTCATTTACTTTTAA-3′ | |
| AP1 | wt: 5′-TTCCTTGACACAAATGACCTTA-3′ | Upper: 5′-TTCCTCAGAACAAACAGACTTA-3′ |
| mut: 5′-TTCCTCAGAACAAACAGACTTA-3′ | Lower: 5′-TAAGTCTGTTTGTTCTGAGGAA-3′ |
Definition of abbreviations: AP1, activator protein-1; EMSA, electrophoretic mobility shift assay.
Mutation sites are indicated with underline. The upper-strand primer and the lower-strand primer are complementary (overlap each other).
Site-Directed Mutagenesis
Three MUC7 promoter mutants were generated from the −138/+30 bp construct using a PCR-based method of primer overlap extension, in which the overlapping primers contain the desired bases (38). Mutation at the NF-κB binding site was made by changing 5′-TCC-3′ (−93/−91) to 5′-GAG-3′, using the primers listed in Table 2. Mutations at the two AP1 binding sites were made by changing 5′-TGAC-3′ (−89/−86 and −80/−77) to 5′-CAGA-3′, using the primers listed in Table 2. The third mutant promoter construct contains mutations at both the NF-κB and AP1 sites. It was generated from the construct that already contains mutations at the AP1 sites by incorporating mutation into the NF-κB site, using same primers as for generation of the NF-κB mutant.
Statistical Analysis
Statistical analyses were performed upon comparisons made between the treated group and the control group using the Student's t test. A P value of < 0.05 was considered statistically significant. Data are expressed as mean ± SE.
RESULTS
Expression and Modulation of Endogenous MUC7 mRNA in A549 Cells
First we evaluated the level of human MUC7 gene transcript in A549 cell line using Northern blot analysis. As shown in Figure 1A, one distinct MUC7 transcript was detected in the RNA sample from these cells. The transcript is identical in size to that detected in the RNA sample from the human salivary glands (previously determined to be ∼ 2.4 kb) (6), but much lower in abundance. This result indicated that the A549 cell line expresses the human MUC7 gene constitutively, and confirmed our hypothesis that it might be used as a model for studying MUC7 gene expression, regulation, and promoter analysis.
Figure 1.
Expression and modulation of endogenous MUC7 mRNA level in A549 cell line. (A) Northern blot analysis. Total RNA from the A549 cells (15 μg) was analyzed. Total RNA from human salivary gland (HSG) (1 μg) served as a positive control. 32P-labeled cDNA probes for human MUC7 and GAPDH genes were used for hybridization as described in Materials and Methods. Only the relevant portion of the Northern blot indicating the MUC7 transcript (∼ 2.4 kb in length) is shown; no other bands were detected in either sample. Three different experiments showed similar results. (B) Effects of P. aeruginosa LPS and human TNF-α on MUC7 gene transcription assessed by real-time RT-PCR. RNA samples were prepared from control cells and the cells treated either with LPS (10 μg/ml) or TNF-α (20 ng/ml) for 12 h. The relative quantities of MUC7 mRNA were evaluated with the quantitative analysis software (Relative Quantification Study Document). GAPDH gene served as an endogenous control for monitoring RNA integrity and the amount of RNA used in each reaction. The MUC7 mRNA amount from each reaction was normalized with that of GAPDH mRNA. Data are representative of three independent experiments and values are means of four replicates ± SD (n = 4). Asterisk indicates the statistically significant difference in the mRNA level of the TNF-α–stimulated cells compared with the control cells (P < 0.0001). Bars represent SE.
Proinflammatory cytokines (IL-1β, IL-6, TNF-α) have been reported to be able to induce airway mucin gene expression (39). In this study we assessed the effects of P. aeruginosa LPS and human TNF-α on MUC7 gene transcription in A549 cells. Quantitative real-time PCR analysis was performed to detect endogenous MUC7 mRNA levels. As shown in Figure 1B, the steady-state level of MUC7 mRNA from the LPS-treated cells was not affected (P > 0.1). This result agrees with the report that LPS cannot activate A549 cells due to the lack of the TLR4 receptor (35). However, the MUC7 mRNA level from TNF-α–treated cells was significantly increased (2.5-fold) as compared with the control cells (Figure 1B). This finding indicated that TNF-α has a potential to upregulate MUC7 gene at the transcriptional level. In addition, no signal was identified in a negative control reaction (data not shown).
Characterization of MUC7 Promoter/Reporter Constructs and Identification of Minimal Functional Region
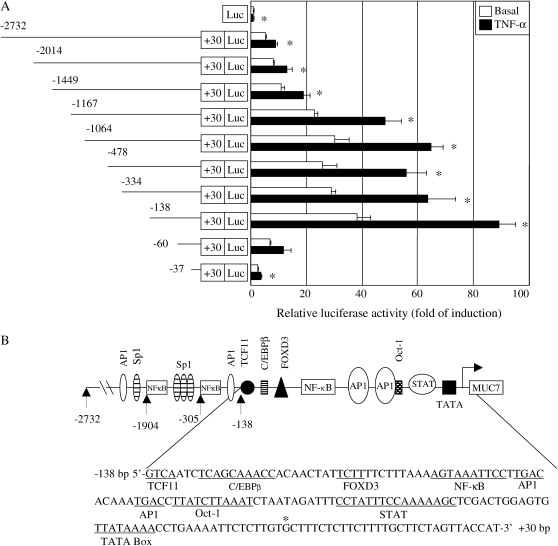
To characterize the transcriptional control region of MUC7 gene promoter, the –2,732/+30 bp fragment was subcloned into a luciferase reporter vector (pGL3-Basic) as described in Materials and Methods. A series of promoter/reporter fusion plasmids containing sequential deletions were also constructed (as depicted on the left of Figure 2A). The constructs were transiently transfected into A549 cells. The luciferase activities, reflecting MUC7 promoter activities, were measured using the cell lysates made from control and TNF-α–treated cells.
Figure 2.
Characterization of MUC7 minimal functional promoter and effect of TNF-α on the promoter activity. (A) Transient transfection analysis of MUC7 gene promoter constructs. The diagram on the left is a schematic representation of the 2.73-kb MUC7 promoter and its deleted variants inserted upstream of the luciferase gene in the reporter plasmid pGL3-Basic. The numbers indicate the 5′-end of the promoter DNA inserts, in relation to the transcription start site (+1), determined previously by a modified RACE procedure (13). The tested plasmids (1 μg) containing promoter/reporter construct were transiently transfected into A549 cells. Renilla luciferase expression plasmid (5 ng) was co-transfected as an internal control. Luciferase activity of each construct was measured and normalized to the R-luciferase activity. On the right, the relative luciferase activity was expressed as fold of induction by comparing to that of the promoterless vector. Values from control cells represent basal level activities. In stimulation, the transfected cells were activated with TNF-α (20 ng/ml). The data represent the average of at least three independent experiments, and each performed in triplicate. Asterisks indicate the values of luciferase activity in TNF-α–stimulated cells significantly different from those of control cells (P < 0.05). Bars represent SE. (B) Schematic representation of the minimal functional MUC7 gene promoter (−138/+30) structure (upper portion), and its nucleotide sequence (lower portion). The transcription start site, determined previously (13), is indicated by the asterisk. TATA box, and the putative and confirmed binding sequences for transcription factors are indicated as underlined.
As shown in Figure 2A (on the right), among all the constructs, the −138/+30 construct exhibited the highest promoting ability in promoting the reporter gene expression. At the basal level, it caused a 38-fold induction of the luciferase activity as compared with the promoterless vector. In TNF-α–treated cells, this construct resulted in an 89-fold induction of the luciferase activity. These results clearly indicate the presence of cis-elements that are crucial for MUC7 transcription at both the basal level and for TNF-α stimulation in the −138/+30 fragment. This proximal promoter region is, therefore, defined as the minimal functional MUC7 promoter. The nucleotide sequence of the proximal promoter (−138/+30 bp), and the putative transcription factor binding elements, including AP1 and NF-κB that were analyzed further, are indicated in Figure 2B.
Characterization of Transcription Factor–Binding Elements in the MUC7 Proximal Promoter Using Electrophoretic Mobility Shift Assay
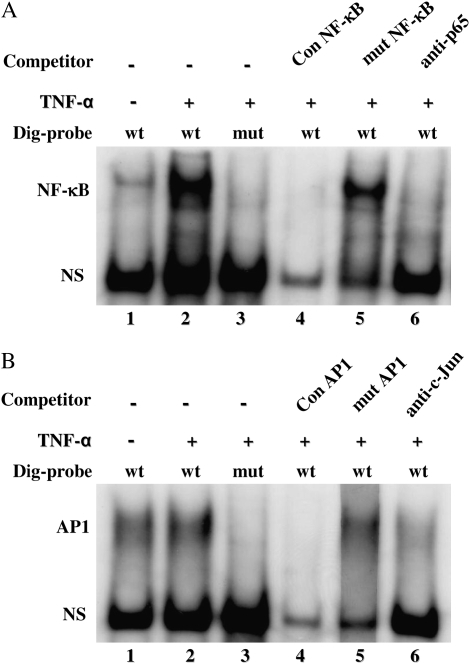
The −100/-91 region (5′-AGTAAATTCC-3′) of the MUC7 proximal promoter was identified as a putative binding sequence for NF-κB, and the −89/−86 (5′-TGAC-3′) and −80/−77 (5′-TGAC-3′) regions as putative binding sequences for AP1 (Figure 2B). To characterize the functions of these putative cis-acting elements, gel mobility shift assays were performed using double-stranded oligonucleotides harboring these elements as probes. Therefore, two oligonucleotide probes, representing respectively the −105/−86 and −94/−73 regions of the MUC7 proximal promoter and their corresponding mutation variants (see Table 2), were synthesized and labeled with Dig. These Dig-probes were incubated with the nuclear proteins extracted from control and TNF-α–treated A549 cells, and analyzed by electrophoretic mobility shift assay (EMSA).
For studying the NF-κB site, the −105/−86 probe was used. As shown in Figure 3A, a specific DNA–protein binding complex was faintly detectable with the nuclear extract from control cells (lane 1), but it was robust with the nuclear extract from TNF-α–treated cells (lane 2), indicating that TNF-α activated a nuclear transcription factor that was able to bind to the putative NF-κB element in the MUC7 promoter. When a mutation was incorporated into the NF-κB element by substituting 5′-TCC-3′ with 5′-GAG-3′, the specific protein–DNA complex disappeared (lane 3). It is clear that the mutant probe failed to interact with the nuclear protein. This result suggests that the three base pair mutation is efficient to abolish the NF-κB element binding ability. To confirm the specificity of the protein–DNA interaction, excess unlabeled NF-κB consensus sequence oligonucleotide was used in a binding competition (lane 4). The protein–DNA complex was displaced by the NF-κB consensus sequence, indicating a specific interaction between the nuclear proteins and the putative NF-κB element. Furthermore, the unlabeled mutant probe was unable to displace the protein–DNA complex (lane 5). To further characterize the interaction between the NF-κB subunit and the −105/−86 probe, NF-κB antibody (anti-p65) was added to the binding reaction. Although the anti-p65 NF-κB antibody failed to demonstrate a super-shift effect on the protein–DNA complex, the complex disappeared (lane 6), suggesting that the DNA–protein complex contains the p65 component. The results from this experiment demonstrated that the nuclear factor NF-κB was activated by TNF-α, and that the putative NF-κB–binding element in the MUC7 gene promoter was functional and responsible for TNF-α stimulation.
Figure 3.
Analyses of transcription factor binding elements in MUC7 proximal promoter using competitive EMSA. (A) Determination of NF-κB binding sequence. A 20-bp oligonucleotide probe (5′-TTAAAAG TAAATTCCTTGAC-3′) representing the −105/−86 region of the MUC7 promoter contains putative NF-κB binding motif shown in underline. The wild-type (wt) probe and its mutation variant (mut, 5′-TTAAAAG TAAATGAGTTGAC-3′) were synthesized and labeled with Dig. Nuclear extracts (10 μg) from either control A549 cells (lane 1) or TNF-α–treated cells (lanes 2–5) were incubated with 60 fmol of the Dig-probes in the absence (lanes 1–3) or presence of 100-fold molar excess of unlabeled competitors (lanes 4–5). Lane 3 contains Dig-labeled Mut-NF-κB oligonucleotide. NF-κB antibody, anti-p65 (0.5 μg) was used for identifying the component involved in protein-DNA complex (lane 6). con NF-κB: NF-κB consensus oligonucleotide (lane 4); mut NF-κB: mutation oligonucleotide (lanes 3 and 5); NS: nonspecific binding. (B) Determination of AP1 binding sequences. A 22-bp oligonucleotide probe (5′-TT CCTTGACACAAATGACCTTA-3′) representing the −94/−73 region of the MUC7 promoter contains two AP1 putative binding motives shown in underline. The wt probe and its mutation variant (mut, 5′- TTCCTCA GAACAAACAGACTTA-3′) were synthesized and labeled with Dig. Nuclear extracts (10 μg) from either control A549 cells (lane 1) or TNF-α–treated cells (lanes 2–5) were incubated with 60 fmol of the Dig-probes in the absence (lanes 1–3) or presence of 100-fold molar excess of unlabeled competitors (lanes 4–5). Lane 3 contains Dig-labeled Mut-AP1 oligonucleotide. AP1 antibody, anti–c-Jun (0.5 μg) was used for identifying the component involved in protein–DNA complex (lane 6). con AP1: AP1 consensus oligonucleotide (lane 4); mut AP1: mutation oligonucleotide (lanes 3 and 5); NS: nonspecific binding.
For studying AP1 sites, the −94/−73 probe was used. As shown in Figure 3B, a specific protein–DNA complex was detectable with nuclear extract from control cells (lane 1), and it was slightly increased in TNF-α–treated cells (lane 2). When mutations were incorporated in this probe by changing the 5′-TGAC-3′ to 5′-CAGA-3′ (at both AP1 sites), the specific protein–DNA complex could not be formed (lane 3), indicating that the 5′-TGAC-3′ motif was necessary for maintaining AP1 element binding ability. When excess unlabeled AP1 consensus sequence oligonucleotide was used as a competitor, the specific complex disappeared (lane 4). This result suggests that the binding complex was formed by specific interaction between nuclear proteins and the putative AP1-binding elements. The specific complex could not be displaced when unlabeled mutant probe was used as the competitor (lane 5), indicating that the mutations (from 5′-TGAC-3′ to 5′-CAGA-3′) at two AP1-binding elements were effective. The specific interaction between the nuclear proteins and the AP1 elements was also confirmed by adding AP1 antibody (anti–c-Jun) into the reaction. As shown in lane 6, the binding complex was displaced by anti–c-Jun, indicating that the complex contained c-Jun protein. Collectively, these findings demonstrate that the two tandemly arrayed AP1-binding elements in the MUC7 proximal promoter are relevant to constitutive transcription of the MUC7 gene.
Functional Analyses of MUC7 Proximal Promoter Using Mutagenesis and Deletion Assay
We further examined the functional roles of the NF-κB and AP1 putative binding elements in the MUC7 proximal promoter by introducing mutations specifically into either the NF-κB element or AP1 elements in the −138/+30 bp construct. A combination of mutation in both NF-κB and AP1 elements was also made. The primers for generating the mutant promoters are listed in Table 2. The wild-type promoter and its mutant variants, shown diagrammatically in Figure 4, were transiently transfected into A549 cells, and their promoting activities were analyzed at basal level and under TNF-α stimulation.
Figure 4.
Mutation and deletion analyses of MUC7 promoter. On the left, schematic diagrams represent the MUC7 proximal promoter and its derivative constructs with cis-elements. Mutations in NF-κB– and AP1-binding sites, either alone or in combination, are indicated. These constructs were transiently transfected into A549 cells, and Renilla luciferase expression plasmid was cotransfected as an internal control. For stimulation, the transfected cells were activated with TNF-α (20 ng/ml). On the right, luciferase activities were expressed as fold activation relative to the basic vector. The data represent the average of at least three independent experiments, and each performed in triplicate. Asterisks indicate the values of mutant promoter activities significantly different from that of wild-type promoter (P < 0.05). Bars represent SE.
As shown in Figure 4, the reporter gene expression (luciferase activity) driven by the wild-type promoter showed a 38-fold induction in control cells, and an 86-fold induction in TNF-α–treated cells. When a mutation was incorporated into the putative NF-κB element, the mutant promoter caused little decrease of luciferase activity in the control cells, but resulted in a substantial decrease in the cells treated with TNF-α. These findings demonstrate that the NF-κB element plays a crucial role in responding to TNF-α stimulation. In conjunction with the result from EMSA that NF-κB was activated by TNF-α (Figure 3A), we conclude that the MUC7 gene is inducible by TNF-α through the NF-κB signaling pathway.
When mutations were made in the two putative AP1 elements, the mutant promoter caused significant decrease of luciferase activity in both the control and TNF-α–treated cells. This result suggests that the AP1 elements appear to play important roles in constitutive transcription of the MUC7 gene. The roles of NF-κB and AP1 elements were further confirmed by using the combination mutant promoter as well as using the deletion construct −37/+30 bp. As shown with the luciferase assay in Figure 4, the combination mutation was able to abrogate both basal level promoter activity and the responsiveness to TNF-α. The −37/+30 bp construct, where these active regions have been deleted, failed to support MUC7 promoter activities at either basal or stimulated level. The combination mutation resulted in a further decrease in luciferase activity when compared with the mutations at either NF-κB or AP1 sites alone, suggesting the functional cooperation of these elements in MUC7 promoter responsiveness. Based on these findings and the results from EMSA experiments, we conclude that, in the human lung epithelial cells, the basal level expression of the MUC7 gene is AP1-dependent, it is TNF-α–inducible, and the induction is mediated via NF-κB activation.
DISCUSSION
MUC7 cDNA was first cloned from the human submandibular gland cDNA library in 1993; it encodes a 377–amino acid protein core of MUC7 (6). A genomic clone, isolated from λGEM11 human genomic library, encompasses the entire MUC7 gene (∼ 10.0 kb), and also includes a 3.0-kb flanking region at both the 5′- and 3′-ends. About 1.5 kb DNA, upstream of the transcription start site, has previously been sequenced (GenBank accession no. L42983). MUC7 gene expression exhibits tissue- and cell-specific patterns. As shown by Northern analyses, MUC7 mRNA (2.5 kb) was largely found in human sublingual gland and in a much lower level in the submandibular gland (6), and a still much lower level in the trachea (40). The tissue-specific expression of MUC7 gene was also found in MUC7 transgenic mice (6, 41). By in situ hybridization, MUC7 gene transcripts were localized to the mucous acinar cells of human salivary glands, and salivary glands of MUC7 transgenic mice (42). More recently, MUC7 expression was detected in normal respiratory tract, and the transcript was localized in serous cells of the submucosal glands (8).
As the first step toward understanding the underlying mechanisms responsible for controlling MUC7 gene expression, we needed to characterize MUC7 gene promoter to identify the transcriptional control region. In our preliminary studies (not shown), we have transiently transfected the pGL3 luciferase reporter vector containing the –2,732/+30 MUC7 fragment into several cell lines, including HSG (human salivary gland cell line), HT-29 (colon carcinoma), NCI-H292 (mucoepidermoid carcinoma), Panc-1 (pancreatic carcinoma), and A549. Only the A549 cell line showed significant increase in the luciferase activity. By assessing the steady-state level of MUC7 mRNA in A549 cell line, we found that these cells express MUC7 gene constitutively, suggesting that this cell line may be an appropriate model system for MUC7 promoter characterization. A549 cell line has been actually used in many laboratories to study various airway epithelial functions, including mucin gene expression. It has been reported that the tumor from which this cell line was derived showed cuboidal epithelial cells in clumps and acini on histologic examination, and its acinar-like spaces were filled with a mucinous material (43). In this study, we also assessed the effect of P. aeruginosa LPS and human TNF-α on MUC7 gene transcription in A549 cells by detecting the steady-state levels of MUC7 mRNA. We found that LPS indeed had no effect on MUC7 transcription (due to the lack of the TLR4 receptor), whereas TNF-α did have a stimulatory effect on the MUC7 gene expression.
Next, we performed promoter activity analysis of MUC7 5′-flanking region and identified the transcriptional control region. The minimal functional MUC7 promoter was defined to the −138/+30 bp region. Several putative transcription factor binding sequences surrounding the TATA box were recognized. They include NF-κB, AP1, C/EBPβ, FOXD3, Oct-1, TCF11, and STATs. In this work, we studied in detail roles of transcription factors NF-κB and AP1 in regulation of the human MUC7 gene expression.
Many genes contain AP1-binding elements that play a role in regulating many biological functions (44). In the human MUC7 gene proximal promoter, we found the presence of two regions, −89/−83 bp (5′-TGACACA-3′) and −80/−77 bp (5′-TGAC-3′), acting as AP1-binding sequences. The 5′-TGA CACA-3′ sequence is very similar to the AP1 consensus sequence (5′-TGACTCA-3′) (45). The results from gel shift assay and luciferase assay (Figure 3B and Figure 4) clearly demonstrated that the two AP1-binding elements are necessary for basal level MUC7 promoter activity. Thus the MUC7 gene is also an AP1-dependent gene. This finding opens a possibility that regulation of MUC7 gene expression might be achieved via regulation of AP1 activity.
NF-κB is an inducible transcription factor that can be activated by a variety of extracellular stimili (46). TNF-α, a proinflammatory cytokine, is one of several NF-κB inducers (47). The transcription factor NF-κB is particularly important in the regulation of gene expression during inflammation process. The TNF-α–mediated transcriptional activation through NF-κB signaling pathway appears to be the common regulatory mechanism of mucin gene expression. In the human airway, MUC2, MUC5AC and MUC5B genes are regulated at a transcriptional level by proinflammatory cytokines (IL-1β, IL-6, and TNF-α) through NF-κB signaling pathway (39). We have shown in this study that the human MUC7 gene is also a TNF-α–inducible gene, as TNF-α was able to induce MUC7 gene both in promoter activity (Figures 2A and 4) and endogenous MUC7 mRNA level in A549 cells (Figure 1B). We further demonstrated that the induction of MUC7 gene by TNF-α is through NF-κB signaling pathway (Figures 3A and 4). Therefore, regulation of the MUC7 gene can be achieved by regulating NF-κB activity. In addition, the NF-κB–binding element, 5′-AGTAAATTCC-3′ (−100/−91), of human MUC7 proximal promoter is very similar to the consensus sequence for binding p65 (RelA), 5′-GGAAATTCC-3′ (48). In our previous study we have reported that MUC7 transcript and glycoprotein product increased upon stimulation of NHTB cells with TNF-α or P. aeruginosa LPS, and upon stimulation of airway tissue of MUC7 transgenic mice with P. aeruginosa LPS. Earlier study reported that MUC7 mucin is present in the airway secretions of most pediatric patients with asthma but is absent in patients without asthma, pointing to a possible clinical relevance of MUC7 upregulation (49).
Except for AP1 and NF-κB, the other transcription factors, such as C/EBPβ, FOXD3, Oct-1, TCF11, and STATs, all having potential binding sites in the proximal promoter, may also play roles in MUC7 gene transcription. Thus, in our future studies, it would be of interest to characterize other transcription factor binding elements. It would also be interesting to study the function of minimal MUC7 promoter (−138/+30 bp) in salivary gland cells, since MUC7 is mainly expressed in salivary glands. This, however, may not be possible yet because, as discussed above, MUC7 is mainly expressed in acinar cells, which are not available. In our preliminary screen, when the −2,732/+30 MUC7 fragment was transfected into many cells lines, including HSG (human salivary gland cell line derived from intercalated ductal cells), there was no significant increase in the luciferase activity. The future studies will also examine the effect of EGF on the MUC7 promoter, as our previous study showed that EGF induced MUC7 expression (30).
Furthermore, the −2,732/+30 bp fragment has a variety of transcription factor potential binding sites in its distal region (Figure 2B). This fragment also contains two more consensus sequences of TATA box around the −600/−450 bp region, suggesting that MUC7 gene may have more than one transcription unit. However, the distal TATA boxes do not seem to be functional in the A549 cells. As shown in Figure 1A, only one distinct MUC7 transcript, identical in size to that detected in the RNA sample from the human salivary glands that is transcribed from the proximal promoter (∼ 2.4 kb), was detected in A549 cells. Interestingly, MUC5B expression has been shown to be under the control of the proximal promoter in airway cells, but the distal promoter in gastric cancer cells (39). Nevertheless, characterizing distal region of the −2,732/+30 bp fragment should also generate valuable information for a better understanding the molecular mechanism of MUC7 gene expression and regulation. Notably, the promoter activity was remarkably decreased (both basal and TNF-α induced expression), when a deletion was made from −1,449 to −1,167, indicating the presence of a potential repressor site in this region (Figure 2B), and this warrants further characterization of this region.
In conclusion, although MUC7 gene expression occurs mainly in human salivary glands and the functions of this mucin in the oral cavity have been well studied, due to the lack of appropriate model system(s), no information is available on the molecular mechanism of MUC7 gene expression in the salivary glands. However, MUC7 gene is also expressed in human airways, and likely contributes to the protection of airway epithelium. With the present study, we have characterized MUC7 gene promoter structure and function using a lung epithelial cell line as a model system. As the MUC7 gene regulatory mechanism begins to be unraveled, it will offer opportunities to exploit the biological implication of MUC7 gene expression.
Acknowledgments
The authors thank Drs. Gaffen, Cho and Scannapieco for valuable advice and helpful discussions, and R. Dunford for help with statistical analyses.
This work was supported by NIH/NIDCR grants DE009820 and T32-DE007034.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0110OC on June 15, 2006
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol 1995;57:607–634. [DOI] [PubMed] [Google Scholar]
- 2.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci 2001;6:D1192–D1206. [DOI] [PubMed] [Google Scholar]
- 3.Van Klinken BJ, Tytgat KM, Buller HA, Einerhand AW, Dekker J. Biosynthesis of intestinal mucins: MUC1, MUC2, MUC3 and more. Biochem Soc Trans 1995;23:814–818. [DOI] [PubMed] [Google Scholar]
- 4.Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res 1999;59:4083–4089. [PubMed] [Google Scholar]
- 5.Levine MJ, Reddy MS, Tabak LA, Loomis RE, Bergey EJ, Jones PC, Cohen RE, Stinson MW, Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res 1987;66:436–441. [DOI] [PubMed] [Google Scholar]
- 6.Bobek LA, Tsai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J Biol Chem 1993;268:20563–20569. [PubMed] [Google Scholar]
- 7.Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol 1998;19:30–37. [DOI] [PubMed] [Google Scholar]
- 8.Copin MC, Devisme L, Buisine MP, Marquette CH, Wurtz A, Aubert JP, Gosselin B, Porchet N. From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. Int J Cancer 2000;86:162–168. [DOI] [PubMed] [Google Scholar]
- 9.Retz M, Lehmann J, Roder C, Plotz B, Harder J, Eggers J, Pauluschke J, Kalthoff H, Stockle M. Differential mucin MUC7 gene expression in invasive bladder carcinoma in contrast to uniform MUC1 and MUC2 gene expression in both normal urothelium and bladder carcinoma. Cancer Res 1998;58:5662–5666. [PubMed] [Google Scholar]
- 10.Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea 2003;22:41–45. [DOI] [PubMed] [Google Scholar]
- 11.Schenkels LCPM, Gururaja TL, Levine JM. Salivary mucins: their role in oral mucosal barrier function and drug delivery. In: Rathbone MJ, editor. Oral mucosal drug delivery. Hamilton, New Zealand: Marcel Dekker; 1996. pp. 191–220.
- 12.Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol 1995;57:547–564. [DOI] [PubMed] [Google Scholar]
- 13.Bobek LA, Liu J, Sait SN, Shows TB, Bobek YA, Levine MJ. Structure and chromosomal localization of the human salivary mucin gene, MUC7. Genomics 1996;31:277–282. [DOI] [PubMed] [Google Scholar]
- 14.Gururaja TL, Levine JH, Tran DT, Naganagowda GA, Ramalingam K, Ramasubbu N, Levine MJ. Candidacidal activity prompted by N-terminus histatin-like domain of human salivary mucin (MUC7)1. Biochim Biophys Acta 1999;1431:107–119. [DOI] [PubMed] [Google Scholar]
- 15.Situ H, Wei G, Smith CJ, Mashhoon S, Bobek LA. Human salivary MUC7 mucin peptides: effect of size, charge and cysteine residues on antifungal activity. Biochem J 2003;375:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei GX, Bobek LA. In vitro synergic antifungal effect of MUC7 12-mer with histatin-5 12-mer or miconazole. J Antimicrob Chemother 2004;53:750–758. [DOI] [PubMed] [Google Scholar]
- 17.Davies JR, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J 1999;344:321–330. [PMC free article] [PubMed] [Google Scholar]
- 18.Perrais M, Pigny P, Buisine MP, Porchet N, Aubert JP, Van Seuningen-Lempire I. Aberrant expression of human mucin gene MUC5B in gastric carcinoma and cancer cells. Identification and regulation of a distal promoter. J Biol Chem 2001;276:15386–15396. [DOI] [PubMed] [Google Scholar]
- 19.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 20.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol 2006;34:661–665. [DOI] [PubMed] [Google Scholar]
- 21.Groenink J, Walgreen-Weterings E, Nazmi K, Bolscher JG, Veerman EC, van Winkelhoff AJ, Nieuw Amerongen AV. Salivary lactoferrin and low-Mr mucin MG2 in Actinobacillus actinomycetemcomitans-associated periodontitis. J Clin Periodontol 1999;26:269–275. [DOI] [PubMed] [Google Scholar]
- 22.Baughan LW, Robertello FJ, Sarrett DC, Denny PA, Denny PC. Salivary mucin as related to oral Streptococcus mutans in elderly people. Oral Microbiol Immunol 2000;15:10–14. [DOI] [PubMed] [Google Scholar]
- 23.Loesche WJ, Rowan J, Straffon LH, Loos PJ. Association of Streptococcus mutants with human dental decay. Infect Immun 1975;11:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajishengallis G, Michalek SM. Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol 1999;14:1–20. [DOI] [PubMed] [Google Scholar]
- 25.Retz M, Lehmann J, Amann E, Wullich B, Roder C, Stockle M. Mucin 7 and cytokeratin 20 as new diagnostic urinary markers for bladder tumor. J Urol 2003;169:86–89. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Zhao YH, Di YP, Wu R. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino-terminal and 5′-flanking region. Am J Respir Cell Mol Biol 2001;25:542–553. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Gallup M, Fan N, Szymkowski DE, Basbaum CB. Cloning of the amino-terminal and 5′-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J Biol Chem 1998;273:6812–6820. [DOI] [PubMed] [Google Scholar]
- 28.Gum JR, Hicks JW, Kim YS. Identification and characterization of the MUC2 (human intestinal mucin) gene 5′-flanking region: promoter activity in cultured cells. Biochem J 1997;325:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Seuningen I, Perrais M, Pigny P, Porchet N, Aubert JP. Sequence of the 5′-flanking region and promoter activity of the human mucin gene MUC5B in different phenotypes of colon cancer cells. Biochem J 2000;348:675–686. [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Intini G, Bobek LA. Modulation of MUC7 mucin expression by exogenous factors in airway cells in vitro and in vivo. Am J Respir Cell Mol Biol 2006;35:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borchers MT, Carty MP, Leikauf GD. Regulation of human airway mucins by acrolein and inflammatory mediators. Am J Physiol 1999;276:L549–L555. [DOI] [PubMed] [Google Scholar]
- 32.Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta 1998;1406:251–259. [DOI] [PubMed] [Google Scholar]
- 33.Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, Nadel JA, Prince A, Basbaum CB. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA 1997;94:967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine SJ, Larivee P, Logun C, Angus CW, Ognibene FP, Shelhamer JH. Tumor necrosis factor-alpha induces mucin hypersecretion and MUC-2 gene expression by human airway epithelial cells. Am J Respir Cell Mol Biol 1995;12:196–204. [DOI] [PubMed] [Google Scholar]
- 35.Tsutsumi-Ishii Y, Nagaoka I. Modulation of human beta-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J Immunol 2003;170:4226–4236. [DOI] [PubMed] [Google Scholar]
- 36.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem 2002;234–235:239–248. [PubMed] [Google Scholar]
- 37.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal 2005;17:1203–1217. [DOI] [PubMed] [Google Scholar]
- 38.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989;77:51–59. [DOI] [PubMed] [Google Scholar]
- 39.Van Seuningen I, Pigny P, Perrais M, Porchet N, Aubert JP. Transcriptional regulation of the 11p15 mucin genes: towards new biological tools in human therapy, in inflammatory diseases and cancer? Front Biosci 2001;6:D1216–D1234. [DOI] [PubMed] [Google Scholar]
- 40.Biesbrock AR, Bobek LA, Levine MJ. MUC7 gene expression and genetic polymorphism. Glycoconj J 1997;14:415–422. [DOI] [PubMed] [Google Scholar]
- 41.Bobek LA, Li H, Rojstaczer N, Jones C, Gross KW, Levine MJ. Tissue-specific expression of human salivary mucin gene, MUC7, in transgenic mice. Transgenic Res 1998;7:195–204. [DOI] [PubMed] [Google Scholar]
- 42.Khan SH, Aguirre A, Bobek LA. In-situ hybridization localized MUC7 mucin gene expression to the mucous acinar cells of human and MUC7-transgenic mouse salivary glands. Glycoconj J 1998;15:1125–1132. [DOI] [PubMed] [Google Scholar]
- 43.Berger JT, Voynow JA, Peters KW, Rose MC. Respiratory carcinoma cell lines: MUC genes and glycoconjugates. Am J Respir Cell Mol Biol 1999;20:500–510. [DOI] [PubMed] [Google Scholar]
- 44.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1991;1072:129–157. [DOI] [PubMed] [Google Scholar]
- 45.Manna PR, Eubank DW, Stocco DM. Assessment of the role of activator protein-1 on transcription of the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol 2004;18:558–573. [DOI] [PubMed] [Google Scholar]
- 46.Sonis ST. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit Rev Oral Biol Med 2002;13:380–389. [DOI] [PubMed] [Google Scholar]
- 47.Das KC, White CW. Activation of NF-kappaB by antineoplastic agents: role of protein kinase C. J Biol Chem 1997;272:14914–14920. [DOI] [PubMed] [Google Scholar]
- 48.Zelivianski S, Glowacki R, Lin MF. Transcriptional activation of the human prostatic acid phosphatase gene by NF-kappaB via a novel hexanucleotide-binding site. Nucleic Acids Res 2004;32:3566–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson A, Troxler RF, Pena M, Kandil A, Berger J, Rose MC. Muc7 mucin glycoprotein is present in airway secretions of asthmatic, but not control, patients [abstract]. Am J Respir Crit Care Med 2003;167:A65. [Google Scholar]