Abstract
Quercetin (3,3′,4′,5,7-pentahydroxyflavone), a dietary flavonoid, is an inhibitor of phosphatidylinositol (PI) 3-kinase and potent antioxidant. We hypothesized that quercetin blocks airway epithelial cell chemokine expression via PI 3-kinase–dependent mechanisms. Pretreatment with quercetin and the PI 3–kinase inhibitor LY294002 each reduced TNF-α–induced IL-8 and monocyte chemoattractant protein (MCP)-1 (also called CCL2) expression in cultured human airway epithelial cells. Quercetin also inhibited TNF-α–induced PI 3-kinase activity, Akt phosphorylation, intracellular H2O2 production, NF-κB transactivation, IL-8 promoter activity, and steady-state mRNA levels, consistent with the notion that quercetin inhibits chemokine expression by attenuating NF-κB transactivation via a PI 3-kinase/Akt-dependent pathway. Quercetin also reduced TNF-α–induced chemokine secretion in the presence of the transcriptional inhibitor actinomycin D, while inducing phosphorylation of eukaryotic translation initiation factor (eIF)-2α, suggesting that quercetin attenuates chemokine expression by post-transcriptional as well as transcriptional mechanisms. Finally, we tested the effects of quercetin in cockroach antigen–sensitized and –challenged mice. These mice show MCP-1–dependent airways hyperresponsiveness and inflammation. Quercetin significantly reduced lung MCP-1 and methacholine responsiveness. We conclude that quercetin blocks airway cell chemokine expression via transcriptional and post-transcriptional pathways.
Keywords: asthma, chemokines, epithelial cells, lung, signal transduction
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a dietary flavonoid found in many plants including onions, broccoli, apples, berries, and tea. Quercetin is the major flavonoid in the human diet, with an estimated average dietary intake in the United States of 25 mg/d (1). It is also present in extracts from Gingko biloba and St. Johns Wort, both popular health supplements.
Flavonoids share a common chemical structure consisting of two phenol rings linked through three carbons. Quercetin has potent antioxidant effects, combining with free radical species to form considerably less reactive phenoxy radicals. Quercetin also has inhibitory effects on several lipid, protein tyrosine, and serine/threonine kinases, including phosphatidylinositol (PI) 3-kinase, AMP-activated kinase, casein kinase 2, p90 ribosomal protein S6 kinase, p70 ribosomal S6 kinase (2), protein kinase C (3), epidermal growth factor receptor tyrosine kinase (4), and IκB kinase (5). Indeed, the design of the synthetic PI 3-kinase inhibitor LY294002 was based on the structure of quercetin (6).
Accordingly, quercetin has been shown to have potent effects in diverse biological systems. These include antiproliferative and proapoptotic effects for many cancer or preneoplastic cell lines, anti-inflammatory effects, and protection against oxidative stress. Focusing on the potential benefit of quercetin in the treatment of airways disease, several studies have demonstrated an inhibitory effect on cytokine or chemokine production in cultured cells. In the most well-studied system, quercetin has been shown to attenuate lipopolysaccharide-induced nitric oxide production, inducible nitric oxide synthase expression, and release of TNF-α and IL-6 in RAW 264.7 macrophages (7–10). In these studies, quercetin strongly reduced activation of mitogen-activated protein kinases and NF-κB (10, 11), a transcription factor complex known to play a critical role in the expression of proinflammatory genes. Further studies showed that luteolin, a related flavonoid, interfered with the phosphorylation of Akt, a downstream effector of PI 3-kinase, as well as NF-κB activation (9). Quercetin has also been noted to have inhibitory effects on mast cell activation and release of histamine, TNF-α, IL-6, and IL-8 (12–14). Quercetin inhibits the induction of IL-8 and monocyte chemoattractant protein (MCP)-1 by TNF-α in cultured human synovial cells (15). EM-X, a quercetin-containing mixture derived from the ferment of unpolished rice, papaya, and seaweed, inhibits both H2O2- and TNF-α–induced IL-8 expression in cultured human alveolar epithelial A549 cells (16). Since TNF-α, a proinflammatory cytokine; IL-8, a potent C-X-C chemokine with the neutrophil chemoattracting E-L-R motif; and MCP-1, a C-C (β) chemokine shown to be an important mediator of monocyte and CD4+/CD8+ lymphocyte recruitment (17), are each increased in the airways of patients with asthma (18–23), these data are consistent with the notion that quercetin may reduce airway inflammation in this disease. We therefore examined the effect of quercetin on chemokine expression in human airway epithelial cells and cockroach antigen–sensitized mice.
MATERIALS AND METHODS
Cell Culture
16HBE14o- human bronchial epithelial cells originating from bronchial epithelial tissue transfected with pSVori-, containing the origin-defective SV40 genome, were provided by Dr. Steven White (University of Chicago, Chicago, IL). Cells were grown in MEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 200 mM of l-glutamine.
Human primary airway epithelial cells obtained from the tracheal trimmings of donor lungs at the time of double lung transplantation (at the University Health Network, Toronto, ON, Canada) were cultured in collagen-coated plates, as previously described (24). Passage one cells were grown under submerged conditions using bronchial epithelial cell culture media (Cambrex, East Rutherford, NJ). Cell viability was determined by trypan blue exclusion.
Measurement of IL-8 and MCP-1 Protein Levels
16HBE14o- or primary cells were grown to 80% confluence, serum-starved for 24 h, and then pretreated with different concentrations of quercetin (0.1–25 μM), the PI 3-kinase inhibitor LY294002 (10 μM), the antioxidant diphenyl iodonium (DPI, 5 μM) (all from Sigma Chemical, St. Louis, MO) or carrier (DMSO). This preparation of quercetin was analyzed by high-performance liquid chromatography (HPLC) and found to be 99.968% pure (Integrated Biomolecules, Phoenix, AZ). After 1 h, cells were stimulated with TNF-α (10 ng/ml). Conditioned medium was collected 24 h after TNF-α treatment, centrifuged to remove cell debris, and then frozen at −80°C. In selected experiments, after overnight stimulation with TNF-α, cells were treated with the transcriptional inhibitor actinomycin D (5 mg/ml) in the presence or absence of quercetin or LY294002, and conditioned medium collected 8 h after actinomycin D treatment. IL-8 and MCP-1 protein levels were measured by ELISA (R&D Systems, Minneapolis, MN).
Northern Analysis
Total RNA, prepared from 16HBE14o- cells using Trizol reagent (Invitrogen, Carlsbad, CA), was electrophoresed on 1.25% SeaKem Gold Agarose gels (Reliant RNA Gel System; Cambrex, Rockland, MN) and then transferred onto Immobilin-NY Plus membranes (Millipore, Bedford, MA). Probe template was prepared from RNA by RT-PCR with the IL-8 primers (sense) 5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′ and (antisense) 5′-TCT CAG CCC TCT TCA AAA ACT TCT C-3′ and the MCP-1 primers (sense) 5′-GCC TTA AGT AAT GTT AAT TCT TAT- 3′and (antisense) 5′-GGT GTA ATA GTT ACA AAA TAT TCA-3′. 32P-radiolabeled probes were prepared by using the Ready-To-Go DNA labeling beads (-dCTP; Amersham-Pharmacia, Piscataway, NJ). Probe hybridization was performed with Ultrahyb buffer as recommended by the manufacturer (Amersham-Pharmacia).
PI 3-Kinase Assay
16HBE14o- cells were immunopreciptated with anti-phosphotyrosine (clone 4G10; Upstate Biotechnology, Waltham, MA). Precipitates were incubated with PI and [γ32P]-ATP for 30 min at room temperature. The reaction was terminated with 20 μl 6N HCl and 150 μl of chloroform/methanol (1:1). The organic phase was then separated by centrifugation at 8,000 rpm for 3 min. Lipids were dissolved in 15 μl of chloroform/methanol (95:5) and exposed to thin layer chromatography. Plates were dried and activity was assessed by autoradiography.
Immunoblotting
16HBE14o- cells were lysed, cellular proteins were resolved by 10% or 7% SDS-PAGE, and proteins transferred to a nitrocellulose membrane. Membranes were probed with antibodies against Ser473 phospho-Akt, Akt, Ser32/36 phospho-IκBα, IκBα, Ser51 phospho-eukaryotic translation initiation factor (eIF)-2α, eIF2α (all from Cell Signaling, Beverly, MA), and β-actin (Sigma Chemical). Signals were amplified and visualized with horseradish peroxidase–conjugated secondary antibody (Bio-Rad, Hercules, CA) and chemiluminescence solution (Pierce, Rockford, IL).
Measurement of Reactive Oxygen Species
96-well plates were seeded with 5,000 16HBE14o- human bronchial epithelial cells per well. After 24 h, the cells were treated with quercetin, LY294002, DPI, or DMSO overnight in serum- and phenol-free Dulbecco's modified Eagle's medium. Subsequently, cells were loaded with 200 μl of HBSS (10 mM HEPES, pH 7.5, 160 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) containing 9 μM 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate, acetyl ester (Molecular Probes, Eugene, OR) dissolved in anhydrous DMSO for 15 min at 37°C. The dye was removed and cells were incubated with 200 μl of serum- and phenol red-free media for another 15 min to stabilize the dye. Cells were then stimulated with TNF-α (10 ng/ml). After 60 min, media were removed, 100 μl HBSS was added to the wells, and the plate was read in a fluorimeter adjusted at an excitation wavelength of 485 nm and emission wavelength of 538 nm. Wells unloaded with dye were used as blank for calculations.
Electrophoretic Mobility Shift Assay
NF-κB consensus oligonucleotide was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and radiolabeled with [γ-32P]-ATP and T4 polynucleotide kinase (New England Biolabs, Ipswich, MA). Nuclear extracts were obtained from 16HBE14o- cells and electrophoretic mobility shift assay (EMSA) was conducted as previously described (25).
Measurement of NF-κB Transactivation and IL-8 Reporter Activity
Reporter plasmids encoding the IL-8 promoter NF-κB site, NF-κB(3)/Luc, and the −162/+44 fragment of the human IL-8 promoter (−162/+44 hIL8/Luc) were provided by Dr. Allan Brasier (University of Texas, Galveston, TX) (26). pRL family Renilla Luciferase plasmid was purchased from Promega (Madison, WI). 16HBE14o- cells were grown to 50% confluence, washed in Optimem and incubated with plasmid DNA, Lipofectamine and Optimem (each from Invitrogen). After 4 h, the solution was replaced with MEM supplemented with 10% FBS. The next morning, cells were serum-starved for 24 h. Cells were then treated with quercetin (0.1–25 μM), LY294002 (10 μM) or vehicle alone for 1 h. Subsequently, cells were incubated with TNF-α (10 ng/ml) for 24 h and harvested for analysis. Luciferase activity was measured using a luminometer. Changes in promoter activity were normalized for transfection efficiency by dividing luciferase light units by renilla luciferase light units.
Cockroach Sensitization and Induction of the Airway Response
Female BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained under standard pathogen-free conditions in the University of Michigan animal facility. Mice were initially sensitized with 0.1 ml of a 1:1 mixture of 100 mg/ml cockroach antigen (Bayer Corporation, Elkhart, IN) and incomplete Freund's adjuvant (Sigma Chemical) both subcutaneously and intraperitoneally on Day 1 (27, 28). To localize the response to the lungs, mice were given an intranasal challenge of 10 μg cockroach allergen in 10 μl diluent on Day 14. Mice were then rechallenged on Day 20 with intratracheal administration of 10 μg cockroach allergen in 50 μl sterile PBS. Mice were treated with quercetin dihydrate (0.06–2 mg per animal) dissolved in propylene glycol or propylene glycol only (vehicle) each morning for 3 d, starting from Day 20 (before intratracheal challenge) to Day 22. Pilot studies demonstrated that propylene glycol increases enteric absorption of quercetin. Quercetin was administered using mouse gavage feeding needle. On the day of challenge, quercetin was given at least 2 h before intratracheal instillation of allergen.
Measurement of Airway Hyperreactivity
Airway hyperreactivity was measured using a Buxco mouse plethysmograph, which is specifically designed for low tidal volumes (Buxco, Troy, NY) as previously described (27). Briefly, mice were anesthetized with sodium pentobarbital and intubated via cannulation of the trachea with an 18-gauge metal tube. Mice were subsequently ventilated with a Harvard pump ventilator and the tail vein was cannulated with a 27-gauge needle for injection of the methacholine challenge. The plethysmograph was sealed, and readings were monitored by computer. To determine an optimal dose of methacholine to be used for the experiment, a dose–response curve (0.001–0.05 mg) was determined. After methacholine challenge, the response was monitored and the peak airway resistance (cm H2O/ml/s) was recorded as a measure of airway reactivity.
Measurement of Lung MCP-1 Levels
Whole lung homogenates prepared from sensitized animals challenged in presence or absence of quercetin were used to determine protein level. Briefly, lung tissue was homogenized on ice with a tissue tearer (Biospec Products, Racine, WI) for 30 s in 1 ml PBS containing 0.05% Triton X-100. Cell-free supernatant obtained after centrifugation (10,000 × g) was applied to flat-bottom 96-well microtiter plate (Nunc, Roskilde, Denmark) coated with 50 μl/well rabbit anti–MCP-1 polyclonal Abs for 1 h at 37°C. Plates were rinsed four times with wash buffer and incubated with strepavidin-peroxidase conjugate (Bio-Rad) for 30 min at 37°C. Plates were washed again and incubated with chromogen substrate (Bio-Rad) at room temperature to the desired extinction. The reaction was terminated with 50 μl/well 3 M H2SO4 solution, and the plates were read at 490 nm in an ELISA reader.
Measurement of Plasma Quercetin Metabolites
Mice were killed and blood withdrawn by cardiac puncture. Blood was collected in tubes with anticoagulant, centrifuged, and the plasma collected and frozen in dry ice for subsequent analysis. The extraction of quercetin and hydrolysis of conjugates in plasma samples was performed by a modification of the procedure described by Erlund and coworkers (29). Quercetin conjugates were hydrolyzed by incubating 0.2 ml of EDTA plasma with 55 μl of 0.78 M sodium acetate buffer (pH 4.8), 50 μl of 0.1 M ascorbic acid, 5 μl of 0.6 M naringenin (internal standard), and 20 μl of nondiluted Helix pomatia (type HP-2; Sigma Chemical) for 17 h at 37°C. Each sample was diluted with 2 ml of phosphate buffer (50 mM, pH 3.0) and added to a Bond Elut C18 solid phase extraction column, pre-conditioned with 6 ml of methanol and 6 ml of phosphate buffer using a multiple manifold vacuum system (Burdick and Jackson, Muskegon, MI). Flavanones from plasma samples were eluted with 4 ml of methanol, dried under a flow of nitrogen, and supplemented with 300 μl of methanol and 100 μl of 5.3 M acetic acid/32 mM oxalic acid (80:20, vol/vol) (pH 2.4). The tubes were centrifuged for 5 min at 15,000 rpm, filtered using a 0.2-μm nylon syringe filter, and placed in 300-μl HPLC vials for analysis. Extraction of tissue samples for quercetin metabolite HPLC has been described previously (30).
The separation and quantification of flavonoids was performed by HPLC with coulometric electrochemical detection using a Supelcosil LC-18-T column heated to 37°C (15 mm × 4.6 mm, 3 μm; Supelco, Belefonte, PA) on the ESA CoulArray Model 5600A dual pump system (ESA, Inc., Chelmsford, MA). The electrochemical detector cell potentials were set as follows: 100, 200, 300, 400, 500, 600, 700, 800 mV. The sample was injected and equilibrated for 5 min with mobile phase A (50 mM phosphate buffer pH 3.0) at 0.5 ml/min. Flavonoids were eluted during a gradient of flow to 40% mobile phase B (acetonitrile: 50 mM phosphate buffer, pH 3.0: methanol, 50:30:20, vol/vol/vol) that occurs at 0.6 ml/min for 40 min. This was followed by a gradient to 100% mobile phase B over 7 min at 0.8 ml/min. Remaining nonpolar constituents were eluted by further wash of the column with mobile phase B for 10 min.
Data Analysis
Statistical significance was assessed by one-way ANOVA. Differences identified by ANOVA were pinpointed by the Student-Newman-Keul's multiple range text.
RESULTS
Effect of Quercetin on Human Bronchial Epithelial Cell Chemokine Release
16HBE14o- cells were pre-treated with quercetin, LY294002, or carrier, and stimulated with TNF-α. Release of IL-8 and MCP-1 was assessed. Quercetin concentrations as low as 0.1 μM blocked TNF-α–induced IL-8 protein abundance (Figure 1A). The inhibitory effect of quercetin was similar to that of LY294002, a specific inhibitor of PI 3-kinase. Neither DMSO, quercetin, nor LY294002 had a significant effect on cell viability (data not shown). DPI, an antioxidant and inhibitor of p47phox and NADPH oxidase (31), also inhibited IL-8 release. Quercetin, LY294002, and DPI blocked MCP-1 protein abundance in a similar manner (Figure 1B). These data suggest that PI 3-kinase activity and reactive oxygen are required for maximal TNF-α–induced airway epithelial cell IL-8 and MCP-1 release, and that quercetin may attenuate chemokine release via inhibition of PI 3-kinase or by its antioxidant effect. We also studied the effects of quercetin in primary human tracheal epithelial cells. Again, quercetin inhibited chemokine release (Figures 1C and 1D).
Figure 1.
Effect of quercetin on human bronchial epithelial cell chemokine expression. 16HBE14o- (A and B) and primary cells (C and D) were pretreated with either quercetin, LY294002, or carrier, and stimulated with TNF-α. Release of IL-8 and MCP-1 was assessed by ELISA. Quercetin concentrations as low as 0.1 μM blocked TNF-α–induced IL-8 protein abundance (A and C). Quercetin blocked MCP-1 protein abundance in a similar manner (B and D) (n = 3–4, mean ± SEM; *different from TNF-α, P < 0.001, ANOVA).
Quercetin Inhibits PI 3-Kinase/Akt Signaling in 16HBE14o- Human Bronchial Epithelial Cells
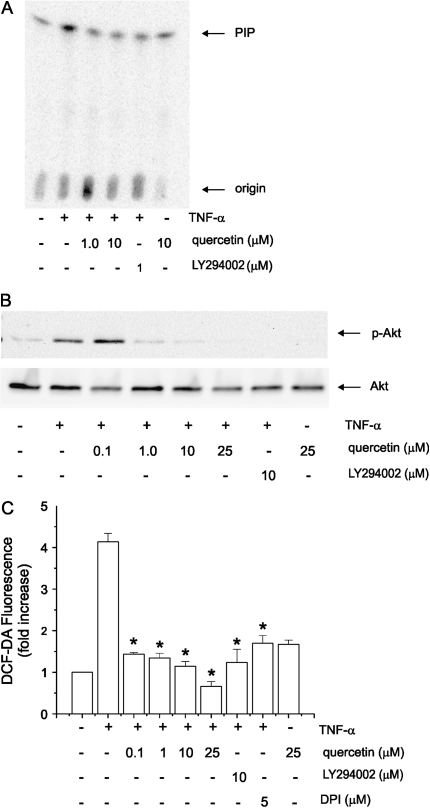
We examined the effects of quercetin on PI 3-kinase activity. Lysates from TNF-α–stimulated cells were immunoprecipitated with anti-phosphotyrosine antibody and the precipitates incubated with PI and [γ32P]-ATP. Lipids were separated by thin layer chromatography. Both quercetin and LY294002 reduced phosphorylation of PI, indicative of PI 3-kinase activity (Figure 2A).
Figure 2.
Quercetin inhibits PI 3-kinase/Akt signaling and intracellular reactive oxygen intermediates. Lysates from TNF-α–stimulated 16HBE14o- cells were immunoprecipitated with anti-phosphotyrosine antibody and the precipitates incubated with PI and [γ-32P]-ATP. Lipids were separated by thin layer chromatography. Both quercetin and LY294002 reduced phosphorylation of PI, indicative of PI 3-kinase activity (A). Quercetin also inhibited Akt phosphorylation in a dose-dependent manner (B). These results were typical of three separate experiments. Pretreatment with LY294002 and quercetin (concentrations as low as 0.1 μM) each decreased TNF-α–induced H2O2 concentration (C) (n = 3, mean ± SEM; *different from TNF-α, P < 0.001, ANOVA).
The serine-threonine kinase Akt, which binds 3,4-phosphorylated PIs through a pleckstrin homology domain, is a downstream effector of class 1A PI 3-kinase. Full Akt activity not only requires membrane localization, but also phosphorylation of Thr308 and Ser473. We therefore assessed the effects of quercetin and LY294002 on Akt Ser473 phosphorylation by immunoblotting. Quercetin inhibited Akt phosphorylation in a dose-dependent manner (Figure 2B). Together, these results suggest that quercetin attenuates PI 3-kinase/Akt signaling in airway epithelial cells.
Effect of Quercetin on Intracellular Reactive Oxygen Intermediates
We tested the effects of quercetin on intracellular H2O2 generation. 16HBE14o- cells were loaded with 2′,7′-dichlorofluorescin diacetate in HBSS buffer for 15 min. Dye was removed and cells were treated with TNF-α. At the end of the time course, the medium was replaced with HBSS buffer and fluorescence measured immediately using an absorbance microplate reader. TNF-α increased intracellular H2O2 production over 4-fold (Figure 2C). Pretreatment with LY294002 and quercetin (concentrations as low as 0.1 μM) each decreased H2O2 concentration.
Effect of Quercetin on NF-κB Signaling in 16HBE14o- Human Bronchial Epithelial Cells
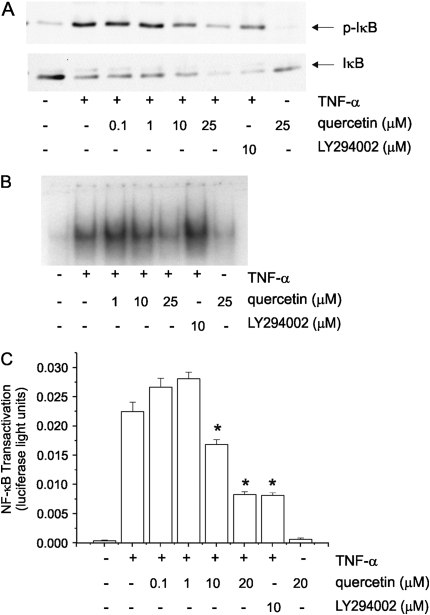
TNF-α stimulation induces phosphorylation of IκBα by IκB kinase. Phosphorylation, ubiquitination, and degradation of IκB, in turn, permits release of NF-κB for translocation to the nucleus and binding to DNA. Maximal NF-κB–mediated gene expression also requires the transactivation of the NF-κB p65 RelA subunit. We therefore examined the effect of quercetin on TNF-α–induced IκBα phosphorylation and degradation. As expected, TNF-α caused Ser32/36 phosphorylation of IκBα and a reduction in the protein abundance of IκBα due to degradation. However, quercetin only minimally reduced IκBα Ser32/36 phosphorylation, and only at high concentrations (Figure 3A). Subsequently, there was no change in the loss of IκBα protein abundance. Consistent with this, quercetin had little effect on TNF-α–induced NF-κB DNA binding, as measured by EMSA (Figure 3B). Using a multimeric reporter plasmid encoding the IL-8 promoter NF-κB site, we found that quercetin attenuated NF-κB transactivation (Figure 3C). Together, these data suggest that quercetin inhibits TNF-α–induced chemokine expression in part by attenuating NF-κB transactivation but not nuclear translocation, likely via a PI 3-kinase/Akt-dependent pathway.
Figure 3.
Effect of quercetin on NF-κB signaling. We examined the effect of quercetin on TNF-α–induced IκB phosphorylation and degradation in 16HBE14o- cells by immunoblotting (A). Quercetin only minimally reduced IκBα Ser32/36 phosphorylation, and only at high concentrations. Subsequently, there was no change in the loss of IκBα protein abundance. Under these circumstances, one would not expect quercetin to attenuate translocation of NF-κB to the nucleus or DNA binding (B). Quercetin and LY294002 in concentration of 10 μM significantly reduced NF-κB promoter activity (C) (n = 3, mean ± SEM; *P < 0.001 compared with TNF-α, ANOVA).
Effect of Quercetin on Chemokine Transcription
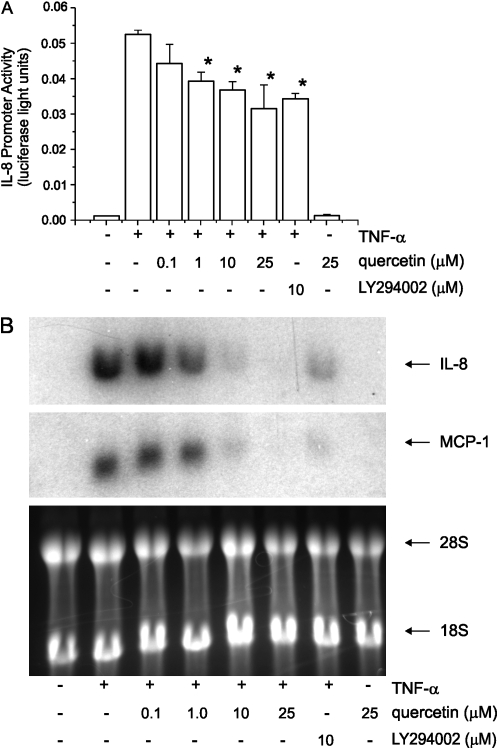
We examined the effects of quercetin on IL-8 promoter activity (Figure 4A) as well as IL-8 and MCP-1 steady-state mRNA levels. Quercetin blocked TNF-α–induced chemokine expression (Figure 4B).
Figure 4.
Effect of quercetin on chemokine transcription. 16HBE14o- bronchial epithelial cells transiently transfected with the −162/+44 fragment of the full-length human IL-8 promoter subcloned into a luciferase reporter, and pretreated either with quercetin (A), LY294002 or vehicle were incubated with TNF-α for an additional 24 h. Quercetin (1μM) significantly reduced IL-8 promoter activity (n = 4, mean ± SEM; *different from TNF-α, P < 0.05, ANOVA). Northern analysis of 16HBE14o- cells pretreated with either quercetin, LY294002, or vehicle, and stimulated with TNF-α for 6 h (B). Quercetin and LY294002 each significantly decreased IL-8 and MCP-1 mRNA levels. 28 and 18 S rRNAs are shown as a loading control. Data are representative of two independent experiments.
Quercetin Inhibits Expression of IL-8 and MCP-1 in a Post-Transcriptional Manner
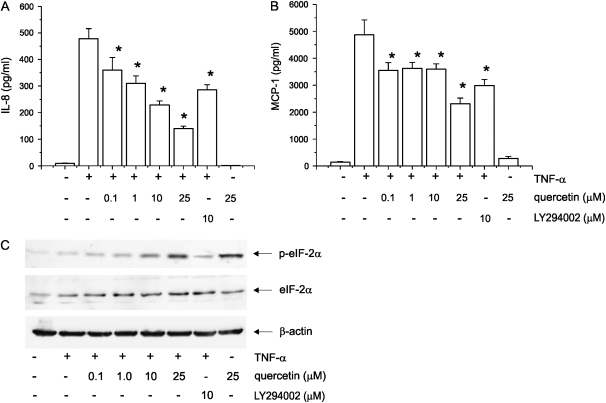
After overnight stimulation with TNF-α (10 ng/ml) to increase the number of IL-8 and MCP-1 transcripts, 16HBE14o- cells were treated with the transcriptional inhibitor actinomycin D (5 mg/ml) in the presence or absence of quercetin. Conditioned medium was collected 8 h later, and IL-8 and MCP-1 protein levels were measured by ELISA (Figure 5A). Quercetin inhibited both IL-8 and MCP-1 expression starting at concentrations of 0.1 μM, suggesting that quercetin attenuates TNF-α–induced chemokine expression in a post-transcriptional manner.
Figure 5.
Effect of quercetin on IL-8 and MCP-1 translation. 16HBE14o- bronchial epithelial cells stimulated with TNF-α overnight and treated with actinomycin D in presence or absence of quercetin, LY294002, and vehicle. Quercetin in low concentrations significantly decreased both IL-8 and MCP-1 release (A) (n = 5, mean ± SEM; *P < 0.01 compared with TNF-α, ANOVA). Immunoblot analysis of 16HBE14o- bronchial epithelial cells treated either with quercetin or LY294002 or vehicle in presence of TNF-α (B). Quercetin induced phosphorylation of eIF-2α. Data are representative of three independent experiments.
Under stressful conditions, phosphorylation of eIF-2α converts it into a competitive inhibitor, reducing global protein synthesis. Quercetin increased phosphorylation of eIF2α (Figure 5B), consistent with the notion that quercetin attenuates chemokine expression by inhibiting protein synthesis. Interestingly, the effects of quercetin on chemokine expression and eIF2α phosphorylation were greater than those of LY294002, suggesting that the inhibitory effect of quercetin on translation is, at least in part, independent of PI 3-kinase.
Quercetin Blocks Airways Hyperresponsiveness and Lung MCP-1 Protein Abundance in Cockroach-Sensitized Mice
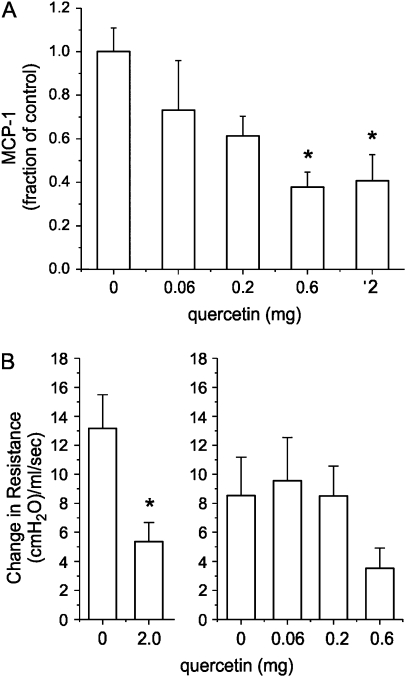
MCP-1 expression is increased in the lungs of cockroach-challenged mice, and is required for airways hyperresponsiveness (28). Further, MCP-1 is sufficient for airways hyperresponsiveness in normal, nonsensitized CBA/J mice. To establish whether quercetin might reduce chemokine expression in vivo as in does in vitro, we examined the effects of quercetin on cockroach allergen–induced MCP-1 expression, as well as on cockroach allergen–induced airway responsiveness. Female adult BALB/c mice were sensitized with cockroach antigen. On the morning of Days 20–22 of the protocol, animals were given 0.06–2 mg quercetin dihydrate in propylene glycol by mouse gavage feeding needle. On Day 21, all mice underwent intratracheal administration of cockroach allergen. On Day 22, animals were killed for analysis of lung MCP-1 and airway reactivity, as described (27). Quercetin reduced lung MCP-1 levels in a concentration-dependent manner (Figure 6A). At the 2 mg dose, average lung MCP-1 level decreased from 21.15 ng/ml/lung for vehicle to 8.64 ng/ml/lung for quercetin. Quercetin also significantly reduced peak airways resistance in response to methacholine (250 μg/kg), a measure of airway reactivity (Figure 6B). At the 2 mg dose, average peak airways resistance in response to methacholine decreased from 13.2 cm H2O/ml/s for vehicle to 5.4 cm H2O/ml/s. Baseline airways resistance before methacholine challenge averaged 0.91 cm H2O/ml/s.
Figure 6.
Quercetin blocks airways hyperresponsiveness and lung MCP-1 protein abundance in cockroach-sensitized mice. (A) Female adult BALB/c mice were sensitized with cockroach antigen. On the morning of Days 20–22 of the protocol, animals were given 0.06– 2 mg quercetin dihydrate in propylene glycol by mouse gavage feeding needle. On Day 21, all mice underwent intratracheal administration of cockroach allergen. On Day 22, mice were killed for measurement of lung MCP-1 and airway reactivity. Quercetin reduced MCP-1 levels in a concentration-dependent manner (A). Because of inter-experiment differences in ovalbumin challenge–induced MCP-1 levels, data are represented as a fraction of the mean MCP-1 level in challenged mice (n = 3–10, mean ± SEM; *different from vehicle alone, P ⩽ 0.011, ANOVA). Quercetin also significantly reduced peak airways resistance in response to methacholine (250 μg/kg), a measure of airway reactivity (n = 4– 11, mean ± SEM; *different from vehicle alone, P = 0.029, ANOVA). (B) Because of inter-experiment differences in methacholine-induced peak airways resistance, data from two cohorts of mice are shown separately.
Measurement of Quercetin Metabolites
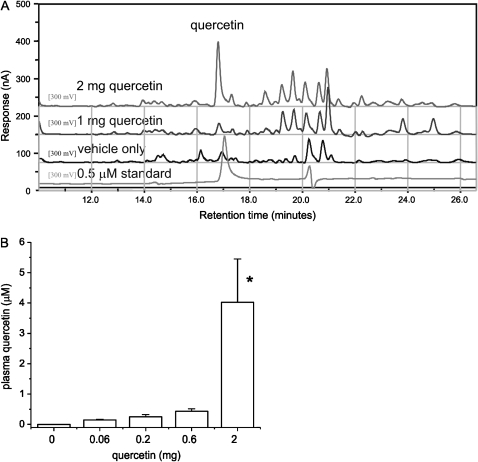
The separation and quantification of flavonoids was performed by HPLC with coulometric electrochemical detection. A typical chromatogram is shown in Figure 7A. Analysis revealed the presence of one large peak corresponding to a quercetin standard. Analysis also showed the presence of minor peaks, which may represent O-methylated or oxidation products of quercetin that would be slightly more hydrophobic and elute at a later times. Doses of 2 mg/d provided serum levels in the μM range, approximating those needed for anti-inflammatory activity in vitro (Figure 7B).
Figure 7.
Measurement of quercetin metabolites. (A) The separation and quantification of flavonoids was performed by HPLC with coulometric electrochemical detection. A typical chromatogram including samples from treated mice and a standard is shown. Analysis revealed the presence of one large peak corresponding to a quercetin standard and minor peaks that may represent O-methylated or oxidation products of quercetin. (B) Serum quercetin concentrations, group mean data (n = 3–5, mean ± SEM; *different from vehicle alone, P = 0.008, ANOVA).
DISCUSSION
We have shown that quercetin reduces TNF-α–stimulated IL-8 and MCP-1 protein abundance in both 16HBE14o- and primary airway epithelial cells. Consistent with its known inhibitory effect on PI 3-kinase (2, 6), quercetin attenuated PI 3-kinase activity and Akt phosphorylation. Quercetin also attenuated intracellular generation of reactive oxygen intermediates, consistent with its antioxidant effect. However, ample evidence exists that PI 3-kinase regulates Rho GTPase activation (32, 33). Of particular interest for airway inflammatory responses is the Rho GTPase Rac1, a constituent of the NADPH oxidase complex that generates reactive oxygen species such as H2O2 (34). It is therefore conceivable that quercetin indirectly attenuates reactive oxygen production by blocking PI 3-kinase.
PI 3-kinase and Akt have been shown to be required for the maximal activation of NF-κB in response to TNF-α and other stimuli (35–41). We therefore examined the effect of quercetin on NF-κB signaling. Quercetin had only minimal effects on IκBα Ser32/36 phosphorylation, IκBα protein abundance, or NF-κB DNA binding, while decreasing NF-κB transactivation. Together, these data suggest that quercetin inhibits TNF-α–induced chemokine expression in part by attenuating NF-κB transactivation but not nuclear translocation, likely via a PI 3-kinase/Akt-dependent pathway.
Based on the different concentrations of quercetin required to block chemokine mRNA expression (Figure 4) and protein expression (Figure 1), we examined the effects of quercetin on IL-8 and MCP-1 protein expression in the presence of the transcriptional inhibitor actinomycin D. The inhibitory effects of quercetin were maintained at concentrations as low as 0.1 μM. These data suggest that quercetin regulates chemokine expression at both the transcriptional and post-transcriptional levels.
eIF-2, a GTPase consisting of α, β, and γ subunits, functions to recruit methionyl tRNA and conduct it as a tRNA–eIF2–GTP ternary complex to the 40S ribosomal subunit, to form the 43S pre-initiation complex. eIF-2 GTP loading is determined by the activity of eIF-2B, a guanine nucleotide exchange factor. Under stressful conditions such as viral infection, heat shock, iron deficiency, and nutrient deprivation, phosphorylation converts eIF-2α into a competitive inhibitor of eIF-2B (42), reducing global protein synthesis by the formation of inactive eIF2α–eIF2B complexes. Quercetin increased phosphorylation of eIF-2α, consistent with the notion that quercetin attenuates chemokine expression by inhibiting protein synthesis. Quercetin has previously been shown to increase eIF-2α phosphorylation in mouse myeloid (43) and neuronal cells (44), though at much higher concentrations (50–100 μM).
In cockroach antigen–sensitized and –challenged mice, enteral administration of quercetin significantly reduced airway responsiveness and lung MCP-1 expression. In a previous study, neutralization of MCP-1 blocked cockroach sensitization–induced airways hyperresponsiveness, and MCP-1 was sufficient for airways hyperresponsiveness in normal, unsensitized mice (28). MCP-1 also elevated airway resistance within 1 h of direct intratracheal instillation, and increased levels of histamine and leukotriene C4 in the bronchoalveolar lavage fluid. In our study, quercetin pretreatment attenuated MCP-1 protein abundance both in vitro and in vivo, strongly suggesting this naturally occurring flavonoid blocks airways hyperresponsiveness by this mechanism. We have not ruled out other mechanisms, however, including the direct inhibition of mast cell mediator release (12–14) or a direct bronchodilatory effect, as has been shown in guinea pig airways (45).
In the present study, the effect of quercetin on airways hyperresponsiveness was achieved at dosages of 0.6 and 2 mg/d. The 2-mg dosage also provided serum levels in the μM range, approximating those needed for anti-inflammatory activity in vitro. Based on the relative surface areas of mice and humans, this dosage translates to ∼ 300 mg/m2, similar to that used previously in the treatment of allergies and prostatitis (46). However, it is conceivable that lower doses of quercetin may achieve μM serum levels in humans. In a previous study examining the absorption of quercetin, peak plasma levels of 0.8 μM were obtained after ingestion of a meal, including only 64 mg of quercetin glycosides (47).
Little information exists regarding the effects of quercetin on airway inflammation in vivo. In adult Swiss albino male mice, oral administration of quercetin (1 mg/d) significantly decreased alveolar macrophage superoxide production during influenza viral infection (48). In another influenza study, oral administration of quercetin to BALB/c male mice (1 mg/d) resulted in a significant decrease in leukocyte infiltration and the levels of both superoxide radicals and lipid peroxidation products (49). Most recently, it has been shown that intraperitoneal 3-O-methylquercetin significantly suppresses the enhanced pause (Penh) value induced by aerosolized methacholine after prolonged ovalbumin challenge in mice (45).
We conclude that quercetin blocks airway epithelial cell IL-8 and MCP-1 expression by attenuating signaling through a PI 3-kinase/Akt/NF-κB pathway. Quercetin also inhibits chemokine expression in a post-transcriptional manner. Finally, quercetin inhibits allergen sensitization–induced MCP-1 expression and airways hyperresponsiveness in vivo. Further animal and human studies examining the use for quercetin for the treatment of asthma appear to be warranted.
Acknowledgments
The authors thank Drs. Steven White (University of Chicago) and Allen Brasier (University of Texas, Galveston) for their gifts of 16HBE14o- cells and NF-κB(3)/Luc, respectively.
These studies were supported by National Institutes of Health grants AT002823 and HL56399, and a grant from the Cystic Fibrosis Foundation (M.B.H.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0149OC on June 22, 2006
Conflict of Interest Statement: S. N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.W.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B.H. is the recipient of a research grant from GlaxoSmithKline.
References
- 1.National Toxicology Program. Toxicology and carcinogenesis studies of quercetin (CAS No. 117–39–5) in F344 rats (feed studies). Natl Toxicol Program Tech Rep Ser 1992;409:1–171. [PubMed] [Google Scholar]
- 2.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000;351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, Payrastre B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol 1997;53:1649–1657. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y-T, Hwang J-J, Lee P-P, Ke F-C, Huang J-H, Huang C-J, Kandaswami C, Middleton E Jr, Lee M-T. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol 1999;128:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peet GW, Li J. IkappaB kinases alpha and beta show a random sequential kinetic mechanism and are inhibited by staurosporine and quercetin. J Biol Chem 1999;274:32655–32661. [DOI] [PubMed] [Google Scholar]
- 6.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H–1-benzopyran-4-one (LY294002). J Biol Chem 1994;269:5241–5248. [PubMed] [Google Scholar]
- 7.Wadsworth TL, Koop DR. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol 1999;57:941–949. [DOI] [PubMed] [Google Scholar]
- 8.Wadsworth TL, McDonald TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signaling pathways involved in the release of tumor necrosis factor-α. Biochem Pharmacol 2001;62:963–974. [DOI] [PubMed] [Google Scholar]
- 9.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther 2001;296:181–187. [PubMed] [Google Scholar]
- 10.Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem 2003;243:153–160. [DOI] [PubMed] [Google Scholar]
- 11.Mu MM, Chakravortty D, Sugiyama T, Koide N, Takahashi K, Mori I, Yoshida T, Yokochi T. The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. J Endotoxin Res 2001;7:431–438. [DOI] [PubMed] [Google Scholar]
- 12.Fox CC, Wolf EJ, Kagey-Sobotka A, Lichtenstein LM. Comparison of human lung and intestinal mast cells. J Allergy Clin Immunol 1988;81:89–94. [DOI] [PubMed] [Google Scholar]
- 13.Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy 2000;30:501–508. [DOI] [PubMed] [Google Scholar]
- 14.Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, Cetrulo CL, Theoharides TC. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol 2005;145:934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato M, Miyazaki T, Kambe F, Maeda K, Seo H. Quercetin, a bioflavonoid, inhibits the induction of interleukin 8 and monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha in cultured human synovial cells. J Rheumatol 1997;24:1680–1684. [PubMed] [Google Scholar]
- 16.Deiana M, Assunta Dessi M, Ke B, Liang Y-F, Higa T, Gilmour PS, Jen L-S, Rahman I, Aruoma OI. The antioxidant cocktail effective microorganism X (EM-X) inhibits oxidant-induced interleukin-8 release and the peroxidation of phospholipids in vitro. Biochem Biophys Res Commun 2002;296:1148–1151. [DOI] [PubMed] [Google Scholar]
- 17.Gunn M, Nelken N, Liao X, Williams L. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol 1997;158:376–383. [PubMed] [Google Scholar]
- 18.Ackerman V, Marini M, Vittori E, Bellini A, Vassali G, Mattoli S. Detection of cytokines and their cell sources in bronchial biopsy specimens from asthmatic patients: relationship to atopic status, symptoms, and level of airway hyperresponsiveness. Chest 1994;105:687–696. [DOI] [PubMed] [Google Scholar]
- 19.Tillie-Leblond I, Pugin J, Marquette C-H, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel A-B, Gosset P. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med 1999;159:487–494. [DOI] [PubMed] [Google Scholar]
- 20.Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, Tonnel AB. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med 1998;157:394–402. [DOI] [PubMed] [Google Scholar]
- 21.Amin K, Ludviksdottir D, Janson C, Nettelbladt O, Bjornsson E, Roomans GM, Boman G, Seveus L, Venge P. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. Am J Respir Crit Care Med 2000;162:2295–2301. [DOI] [PubMed] [Google Scholar]
- 22.Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med 1996;153:1398–1404. [DOI] [PubMed] [Google Scholar]
- 23.Jahnz-Rozyk KM, Kuna P, Pirozynska E. Monocyte chemotactic and activating factor/monocyte chemoattractant protein (MCAF/MCP-1) in bronchoalveolar lavage fluid from patients with atopic asthma and chronic bronchitis. J Investig Allergol Clin Immunol 1997;7:254–259. [PubMed] [Google Scholar]
- 24.Sajjan U, Keshavjee S, Forstner J. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infect Immun 2004;72:4188–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Johnson XD, Iazvovskaia S, Tan A, Lin A, Hershenson MB. Signaling intermediates required for NF-kappa B activation and IL-8 expression in CF bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2003;284:L307–L315. [DOI] [PubMed] [Google Scholar]
- 26.Casola A, Garofalo RP, Jamaluddin M, Vlahopoulos S, Brasier AR. Requirement of a novel upstream response element in respiratory syncytial virus-induced IL-8 gene expression. J Immunol 2000;164:5944–5951. [DOI] [PubMed] [Google Scholar]
- 27.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol 1998;161:7047–7053. [PubMed] [Google Scholar]
- 28.Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J Immunol 1999;163:2160–2167. [PubMed] [Google Scholar]
- 29.Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr 2001;131:235–241. [DOI] [PubMed] [Google Scholar]
- 30.Mullen W, Graf BA, Caldwell ST, Hartley RC, Duthie GG, Edwards CA, Lean ME, Crozier A. Determination of flavonol metabolites in plasma and tissues of rats by HPLC-radiocounting and tandem mass spectrometry following oral ingestion of [2-(14)C]quercetin-4'-glucoside. J Agric Food Chem 2002;50:6902–6909. [DOI] [PubMed] [Google Scholar]
- 31.Radeke H, Cross A, Hancock J, Jones O, Nakamura M, Kaever V, Resch K. Functional expression of NADPH oxidase components (alpha- and beta- subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem 1991;266:21025–21029. [PubMed] [Google Scholar]
- 32.Wennstrom S, Hawkins P, Cooke F, Hara K, Yonesawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol 1994;4:385–393. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins PT, Eguinoa A, Qiu R-G, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol 1995;5:393–403. [DOI] [PubMed] [Google Scholar]
- 34.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 1991;353:668–670. [DOI] [PubMed] [Google Scholar]
- 35.Beraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappa B activation. Proc Natl Acad Sci USA 1999;96:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 1999;401:82–85. [DOI] [PubMed] [Google Scholar]
- 37.Romashkova JA, Makarov SS. NF-kappaB is a target of Akt in anti-apoptotic PDGF signalling. Nature 1999;401:86–90. [DOI] [PubMed] [Google Scholar]
- 38.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappa B p65/RelA subunit. Mol Cell Biol 1999;19:4798–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy SAG, Huang JH, Liao WS-L. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-κB activation. J Immunol 2000;164:1355–1363. [DOI] [PubMed] [Google Scholar]
- 40.Madrid LV, Wang C-Y, Guttridge DC, Schottelius AJG, Baldwin AS Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappa B. Mol Cell Biol 2000;20:1626–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madrid LV, Mayo MW, Reuther JY, Baldwin AS Jr. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the I kappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem 2001;276:18934–18940. [DOI] [PubMed] [Google Scholar]
- 42.Kimball SR, Heinzinger NK, Horetsky RL, Jefferson LS. Identification of interprotein interactions between the subunits of eukaryotic initiation factors eIF2 and eIF2B. J Biol Chem 1998;273:3039–3044. [DOI] [PubMed] [Google Scholar]
- 43.Ito T, Warnken SP, May WS. Protein synthesis inhibition by flavonoids: Roles of eukaryotic initiation factor 2α kinases. Biochem Biophys Res Commun 1999;265:589–594. [DOI] [PubMed] [Google Scholar]
- 44.Chang RC-C, Suen K-C, Ma C-H, Elyaman W, Ng H-K, Hugon J. Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2α; in neuronal degeneration. J Neurochem 2002;83:1215–1225. [DOI] [PubMed] [Google Scholar]
- 45.Ko WC, Shih CM, Chen MC, Lai YH, Chen JH, Chen CM, Lin CN. Suppressive effects of 3-O-methylquercetin on ovalbumin-induced airway hyperresponsiveness. Planta Med 2004;70:1123–1127. [DOI] [PubMed] [Google Scholar]
- 46.Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology 1999;54:960–963. [DOI] [PubMed] [Google Scholar]
- 47.Hollman PC, van Trijp JM, Mengelers MJ, de Vries JH, Katan MB. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett 1997;114:139–140. [DOI] [PubMed] [Google Scholar]
- 48.Raju TAN, Lakshmi ANV, Anand T, Rao LV, Sharma G. Protective effects of quercetin during influenza virus-induced oxidative stress. Asia Pac J Clin Nutr 2000;9:314–317. [DOI] [PubMed] [Google Scholar]
- 49.Kumar P, Sharma S, Khanna M, Raj HG. Effect of Quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infection. Int J Exp Pathol 2003;84:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]