Abstract
The amine- and peptide-producing pulmonary neuroendocrine cells (PNEC) are widely distributed within the airway mucosa of mammalian lung as solitary cells and innervated clusters, neuroepithelial bodies (NEB), which function as airway O2 sensors. These cells express Cftr and hence could play a role in the pathophysiology of cystic fibrosis (CF) lung disease. We performed confocal microscopy and morphometric analysis on lung sections from Cftr−/− (null), Cftr+/+, and Cftr+/− (control) mice at developmental stages E20, P5, P9, and P30 to determine the distribution, frequency, and innervation of PNEC/NEB, innervation and cell mass of airway smooth muscle, and neuromuscular junctions using synaptic vesicle protein 2, smooth muscle actin, and synaptophysin markers, respectively. The mean number of PNEC/NEB in Cftr−/− mice was significantly reduced compared with control mice at E20, whereas comparable or increased numbers were observed postnatally. NEB cells in Cftr null mice showed a significant reduction in intracorpuscular nerve endings compared with control mice, which is consistent with an intrinsic abnormality of the PNEC system. The airways of Cftr−/− mice showed reduced density (∼ 20–30%) of smooth muscle innervation, decreased mean airway smooth muscle mass (∼ 35%), and reduced density (∼ 20%) of nerve endings compared with control mice. We conclude that the airways of Cftr−/− mice exhibit heretofore unappreciated structural alterations affecting cellular and neural components of the PNEC system and airway smooth muscle and its innervation resulting in blunted O2 sensing and reduced airway tonus. Cftr could play a role in the development of the PNEC system, lung innervation, and airway smooth muscle.
Keywords: autonomic nervous system, CF, Cftr null mice, lung disease
Pulmonary neuroendocrine cells (PNEC) play a multifunctional role in lung homeostasis because they are widely distributed within the airway mucosa and produce amine(serotonin,5-HT) and a variety of neuropeptides (e.g., bombesin in humans and calcitonin gene-related peptide in rodents) (1). Recent studies suggest an important role for these cells during lung development (2), in O2 sensing (3, 4), and in the regeneration of distal pulmonary epithelium (5). Innervated clusters of PNEC, called neuroepithelial bodies (NEB), may function as airway O2 sensors because they express a membrane-bound molecular complex comprised of a multicomponent NADPH oxidase coupled to an O2-sensitive K+ channel (6, 7). Hypoxia, within a physiologic range of Po2, triggers a dose-dependent release of 5-HT from NEB cells that is modulated by L-type Ca2+ channels (8). Studies using multilabel immunohistochemistry and confocal microscopy have identified the complex innervation of NEB in keeping with their postulated role as multifunctional receptors (9). Recent studies of innervation in the developing rabbit lung revealed an extensive network of nerve fibers surrounding the airway smooth muscle with only occasional nerve fibers reaching the airway epithelium during the early stages of lung development (10). In developing lung, NEB and solitary PNEC became gradually innervated while the density of nerve fibers increases with progressing gestation peaking postnatally, consistent with a process of maturation. It has been observed that most intraepithelial nerve fibers exclusively contact PNEC/NEB cells, suggesting that specific neurotrophins could be secreted by these cells, thus directing their innervation (10).
It has been suggested that PNEC/NEB and their amine/peptide mediators could be involved directly or indirectly in the pathogenesis of cystic fibrosis (CF) lung disease (11, 12). In earlier studies, the expression of Cftr has been identified in NEB cells of rabbit lung (11) and in human tumor cell lines representative of PNEC/NEB in native lung (12). It has been demonstrated that downregulation of Cftr in a PNEC/NEB tumor cell model abolished hypoxia-induced 5-HT release, indicating possible involvement of Cftr in the process of neurosecretion and O2 sensing (12).
In the present study, we have investigated the distribution and frequency of PNEC/NEB and their innervation in a Cftr−/− mouse model (13) using single or multilabel immunohistochemistry combined with confocal microscopy and computer-assisted morphometry. We used immunostaining for synaptic vesicle protein 2 (SV2), a pan-neural marker, to demonstrate PNEC/NEB cells and airway neural networks (10). Because we have observed reduced tonus of the trachea in Cftr−/− mice compared with Cftr+/− or wild-type littermates (unpublished observation), we assessed the relative density of innervation of airway smooth muscle on lung sections immunostained for SV2 and airway smooth muscle mass using smooth muscle actin antibody and an antibody against synaptophysin to determine the density of neuromuscular junctions. Our studies show that lungs of Cftr null mice exhibit widespread alterations in airway neuroendocrine cell and neural components with decreased density of airway smooth muscle innervation, decreased airway smooth muscle mass, and decreased neuromuscular junctions. The potential significance of our findings in terms of possible alterations in airway function and pathogenesis of CF lung disease is discussed.
MATERIALS AND METHODS
Animals and Tissue Collection
Congenic C57BL/6J Cftr heterozygous breeding pairs were maintained on regular mouse chow and continuously bred. To maintain congenic status and to prevent genetic drift, each new generation of mice was bred to wild-type C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). Timed pregnant C57BL/6J CF mice at 15 or 20 d of gestation (E15, E20) and neonates on Days 5 and 9 (P5, P9) were maintained in the hospital animal facility. To minimize bowel obstruction and to optimize long-term viability, 30-d-old (P30) Cftr−/− mice and their Cftr+/− littermates were weaned on a liquid diet (Liquidet F3107; Bioserve, Frenchtown, NJ) using glass liquid mouse feeders. The liquid diet was prepared in sterile water according to the manufacturer's instructions (13, 14). Experimental protocols were conducted after review and approval by the institutional Animal Care Committee. Groups of Cftr−/− animals and their wild-type littermates were killed at different developmental stages (E15 [Cftr−/−, n = 4; Cftr+/−, n = 4], E20 [Cftr−/−, n = 3; Cftr+/−, n = 4], P5 to P9 [Cftr−/−, n = 5; Cftr+/−, n = 4], and P30 [Cftr−/−, n = 2; Cftr+/−, n = 2]). Mice were killed by intraperitoneal injection of sodium pentobarbital (30 mg/kg), and the fetuses were killed by decapitation. Dissected lungs were washed three times in CO2-independent medium and embedded in polyethylene glycol (OCT medium) (Lab-Tek Products; Naperville, IL) and then snap-frozen on dry ice. All tissue blocks were sealed and stored at −80°C until examination.
Tissue Preparation and Immunolabeling
The methods for tissue preparation and immunolabeling protocols were similar to those previously published (10). Briefly, cryostat sections of medial lobes were cut at 100 μm under low working temperature (−14°C), and the sections were immediately transferred to a dish containing zinc formalin fixative (Newcome Supply, Middleton, WI) at room temperature (RT). After three changes of fresh fixative (10 min each at RT), lung sections were washed in PBS and stored at 4°C. Immunofluorescence labeling was performed on sections permeabilized with 1% Triton X-100 in PBS for 10 min and incubated with 0.3% of H2O2 in PBS for 10 min to quench endogenous peroxidase. To block nonspecific binding and endogenous mouse IgG from tissues, the slices were incubated in 20% normal donkey serum in 4% BSA plus avidin/biotin blocking solution and mouse-on-mouse blocker (M.O.M.; Vector Labs, Burlingame, CA) for 60 min at RT. This was followed by incubation with primary antibodies, mouse monoclonal anti-SV2 antibody (1:200 dilution) (Developmental Studies Hybridoma Bank, Iowa City, IA), or rabbit monoclonal antisynaptophysin (1:100 dilution) (LabVision Corp., Fremont, CA) at 4°C overnight using an orbital shaker. As secondary antibodies we used donkey antibodies conjugated with horseradish peroxidase (HRP) (1:1,000 dilution) for SV2 or fluorescein isothiocyanate (FITC) (1:100 dilution) for 2 h at RT. To demonstrate airway smooth muscle cells, a mouse monoclonal anti–smooth muscle cell actin conjugated with FITC (1:100) (Sigma-Aldrich, Oakville, ON, Canada) was used.
The signal for SV2 immunolabeling was enhanced by a catalyzed reporter deposition amplification system as previously reported (10). The N-hydroxysuccinimide ester of biotin (sulfosuccinimidyl-6-[biotinimide] hexanoate) (BIO-NHS; Boehringer-Mannheim; Mannheim, Germany) was coupled to tyramide-HCl (Sigma) to produce a biotin/tyramide substrate for the HRP reaction. After incubation with HRP-conjugated secondary antimouse IgG antibody, this complex was amplified with the biotin/tyramide substrate in sodium borate buffer (pH 8.3) with 0.0003% H2O2 for 10 min at RT. To visualize the signal, we applied a 1:400 dilution of streptavidin-Texas Red X conjugate (Molecular Probes, Eugene, OR) for 30 min at RT.
Confocal Microscopy
Fluorescent images of PNEC/NEB cells, the airway nerves, and smooth muscle in the double-stained (FITC/Texas Red) whole mounts were obtained using a Leica confocal laser scanning microscope (model TCS 4D; Leica Lasertechnik, Heidelberg, Germany) with SCANWARE software (Leica). The excitation wavelengths of the krypton/argon laser were 488 nm for FITC and 568 nm for Texas Red. The whole lung slices were optically sectioned by scanning at increasing depths of focus (typically in steps of 1 μm) under a × 25 objective lens to follow the path of the neural network toward NEB or PNEC in relation to the airway smooth muscle. Many fields of each slice were scanned, and multiple areas were selected for detailed study of the NEB innervation under a ×64 objective lens. Representative images of PNEC/NEB at various gestational ages were acquired. The maximum intensity of the corresponding pixels in each optical section was used to generate a single image (2-D projection) from a stack of images obtained at certain depths (40 μm on average). The image of the nerves (converted to green color in Adobe Photoshop; Adobe Systems, San Jose, CA) was merged with that of the smooth muscle (converted to red color) to form a composite nerve/muscle image. FITC- or Texas Red–labeled images of nerves (including PNEC/NEB and ganglia) were merged with images of airway smooth muscle (Texas Red or FITC) to produce a dual-color nerve/smooth muscle airway image. Image processing, including merging and montage of fields. was performed using Adobe PhotoShop 6.0 software. Single fields were collated and assembled to create a comprehensive bronchial nerve/smooth muscle airway image. All airway diameters refer to the external diameter of the smooth muscle layer unless stated otherwise.
Morphometric Analysis
For quantification of PNEC/NEB, we used a modified method of Van Lommel and Lauweryns (14). The integrated surface area of bronchioles of different sizes, expressed in mm2 of the section (100 μm thickness), was measured using the NIH-Scion program (Scion Corp., Frederick, MD; http://rsb.info.nih.gov/nih-image) standardized by an internal scale bar in each counted confocal image. The numbers of PNEC/NEB were assessed in three sections of medial lobe immunostained for SV2. The total number of NEB and PNEC in each section was divided by the integrated surface area and the relative number expressed per mm3 of lung tissue based on the calculated volume of three 100-μm slices in each case. The actual standard areas measured were normalized by an internal scale bar in each confocal image. We also assessed NEB innervation because NEB are localized within the airway mucosa and are the only well defined epithelial cell component invested with a complex neural network (10). As a measure of intracorpuscular nerve density in NEB, we compared the percentage of NEB without apparent nerve fibers entering the corpuscle, NEB with a single nerve fiber, and NEB with multiple nerve fibers.
The densities of airway smooth muscle fibers at different developmental stages were measured by a line plot tool in the program. Morphometric analysis of the density of airway innervation was performed using NIH-Scion Image software with modifications (10, 15). The density of innervation of airway smooth muscle and submucosa was defined by the rectangular selection tool as integrated reading density per 50 μm2 of nerve immunoreactivity in airways of different sizes. The sizes of airways were determined from measurement of the internal diameter of the smooth muscle coat. The selected measured areas excluded large nerve trunks and PNEC/NEB. The color images were converted to black and white field and measured using NIH-Scion Image. The actual standard areas measured were normalized by an internal scale bar in each confocal image. The densities of airway smooth muscle fibers in lung samples from different ages were measured by a line plot tool in the program. The quantification of muscle fibers in 100 μm length was plotted and expressed as relative density.
The density of synapses (synaptophysin positive) was determined relative to the density of airway smooth muscle cells quantified using SimplePCI Advanced Image Software (Complix Image system, Cranberry Township, PA). Results were statistically analyzed as described below.
Statistical Analysis
One-way ANOVA (with repeated measures was used for statistical analysis of Cftr+/− and Cftr−/− nerve densities with respect to the different stages in development. One-way ANOVA tests with repeated measures were also used for comparison of PNEC/NEB numbers and integrated density of smooth muscle fiber in Cftr+/− and Cftr−/− animals. All data are expressed as means ± SEM or as means ± SD of the mean where appropriate.
RESULTS
PNEC/NEB Component
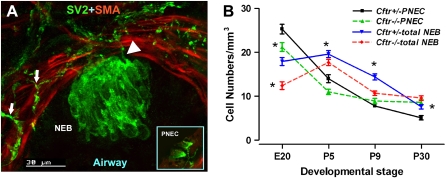
We used immunostaining with a pan neural/neuroendocrine marker SV2, to visualize single PNEC and NEB and the entire airway innervation (Figures 1A and 2A) (10). The first PNEC/NEB were detected within the primitive airway epithelium at E20 in Cftr null mice and in control mice. In lung sections from Cftr−/− mice at E20, the total number of single PNEC (21.2 ± 1.6 versus 25.3 ± 3.4; P < 0.05) and NEB (12.4 ± 2.1 versus 17.9 ± 2.5; P < 0.05) per mm3 of lung tissue was significantly reduced compared with age-matched control mice (Figure 1B). Postnatally, the total number of single PNEC in lungs of Cftr−/− and Cftr+/− mice were comparable, with no significant differences noted at P5 (17.1 ± 2.5 versus 15.5 ± 2.2; not significant [ns]) and P9 (8.9 ± 1.1 versus 7.8 ± 1.7; ns). At P30, the number of single PNEC was increased in the lungs of Cftr−/− mice (8.6 ± 0.9) compared with Cftr+/− littermates (5.1 ± 0.9; P < 0.05) (Figure 1B). The total number of NEB in lungs of Cftr−/− mice at P5 (17.7 ± 1.8) was less, but the difference was not statistically significant compared with their Cftr+/− littermates (19.6 ± 2.8; ns). The number then decreased significantly at P9 (10.7 ± 1.6 versus 14.4 ± 1.5; P < 0.05) (Figure 1B). As with single PNEC, the total number of NEB at postnatal age P30 was significantly higher in the lungs of Cftr−/− mice (9.6 ± 1.0) compared with Cftr+/− littermates (7.6 ± 0.8; P < 0.05) (Figure 1B).
Figure 1.
(A) Confocal microscopy image of section from control (Cftr+/−) mouse lung at P5 immunostained with antibodies against SV2 (green) and SMA (red). A large corpuscular NEB is located within the airway mucosa, and a complex network of nerve fibers (arrows) interdigitates between bundles of airway smooth muscle with some entering the NEB basal area (arrowhead). (Insert) Two triangular single PNEC within the mucosa of a larger airway in the same section. (B) Frequency of PNEC and NEB/mm3 in lungs of Cftr+/− compared with Cftr−/− mice during different developmental stages expressed as means ± SEM (*P < 0.05, Cftr+/− versus Cftr−/−; one-way ANOVA analysis, Newman-Keuls multiple comparison).
Airway Innervation
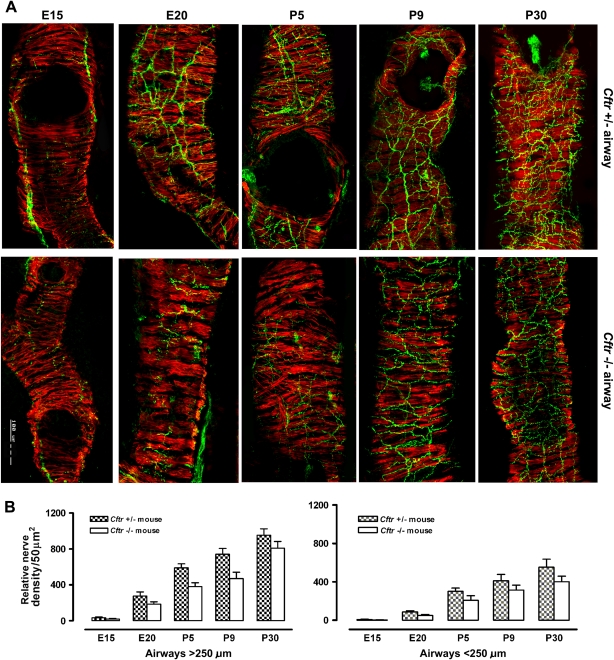
In our initial study of the nerve fiber density in the airways using SV2 as a marker, we compared wild-type (Cftr+/+) and heterozygous (Cftr+/−) with homozygous Cftr−/− mice. No differences were found between wild-type and heterozygous mice, whereas Cftr−/− mice showed significant reductions (Figure 2A). Because of the ready availability of heterozygous mice littermates, in all subsequent analyses we compared lung samples from Cftr+/− mice with Cftr−/− mice to determine whether these differences persisted throughout gestation. Lungs from midgestation (E15) through late gestation (E20) to postnatal days P5, P9, and P30 were examined. At E15, only the main airways in Cftr−/− and control lungs contained occasional nerve fibers cursing along the airway smooth muscle (Figure 2A). From E20 on, the overall density and complexity of the neural network in airways of Cftr−/− mice and their Cftr+/− littermates increased, but in Cftr−/− mice it seemed to be reduced compared with the age-matched control mice (Figure 2A). Morphometric analysis confirmed this impression, showing ∼ 25–30% reduction of the nerve fiber density in airways of different sizes (> < 250 μm) in the lungs of Cftr−/− mice compared with Cftr+/− littermates throughout the gestation and postnatally (Figure 2B). These findings indicate that the airway innervation in Cftr−/− mice is deficient from the earliest stages of lung development and persists into adulthood.
Figure 2.
(A) Composite photograph of confocal microscopy images of medium-size airways (100–200 μm) in the lungs of control (Cftr+/−) and Cftr null (Cftr−/−) mice during different developmental stages (E15, E20, P5, P9, P30) immunostained with SV2 and SMA antibodies. Well developed airway smooth muscle coat (red) is apparent even at an early (E15) developmental stage when only sparse nerve fibers (green) are noted. With progressing developmental stages, density and complexity of airway neural network increases in control but increases less in Cftr null mice. (B) Comparison of morphometric assessment of relative nerve density/50 μm2 within airway smooth muscle in different size airways in lungs of Cftr+/− versus Cftr−/− mice during development. Relative nerve density, expressed as means ± SD, in the airways of Cftr−/− mice is significantly reduced compared with Cftr+/− control mice (P < 0.001) in both airway sizes and during all developmental stages examined.
Innervation of NEB
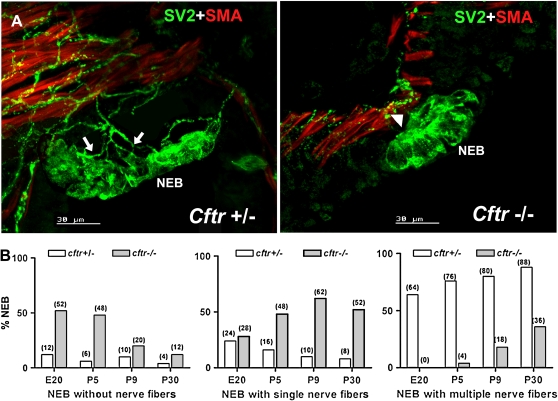
During all developmental stages, the innervation of NEB in the airways of Cftr−/− mice seemed to be reduced compared with control mice. This difference was most striking at P5, when NEB innervation in normal neonatal lung is fully developed (Figures 1A and 3A, left image). Lungs of Cftr−/− mice showed a higher percentage of NEB without apparent innervation and more numerous NEB with only a single nerve fiber entering it (Figures 3A, right image). Semiquantitative assessment of NEB innervation in Cftr−/− versus Cftr+/− mice confirmed globally reduced innervation of NEB in the former, with a higher percentage of NEB without apparent innervation or the presence of only a single nerve fiber (Figure 3B).
Figure 3.
(A) Pattern of innervation of individual NEB in control (Cftr+/−) compared with Cftr−/− mice lungs at the P5 stage. Numerous nerve fibers originating in a complex submucosal network are seen entering the NEB base (arrows) in a Cftr+/− lung (left image), whereas a sparse submucosal nerve fiber network with only a single fiber (arrowhead) at the NEB base is noted in a Cftr−/− lung (right) (double immunostaining for SV2 and SMA). (B) Semi-quantitative evaluation of NEB innervation (expressed as % NEB) in Cftr+/− compared with Cftr−/− mice during different developmental stages (E20, P5, P9, P30). NEB in the lungs of Cftr−/− mice are characterized by a higher percentage of NEB without nerve fibers or single nerve fiber, whereas in control (Cftr+/−) mice, the majority of NEB possess multiple nerve fibers throughout development.
Smooth Muscle Component
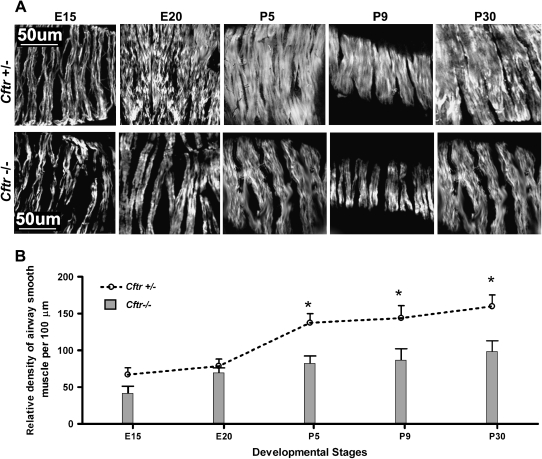
On cursory examination, the density of airway smooth muscle fibers, as defined by immunostaining for smooth muscle actin (SMA), also seemed to be reduced in Cftr−/− mice compared with age-matched control animals (Figure 4A). This impression was substantiated by morphometric analysis that showed differences from the earliest stages of lung development reaching statistical significance (P < 0.05) during the postnatal period and into adulthood. This finding supports our observation that tracheas of Cftr−/− mice have reduced muscle tone (unpublished observation).
Figure 4.
(A) Series of confocal microscopy images of airway smooth muscle immunostained for SMA in developing lungs from stages E15 to P30.Visually reduced smooth muscle mass in the airways (< 250 μm) of Cftr−/− mice compared with Cftr+/− control mice is evident at postnatal Days P5 through P30. (B) Morphometric assessment of relative density of smooth muscle fibers/100 μm expressed as means ± SEM during different developmental stages (E15, E20, P5, P9, P30). Statistically significant differences are noted at postnatal Days P5, P5, and P30 (*P < 0.05).
Density of Synapses and Neuromuscular Junctions
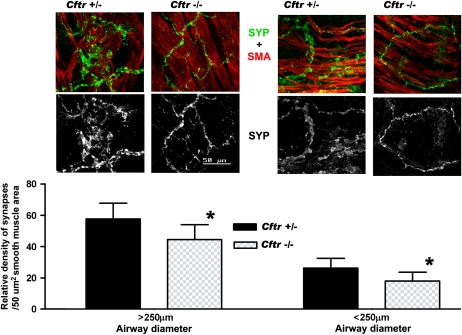
The deficiency in airway neural networks in Cftr−/− mice raised the possibility that neuromuscular synapses could be similarly affected. To examine this aspect, lung sections from mice at P5 were immunostained for synaptophysin alone or in combination with SMA (Figure 5A). Morphometric analysis of lung sections from Cftr−/− mice that were double immunostained for synaptophysin and SMA revealed a significant reduction in synaptophysin-positive neuromuscular synapses per defined airway smooth muscle area (50 μm) in large (> 250 μm) and small airways (< 250 μm) compared with their Cftr+/− littermates (Figure 5B).
Figure 5.
(A) Confocal microscopy images from different size airways (> 250 μm; < 250 μm) of Cftr+/− versus Cftr−/− mice at postnatal age P5 double immunostained for SMA to visualize airway smooth muscle and synaptophysin (SYP) to demonstrate neuromuscular junctions. Note the sparse synapses contacting smooth muscle fibers in the airway of the Cftr−/− mouse compared with its Cftr+/− littermate. Images for SYP immunolabeling were converted to black and white signal (lower photographs) and analyzed morphometrically (shown in B). (B) Quantitative assessment of relative density of synapses/50 μm of airway smooth muscle area expressed as means ± SD. Note the significant reduction in synapses in the airways of different diameters of Cftr−/− mice compared with Cftr+/−control mice (*P < 0.01).
DISCUSSION
CF is a multiorgan disease that affects predominantly the respiratory system, the pancreas, the gastrointestinal tract, and male reproductive organs (16). The basic molecular defect in CF involves a dysfunction or loss of Cftr chloride transport function, but modifier genes may play important roles in determining the severity of the disease phenotype associated with particular Cftr mutations (17). In spite of the progress made in discovering the gene defect in CF, our understanding of the pathobiology of CF lung disease is largely incomplete. In the present study, we have examined the Cftr null mouse model, which exhibits some of the histologic and physiologic changes found in humans with CF, including changes in lung structure and function (13). Our focus was the investigation of the possible role of the PNEC system and recently described extensive network of nerve fibers innervating the airways and PNEC/NEB (10) in the pathobiology of CF lung disease.
In earlier studies, hyperplasia of PNEC in lungs of patients with CF with chronic severe lung disease has been reported (18), as have increased urine levels of bombesin (19), a secretory product of PNEC/NEB. The hyperplastic response of PNEC/NEB in this setting was interpreted as secondary to the effects of chronic hypoxia/hypercapnia due to airway obstruction characteristic of late-stage CF lung disease.
In the present study, we found that in the lungs of Cftr null mice the number of single PNEC and NEB was reduced during early stages of lung development, suggesting an intrinsic abnormality in the PNEC system unrelated to the chronic hypoxia/hypercapnia and inflammatory process seen in patients with late-stage CF lung disease (18). Based on our findings of reduced numbers of NEB and their sparse innervation, we predict that the response to acute hypoxia stimulus in NEB cells of Cftr null mice will be blunted, leading to functional deficits. In addition, we expect that hypoxia-induced 5-HT release in NEB cells of Cftr−/− mice will be affected because downregulation of Cftr expression by antisense to Cftr inhibits hypoxia-induced serotonin release (12). Because NEB represent airway O2 sensors (analogous to carotid body chemoreceptors) involved in the control of breathing (2, 4), the previously mentioned abnormalities could partly account for recent observation of decreased ventilatory responses to hypoxia observed in Cftr−/− mice using whole-body plethysmography (20) and decreased compliance and increased airway resistance in the S489X Cftr−/− mouse (21). In the latter study, heterozygous mice showed increased lung compliance and decreased airway resistance, suggesting a possible developmental role for Cftr.
To what extent these findings relate to the clinical manifestations of CF is unknown. Patients with CF show impaired ventilatory control with blunted ventilatory response to hypoxia and hypercapnia (22). However, in this clinical setting these reduced ventilatory responses could be secondary to the abnormal respiratory load caused by chronic airway disease.
One of the limitations of the present study is that the immunolabeling with SV2 does not discriminate between different nerve subtypes or their origin. Innervation of the lung is complex because it includes several nerve subtypes and is subject to species variation (9). In the mouse lung, a variety of nerve fibers innervating the airways have been described, including peptidergic components (23), but their precise function is unknown. Future studies are required to further define specific nerve fiber subpopulations affected in the airways of Cftr null mice. Similarly, examination of other tissues and organs in Cftr−/− mice is warranted to determine if a generalized defect in innervation underlies a defect in Cftr function.
The developing airway, from the earliest stage, is invested by a smooth muscle coat that becomes progressively innervated (24). Maturation of this neural network, including the formation of functional neuromuscular junctions, generally follows the development of the smooth muscle coat (24). In Cftr−/− mice, we observed generalized reduction in the density of the nerve fiber network innervating airway smooth muscle and reduced smooth muscle mass, suggesting that the two processes may be interrelated, but the precise mechanism is unknown. Mhanna and colleagues (25) reported abnormal airway smooth muscle responses in Cftr−/− mice, showing impaired relaxation of a precontracted tracheal segment. These authors postulated that nitric oxide deficiency may contribute to this abnormality, but the precise mechanism is unknown. We can only speculate on possible explanations for our findings and on a unifying hypothesis that could account for these heretofore unappreciated structural airway abnormalities in Cftr−/− mice.
The potential role for Cftr in excitable cells, such as neurons, has been suggested by the finding of its expression in the medial preoptic area and the hypothalamus (26). At these sites, defective Cftr function could affect neurotransmission/neurosecretion because chloride conductances are critical for membrane repolarization (27). Whether Cftr is also involved in the development of the central and peripheral nervous system and/or the maturation of neural function remains to be explored. Nevertheless, our finding that the absence of functional Cftr affects lung innervation and potentially synapse formation could implicate Cftr in the process of neurogenesis. One possibility that could explain most of our findings could involve the process of neurosecretion, where inhibition of neurotrophin expression and/or secretion from a variety of lung cells that produce them (including PNEC/NEB) could lead to reduced airway innervation. The airway smooth muscle may be also affected because these cells are known to expresses glial-derived neurotrophic growth factor (28), whereas nerve growth factor and brain-derived nerve growth factor are produced by airway epithelium and immune cells, especially during inflammatory conditions (29). As to the relevance of our findings to the pathobiology of CF lung disease, the striking alterations in airway structure leading to altered airway tonus could directly contribute to persistent airway obstruction. In addition, innate airway immunity modulated by neurogenic signals (30) could also be affected in Cftr null mice because the innervation of their airway wall and mucosa is deficient. Further studies are required to determine if these changes observed in Cftr null mice are also found in the lungs of patients with CF, particularly during the early preinfectious stage. Because such tissue material is difficult to obtain, examination of transgenic mouse models with specific Cftr mutations (e.g., ΔF508) (31) may provide an excellent alternative and may provide a possibility for experimental approaches.
This work was supported by the Canadian Cystic Fibrosis Foundation. The mouse colony work was partially supported by a NIH SCOR grant to GK (NIDDK-2P50).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0468OC on April 13, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.VanLommel A, Bolle T, Faunes W, Lauweryns JM. The pulmonary neuroendocrine system: the past decade. Arch Histol Cytol 1999;62: 1–16. [DOI] [PubMed] [Google Scholar]
- 2.Sunday ME, Cutz E. The role of neuroendocrine cells in fetal and post-natal lung. In: Mendelson CR, editor. Endocrinology of the lung. Totowa, NJ: Humana Press; 2000. pp. 209–336.
- 3.Cutz E, Jackson A. Neuroepithelial bodies as airway oxygen sensors. Respir Physiol 1999;115:201–214. [DOI] [PubMed] [Google Scholar]
- 4.Cutz E, Fu XW, Yeger H, Peers C, Kemp PJ. Oxygen sensing in pulmonary neuroepithelial bodies and related tumour cell model. In: Lahiri S, Prabhakar H. Semenza G, editors. Oxygen sensing: responses and adaptation to hypoxia. Lung biology in health and disease. New York, NY: Marcel Dekker; 2003. pp. 567–602.
- 5.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara Cell Secretory Protein-expressing cells of the airway neuroepithelial body environment include a label retaining subset and are critical for epithelial renewal after progenitor depletion. Am J Respir Cell Mol Biol 2002;24:671–681. [DOI] [PubMed] [Google Scholar]
- 6.Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors: demonstration of hypoxia-sensitive K+ current and O2-sensor protein. Nature 1993;365:153–155. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Youngson C, Wong V, Yeger H, Dinauer MC, Vega-Saenz de Miera E, Rudy B, Cutz E. NADPH-oxidase and a hydrogen peroxide-sensitive K+ channel may function as an oxygen sensor complex in airway chemoreceptors and small cell lung carcinoma cell lines. Proc Natl Acad Sci USA 1996;93:13182–13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu XW, Nurse CA, Wong V, Cutz E. Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbits. J Physiol 2002;539:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adriaensen D, Brouns I, Van Genechten J, Timmermans J-P. Functional morphology of pulmonary neuroepithelial bodies: extremely complex airway receptors. Anat Rec 2003;270A:25–40. [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Yeger H, Cutz E. Innervation of pulmonary neuroendocrine cells and neuroepithelial bodies in developing rabbit lung. J Histochem Cytochem 2004;53:379–389. [DOI] [PubMed] [Google Scholar]
- 11.Yeger H, Pan J, Fu XW, Bear C, Cutz E. Expression of CFTR and Cl- conductances in cells of pulmonary neuroepithelial bodies. Am J Physiol 2001;281:L713–L721. [DOI] [PubMed] [Google Scholar]
- 12.Pan J, Bear C, Farragher S, Cutz E, Yeger H. Cystic fibrosis transmembrane conductance regulator modulates neurosecretory function in pulmonary neuroendocrine cell-related tumor cell line models. Am J Respir Cell Mol Biol 2002;27:553–560. [DOI] [PubMed] [Google Scholar]
- 13.Kent G, Iles R, Bear CE, Huan LJ, Griesenbach U, McKerlie C, Frndova H, Ackerley C, Gosselin D, Radzioch D, et al. Lung disease in mice with cystic fibrosis. J Clin Invest 1997;100:3060–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Lommel A, Lauweryns JM. Postnatal development of the pulmonary neuroepithelial bodies in various species. J Auton Nerv Syst 1997; 65:17–24. [DOI] [PubMed] [Google Scholar]
- 15.Baluk PJ, Nadel A, McDonald DM. Substance P immunoreactive sensory axons in the respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol 1992;319:568–598. [DOI] [PubMed] [Google Scholar]
- 16.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352: 1992–2002. [DOI] [PubMed] [Google Scholar]
- 17.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev 1999;79:S215–S255. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DA, Wobken JD, Landrum BG. Changes in bombesin, calcitonin, and serotonin immunoreactive pulmonary neuroendocrine cells in cystic fibrosis and after prolonged mechanical ventilation. Am Rev Respir Dis 1998;137:123–131. [DOI] [PubMed] [Google Scholar]
- 19.Scher H, Miller YE, Aguayo SM, Johnson KJ, Miller JE, McCray PB Jr. Urinary bombesin-like peptide levels in infants and children with bronchopulmonary dysplasia and cystic fibrosis. Pediatr Pulmonol 1998;26:326–331. [DOI] [PubMed] [Google Scholar]
- 20.Bonora M, Bernaudin JF, Guernier C, Brahimi-Horn MC. Ventilatory responses to hypercapnia and hypoxia in conscious cystic fibrosis knock-out mice Cftr−/−. Pediatr Res 2004;55:738–746. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JC, Lundblad LKA, Bates JHT, Levitzky M, Larson JE. The “Goldilocks Effect” in cystic fibrosis: identification of a lung phenotype in the cftr knockout and heterozygous mouse. BMC Genet 2004;5: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bureau MA, Lupien L, Begin R. Neural drive and ventilatory strategy of breathing in normal children and in patients with cystic fibrosis and asthma. Pediatrics 1981;68:187–194. [PubMed] [Google Scholar]
- 23.Verastegui C, Fernandez-Vivero J, Prada A, Rodriguez F, Romero A, Gonzalez-Moreno M, de Castro JM. Presence and distribution of 5HT-, VIP-, NPY-, and SP-immunoreactive structures in adult mouse lung. Histol Histopathol 1997;12:909–918. [PubMed] [Google Scholar]
- 24.Sparrow MP, Lamb JP. Ontogeny of airway smooth muscle: structure, innervation, myogenesis and function in the fetal lung. Respir Physiol Neurobiol 2003;137:361–372. [DOI] [PubMed] [Google Scholar]
- 25.Mhanna MJ, Ferkol T, Martin RJ, Dreshaj IA, van Heeckeren AM, Kelley TJ, Haxhiu MA. Nitric oxide deficiency contributes to impairment of airway relaxation in cystic fibrosis mice. Am J Respir Cell Mol Biol 2001;24:621–626. [DOI] [PubMed] [Google Scholar]
- 26.Weyler RT, Yurko-Mauro KA, Rubenstein R, Kollen WJ, Reenstra W, Altschuler SM, Egan M, Mulberg AE. CFTR is functionally active in GnRH-expressing GT1–7 hypothalamic neurons. Am J Physiol 1999; 277:C563–C571. [DOI] [PubMed] [Google Scholar]
- 27.Pusch M, Jentsch TJ. Molecular physiology of voltage-gated chloride channels. Physiol Rev 1994;4:813–827. [DOI] [PubMed] [Google Scholar]
- 28.Tollet J, Everett AW, Sparrow MP. Development of neural tissue and airway smooth muscle in fetal mouse lung explants: a role for glial-derived neurotrophic factor in lung innervation. Am J Respir Cell Mol Biol 2002;26:420–429. [DOI] [PubMed] [Google Scholar]
- 29.Nassenstein C, Kerzel S, Braun A. Neurotrophins and neurotrophin receptors in allergic asthma. Prog Brain Res 2004;146:346–367. [DOI] [PubMed] [Google Scholar]
- 30.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 31.Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB Jr, Cappechi MR, Welsh MJ, Thomas KR. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest 1995;96:2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]