Abstract
We investigated the therapeutic potential of a newly developed antifibrotic agent, pirfenidone, to regulate airway remodeling and the development of allergic airway inflammation and airway hyperresponsiveness after chronic allergen challenge. Administration of pirfenidone after sensitization but during the period of ovalbumin challenge significantly prevented the development of airway hyperresponsiveness and prevented eosinophil and lymphocyte accumulation in the airways. IL-4, IL-5, and IL-13 levels in bronchoalveolar lavage fluid and ovalbumin-specific serum IgE antibody levels were also significantly reduced. Treatment with pirfenidone significantly reduced transforming growth factor-β1 and platelet-derived growth factor levels in bronchoalveolar lavage fluid. Pirfenidone reduced the expression of transforming growth factor-β1, the development of goblet cell hyperplasia and subepithelial collagenization, and the increases in contractile elements in the lung. These data indicate that pirfenidone may play an important role in the treatment of asthma and has the potential reduce or prevent airway remodeling.
Keywords: airway hyperresponsiveness, airway inflammation, airway remodeling, pirfenidone
Bronchial asthma is a syndrome associated with allergen-induced chronic airway inflammation and airway hyperresponsiveness (AHR). The pathophysiology of AHR is complex, and many factors contribute to its development. Airway mucosal inflammation is characterized by an influx of activated eosinophils and T lymphocytes (1), and numerous investigations have shown that Th2 cytokines (particularly IL-4, IL-5, and IL-13) play critical roles in orchestrating the allergic inflammatory response leading to AHR (2–6). Airway structural changes occur in response to persistent inflammation and include subepithelial fibrosis, hyperplasia of mucus glands, myofibroblast and smooth muscle proliferation, and vascular changes (7). Collectively, these responses are termed airway remodeling, which is thought to occur as a result of an imbalance in the mechanism of regeneration and repair. Transforming growth factor (TGF)-β1, which accelerates fibrotic changes through the accumulation of extracellular matrix, may play a key role in this airway remodeling process. TGF-β1 expression correlates with basement membrane thickness and fibroblast number (7, 8). Furthermore, although TGF-β1 is reported to be an important factor in the regulation of acute pulmonary inflammation, as in pneumonia (9), its role in asthma remains to be defined.
Pirfenidone (5 methyl-1-phenyl-2-(1H)-pyridone) (PFD), a newly developed antifibrotic agent, has proven effective in preventing and resolving the accumulation of fibrous tissue in experimental models of lung (10, 11), kidney (12, 13), and hepatic fibrosis (14, 15). In patients with end-stage pulmonary fibrosis, pirfenidone improved survival and restored pulmonary function (16). Furthermore, pirfenidone attenuated ischemia-reperfusion injury in rat small intestine (17), staphylococcal enterotoxin B–induced cytokines levels (e.g., IL-6, IFN-γ, and TNF-α) (18), and amiodarone-induced over-expression of pulmonary TGF-β1 (19). In this context, pirfenidone may have potential as a treatment in asthma where airway structural changes occur in response to persistent inflammation. This possibility was tested in sensitized mice exposed to repeated allergen challenge.
MATERIALS AND METHODS
Animals
Female BALB/c mice (8–10 wk of age) were purchased from Charles River Japan, Inc. (Yokohama, Japan). The mice were maintained on diets free of ovalbumin (OVA). All experimental animals used in this study were housed under constant temperature and light cycles and under a protocol approved by the Institutional Animal Care and Use Committee of Okayama University Medical School.
Sensitization and Airway Challenge
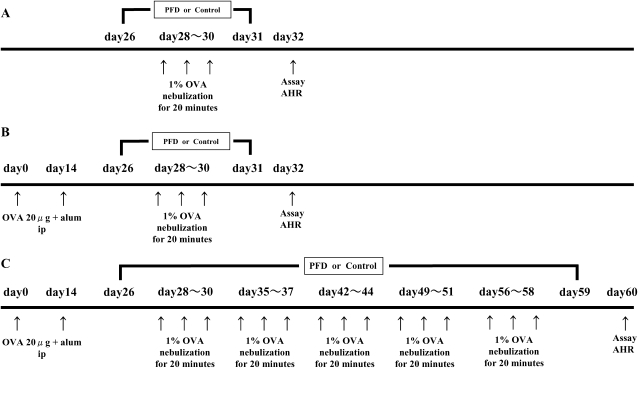
Mice (four mice/group/experiment) receiving the following treatments were studied: (1) nonsensitized and OVA airway-challenged mice and (2) OVA-sensitized and OVA airway-challenged mice. Mice were immunized by intraperitoneal injection of 20 μg of OVA (Grade V) (Sigma, St. Louis, MO) emulsified in 2.25 mg alum (AlumImuject; Pierce, Rockford, IL) in a total volume of 100 μl on Days 0 and 14. Mice were challenged via the airways with OVA (1% in saline) for 20 min on Days 28, 29, and 30 by ultrasonic nebulization (Figure 1). AHR was assessed 48 h after the last challenge, and tissues and cells were obtained for further assays (20, 21).
Figure 1.
Experimental protocols. (A) Mice were nonsensitized and received 3 consecutive days of aerosolized OVA challenge (nonsensitized/OVA). (B) Mice were sensitized by two intraperitoneal injections of OVA/alum and then received three consecutive days of aerosolized 1% OVA challenge (OVA/OVA). To evaluate the effect of pirfenidone on AHR and airway inflammation, mice received subcutaneous injections of pirfenidone (125, 250, or 500 mg/kg/d versus saline as vehicle) from Days 26 to 31. (C) To define the effect of pirfenidone on the airways after repeated OVA challenge, mice were exposed to aerosolized 1% OVA for 20 min 3 d per week for 4 wk (4 wk-OVA/OVA) and received subcutaneous injections of pirfenidone (500 mg/kg/d) from Days 26 to 59.
In a repeated-exposure model, mice were further exposed to aerosolized 1% OVA for 20 min, 3 d per week for an additional 4 wk (Figure 1). AHR and immunologic examinations were assessed 48 h after the last challenge.
Administration of Pirfenidone
Mice received subcutaneous injections of pirfenidone (Shionogi, Tokyo, Japan) (125, 250, or 500 mg/kg daily versus saline as vehicle) from Days 26 to 31 in the short exposure model (Figure 1) (14, 15, 22). In the repeated exposure model, mice received subcutaneous injections of pirfenidone (500 mg/kg/d) from Days 26 to 59. As controls, mice were administered saline subcutaneously (Figure 1).
Determination of Airway Responsiveness
Airway responsiveness was assessed as a change in airway function after challenge with aerosolized methacholine (MCh) using barometric plethysmography (Buxco Electronics Inc., Troy, NY) as described (23). Pressure differences were measured between the main chamber of the plethysmograph, containing conscious, spontaneously breathing animals, and the reference chamber (box pressure signal). Mice were challenged with aerosolized saline (for baseline measurements) or MCh (3.125–25 mg/ml) for 3 min, and readings were taken and averaged for 3 min after each nebulization. Data were expressed using the dimensionless parameter, enhanced pause (Penh) as described (24).
Cell Preparation and Assessment of Peribronchial Lymph Node Mononuclear Cell Proliferation
After OVA sensitization and airway challenge, peribronchial lymph nodes (PBLN) were removed aseptically. PBLN mononuclear cells were purified by passing the tissue through a stainless steel mesh, followed by density-gradient centrifugation (Sigma Diagnostics, St. Louis, MO). Cells were washed three times with PBS and resuspended in culture medium (GIBCO, Grand Island, NY). Mononuclear cells were cultured for 72 h in 96-well, round-bottom plates at a concentration of 1 × 106 cells/well in the presence or absence of OVA (1, 10, or 100 μg/ml) in proliferation assays. 3H-thymidine (0.5 μCi/well) was added to the wells before harvesting, and incorporation was measured in a liquid scintillation counter. PBLN mononuclear cell proliferation was analyzed in the presence or absence of pirfenidone (0.1 mg/ml). Results were expressed as mean counts per minute of triplicate cultures.
Measurement of Bronchoalveolar Lavage Fluid Cytokines and In Vitro Cytokine Production
After assessment of Penh, lungs were lavaged via the tracheal tube with saline (2 × 1 ml, 37°C). The volume of the collected bronchoalveolar lavage fluid (BALF) was measured in each sample, and the number of cells in the BALF was counted. Cytospin slides were stained and differentiated in a blinded fashion by counting at least 300 cells under light microscopy. Cytokine concentrations in the BALF and cytokine levels in OVA-stimulated (10 μg/ml) culture supernatants in the presence or absence of pirfenidone (0.1 mg/ml) were measured by ELISA according to the manufacturer's instructions. The limits of detection were 4 pg/ml for IL-4, IL-5, IL-12, IL-13, IFN-γ, and platelet-derived growth factor (PDGF) (R&D Systems, Minneapolis, MN). TGF-β1 levels in the BALF were assayed using a TGF-β1 ELISA kit (TGF-β1 E max ImmunoAssay System; Promega, Madison, WI). The assay detects only the active form of TGF-β1. Each sample was directly measured for the detection of the active form or was activated before measuring, according to the manufacturer's recommendations, for the detection of total amount of TGF-β1. The limit of detection was 10 pg/ml for TGF-β1.
Measurement of Serum Anti-OVA Antibody
Anti-OVA IgE and IgG1 antibody levels were measured by ELISA 48 h after the last airway challenge as previously described (25). The antibody titers of samples were related to pooled standards that were generated in the laboratory and expressed as ELISA units per milliliter (EU/ml).
Histologic and Immunohistochemistry Studies
After obtaining the BALF, right lungs were inflated through the tracheal tube with 2 ml air and fixed in 10% formalin. The transpulmonary pressure at which the lungs were fixed inflated was 25 cm H2O static pressure by intratracheal instillation (26). Blocks of lung tissue were cut around the main bronchus and embedded in paraffin blocks. Tissue sections 4 μm thick were affixed to microscope slides and deparaffinized. The slides were stained with hematoxylin-eosin and periodic acid Schiff (PAS) for identification of mucus-containing cells and examined under light microscopy. In hematoxylin and eosin–stained lung sections, the numbers of total leukocytes and eosinophils per square millimeter in the peribronchial and perivascular tissue were analyzed using the NIH Image Analysis system (National Institutes of Health, Bethesda, MD). Bronchioles < 200 μm, 200–400 μm, and > 400 μm in diameter were selected (27). The slides were randomly examined in more than 30 bronchioles in a minimum of 10 fields in a blinded fashion. To quantitate the level of mucus expression in the airway, the PAS-positive and PAS-negative epithelial cells in individual bronchioles were counted as previously described in our laboratory (21, 28). The numbers of mucus-containing, PAS-positive cells (goblet cells) were counted in at least 30 bronchioles in a minimum of 10 fields by measuring the length of epithelium defined along the 1-mm basement membrane and luminal area using the NIH Image Analysis system. Bronchioles < 200 μm, 200–400 μm, and > 400 μm in diameter that demonstrated mucus-containing cells were selected.
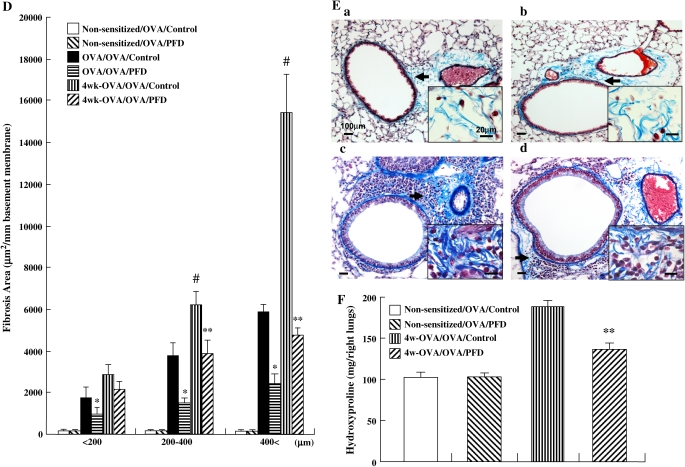
Left lung tissues were fixed with ethanol for 12 h, dehydrated, and embedded in paraffin. Each section was cut to a thickness of 4 μm. Masson's trichrome-stained sections were used for assessment of subepithelial fibrosis as described previously (29–31). Briefly, two to four specimens of the Masson's trichrome-stained histologic preparations of the left lobe, in which the total length of the epithelial basement membrane of the bronchioles (< 200 μm, 200–400 μm, and > 400 μm in diameter) was 1.0–2.5 mm, were selected, and the fibrotic area (stained in blue) beneath the basement membrane at 50–100 μm depth (depending on the size of bronchioles) was measured. The mean scores of the fibrotic area divided by basement membrane length in two to four preparations of one mouse were calculated, and the mean scores of subepithelial fibrosis were calculated in each group. Results are expressed as the area of trichrome staining per millimeter length of basement membrane of bronchioles.
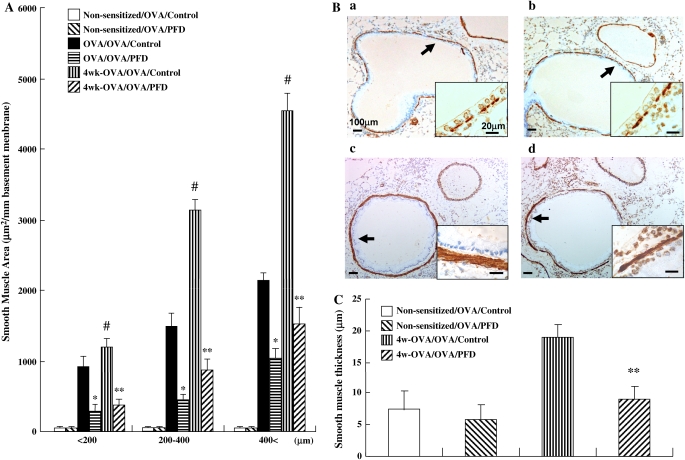
To identify myofibroblasts, a direct technique using a kit of peroxidase-conjugated mouse monoclonal IgG against human α-smooth muscle actin (α-SMA) (DAKO, Grostrup, Denmark) was used. The area of peribronchial α-SMA immunostaining was outlined and quantified using a light microscope attached to an image-analysis system as described previously (31). Results are expressed as the area of α-SMA immunostaining per millimeter length of basement membrane of bronchioles. The thickness of the airway smooth muscle layer (the transverse diameter) was measured from the innermost aspect to the outermost aspect using an image-analysis system. The smooth muscle layer thickness was assessed at four predetermined bronchiole sites (12, 3, 6, and 9 o'clock) in at least 10 bronchioles of similar size (400–600 μm) on each slide (32).
The NIH Image Program allows for manual outlining of the trichrome-stained collagen layer or α-SMA–stained smooth muscle layer and computes the area within the outlined ring of tissue; the perimeter was defined as the airway basement membrane circumference. The slides were randomly examined in more than 30 bronchioles in a minimum of 10 fields in a blinded fashion.
Measurement of Hydroxyproline Content in the Right Lungs
Whole collagen content of the right lung was evaluated by determining hydroxyproline (HP) content as described previously (33, 34). Briefly, the right lung lobes were removed and cut into sections (1 mm thick). The chopped lungs were dried with acetone. The dried lung samples were hydrolyzed with 2 ml of 6N HCl at 120°C for 24 h in sealed glass tubes. The amount of HP in the hydrolysate was measured according to Kivirikko and coworkers (35). Authentic HP (hydroxy-L-proline) was used to establish a standard curve.
Statistical Analysis
All results are expressed as the mean ± SEM. ANOVA was used to determine the levels of difference among all groups. Pairs of groups were compared with unpaired two-tailed Student's t test or the Tukey-Kramer honest significant difference test. Statistical significance was set at P < 0.05.
RESULTS
Treatment with Pirfenidone Attenuates AHR and Inflammatory Cell Accumulation in BALF
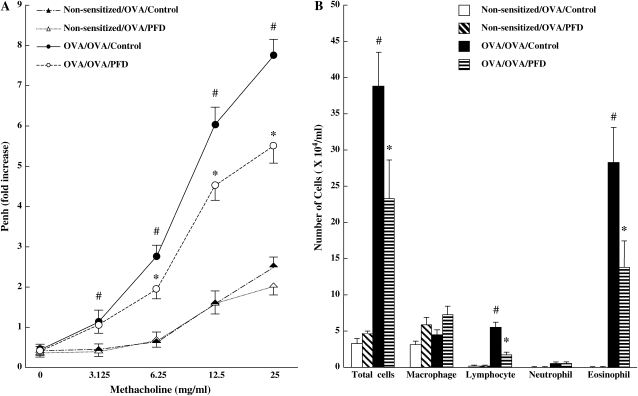
To determine the effects of pirfenidone on the development of altered airway function to inhaled MCh after OVA sensitization and challenge, mice received subcutaneous injections of pirfenidone (125, 250, or 500 mg/kg/d) or saline, from Days 26 to 31, and AHR was assessed on Day 32. There were no significant differences in baseline Penh values between nonsensitized and OVA-challenged mice receiving saline (0.50 ± 0.07) and OVA-sensitized and OVA-challenged mice receiving saline (0.58 ± 0.11). After OVA sensitization and challenge, AHR to inhaled MCh significantly increased in a dose-dependent manner compared with nonsensitized and challenged mice. Administration of pirfenidone daily from 2 d before the first OVA challenge to 24 h after the last challenge to OVA-sensitized and OVA-challenged mice significantly prevented the increases in AHR throughout the MCh dose–response curve (Figure 2A) in a dose-dependent manner (7.11 ± 0.25, 6.22 ± 0.33, and 5.31 ± 0.34 fold increase in Penh at 25 mg/ml by the treatment with pirfenidone 125, 250, or 500 mg/kg, respectively).
Figure 2.
Treatment with pirfenidone in a dose-dependent manner prevents the development of AHR (A) and inflammatory cell accumulation in BALF (B). Penh values to increasing concentrations of inhaled MCh were measured as described in Materials and Methods in nonsensitized/challenged mice receiving saline, OVA-sensitized/OVA-challenged mice receiving saline, nonsensitized/challenged mice receiving pirfenidone, and OVA-sensitized/OVA-challenged mice receiving pirfenidone. Results for each group are expressed as the mean ± SD. n = 16 in each group. #Significant differences (P < 0.05) between nonsensitized/OVA-challenged mice (Non-sensitized/OVA/Control) and OVA-sensitized/OVA-challenged mice (OVA/OVA/Control). *Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged control mice (receiving saline) (OVA/OVA/Control) and pirfenidone-treated mice (OVA/OVA/PFD).
The numbers and types of inflammatory cells in the airways were determined in BALF 48 h after the last of the three consecutive allergen challenges (Day 32). In nonsensitized and challenged mice, over 95% of the detected cells were macrophages. Sensitization and challenge with OVA resulted in a marked increase in the number of eosinophils and lymphocytes in BALF. Treatment with pirfenidone significantly suppressed the number of eosinophils and lymphocytes and the total cell numbers in BALF (Figure 2B) in a dose-dependent manner (eosinophils: a 25.6%, 42.5%, and 50.2% decrease compared with OVA-sensitized and OVA-challenged control mice after treatment with pirfenidone 125, 250, or 500 mg/kg, respectively). These findings suggest that administration of pirfenidone can suppress the development of AHR and airway inflammation.
Treatment with Pirfenidone Decreases Cytokine and Growth Factor Levels in BALF
Concentrations of cytokines (IL-4, IL-5, IL-13, IL-12, and IFN-γ) and growth factors (TGF-β1 and PDGF) in BALF were measured by ELISA. In sensitized and challenged mice, the levels of Th2 cytokines, IL-4, IL-5, and IL-13, and growth factors such as TGF-β1 and PDGF in BALF were significantly increased compared with nonsensitized and challenged mice. In contrast, IL-12 and IFN-γ levels were not significantly changed (Table 1). Administration of pirfenidone daily from 2 d before the first OVA challenge to 24 h after the last challenge significantly reduced IL-4, IL-5, and IL-13, TGF-β1, and PDGF levels in BALF compared with control mice. Pirfenidone showed no significant effect on BALF IL-12 and IFN-γ levels (Table 1).
TABLE 1.
CYTOKINE AND GROWTH FACTOR LEVELS IN BALF
| Groups | IL-4 (pg/ml) | IL-5 (pg/ml) | IL-13 (pg/ml) | IL-12 (pg/ml) | IFN-γ (pg/ml) | TGF-β1 (pg/ml) | PDGF (pg/ml) |
|---|---|---|---|---|---|---|---|
| Nonsensitized/OVA/Control | 3.9 ± 0.9 | 8.8 ± 3.6 | 4.2 ± 1.1 | 1.2 ± 0.6 | 0.4 ± 0.1 | 22.4 ± 16.3 | 4.3 ± 0.9 |
| Nonsensitized/OVA/PFD | 4.1 ± 0.8 | 9.2 ± 1.8 | 3.6 ± 0.7 | 0.9 ± 0.4 | 0.3 ± 0.2 | 4.1 ± 1.0 | 4.6 ± 1.1 |
| OVA/OVA/Control | 17.2 ± 1.9* | 32.2 ± 2.9* | 43.5 ± 5.7* | 1.4 ± 0.6 | 0.7 ± 0.4 | 183.6 ± 50.7* | 32.8 ± 0.8* |
| OVA/OVA/PFD | 8.6 ± 2.2# | 15.7 ± 2.8# | 23.4 ± 6.5# | 1.7 ± 0.4 | 0.6 ± 0.3 | 42.5 ± 11.8# | 25.2 ± 3.9# |
Definition of abbreviations: BALF, bronchoalveolar lavage fluid; OVA, ovalbumin; PFD, pirfenidone; TGF-β1, transforming growth factor-beta 1; PDGF, platelet-derived growth factor.
Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged control mice (receiving saline) (OVA/OVA/Control) and pirfenidone-treated mice (OVA/OVA/PFD). The results for each group are expressed as mean ± SD. n = 16 in each group.
Significant differences (P < 0.05) between nonsensitized/challenged mice (Nonsensitized/OVA/Control) and OVA-sensitized/OVA-challenged mice (OVA/OVA/Control).
Treatment with Pirfenidone Inhibits Serum Anti-OVA IgE Antibody Levels
After OVA sensitization and airway challenge, serum anti-OVA IgE and IgG1 levels were significantly increased compared with nonsensitized and OVA-challenged mice. Treatment with pirfenidone daily from 2 d before the first OVA challenge to 24 h after the last challenge significantly reduced serum OVA-specific IgE levels compared with control mice (Table 2).
TABLE 2.
SERUM OVA-SPECIFIC ANTIBODY LEVELS
| Groups | OVA-specific IgE Levels (EU/ml) | OVA-specific IgG1 Levels (EU/ml) |
|---|---|---|
| Nonsensitized/OVA/Control | 31.4 ± 11.7 | 18.2 ± 8.1 |
| Nonsensitized/OVA/PFD | 26.2 ± 10.4 | 16.7 ± 9.5 |
| OVA/OVA/Control | 1046.8 ± 158.9* | 418.0 ± 60.6* |
| OVA/OVA/PFD | 582.7 ± 94.0# | 267.2 ± 35.5 |
Definition of abbreviations: OVA, ovalbumin; PFD, pirfenidone.
Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged control mice (receiving saline) (OVA/OVA/Control) and pirfenidone-treated mice (OVA/OVA/PFD). The results for each group are expressed as mean ± SD. n = 16 in each group.
Significant differences (P < 0.05) between nonsensitized/challenged mice (Nonsensitized/OVA/Control) and OVA-sensitized/OVA-challenged mice (OVA/OVA/Control).
Effect of Pirfenidone on Mononuclear Cell Proliferation and Cytokine Production In Vitro
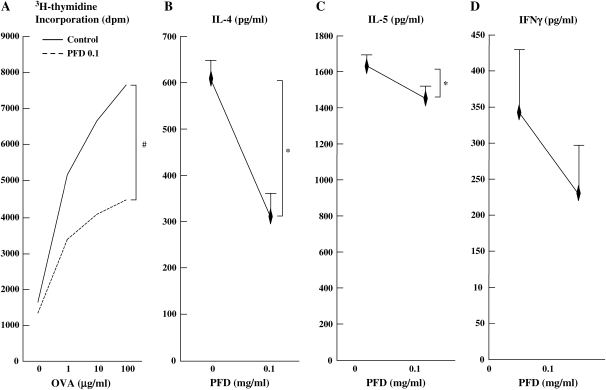
To investigate whether T cells from OVA-sensitized and airway-challenged mice could respond to the antigen, proliferation of local PBLN mononuclear cells was measured. PBLN mononuclear cells showed a dose-dependent proliferative response to OVA. Treatment with pirfenidone (0.1 mg/ml) significantly inhibited the proliferation of PBLN mononuclear cells (Figure 3A). There was no evidence of drug-induced cytotoxicity, as determined by trypan blue exclusion, with > 95% viability during these experiments.
Figure 3.
Treatment with pirfenidone suppresses mononuclear cell proliferation and in vitro cytokine production. OVA-stimulated PBLN mononuclear cell proliferation (A) and IL-4 (B), IL-5 (C), and IFN-γ (D) supernatant levels in mice after OVA sensitization and airway challenge were measured as described in Materials and Methods. The results for each group are expressed as the mean ± SD. n = 16 in each group. #Significant differences (P < 0.05) in treated PBLN mononuclear cell proliferation between the saline group and the pirfenidone-treated group. *Significant differences (P < 0.05) between the control group and the pirfenidone-treated group.
IL-4, IL-5, and IFN-γ were detected in the culture supernatant of OVA (10 μg/ml)-stimulated PBLN from OVA-sensitized and OVA-challenged mice (Figures 3B and 3D). Treatment with pirfenidone significantly suppressed IL-4 and IL-5 but not IFN-γ in culture supernates.
Treatment with Pirfenidone Attenuates AHR, Inflammatory Cell Infiltrates, and Cytokine and Growth Factor Levels in BALF of Chronically Exposed Mice
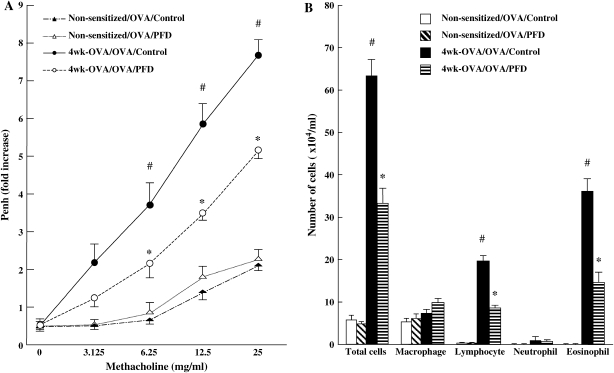
In OVA-sensitized and OVA-challenged mice after 4 wk of additional allergen exposure, baseline (saline) Penh values remained low (0.55 ± 0.08) on Day 60, but the responses to inhaled MCh were still significantly increased (Figure 4A). The number of eosinophils in the BALF also remained high (Figure 4B). In contrast, the number of lymphocytes was significantly increased when compared with OVA-sensitized mice that received only three challenges (Figures 2 and 4B).
Figure 4.
Treatment with pirfenidone prevents the development of AHR (A) and inflammatory cell accumulation (B) in BALF in chronically exposed mice with OVA. Data represent the mean ± SD. n = 16 in each group. #Significant differences (P < 0.05) between nonsensitized/OVA-challenged mice (Non-sensitized/OVA/Control) and OVA-sensitized/OVA-challenged mice after repeated OVA exposure (receiving saline) (4 wk-OVA/OVA/Control). *Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged mice after repeated OVA exposure (4 wk-OVA/OVA/Control) and pirfenidone-treated chronically OVA-exposed mice (4 wk-OVA/OVA/PFD).
To determine whether pirfenidone remained effective in modifying AHR and inflammatory cell accumulation after 4 wk of additional allergen exposure, mice received subcutaneous injections of pirfenidone from Days 26 to 59 (500 mg/kg/d or saline) (Figure 1). Administration of pirfenidone to repeatedly challenged mice significantly prevented the development of AHR (Figure 4A) and reduced the number of eosinophils and lymphocytes in BALF compared with the control groups (Figure 4B).
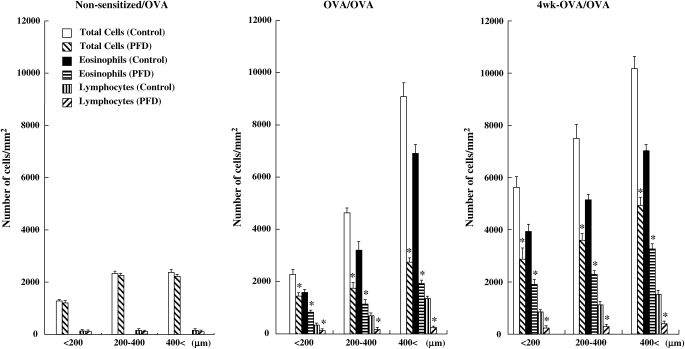
The effects of pirfenidone on inflammatory cell accumulation were further investigated by histologic examination of hematoxylin-eosin–stained slides. In nonsensitized and OVA-challenged mice, few eosinophils or lymphocytes were detected in the peribronchial and perivascular areas. Sensitization and subsequent challenge with OVA via the airways increased the numbers of eosinophils and lymphocytes. Examination of tissue sections showed that treatment with pirfenidone significantly reduced eosinophil and lymphocyte infiltration around all sizes of bronchioles in sensitized mice subject to three or repeated OVA challenges (Figure 5).
Figure 5.
Effect of pirfenidone on inflammatory cell accumulation in peribronchial and perivascular tissue in nonsensitized/OVA-challenged mice (Non-sensitized/OVA), OVA-sensitized/OVA-challenged mice (OVA/OVA), and OVA-sensitized/OVA-challenged mice after repeated OVA exposure (4 wk-OVA/OVA). Evidence of inflammatory cell infiltration was investigated by histologic examination of hematoxylin-eosin–stained tissue as described in Materials and Methods (final magnification: ×400; inset: ×1,000). Bronchioles < 200 μm, 200–400 μm, and > 400 μm in diameter were selected. *Significant differences (P < 0.05) in the number of total cells, eosinophils, and lymphocytes in peribronchial and perivascular tissue between the saline-treated control group and the pirfenidone-treated group.
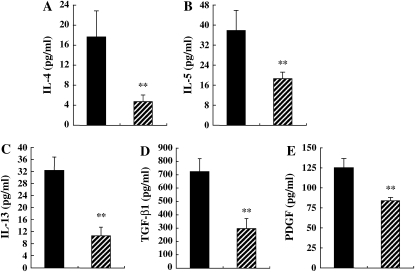
In OVA-sensitized and repeatedly challenged mice, the levels of IL-4, IL-5, and IL-13 in BALF remained elevated, and TGF-β1 and PDGF levels were increased compared with short-term–challenged mice (Table 1, Figures 6A–6E). IL-12 and IFN-γ were not detected. Pirfenidone significantly reduced TGF-β1, PDGF, IL-4, IL-5, and IL-13 levels in BALF compared with control mice (Figure 6).
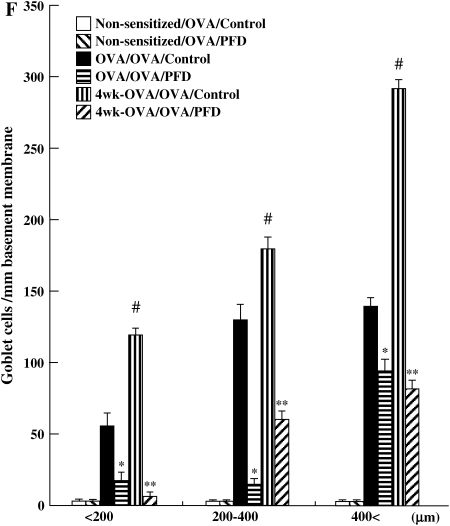
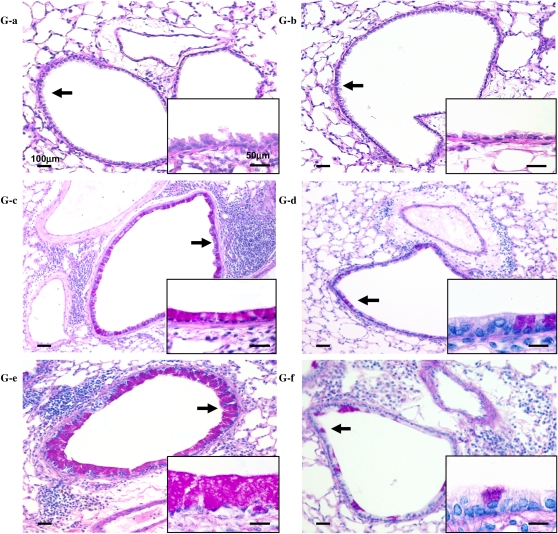
Figure 6.
Treatment with pirfenidone reduces cytokine and growth factor levels in BALF of chronically exposed mice to OVA. The levels IL-4 (A), IL-5 (B), IL-13 (C), TGF-β1 (D), and PDGF (E) in BALF and goblet cell numbers (F) were determined as described in Materials and Methods. (G) PAS staining: (a) Nonsensitized/OVA/Control, (b) Nonsensitized/OVA/PFD, (c) OVA/OVA/Control, (d) OVA/OVA/PFD, (e) 4 wk-OVA/OVA/Control, and (f) 4 wk-OVA/OVA/PFD. The results for each group are expressed as mean ± SD. n = 16 in each group. *Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged, saline-treated control mice (OVA/OVA/Control; solid bars) and pirfenidone-treated mice (OVA/OVA/PFD; striped bars). **Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged mice after repeated OVA exposure (receiving saline) (4 wk-OVA/OVA/Control) and pirfenidone-treated, chronically OVA-exposed mice (4 wk-OVA/OVA/PFD).
Treatment with Pirfenidone Inhibits Airway Remodeling after Chronic Allergen Exposure
Lung sections were stained with PAS to identify mucus-containing cells in the airway epithelium (Figures 6F and 6G). A significant increase in numbers of PAS+ cells was found in OVA-sensitized and OVA-challenged mice compared with nonsensitized and challenged mice. The number of cells staining positive for mucus increased with increasing allergen exposure, and treatment with pirfenidone daily from Days 26 to 31 or from Days 26 to 59 significantly reduced the number of PAS+ cells.
The areas of α-SMA–stained smooth muscle layer (Figures 7A and 7B) and Masson's trichrome-positive peribronchiolar collagen layer (Figures 7D and 7E) in OVA-sensitized and OVA-challenged mice after 4 wk of additional allergen exposure were also increased. Treatment with pirfenidone significantly inhibited the increase of thickness in the smooth muscle layer in all bronchioles (Figure 7A) and collagen deposition/fibrosis surrounding the airways in bronchioles > 200 μm in diameter (Figure 7D) after the additional allergen exposure. The thickness of the peribronchial smooth muscle layer in OVA-sensitized and repeatedly challenged control mice was significantly greater than in nonsensitized and challenged control mice. Pirfenidone significantly reduced the thickness of the peribronchial smooth muscle layer in mice repeatedly challenged with OVA compared with control mice (Figure 7C). Treatment with pirfenidone significantly reduced the total lung hydroxyproline content compared with control group in OVA-sensitized and OVA-challenged mice after 4 wk of additional allergen exposure (Figure 7F).
Figure 7.
Treatment with pirfenidone inhibits airway remodeling in mice receiving repeated (4 wk) airway challenges. (A, B) Area of α-SMA–stained smooth muscle layer: (a) Non-sensitized/OVA/Control, (b) Non-sensitized/OVA/PFD, (c) 4 wk-OVA/OVA/Control, (d) 4 wk-OVA/OVA/PFD. (C) Thickness of the airway smooth muscle. (D, E) Masson's trichrome-positive peribronchiolar collagen layer: (a) Non-sensitized/OVA/Control, (b) Non-sensitized/OVA/PFD, (c) 4 wk-OVA/OVA/Control, (d) 4 wk-OVA/OVA/PFD. n = 16 in each group. Total lung hydroxyproline content in the right lung (F) was determined as described in Materials and Methods. n = 8 in each group. Data represent mean ± SD. #Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged mice (OVA/OVA/Control) and OVA-sensitized/OVA-challenged mice after 4 wk of additional allergen exposure (4 wk-OVA/OVA/Control). *Significant differences (P < 0.05) between OVA-sensitized/OVA-challenged saline-treated control mice (OVA/OVA/Control) and pirfenidone-treated mice (OVA/OVA/PFD). **Significant differences (P < 0.05) between saline-treated control mice (4 wk-OVA/OVA/Control) and pirfenidone-treated mice (4 wk-OVA/OVA/PFD).
DISCUSSION
Pirfenidone is a recently developed broad spectrum antifibrotic agent with efficacy in preventing and resolving several kinds of fibrotic disease, including lung (11) and renal fibrosis (13), liver cirrhosis (15), cardiac remodeling, and increased cardiac stiffness (36). Several recent studies have been published about the efficacy of pirfenidone on idiopathic pulmonary fibrosis (IPF). Although their study design and methodologic issues are controversial, Azuma and colleagues demonstrated that treatment with pirfenidone improved vital capacity and prevented acute exacerbation of IPF during the 9 mo of follow-up in a double-blind, placebo-controlled, multicenter trial Japanese patients with IPF (phase II clinical study) (37–40). Adverse events, such as skin photosensitivity associated with pirfenidone, were similar to those of a previous report (16, 41, 42); however, most of the events disappeared when the dosage was decreased or when the medication was stopped temporarily, and readministration of pirfenidone at a lower dose based on the prespecified protocol was well tolerated (37). Because IPF is a progressive and fatal disorder and because some studies have suggested that no treatment exists that modifies its course (43, 44), it is hoped that the results of an ongoing placebo-controlled phase III clinical trial of pirfenidone will clarify the overall safety and efficacy in IPF.
The mechanism of action of pirfenidone remains unclear. Experimental and clinical studies have shown that pirfenidone reduces fibroblast proliferation and synthesis of collagen (45) and reduces the formation and accumulation of fibrotic matrix (12). Shihab and coworkers demonstrated that pirfenidone treatment decreased TGF-β1 protein expression in chronic, cyclosporine-induced nephrotoxicity (13). Garcia and coworkers demonstrated that gene expression of collagens I, III, and IV, TGF-β1, Smad7, and TIMP-1 decreased in pirfenidone-treated cirrhotic rats (15). On the other hand, in LPS-induced acute lung injury, pretreatment of mice with pirfenidone reduced neutrophil recruitment, TNF-α and TGF-β levels, and matrix metalloproteinase-9 secretion in the BALF (46). Hale and coworkers reported that pirfenidone reduced staphylococcal enterotoxin B–induced cytokine levels and T-cell proliferation in a murine shock model and in vitro human peripheral blood lymphocyte assays (18). Moreover, pirfenidone induced intracellular adhesion molecule-1 downregulation on cultured human synovial fibroblasts (47). These results suggest that pirfenidone may be promising as a new therapeutic strategy for patients with asthma who have airway structural changes and remodeling in response to persistent inflammation.
A key question is whether the airway structural changes in patients with asthma are reversible by drug intervention. Therapeutic approaches to reverse allergic airway remodeling have been examined in animal models of asthma and airway remodeling. Henderson and coworkers demonstrated that cysteinyl leukotriene 1 receptor antagonist significantly reduced the eosinophil infiltration, mucus plugging, smooth muscle hyperplasia, and subepithelial fibrosis in the OVA-sensitized/challenged chronic asthma model, whereas it had no effect on airway hyperreactivity to aerosolized MCh or intravenous MCh as determined by noninvasive and invasive plethysmography, respectively (30). Christie and coworkers reported that treatment of OVA-sensitized/challenged mice with dexamethasone reduced airway expression of laminin and laminin-1 receptor in OVA-treated mice but not AHR to MCh by noninvasive plethysmography (48). In a rat chronic asthma model with development of airway wall thickening, the increased fibronectin deposition persisted after the final OVA challenge and was not reversed by fluticasone treatment (49). Thus, the relationship of airway inflammation, remodeling, and lung function is complex. The clinical relevance of remodeling and the therapeutic modalities controlling these features of asthma remain to be defined. In the present study, we showed that in short-term airway challenge and after repeated airway challenge of sensitized BALB/c mice, treatment with pirfenidone significantly reduces the numbers of eosinophils and lymphocytes; decreases IL-4, IL-5, and IL-13 levels in the BALF; and alters airway function in airway challenge of sensitized BALB/c mice and airway remodeling mice. Furthermore, in short-term OVA-challenged mice, pirfenidone significantly reduced the serum levels of OVA-specific IgE but showed no significant effect on IL-12 and IFN-γ production in the BALF.
The pathophysiology of asthma is complex, with allergens triggering a cascade of cellular interactions and the release of cytokines and mediators in sensitized individuals, resulting in acute and late or delayed symptoms. Th2 cells, through the release of cytokines and chemokines, regulate inflammatory cell recruitment to the lung, leading to AHR. Airway inflammation is a fundamental characteristic of asthma and over time may lead to airway remodeling. The structural alterations induced during remodeling may play an important role in eliciting the airway functional changes. Subepithelial fibrosis, goblet cell hyperplasia, mucus hypersecretion, and myofibroblast hypertrophy are components of the remodeling response (7). Recent studies suggest that inhibition of IL-5 or IL-13 significantly suppressed AHR, eosinophil infiltration in the airways, and airway remodeling in a mouse model (29, 50). On the contrary, treatment with an IL-5–blocking monoclonal antibody in patients with mild asthma was effective in inhibiting sputum and blood eosinophilia but had no effects on the late-phase response to allergen challenge or AHR to MCh (51). Accordingly, many factors play crucial roles in the development of AHR and remodeling.
In the present study, we showed that OVA-sensitized and short-term airway–challenged BALB/c mice showed increases in airway responsiveness to inhaled MCh in a dose-dependent manner. Eosinophil and lymphocyte accumulation in BALF and lung tissue and concentrations of IL-4, IL-5, and IL-13 levels in BALF were increased when compared with nonsensitized and challenged mice. After 4 wk of additional allergen exposure, altered airway function persisted, the number of eosinophils in the BALF remained increased, and lymphocyte numbers were significantly increased. The levels of IL-4, IL-5, and IL-13 remained increased after 4 wk of repeated OVA challenges when compared with nonsensitized and OVA-challenged mice. Compared with OVA-sensitized and short-term challenged mice, IL-5 levels in BALF were significantly increased, but IL-13 levels were significantly decreased.
In contrast to defining the role of cytokines in allergic inflammation, the function of growth factors in asthma has been less fully delineated, and the results have not been consistent. Richter and coworkers demonstrated that stimulation of human fibroblasts with IL-4 or IL-13 resulted in proliferation and an increase in eotaxin and MCP-1 release (52). Furthermore, it has been shown that IL-13 induces tissue fibrosis by selectively stimulating and activating TGF-β1 and that IL-13 acting in concert with TGF-β1 may increase the release of eotaxin from human fibroblasts (53, 54). Minshall and coworkers demonstrated that TGF-β1 may play a role in the fibrotic changes occurring within asthmatic airways, and that activated eosinophils are a major source of this cytokine (55). TGF-β1, which accelerates fibrotic changes through the accumulation of extracellular matrix, may play an important role in this airway remodeling process; TGF-β1 expression correlates with basement membrane thickness and fibroblast number (7, 8). PDGF was also involved in the augmentation of airway responsiveness through remodeling of airways in diesel exhaust particulate-exposed mice (56). Consequently, IL-4 and IL-13 produced after the acute phase may stimulate the production of growth factors, especially TGF-β1, which may contribute to the collagen deposition observed during chronic allergen challenge. In this study, we demonstrated that growth factors, especially TGF-β1 levels in BALF, in OVA-sensitized and OVA-challenged mice after 4 wk of additional allergen exposure were significantly increased compared with nonsensitized and OVA-challenged mice and OVA-sensitized and OVA-challenged mice, even though IL-13 levels were significantly decreased. Histologic studies revealed that the numbers of cells staining positive for mucus, collagen deposition and fibrosis, and the thickness of the smooth muscle layer surrounding the airway increased in sensitized mice exposed to repeated OVA challenge. These results show that not only do the levels of Th2 cytokines increase, but also a number of growth factors that have the potential to contribute to the pathogenesis of altered airway function are increased. We previously demonstrated that not only Th2 cytokines, but also growth factors such as TGF-β1 have the potential contribution to the pathogenesis of altered airway function. Another growth factor, hepatocyte growth factor, seems to be an important regulator of allergic airway inflammation, hyperresponsiveness, and remodeling (57). Consequently, to control and cure chronic severe asthma, we have to regulate airway inflammation, including Th2 cytokines and growth factors, and airway remodeling leading to AHR. Current medications to reverse established structural airway changes have had limited effect in animal asthma models and in patients with asthma (58, 59).
We demonstrate for the first time that administration of pirfenidone daily from Days 26 to 59 significantly suppressed TGF-β1 and PDGF levels, which significantly increased in BALF after repeated allergen challenge. In addition, the expression of TGF-β1, the development of goblet cell hyperplasia, subepithelial collagenization, and the increases in contractile elements in the lung were significantly reduced by pirfenidone in OVA-sensitized and chronically challenged mice. Given its purported mechanism of action, it was surprising that treatment with pirfenidone significantly prevented AHR and eosinophil and lymphocyte accumulation in the airways in sensitized and chronically challenged mice. Furthermore, IL-4, IL-5, and IL-13 levels in the BALF and OVA-specific serum IgE levels were significantly reduced. To investigate whether T cells from sensitized and challenged mice could respond to OVA, we measured the proliferative responses in PBLN mononuclear cells and IL-4, IL-5, and IFN-γ production in the culture supernatants of OVA-stimulated PBLN mononuclear cells. PBLN mononuclear cells showed a dose-dependent proliferative response to OVA. Treatment with pirfenidone significantly inhibited antigen-specific proliferation of PBLN cells. We showed that administration of pirfenidone significantly suppressed OVA-stimulated IL-4 and IL-5 levels, whereas there was no significant change of IFN-γ levels in cell culture supernatants. These data suggest that the potential mechanisms underlying pirfenidone activities may be not only the immunomodulatory effects on the augmented Th2 cytokine and TGF-β1 response in the pathogenesis of key features of allergen–induced asthma, but also the inhibitory effects on the development of goblet cell hyperplasia, subepithelial collagenization, and the increases in contractile elements in the lung, which is thought to occur as a result of an imbalance in the mechanism of regeneration and repair caused by chronic allergic airway inflammation in patients with persistent asthma.
In summary, these studies identify the potential of pirfenidone to interfere with the development of allergic inflammation and AHR. Repeated allergen challenge is associated with an increase in TGF-β1. We demonstrate that administration of pirfenidone significantly suppressed TGF-β1 levels in BALF. Pirfenidone significantly attenuated the development of goblet cell hyperplasia, subepithelial collagenization, and the increases in contractile elements in the airways. A number of these inhibitory activities seemed to be the consequence of inhibiting allergen-specific T-cell responses and cytokine and growth factor responses. Although additional studies are needed to further elucidate the mechanism(s), the data presented here show that pirfenidone exhibits potent antifibrotic effects, which may protect the airways by reducing inflammation, AHR, and airway remodeling.
Acknowledgments
The authors thank Ms. Diana Nabighian for her help in the preparation of this manuscript.
This work was supported in part by a grant from the Ministry of Education, Science and Culture of Japan, by National Institutes of Health grants HL-36577, HL-61005, and by Environmental Protection Agency grant R825702.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0452OC on May 4, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham S, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis 1990;142:1407–1413. [DOI] [PubMed] [Google Scholar]
- 2.Ying S, Humbert M, Barkans J, Corrigan CJ, Pfister R, Menz G, Larche M, Robinson DS, Durham SR, Kay AB. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol 1997;158:3539–3544. [PubMed] [Google Scholar]
- 3.Kotsimbos TC, Ernst P, Hamid QA. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proc Assoc Am Physicians 1996;108: 368–373. [PubMed] [Google Scholar]
- 4.Minty A, Asselin S, Bensussan A, Shire D, Vita N, Vyakarnam A, Wijdenes J, Ferrara P, Caput D. The related cytokines interleukin-13 and interleukin-4 are distinguished by differential production and differential effects on T lymphocytes. Eur Cytokine Netw 1997;8:203–213. [PubMed] [Google Scholar]
- 5.Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol 1998;161:1501–1509. [PubMed] [Google Scholar]
- 6.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest 1999;103:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 1999;104:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-β expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med 1997;156:591–599. [DOI] [PubMed] [Google Scholar]
- 9.Buhling F, Tholert G, Kaiser D, Hoffmann B, Reinhold D, Ansorge S, Welte T. Increased release of transforming growth factor (TGF)-beta1, TGF-beta2, and chemoattractant mediators in pneumonia. J Interferon Cytokine Res 1999;19:271–278. [DOI] [PubMed] [Google Scholar]
- 10.Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med 1995;125:779–785. [PubMed] [Google Scholar]
- 11.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999;291:367–373. [PubMed] [Google Scholar]
- 12.Shimizu T, Kuroda T, Hata S, Fukagawa M, Margolin SB, Kurokawa K. Pirfenidone improves renal function and fibrosis in the post-obstructed kidney. Kidney Int 1998;54:99–109. [DOI] [PubMed] [Google Scholar]
- 13.Shihab FS, Bennett WM, Yi H, Andoh TF. Pirfenidone treatment decreases transforming growth factor-β1 and matrix proteins and ameliorates fibrosis in chronic cyclosporine nephrotoxicity. Am J Transplant 2002;2:111–119. [DOI] [PubMed] [Google Scholar]
- 14.Tada S, Nakamuta M, Enjoji M, Sugimoto R, Iwamoto H, Kato M, Nakashima Y, Nawata H. Pirfenidone inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Clin Exp Pharmacol Physiol 2001;28: 522–527. [DOI] [PubMed] [Google Scholar]
- 15.Garcia L, Hernandez I, Sandoval A, Salazar A, Garcia J, Vera J, Grijalva G, Muriel P, Margolin S, Armendariz-Borunda J. Pirfenidone effectively reverses experimental liver fibrosis. J Hepatol 2002;37:797–805. [DOI] [PubMed] [Google Scholar]
- 16.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, Pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med 1999;159:1061–1069. [DOI] [PubMed] [Google Scholar]
- 17.Arumugam TV, Shiels IA, Margolin SB, Taylor SM, Brown L. Pirfenidone attenuates ischaemia-reperfusion injury in the rat small intestine. Clin Exp Pharmacol Physiol 2002;29:996–1000. [DOI] [PubMed] [Google Scholar]
- 18.Hale ML, Margolin SB, Krakauer T, Roy CJ, Stiles BG. Pirfenidone blocks the in vitro and in vivo effects of staphylococcal enterotoxin B. Infect Immun 2002;70:2989–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Card JW, Racz WJ, Brien JF, Margolin SB, Massey TE. Differential effects of pirfenidone on acute pulmonary injury and ensuring fibrosis in the hamster model of amiodarone-induced pulmonary toxicity. Toxicol Sci 2003;75:169–180. [DOI] [PubMed] [Google Scholar]
- 20.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Kohler G, O'Brien R, Gelfand EW, Born W. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nat Med 1999;5:1150–1156. [DOI] [PubMed] [Google Scholar]
- 21.Kanehiro A, Takeda K, Joetham A, Tomkinson A, Ikemura T, Irvin CG, Gelfand EW. Timing of administration of anti-VLA-4 differentiates airway hyperresponsiveness in the central and peripheral airways in mice. Am J Respir Crit Care Med 2000;162:1132–1139. [DOI] [PubMed] [Google Scholar]
- 22.Oku H, Nakazato H, Horikawa T, Tsuruta Y, Suzuki R. Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol 2002;446:167–176. [DOI] [PubMed] [Google Scholar]
- 23.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997;156:766–775. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med 1997;186:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temann UA, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol 1997;16:471–478. [DOI] [PubMed] [Google Scholar]
- 26.Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2001;280:L39–L49. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn C III, Homer RJ, Zhu Z, Ward N, Flavell RA, Geba GP, Elias JA. Airway hyperresponsiveness and airway obstruction in transgenic mice: morphologic correlates in mice overexpressing interleukin (IL)-11 and IL-6 in the lung. Am J Respir Cell Mol Biol 2000;22:289–295. [DOI] [PubMed] [Google Scholar]
- 28.Kanehiro A, Ikemura T, Makela MJ, Lahn M, Joetham A, Dakhama A, Gelfand EW. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am J Respir Crit Care Med 2001;163:173–184. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 2004;31:62–68. [DOI] [PubMed] [Google Scholar]
- 30.Henderson WR Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med 2002;165:108–116. [DOI] [PubMed] [Google Scholar]
- 31.Bonner JC, Rice AB, Moomaw CR, Morgan DL. Airway fibrosis in rats induced by vanadium pentoxide. Am J Physiol Lung Cell Mol Physiol 2000;278:L209–L216. [DOI] [PubMed] [Google Scholar]
- 32.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest 2004;113:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komai M, Tanaka H, Masuda T, Nagao K, Ishizaki M, Sawada M, Nagai H. Role of Th2 responses in the development of allergen-induced airway remodeling in a murine model of allergic asthma. Br J Pharmacol 2003;138:912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagao K, Tanaka H, Komai M, Masuda T, Narumiya S, Nagai H. Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am J Respir Cell Mol Biol 2003;29:314–320. [DOI] [PubMed] [Google Scholar]
- 35.Kivirikko KI, Laitinen O, Prockop DJ. Modifications of a specific assay for hydroxyproline in murine. Anal Biochem 1967;19:249–255. [DOI] [PubMed] [Google Scholar]
- 36.Mirkovic S, Seymour AM, Fenning A, Strachan A, Margolin SB, Taylor SM, Brown L. Attenuation of cardiac fibrosis by pirfenidone and amiloride in DOCA-salt hypertensive rats. Br J Pharmacol 2002;135: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040–1047. [DOI] [PubMed] [Google Scholar]
- 38.du Bois RM. Is idiopathic pulmonary fibrosis now treatable? Am J Respir Crit Care Med 2005;171:939–940. [DOI] [PubMed] [Google Scholar]
- 39.Mathai SC, Polito AJ. The questionable efficacy of pirfenidone in IPF. Am J Respir Crit Care Med 2005;172:1228. [DOI] [PubMed] [Google Scholar]
- 40.Levitt J, Gould MK. Poor choice of primary outcome in a clinical trial of pirfenidone in patients with IPF. Am J Respir Crit Care Med 2005; 172:1228–1229. [DOI] [PubMed] [Google Scholar]
- 41.Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med 2002;41:1118–1123. [DOI] [PubMed] [Google Scholar]
- 42.Gahl WA, Brantly M, Troendle J, Avila NA, Padua A, Montalvo C, Cardona H, Calis KA, Gochuico B. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab 2002;76:234–242. [DOI] [PubMed] [Google Scholar]
- 43.Fellrath JM, du Bois RM. Idiopathic pulmonary fibrosis/cryptogenic fibrosing alveolitis. Clin Exp Med 2003;3:65–83. [DOI] [PubMed] [Google Scholar]
- 44.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 45.Lee BS, Margolin SB, Nowak RA. Pirfenidone: a novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab 1998;83:219–223. [DOI] [PubMed] [Google Scholar]
- 46.Corbel M, Lanchou J, Germain N, Malledant Y, Boichot E, Lagente V. Modulation of airway remodeling-associated mediators by the antifibrotic compound, pirfenidone, and the matrix metalloproteinase inhibitor, batimastat, during acute lung injury in mice. Eur J Pharmacol 2001;426:113–121. [DOI] [PubMed] [Google Scholar]
- 47.Kaneko M, Inoue H, Nakazawa R, Azuma N, Suzuki M, Yamauchi S, Margolin SB, Tsubota K, Saito I. Pirfenidone induces intercellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin Exp Immunol 1998;113:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christie PE, Jonas M, Tsai CH, Chi EY, Henderson WR Jr. Increase in laminin expression in allergic airway remodelling and decrease by dexamethasone. Eur Respir J 2004;24:107–115. [DOI] [PubMed] [Google Scholar]
- 49.Vanacker NJ, Palmans E, Kips JC, Pauwels RA. Fluticasone inhibits but does not reverse allergen-induced structural airway changes. Am J Respir Crit Care Med 2001;163:674–679. [DOI] [PubMed] [Google Scholar]
- 50.Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, Shang X, Li J, Das AM, Shealy D, Griswold DE, et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine 2004;28:224–232. [DOI] [PubMed] [Google Scholar]
- 51.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000; 356:2144–2148. [DOI] [PubMed] [Google Scholar]
- 52.Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST, Davies DE. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol 2001;25:385–391. [DOI] [PubMed] [Google Scholar]
- 53.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta (1). J Exp Med 2001;194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Asmin CJ, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts: implication in asthma. J Clin Invest 1998;101:2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minshall EM, Leung DYM, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1997; 17:326–333. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita N, Sekine K, Miyasaka T, Kawashima R, Nakajima Y, Nakano J, Yamamoto T, Horiuchi T, Hirai K, Ohta K. Platelet-derived growth factor is involved in the augmentation of airway responsiveness through remodeling of airways in diesel exhaust particulate-treated mice. J Allergy Clin Immunol 2001;107:135–142. [DOI] [PubMed] [Google Scholar]
- 57.Ito W, Kanehiro A, Matsumoto K, Hirano A, Ono K, Maruyama H, Kataoka M, Nakamura T, Gelfand EW, Tanimoto M. Hepatocyte growth factor attenuates airway hyperresponsiveness, inflammation, and remodeling. Am J Respir Cell Mol Biol 2005;32:268–280. [DOI] [PubMed] [Google Scholar]
- 58.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J; NHLBI Working Group. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med 2004;170:683–690. [DOI] [PubMed] [Google Scholar]
- 59.Inman M. Is there a place for anti-remodelling drugs in asthma which may not display immediate clinical efficacy? Eur Respir J 2004;24:1–2. [DOI] [PubMed] [Google Scholar]