Abstract
Lung development is associated with a surge in surfactant phosphatidylcholine (PC) production to prepare the newborn for extrauterine breathing. This process is associated with a marked increase in the activity of the rate-regulatory surfactant enzyme, CTP:phosphocholine cytidylyltransferase (CCTα). To investigate the molecular basis for developmental activation of CCTα, we analyzed expression of endogenous CCTα and a reporter gene, β-galactosidase, in fetal, newborn, and adult promoter-reporter transgenic mice. Transgenics harboring ∼ 2 kb of the CCTα promoter linked upstream of a β-galactosidase reporter gene displayed relatively high expression in distal lung epithelia. Endogenous lung CCTα and β-galactosidase activities, protein content, and transcript levels displayed maximal expression within the newborn period. CCTα and β-galactosidase activities and enzyme levels increased with time in cultured fetal lung explants isolated from transgenics. Transfectional analysis using CCTα promoter–reporter constructs in developing rat type II cells revealed that a region encompassing −169/+71 contained the DNA elements required for perinatal activation. The studies demonstrate that developmental induction of surfactant phospholipid is due, at least in part, to transcriptional activation of the CCTα gene.
Keywords: surfactant, development, pulmonary
One characteristic feature of the developing lung is the surge in biosynthesis of surfactant during the perinatal period that is critical to prepare the fetus for ambient breathing after parturition (1). Pulmonary surfactant's ability to lower alveolar surface tension is attributed to a mixture of phosphatidylcholine (PC), the major phospholipid component, and key surfactant-associated proteins secreted by alveolar type II epithelia (2). In preterm infants with the neonatal respiratory distress syndrome (RDS), the lungs have not matured enough to synthesize sufficient quantities of surfactant phospholipid to sustain adequate ventilation during extrauterine life. In addition, it is increasingly apparent that the synthesis of surfactant components, specifically PC, may be impaired in other pulmonary disorders acquired later in life (3–5). Thus, an understanding of the biochemical mechanisms underlying surfactant PC metabolism during development may be important in devising newer strategies for therapeutic intervention.
In mammals, PC is produced primarily via the CDP-choline pathway (6). This pathway involves a series of four sequential steps: (1) cellular uptake of choline from the circulation via the diet, (2) phosphorylation of choline to cholinephosphate by choline kinase (CK), (3) transfer of cytidine to cholinephosphate generating CDP-choline by cytidylyltransferase (CCT), and (4) transfer of the phosphocholine moiety of CDP-choline to diacylglycerol by cholinephosphotransferse (CPT) generating the final product, PC. Of these steps, the reaction catalyzed by CCT is very slow and rate-regulatory (7). Thus, efforts at uncovering CCT's regulation at the molecular level has drawn significant interest.
The predominant isoform of CCT in lung is CCTα, a 367 AA protein that contains a catalytic core flanked by a nuclear targeting aminoterminus, membrane-binding, and carboxyl-terminal phosphorylation domains (8). Although CCTα is a ubiquitous, essential enzyme, relatively high activities are detected in lung epithelia relative to other tissues as these cells synthesize surfactant PC (9–11). It is not known whether this high-level pulmonary CCTα expression is secondary to increased transcriptional activity compared with other organs. However, maneuvers designed to overexpress wild-type CCTα result in increased PC synthesis, whereas knockdown or knockout of CCTα expression result in apoptosis and embryonic lethality, respectively (12–15). CCT activity is extensively controlled by both activating and inhibitory lipids (6). CCTα is also a phosphoenzyme, and recent studies in our laboratory indicate that enzyme function is inhibited by site-specific phosphorylation by mitogen-activated protein kinases (8). In contrast to regulation by lipids and phosphorylation events, less is known about CCTα control at the level of gene transcription. Further, very limited data are available regarding the molecular regulation of CCT during ontogeny.
It has been demonstrated that the developmental increase in PC in the lung is paralleled by a marked increase in the activity of CCT (16–19). Enzyme activity increases nearly 7-fold postnatally, and the increase in activity is nearly linear with time for several hours after birth (16, 20). These findings suggest that enhanced activity of CCT is required for the fetal lung to more actively synthesize and secrete surfactant lipids. Further, Hogan and coworkers (21) and Fraslon and Batenburg (22) demonstrated a gestational increase in CCTα protein and mRNA in rat fetal type II cells, and others showed a spontaneous increase in CCTα mRNA using fetal rat lung explants. Additional work suggests that developmental increases in CCTα expression are due to increases in the stability of the CCTα transcript (23). In contrast, there also appears to be evidence of activation of preformed CCTα by lipids in mature lung (24). Overall, the data to date suggest that there are both pre-translational mechanisms involved in expression of the CCTα enzyme during critical phases of lung maturation and post-translational activation of this enzyme later in life. However, one limitation of some prior studies is that investigators performed analysis using cultured type II cells or lung organ cultures, while other studies used whole lung. While each of these systems have their own virtues, it is unclear if observations in vitro are recapitulated in an intact animal model. Further, some of these studies were performed before the availability of newer transgenic systems to monitor gene transcriptional activity.
In the present study we investigated the hypothesis that developmental increases in CCT activity are due to increases in CCTα gene transcription. To evaluate this hypothesis, we exploited a promoter-reporter murine transgenic model system in which ∼ 2 kb of the proximal 5′ flanking region of the CCTα gene was coupled to a β-galactosidase reporter gene (25). By comparing endogenous CCTα gene activity with activity of this reporter gene, we observed that CCTα expression during pulmonary development is attributed, at least in part, to increased transcription of the gene for this key regulatory enzyme.
MATERIALS AND METHODS
Materials
Waymouth's medium and BGjb medium were obtained from InVitrogen (San Diego, CA). The murine lung epithelial (MLE)-12 cell line was obtained from American Type Culture Collection (Manassas, VA). All radiochemicals were purchased from DuPont New England Nuclear Chemicals (Boston, MA). The Basic Nucleofector Solution for primary mammalian epithelial cells, a plasmid construct encoding green fluorescence protein (GFP) driven by CMV (pmaxGFP), and electroporation apparatus were provided by Amaxa (Gaithersburg, MD). The luciferase assay system, including the reporter lysis buffer and polyclonal antibody to β-galactosidase, was obtained from Promega (Madison, WI) and the Galacto-light plus kit was from TROPIX (St. Louis, MO). Luciferase and β-galactosidase activities were determined using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Immunoblotting membranes were obtained from Millipore (Bedford, MA). The ECL Western blotting detection system was from Pierce Biotechnology, (Rockford, IL). Anti-CCTα rabbit polyclonal antiserum to synthetic peptide was generated by Covance Research Products Inc. (Richmond, CA). The β-actin antibody was purchased from R&D Systems (Minneapolis, MN). The random primed labeling kit used was Redprime II (Buckinghamshire, UK). The Advantage cDNA polymerase was from Clontech, (Palo Alto, CA). The Geneclean2 Kit was obtained from Bio101 (Carlsbad, CA). QIAprep Miniprep kits were obtained from QIAGEN (Valencia, CA). All DNA sequencing was performed by the University of Iowa DNA Core Facility.
Construction of the CCTα Promoter-β−Galactosidase cDNA Cassette
Cloning procedures were performed on a modified vector made from pCR2.1 (Invitrogen) and pCI (Promega) (pStec-TgDNA2) kindly provided by David Stec, U. of Iowa). The Escherichia coli lacZ gene was obtained from a pBS401 vector. After amplifying the 1,938-bp fragment of the CCTα promoter (−1,867/+71), it was cloned into the Sac I site of the modified vector. The E. coli LacZ reporter gene (3.2 kb) was then cloned into the Sal 1 site generating pStecCCTαβgal. After cloning procedures, DNA sequences and orientation were verified by DNA sequencing. In addition, CCTα promoter fragments (−169/+71 and −1,867/+71) coupled to luciferase were constructed as described previously (26).
Generation and Screening of CCTα Promoter–Reporter Murine Transgenics
Transgenic mice were generated at the University of Iowa Transgenic Animal Facility, where microinjections of the cDNA cassette into oocytes were performed to generate transgenic founders. Transgenic offspring were identified by PCR analysis using tail DNA with β-galactosidase primers. To detect transgene integration and copy number, genomic DNA (20 μg) from mice was digested with Nhe I, resolved by agarose gel electrophoresis and transferred to nylon membranes (Zetaprobe GT; Bio-Rad, Hercules, CA). An 899-bp genomic DNA fragment of the murine CCTα gene that corresponded to the first exon and adjacent sequence from the 5′ flanking region and the first intron was used as a hybridization probe. Briefly, the DNA fragment was labeled by random primer extension with Klenow DNA polymerase and [α-32P] dCTP (Decaprime II DNA labeling kit; Ambion, Austin, TX) and then hybridized with the transferred membrane as previously described (25).
Animals and Lung Explant Culture
Timed pregnant CCTα promoter–reporter transgenic mice at Days 16 and 18 (gestational age; Day 0 designated by presence of vaginal sperm plug) were generated at the University of Iowa by crossing transgene-positive males with females. Newborn (2–4 d old), 2-wk-old, and 8-wk-old (adult) mice were first screened by tail clip analysis, and transgene-positive animals were used for all studies. After killing with phenobarbital (150 mg/kg intraperitoneally), fetal mice were delivered by Cesarean section from their dams. The fetal lungs, liver, heart, skeletal muscle, small intestine, and kidneys were resected, separately pooled, homogenized, and used directly for biochemical studies. The isolation of lung newborn mouse type II cells, alveolar macrophages, and fibroblasts was performed using methods as described previously (27). In separate studies, Day 17 gestation fetal pooled tissues were placed in calcium- and magnesium-free Hank's Balanced Salt Solution (HBSS) and subsequently minced into ∼ 1-mm pieces in HBSS for preparation of lung explant cultures as described (28). Lung explants were cultured in BGjb (Fitton-Jackson modification) with L-glutamine, penicillin, and streptomycin for up to 48 h before harvest for biochemical analysis.
In other studies, adult, newborn, and timed pregnant Sprague-Dawley rats (Harlan) at 18 d gestational age (term gestation = 21 d) were also killed with phenobarbitol (150 mg/kg intraperitoneally). Fetal rats were delivered by Caesarean section from their mothers. Newborn and adult rats were also anesthetized, and their lungs dissected free from proximal hila and airways. Primary alveolar type II epithelial cells were isolated at each stage of maturation as we described (27). The newborn and adult epithelial cells were predominantly mature alveolar type II cells (> 90%) as determined by tannic acid staining. Type II cells were counted using a Coulter Z1 particle counter. All procedures were approved by the University of Iowa Animal Care and Use Committee.
CCT and β-Galactosidase Activities
CCT activities were determined by measuring the rate of incorporation of [methyl-14C] phosphocholine into CDP-choline using a charcoal extraction method (29). CCT activities were determined without addition of exogenous lipid activator in the reaction mixture.
β-Galactosidase activities in murine tissues isolated from CCTα promoter–reporter transgenic mice was measured after preheating samples at 48°C for 55 min to inactivate endogenous β-galactosidase activity (25).
Immunoblot Analysis
Immunoblotting was performed using polyclonal antibodies to CCTα, β-actin, or β-galactosidase as described (25). For these studies, equal amounts of lung protein were loaded on 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane. Immunoreactive products were detected using an ECL Western blotting detection system. Dilution factors of all antibodies used for probing membranes were 1:1,000.
Detection of mRNAs Using Real-Time PCR Analysis
After RNA isolation, Taqman reverse transcription reagents (Applied Biosystems) were used to generate cDNA from cellular RNA (30). Real-time PCR was then performed on cDNA using the Applied Biosystems 7700 real-time PCR instrument and the SYBR Green PCR master mix. CCTα mRNA detection primers were: 5′-cct gga aat gtt tgg tcc aga-3′, and 5′-ctc tgc ttg gga ctg atg g-3′ and β-galactosidase mRNA detection primers were: 5′- cga gta aca acc cgt cgg at-3′ and 5′-cga gtg cga tct tcc tga gg-3′. Taqman rodent 18S was used as the internal control using the following primers: 5′-taa gtc cct gcc ctt tgt aca ca-3′, and 3′ primer 5′-gat ccg agg gcc tca cta aac-3′.
Transient Transfectional Analysis
Nucleofection was performed on 2 × 106 freshly isolated type II cells using the Amaxa Nucleofector Device (Program T-13). Suspended cells were incubated with 100 μl of Amaxa Basic Nucleofector Solution for primary mammalian epithelial cells, 1.5 μg of one of two CCTα promoter fragments (−1,867/+71) or (−169/+71) coupled to luciferase, and 0.5 μg pSV-β−galactosidase plasmid DNA to normalize for transfection efficiency. As an additional control, nucleofection was performed as above but using 2 μg of Amaxa pmaxGFP. After electroporation using the Amaxa Nucleofector Device, cells were transferred to 12-well culture plates and allowed to recover for 24 h in Waymouth's medium containing 10% carbon-stripped FBS. GFP-tagged cells were trypsinized for flow cytometry to determine transfectional efficiency. All other cells were harvested in reporter lysis buffer. Luciferase was determined using the Promega Luciferase Assay System as described (25). Promoter activities in primary type II cells were calculated as ratios of luciferase to β−galactosidase and additionally corrected for transfectional efficiencies in fetal, newborn, and adult type II cells as determined by GFP labeling.
Immunohistochemistry
Mice were killed and their heart and lungs perfused with 30 ml PBS to remove blood. The lungs were instilled with 4% paraformaldehyde in phosphate buffer (pH 7.4) and then immersed in this fixative for 18 h at 25°C. The fixed lungs were exposed to a graded series of sucrose (10–30% sucrose in PBS), embedded in OCT compound, and frozen at −80°C. Tissue sections (8 μm) were cryostat-cut and placed on glass slides.
Sections were permeabilized with 0.1% Triton X-100 (10 min), treated with 3% hydrogen peroxide (10 min), and blocked for 1 h using 5% bovine serum albumin in PBS. Sections were then incubated for 18 h at 4°C with either no primary antibody (negative control) or with rabbit polyclonal antibodies against β-galactosidase (Abcam, Inc., Cambridge, MA) or pro–surfactant protein C (pro–SP-C; Abcam, Inc.) using a 1:500 dilution in PBS. Sections were washed three times and then incubated with a goat anti-rabbit IgG-horseradish perioxidase conjugate at 1:1,000 dilution for 1 h. Immunohistochemical staining was performed using a DAB substrate peroxidase kit (Vector Laboratories, Burlingame, CA) plus nickel chloride, and tissues were counterstained with nuclear fast red.
Statistical Analysis
All data were analyzed using statistical software SPSS 11.5. Data are presented as means ± SEM. Statistical significance was accepted at the P < 0.05 level by unpaired samples t test or one-way ANOVA for multiple group analysis using a Bonferroni adjustment.
RESULTS
Analysis of CCTα Promoter-Reporter Trangenic Mice
We first generated a CCTα–β-galactosidase fusion gene cassette that contained ∼ 2 kb of the proximal 5′ flanking region of the mouse CCTα gene. Injection of this construct into mouse oocytes led to production of transgenic founders that were bred to ultimately establish lines of CCTα-promoter reporter mice. Tail clip DNA analysis using PCR revealed the generation of three transgene-positive lines (Figure 1A). Offspring from one of these lines (F595) was tested for transgene integration and copy number by Southern blotting, which confirmed that the transgene was integrated as two copies in the founder genome (25). Germ lines from two independent founder lines (F595 and F66/2) of CCTα promoter–reporter transgenic mice were established and examined for expression of the reporter gene, β-galactosidase. In MLE cell lines transfected with a CCTα promoter–reporter construct used for generation of transgenics, adequate β-galactosidase protein was detected by immunoblot analysis versus untransfected cells (Figure 1B, lanes 1–2). Comparable levels of the lung immunoreactive β-galactosidase product were seen on immunoblots in transgene-positive founders (F595) unlike wild-type negative control littermates (Figure 1B, lanes 3–6). Further analysis of β-galactosidase expression revealed high-level activity within adult whole lung, with lower levels observed in the heart and kidney (Figure 1C). Thus, the reporter gene in CCTα promoter transgenic mice generated using these methods was sufficiently expressed to allow for further analysis during pulmonary development.
Figure 1.
Expression of a β-galactosidase transgene in CCTα promoter–reporter mice. (A) A murine transgenic harboring a large fragment (−1,867/+71) of the CCTα promoter was linked to a β-galactosidase reporter gene and used for the generation of transgenic mice. Tail clip analysis of genomic DNA from three independent founder lines tested positive (lanes 1, 6, and 7) for the reporter gene. Lane 11 is a molecular weight ladder, and lanes 12–13 are positive control lanes. (B) Immunoblotting was performed to detect the reporter product, β-galactosidase, that was driven by the CCTα promoter. Lane 1, untransfected MLE cells; lane 2, MLE cells transfected with a −1,867/+71 CCTα–β-galactosidase construct used for generation of transgenics. Lanes 3–5: lungs from three different transgene positive founders (F1) and a wild-type (Wt) negative control littermate (lane 6) all from one line. (C) Various whole organs were isolated from transgene positive founders (F1) and wild-type (negative control) littermates from one line. Relative expression of β-galactosidase activity was assayed from three mice in each group. The values are expressed as relative light units/mg of protein. Minimal activity was detected in control organs.
Comparative Analysis of Endogenous CCTα and β-Galactosidase in Promoter–Reporter Newborn Transgenics
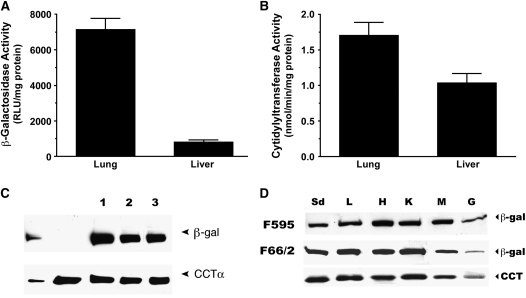
We next investigated whether the reporter gene faithfully mirrored expression of the endogenous gene in newborn transgenics, in which we hypothesized that both CCTα and β-galactosidase would be detected at higher levels compared with the adults (Figure 1C). Indeed, pulmonary and hepatic β-galactosidase activities were higher in newborn lung compared with adult lung and liver (Figures 1C and 2A). Moreover, both CCTα and β-galactosidase activities were higher in lung versus liver in newborns (Figures 2A and 2B). In three separate mice, immunoreactive β-galactosidase levels in the lung generally mirrored levels of CCTα mass (Figure 2C). Concordant expression in various tissues between CCTα and β-galactosidase levels was also seen in the two founder lines, with high-level expression of each enzyme in all organs surveyed in newborns with the exception of small intestine (Figure 2D). The data as a whole indicate that the β-galactosidase reporter gene in the transgenics can be used to investigate pulmonary CCTα transcriptional regulation.
Figure 2.
Correlation of CCTα and β-galactosidase expression in CCTα transgenic lines. β-Galactosidase activity (A) and CCTα activity (B) were assayed in lung and livers of positive promoter–reporter transgenics. (C) Immunoblotting for lung β-galactosidase (top row) and CCTα levels (bottom row) from three different transgene positive littermates. Lungs from Mouse 1 displayed higher levels of the endogenous and transgene compared with littermate Mice nos. 2 and 3. The far left lanes represent protein standards. The second lane represents a transgene negative founder. (D) Distribution of immunoreactive β-galactosidase (top two rows) and CCTα (bottom row) in two independent founder lines (F595 and F66/2). Organs analyzed include lung (L), heart (H), kidney (K), skeletal muscle (M), and gut (G). The far left lane represents protein standards (Sd). A, B, and D represent data from at least n = 3 independent experiments or murine tissues.
CCTα Is Developmentally-Regulated in Promoter–Reporter Transgenic Mice
We next isolated fetal, newborn, and adult murine lungs from transgene-positive mice and assayed CCT and β-galactosidase activities. As shown in Figure 3A, CCT activity increased from Day 16 to Day 18 gestation ∼ 5- to 6-fold. Importantly, enzyme specific activity during the newborn period was nearly 11-fold greater compared with the Day 16 fetus. After birth, CCT activity significantly dropped to lower levels comparable to activities observed in the developing fetal lung (Figure 3A). In similar tissues, we assayed β-galactosidase activity (Figure 3B). Activity of β-galactosidase also exhibited a robust, ∼ 70-fold increase in expression between the fetal (Day 16) and newborn period. Enzyme activity then plummeted to relatively low levels with lung maturation. Thus, these data also indicate that as a whole, functional expression of the endogenous (CCTα) gene mirrors that of the reporter gene, β-galactosidase, both of which peak after birth in developing mouse lung.
Figure 3.
CCTα and β-galactosidase activities in developing CCTα transgenic mice. CCTα activity (A) and β-galactosidase activity (B) were assayed during various periods of lung development in whole lung tissue. These periods include Day 16 and Day 18 fetal gestation, newborn (N), 2-wk-old, and adult lung. Enzyme activities are expressed as pmoles/min/mg of protein for CCTα and relative light units/mg protein for β-galactosidase. CCT activity was assayed in the absence of exogenous lipid activator in the assay mixture. Each period represents activity values assayed in at least six pups and three adults. †P < 0.01 or *P < 0.05 versus fetal (Day 16) by ANOVA.
Expression of Immunoreactive CCTα and β-Galactosidase
To determine if developmental changes in activities observed for CCT and β-galactosidase were due to increased enzyme levels, we performed immunoblotting. CCTα mass increased during gestation, with a peak in expression of the 42-kD product in newborn lung (Figure 4A). In addition, multiple bands were detected in whole lung after birth using our polyclonal CCTα antibody, suggesting reactivity with other CCT isoforms, the presence of CCTα dimeric subunits, or presence of degradation products. However, only the 42-kD product was competed off using excess recombinant CCTα in the immunoblotting solution (data not shown). β-Galactosidase protein also reached highest levels during the perinatal period (Figure 4B). Relatively low levels of the reporter gene product were observed in adult lung. Because insertion sites of plasmid constructs can sometimes regulate developmental expression of transgenes, we analyzed β-galactosidase expression in another founder line (F66/2). Although this second transgenic line exhibited lower levels of transgene expression, β-galactosidase protein and activities also peaked in newborn lung (Figure 4C). Overall, the data indicate that expression of both CCTα and β-galactosidase are upregulated during perinatal lung maturation.
Figure 4.
Expression of CCTα and β-galactosidase protein and mRNAs in developing CCTα transgenic mice. CCTα (A) and β-galactosidase levels (B) were determined during various periods of lung development in whole lung tissue. The far left band in each panel represents purified CCTα or β-galactosidase standard. Each gel was loaded with equal amounts of total homogenate protein. The data represent n = 3 separate experiments. (C) Left: Immunoblotting for β-galactosidase levels in fetal (F), newborn (N), and adult (Ad) lungs in two separate transgenic lines (F66/2 and F595). Right: β-galactosidase activities were also assayed in the F66/2 line. *P < 0.05, newborn versus fetal activity. (D) Real-time PCR was used to detect mRNAs for CCTα (closed bars) and β-galactosidase levels (open bars) in CCTα promoter–reporter transgenics during lung development. Data represent mean ± SEM, and from analysis in lungs from at least three fetal, newborn, or adult mice; data are normalized to the housekeeping gene, 18S. For CCTα, *P < 0.01, newborn versus 18 d or 2-wk-old mice; +P = 0.06, newborn versus adult mice; and *P < 0.01, 16 d versus 2-wk-old mice. For β-galactosidase, *P < 0.01 newborn versus 18 d, 2 wk old, or adult mice by ANOVA.
Profile of CCTα and β-Galactosidase mRNAs
Analysis of mRNA content by real-time PCR in the promoter–reporter transgenic mice revealed that CCTα transcript levels tended to be relatively high in Day 16 fetal lung (Figure 4D). Nevertheless, CCTα mRNAs increased to highest levels within newborn lung tissue and thereafter decreased to lower levels postnatally (Figure 4D). Levels of β-galactosidase mRNA mirrored the pattern of CCTα transcripts, with the highest level of expression in newborns (Figure 4D). In fact, β-galactosidase mRNA levels in newborn lung were significantly greater than mRNAs for the reporter gene in either fetal, 2-wk-old, or adult lung. These data were confirmed using Northern blot analysis (data not shown). A high level of expression of CCTα and β-galactosidase in early fetal (Day 16) lung suggests additional mechanisms of control such as changes in mRNA stability. As a whole, however, the data are consistent with protein data, where expression of both the endogenous gene and transgene peak in the newborn stage.
Immunolocalization of β-Galactosidase in Developing Lung
We next attempted to localize β-galactosidase expression in murine transgenic lungs by immunohistochemical staining for the transgene (Figure 5). Reporter gene expression in adult lung was localized to the alveolar epithelia consistent with the pattern of expression of pro–SP-C (Figures 5A–5C, arrows, shown by black staining). Overall, the level of expression was more dispersed and intense along distal lung epithelia in newborn lung (Figures 5D–5F, arrows). Importantly, in newborn transgene-positive lungs, freshly isolated alveolar type II cells exhibited a several-fold higher level of β-galactosidase activity compared with fibroblasts or alveolar macrophages, indicating that immunohistochemical labeling observed was likely originating from surfactant-producing epithelia (Figure 5D1). In fetal lung, relatively modest expression was detected sporadically throughout primitive lung tubules (Figures 5G–5I).
Figure 5.
Immunolocalization of lung β-galactosidase in developing murine transgenics. Adult (A–C), newborn (D–F), and fetal (G–I) lung tissues from transgenic promoter–reporter mice were isolated and processed for β-galactosidase immunostaining. A, D, and G represent negative controls, and C, F, and I were processed for pro–SP-C staining (positive control). B, E, and H were immunostained for the transgene, β-galactosidase. Arrows indicate black transgene-positive staining cells in distal lung alveolar epithelia. The unlabeled panels under panels B and H represent β-galactosidase staining at higher magnification in adult and fetal transgenic lungs, respectively. D1 shows relative β-galactosidase activity in newborn alveolar macrophages, fibroblasts, and alveolar type II cells isolated from transgenic promoter–reporter mice. Data are from four independent experiments, *P < 0.01 versus other groups by ANOVA.
Expression of CCTα and β-Galactosidase in Explant Cultures
Because fetal lung tissue undergoes maturation in vitro using conditioned medium, we used lung explant cultures as a model to study transcriptional expression of CCTα. Thus, lung explants were generated after isolating lungs from Day 17 gestation transgene CCTα-positive promoter–reporter mice. Explants were cultured in chemically defined medium and harvested after 24 and 48 h for functional assays (Figure 6). As shown in Figure 6A, culturing explants from 24–48 h resulted in increases in expression of CCT and β-galactosidase activities. These effects were associated with increases in enzyme levels (Figure 6B). The data suggest that these enzymes may serve as biochemical markers of in vitro maturation of the surfactant system as reflected in vivo.
Figure 6.
CCTα transcriptional activity in vitro. (A and B) Fetal lung explants isolated from CCTα promoter–reporter transgenic mice were cultured in chemically defined medium. After 24 or 48 h, tissues were harvested for analysis of CCTα and β-galactosidase activities (A) or CCTα, β-galactosidase, and β-actin content by immunoblotting (B). In A, CCTα and β-galactosidase activities are expressed as pmoles/min/mg protein and RLU/mg protein, respectively. In B, the far left lanes in the top and middle rows represent β-galactosidase and CCTα standards, respectively. In C, rat fetal (Day 18), newborn, and adult type II alveolar epithelial cells were isolated, and nucleofection was performed on 2 × 106 freshly isolated cells by electroporation. Suspended cells were co-transfected with CCTα promoter fragments (−1,867/+71 or −169/+71) coupled to luciferase (1.5 μg), and a pSV–β-galactosidase (0.5 μg) plasmid to normalize for transfection efficiency. After electroporation, cells were cultured for an additional 24 h before analysis for luciferase and β-galactosidase activities. Promoter activities in primary type II cells are expressed as relative light units calculated as ratios of luciferase to β-galactosidase and additionally corrected for transfectional efficiencies as determined by GFP labeling. The data are from type II cells isolated from 20 fetal pups, 22 neonates, and two adult rats. (D) Immunoreactive CCTα levels and CCT activity was assayed in freshly isolated rat primary type II cells during development. *P < 0.05 versus other groups. In A, †P = 0.08 or *P < 0.05 versus 24 h samples by t test. In C, **P < 0.01 versus all other groups and *P < 0.05 versus fetal (Day 18) or adult promoter activity by ANOVA.
Analysis of CCTα Transcription in Primary Alveolar Type II Cells
Our investigations of CCTα transcriptional activity were next extended to primary type II alveolar epithelia. As methods for isolation of fetal murine type II cells have not been adequately developed, we used rat type II cells for these studies. Fetal Day 18 gestation type II cells, newborn, and adult type II cells were isolated and electroporated in suspension with plasmids containing either a 1,938-bp fragment of the CCTα promoter coupled to luciferase or a 240-bp construct together with a β-galactosidase reporter plasmid (Figure 6C). Cells were cultured for 24 h and then harvested for determination of promoter activity by measuring luciferase and β-galactosidase activities. Cells were additionally corrected for transfectional efficiency as determined by cellular expression of GFP. Indeed, transfectional efficiencies varied significantly depending on the maturational age of cells, as values ranged from for 90 ± 2.4%, 61 ± 2.3%, and 13 ± 1.5% for fetal, newborn, and adult type II cells, respectively. As shown in Figure 6C, promoter activity for the ∼ 2-kb CCTα promoter–luciferase construct increased from 1.05 ± 0.05 units to 7.03 ± 0.49 units from the fetal to newborn period in cells. Promoter activity then decreased to 1.44 ± 0.17 units in adult type II cells. In separate studies, cells were transfected as above except with a smaller CCTα promoter–luciferase construct (−169/+71). Indeed, promoter activity in this smaller construct mirrored that of the larger promoter fragment, with activity in newborn cells at highest levels. As described previously, these changes in CCTα transcriptional activity in developing rat type II cells were associated with a coordinate induction in enzyme activity and mass (Figure 6D) (31). Thus, the results strongly indicate that regulatory elements required for transcription of the CCTα promoter in type II cells during ontogeny reside within ∼ 240-bp of the CCTα gene.
DISCUSSION
The molecular mechanisms that underlie the burst in surfactant phospholipid synthesis with perinatal lung development have not been fully elucidated. These studies demonstrate that the developmental increase in PC synthesis is attributed, at least in part, to increased transcription of the CCTα gene. Unlike prior studies, a unique contribution of our study was that observations were made using a murine transgenic promoter–reporter system in which ∼ 2 kb of the proximal 5′ flanking sequence of the CCTα gene was linked upstream of a β-galactosidase reporter. We specifically coupled a relatively large region of the CCTα promoter region upstream of the reporter gene for generation of these mice to ensure a high likelihood that the DNA introduced contained relevant regulatory elements for developmental control. This system allows us to readily assay CCTα gene transcriptional activity during lung maturation in an animal model in which cell-to-cell interactions and neuro-humoral reflexes are preserved. Indeed, when using these transgenic mice, the highest level of CCTα and β-galactosidase expression was detected during the perinatal (newborn) period, with lower activities in the fetus (Day 16) and mature adult lung. Overall, developmental expression of CCTα paralleled that of β-galactosidase in two independent founder lines, suggesting that expression of the reporter gene faithfully mirrors the endogenous gene.
A somewhat surprising result from our work was relatively low β-galactosidase expression in the liver, an organ that generally exhibits high levels of CCT activity and phospholipid synthesis. It is possible that either hepatic CCT activity is regulated post-transcriptionally or uses a transcriptional mechanism primarily under nutritional or environmental stress as described elseware (32, 33). The liver, unlike the lung, also synthesizes PC using an auxiliary pathway via sequential methylation of phosphatidylethanolamine catalyzed by phosphatidylethanolamine N-methyltransferase (6). Thus, if this pathway is constitutively active, additional levels of control of CCTα by transcriptional mechanisms may not be necessary to maintain hepatic PC homeostasis.
Although there is some variability in the temporal patterns for surfactant production in developing lung among different species, as a whole there is general agreement that induction of PC synthesis at or near term in pregnant animals correlates with increases in CCT activity (1). In particular, a surge in PC synthesis and CCT activity is observed by several laboratories immediately after birth in the rodent model (16, 20, 31). Many of these studies were executed in intact whole lung (16, 20, 31). Other reports using primary fetal rat type II alveolar epithelial cells show increased PC synthesis coupled to increases in CCT protein and mRNA with advancing gestation (21, 34). Mechanistic studies in these cells suggest that induction of CCT transcripts with fetal development are associated with ∼ 3-fold increase in CCTα message stability without significant alterations in mRNA synthesis (23). Although these latter studies were carefully conducted, transcription rates relied solely on analysis performed using isolated nuclei from fetal type II cells in transcription run-ons, and analysis was not performed in neonatal tissues or more mature lung epithelia for comparative analysis (23). Our studies differ in that we performed a broader range of analysis at different stages of lung maturation and employed genetic approaches using transgenic mice complemented with transient transfectional analysis of promoter–reporter plasmids in developing type II cells. Studies were also supplemented using pulmonary fetal lung explants in which surfactant synthesis increases in vitro similar to that which occurs in whole lung (35). By using this combinatorial approach, we uncovered transcriptional activation of the CCTα gene during a window period shortly after birth.
The ∼ 7-fold increase in CCT activity in mouse lung from the fetal (Day 16) to the newborn period observed here is highly consistent with the ∼ 5-fold increase in enzyme activity described previously (19). This activation pattern was associated with a robust increase in CCTα protein, β-galactosidase activity, and β-galactosidase protein content. We did detect a trend for high-level CCTα and β-galactosidase mRNAs in early fetal lung, suggesting additional levels of control. For example, mRNAs for both CCTα and β-galactosidase might be more stable in Day 16 fetal lung and yet not be efficiently translated to protein, accounting for relatively low enzyme activities that were detected (Figure 3). Regardless, the highest levels of CCTα and β-galactosidase transcripts were also observed in newborn lung, consistent with our protein and functional data.
Fetal lung explants generated from transgenics exhibited coordinate increases in CCTα and β-galactosidase activities and protein with culturing suggesting that increased PC synthesis in explants described elseware is due to increased mRNA synthesis of CCTα (35). Our results do not exclude other mechanisms that might result in increased CCTα activity. Post-translational activation of CCTα might also play a role as the fold increase in enzyme activities between fetal and newborn mice for the endogenous and reporter genes exceeded the magnitude of induction of protein and transcript levels during this time frame. In two systems, whole lung and explant culture where cell–cell and cell–matrix interactions are intact, the ∼ 2-kb CCTα promoter was sufficient to interact with DNA binding elements such as enhancers that might transactivate the CCTα gene during late gestation and shortly after birth.
Finally, we assessed gene transcriptional activity in cells of interest: fetal, newborn, and adult rat type II cells. It is likely that distal lung (type II cell) epithelia are highly active in CCTα transcriptional activity, as our immunostaining and comparative analysis of different cell types show that β-galactosidase expression was mainly detected within these cells (Figure 5). Transfectional studies were then performed using type II cell isolates (Figure 6). To date, these studies are limited by inherently low transfectional efficiencies in primary lung epithelia and poor cell yields in fetal mouse type II cells. Recently, Amaxa developed a new strategy for delivering plasmid directly into the cell nucleus in primary cells using a Nucleofector electroporation system. By using this system with enhanced GFP as a reporter gene, primary alveolar type II epithelia were transfectable with efficiencies of ∼ 90% and 61% in fetal and newborn type II cells, respectively. Adult type II cells, however, exhibited much lower transfection efficiencies. To circumvent the issue of low numbers of type II cells, we used rat type II cells, in which greater cell yields have been obtained. We selected Day 18 gestation in the fetal rat lung because it corresponds to Day 16 gestation in lungs of fetal mice. This time represents a relatively immature phase, with completion of only proximal airways in both species (i.e., late glandular stage) (1). Cells were co-transfected with the ∼ 2-kb CCTα promoter linked to a luciferase reporter, a β-galactosidase plasmid, or a GFP reporter driven by a strong viral CMV promoter to correct for transfectional efficiency. Promoter activity increased ∼ 7-fold from rat fetal (Day 18) gestation to the newborn period, and activity then decreased to lower levels in mature lung. Additional transient transfection experiments in type II cells using a smaller core promoter (−169/+71) showed even higher level of promoter activity in newborn cells compared with fetal cells. A robust increase in transcriptional activity in newborn type II cells versus fetal and adult type II cells indicates that the ∼ 240-bp core promoter contains the necessary cis-acting regulatory elements within the CCTα gene for perinatal activation in primary alveolar epithelia. However, because of very low transfectional efficiencies observed in mature lung epithelia, it is difficult to conclude that CCTα transcriptional activity is repressed in adult lung type II cells. It is conceivable that culture conditions were not optimized for mature type II cells, that kinetics for luciferase expression differs in adult cells from newborns, or that stimulatory factors that drive CCTα gene transcription in newborn cells are not expressed in more mature type II cells. Nevertheless, these results indicate that high-level CCTα expression in newborn whole lung and pulmonary explants are recapitulated for the most part in surfactant-producing newborn alveolar type II cells, in which a transcriptional mechanism plays a role. Future studies localizing the specific regions of the CCTα promoter and relevant DNA-binding partners will be important in elucidating the molecular basis for CCTα transcriptional activation during ontogeny.
This study was supported by a Merit Review Award, the Department of Veterans Affairs, the Cystic Fibrosis Foundation, and NIH R01 Grants HL071040, HL081784, HL068135, and HL080229 (to R.K.M.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0401OC on April 27, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ballard PL. Hormones and lung maturation, monogr. endocrinol., 1st ed. Berlin: Springer-Verlag; 1986. [PubMed]
- 2.Rooney SA, Young SL, Mendelson CR. Molecular and cellular processing of lung surfactant. FASEB J 1994;8:957–967. [DOI] [PubMed] [Google Scholar]
- 3.Oldham KT, Guice KS, Stetson PS, Wolfe RR. Bacteremia-induced suppression of alveolar surfactant production. J Surg Res 1989;47:397–402. [DOI] [PubMed] [Google Scholar]
- 4.Babu KS, Woodcock DA, Smith SE, Staniforth JN, Holgate ST, Conway JH. Inhaled synthetic surfactant abolishes the early allergen-induced response in asthma. Eur Respir J 2003;21:1046–1049. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Wang L, Holm BA, Enhorning G. Dysfunction of guinea-pig pulmonary surfactant and type II pneumocytes after repetitive challenge with aerosolized ovalbumin. Clin Exp Allergy 1997;27:802–807. [DOI] [PubMed] [Google Scholar]
- 6.Jackowski S, Fagone P. CTP: Phosphocholine cytidylyltransferase: paving the way from gene to membrane. J Biol Chem 2005;280:853–856. [DOI] [PubMed] [Google Scholar]
- 7.Post M, Batenburg JJ, Schuurmans EA, Van Golde LM. The rate-limiting step in the biosynthesis of phosphatidylcholine by alveolar type II cells from adult rat lung. Biochim Biophys Acta 1982;712:390–394. [DOI] [PubMed] [Google Scholar]
- 8.Agassandian M, Zhou J, Tephly LA, Ryan AJ, Carter AB, Mallampalli RK. Oxysterols inhibit phosphatidylcholine synthesis via ERK docking and phosphorylation of CTP:Phosphocholine cytidylyltransferase. J Biol Chem 2005;280:21577–21587. [DOI] [PubMed] [Google Scholar]
- 9.Feldman DA, Dietrich JW, Weinhold PA. Comparison of the phospholipid requirements and molecular form of CTP: phosphocholine cytidylyltransferase from rat lung, kidney, brain and liver. Biochim Biophys Acta 1980;620:603–611. [DOI] [PubMed] [Google Scholar]
- 10.Weinhold PA, Rounsifer ME, Charles L, Feldman DA. Characterization of cytosolic forms of CTP: choline-phosphate cytidylyltransferase in lung, isolated alveolar type II cells, A549 cell and Hep G2 cells. Biochim Biophys Acta 1989;1006:299–310. [DOI] [PubMed] [Google Scholar]
- 11.Miller BE, Hoo KG. Regulation of phosphatidylcholine biosynthesis in activated alveolar type II cells. Am J Respir Cell Mol Biol 1989;1:127–136. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Marsh JJ, Spragg RG. Effect of CTP:phosphocholine cytidylyltransferase overexpression on the mouse lung surfactant system. Am J Respir Cell Mol Biol 2002;26:709–715. [DOI] [PubMed] [Google Scholar]
- 13.Ridsdale R, Tseu I, Roth-Kleiner M, Wang J, Post M. Increased phosphatidylcholine production but disrupted glycogen metabolism in fetal type II cells of mice that overexpress CTP:phosphocholine cytidylyltransferase. J Biol Chem 2004;279:55946–55957. [DOI] [PubMed] [Google Scholar]
- 14.Cui Z, Houweling M, Chen MH, Record M, Chap H, Vance DE, Terce F. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J Biol Chem 1996;271:14668–14671. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Magdaleno S, Tabas I, Jackowski S. Early embryonic lethality in mice with targeted deletion of the CTP:phosphocholine cytidylyltransferase alpha gene (Pcyt1a). Mol Cell Biol 2005;25:3357–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan F, Harding PG, Wong T, Fellows GF, Possmayer F. Cellular distribution of enzymes involved in phosphatidylcholine synthesis in developing rat lung. Can J Biochem Cell Biol 1983;61:107–114. [DOI] [PubMed] [Google Scholar]
- 17.Oldenborg V, Van Golde LM. The enzymes of phosphatidylcholine biosynthesis in the fetal mouse lung. Effects of dexamethasone. Biochim Biophys Acta 1977;489:454–465. [DOI] [PubMed] [Google Scholar]
- 18.Casola PG, Chan F, Macdonald PM, Ryan S, McMurray WC, Possmayer F. Coordinate increases in the enzyme activities responsible for phosphatidylglycerol synthesis and CTP:cholinephosphate cytidylyltransferase activity in developing rat lung. Biochem Biophys Res Commun 1980;96:1209–1215. [DOI] [PubMed] [Google Scholar]
- 19.Brehier A, Rooney SA. Phosphatidylcholine synthesis and glycogen depletion in fetal mouse lung: developmental changes and the effects of dexamethasone. Exp Lung Res 1981;2:273–287. [DOI] [PubMed] [Google Scholar]
- 20.Stern W, Kovac C, Weinhold PA. Activity and properties of CTP: cholinephosphate cytidylyltransferase in adult and fetal rat lung. Biochim Biophys Acta 1976;441:280–293. [DOI] [PubMed] [Google Scholar]
- 21.Hogan M, Zimmermann LJ, Wang J, Kuliszewski M, Liu J, Post M. Increased expression of CTP:phosphocholine cytidylyltransferase in maturing type II cells. Am J Physiol 1994;267:L25–L32. [DOI] [PubMed] [Google Scholar]
- 22.Fraslon C, Batenburg JJ. Pre-translational regulation of lipid synthesizing enzymes and surfactant proteins in fetal rat lung in explant culture. FEBS Lett 1993;325:285–290. [DOI] [PubMed] [Google Scholar]
- 23.Hogan M, Kuliszewski M, Lee W, Post M. Regulation of phosphatidylcholine synthesis in maturing type II cells: increased mRNA stability of CTP:phosphocholine cytidylyltransferase. Biochem J 1996;314:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallampalli RK, Hunninghake GW. Expression of immunoreactive cytidine 5′-triphosphate: cholinephosphate cytidylyltransferase in developing rat lung. Pediatr Res 1993;34:502–511. [DOI] [PubMed] [Google Scholar]
- 25.Ryan AJFK, Thomas CP, Mallampalli RK. Transcriptional repression of the CTP:phosphocholine cytidylyltransferase gene by sphingosine. Biochem J 2004;382:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, You Y, Zabner J, Ryan AJ, Mallampalli RK. The CCT promoter directs high-level transgene expression in distal lung epithelial cell lines. Am J Respir Cell Mol Biol 2004;30:61–68. [DOI] [PubMed] [Google Scholar]
- 27.Longo CA, Tyler D, Mallampalli RK. Sphingomyelin metabolism is developmentally regulated in rat lung. Am J Respir Cell Mol Biol 1997; 16:605–612. [DOI] [PubMed] [Google Scholar]
- 28.McCoy DM, Salome RG, Kusner DJ, Iyar SS, Mallampalli RK. Identification of sex-specific differences in surfactant synthesis in rat lung. Pediatr Res 1999;46:722–730. [DOI] [PubMed] [Google Scholar]
- 29.Salome RG, McCoy DM, Ryan AJ, Mallampalli RK. Effects of intratracheal instillation of TNF-alpha on surfactant metabolism. J Appl Physiol 2000;88:10–16. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Ryan AJ, Medh J, Mallampalli RK. Oxidized lipoproteins inhibit surfactant phosphatidylcholine synthesis via calpain mediated cleavage of CTP:phosphocholine cytidylyltransferase. J Biol Chem 2003;278: 37032–37040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viscardi RM, McKenna MC. Developmental changes in cholinephosphate cytidylyltransferase activity and microsomal phospholipid fatty acid composition in alveolar type II cells. Life Sci 1994;54:1411–1421. [DOI] [PubMed] [Google Scholar]
- 32.Houweling M, Tijburg LB, Jamil H, Vance DE, Nyathi CB, Vaartjes WJ, van Golde LM. Phosphatidylcholine metabolism in rat liver after partial hepatectomy. Evidence for increased activity and amount of CTP:phosphocholine cytidylyltransferase. Biochem J 1991;278:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao ZM, Jamil H, Vance DE. Choline deficiency causes translocation of CTP:phosphocholine cytidylyltransferase from cytosol to endoplasmic reticulum in rat liver. J Biol Chem 1990;265:4326–4331. [PubMed] [Google Scholar]
- 34.Zimmermann LJ, Hogan M, Carlson KS, Smith BT, Post M. Regulation of phosphatidylcholine synthesis in fetal type II cells by CTP:phosphocholine cytidylyltransferase. Am J Physiol 1993;264:L575–L580. [DOI] [PubMed] [Google Scholar]
- 35.Gross I, Smith GJ, Maniscalco WM, Czajka MR, Wilson CM, Rooney SA. An organ culture model for study of biochemical development of fetal rat lung. J Appl Physiol 1978;45:355–362. [DOI] [PubMed] [Google Scholar]