Abstract
Degradation of preexisting and newly synthesized extracellular matrix is thought to play an important role in tissue remodeling. The current study evaluated whether thrombin and TNF-α/IL-1β could collaboratively induce collagen degradation by human fetal lung fibroblasts (HFL-1) and adult bronchial fibroblasts cultured in three-dimensional collagen gels. TNF-α/IL-1β alone induced production of matrix metalloproteinases (MMPs)-1, -3, and -9, which were released in latent form. With the addition of thrombin, the latent MMPs were converted into active forms and this resulted in collagen gel degradation. Part of the activation of MMPs by thrombin resulted from direct activation of MMP-1, MMP-2, MMP-3, and MMP-9 in the absence of cells. In addition, tissue inhibitor of metalloproteinase-1 production was inhibited by the combination of thrombin and TNF-α/IL-1β. These results suggest that thrombin and TNF-α/IL-1β synergize to induce degradation of three-dimensional collagen gels through increasing the production and activation of MMPs, and that this effect is mediated through both direct activation of MMPs by thrombin and indirectly by thrombin activation of fibroblasts. Through such mechanisms, thrombin could contribute to many chronic lung disorders characterized by tissue remodeling.
Keywords: thrombin, matrix metalloproteinase, collagen degradation
Alterations in tissue structure are characteristic of many chronic inflammatory diseases. In the lung, tissue remodeling is believed to be the major cause for fixed airflow limitation that is the defining feature of chronic obstructive pulmonary disease and may be present in asthma (1, 2). Tissue remodeling with loss of function also characterizes interstitial lung diseases. It is now clear that the lung can both produce new extracellular matrix (ECM) and cause its degradation. Tissue remodeling, therefore, can result from an imbalance between ECM production and destruction.
The mechanisms that lead to ECM degradation are only partially defined. In this context, the matrix metalloproteinases (MMPs) are believed to play a central role (3, 4). This family of enzymes includes more than 20 different members that have a variety of substrate affinities (5). The production of these enzymes is increased in many chronic diseases (6–8). Their activity, however, is regulated at several levels. Many proteases, which do not directly degrade ECM, for example, may contribute to matrix degradation by activating the MMPs (9–11).
Thrombin is a serine protease activated in the final stages of the coagulation cascade. Thrombin activation can occur as a result of intravascular coagulation and as a result of tissue injury (12). Thrombin not only cleaves fibrinogen to fibrin but also has a number of other biological activities. In this context, thrombin can activate cell surface receptors and can modulate potential repair responses in a variety of cell types (13–15).
Since thrombin, which does not directly degrade collagen, is likely to be present in inflammatory tissue injury where cytokines, such as IL-1β and TNF-α, may drive the production of MMPs (16, 17), the current study was designed to determine if an interaction between these pathways may occur in an in vitro model of tissue remodeling. Specifically, the cytokines IL-1β and TNF-α were used to induce fibroblasts cultured in three-dimensional collagen gels to produce MMPs. Thrombin was then tested for its ability to activate these MMPs and lead to degradation of ECM. In addition, whether the actions of thrombin were mediated directly on the MMPs or indirectly through a cellular-mediated process were explored. The current study provides support for a synergistic interaction between thrombin and IL-1β/TNF-α in regulating ECM degradation by human lung fibroblasts.
MATERIALS AND METHODS
Materials
Type I collagen was extracted from rat tail tendons (RTTC) as previously described (18, 19). Briefly, tendons were excised from rat tails, and the tendon sheath and other connective tissues were removed carefully. Repeated washing with Tris-buffered saline (0.9% NaCl, 10mM Tris, pH7.5) was followed by dehydration and sterilization with 50%, 75%, 95% and pure ethanol. Type I collagen was extracted in 6 mM hydrochloric acid at 4°C for 24 h. The supernatant was harvested by centrifugation at 2,000 × g for 2 h at 4°C. Protein concentration was determined by weighing a lyophilized aliquot from each lot of collagen solution. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) routinely demonstrated no detectable proteins other than type I collagen. The RTTC was stored at 4°C until use.
Thrombin from human plasma (Cat #T6884) and hirudin (Cat #7380) was purchased from Sigma (St. Louis, MO). MMP-1 and MMP-3 standards for immunoblotting, TNF-α, and IL-1β were purchased from R&D Systems, Inc. (Minneapolis, MN). Tissue culture supplements, fetal calf serum (FCS), and media were purchased from Invitrogen (Carlsbad, CA).
Cell Culture
Human fetal lung fibroblasts (HFL-1) were obtained from the American Type Culture Collection (Rockville, MD). Human adult bronchial fibroblasts (HBF) were isolated as primary cultures from bronchial biopsies tissues obtained from normal smokers. After expansion, the cells were cultured in 100-mm tissue culture dishes (FALCON; Becton-Dickinson Labware, Franklin Lakes, NJ) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 1 μg/ml fungizone. The fibroblasts were passaged every 3–5 d, and at passage 2–3 were stored under liquid nitrogen vapor until use. Frozen cells were thawed, cultured as described above, and subconfluent fibroblasts were trypsinized (trypsin-EDTA; 0.05% trypsin, 0.53mM EDTA-4Na) and used for collagen gel culture. HFL-1 fibroblasts used in these experiments were between cell passages 14 and 19, and HBFs were between passages 4 and 6 after initial isolation.
Collagen Gel Preparation
Collagen gels were prepared as described previously (19). Briefly, the appropriate amount of RTTC was mixed with distilled water, 4× concentrated DMEM, and the cell suspension so that the final mixture resulted in 0.75 mg/ml of collagen, 4.5 × 105 cells/ml, and a physiologic ionic strength. Fibroblasts were routinely added last to minimize damage during the preparation of collagen gels. One half milliliter of the mixture, which contains approximately 375 μg collagen, was cast into each well of 24-well tissue culture plates (FALCON; Becton Dickinson Labware). Gelation occurred in about 20 min at room temperature, after which the gels were released and transferred to 60-mm tissue culture dishes containing 5 ml of serum-free DMEM with or without thrombin or the cytokines (TNF-α and IL-1β). The floating gels were then incubated at 37°C in a 5% CO2 atmosphere for various periods of time. In order to determine if thrombin catalytic activity was required, thrombin (500 nM, which equals 56 U/ml) was neutralized with the specific inhibitor hirudin (2.5 μM, which equals 84 U/ml). To accomplish this, thrombin and hirudin were added to the culture medium and incubated at 37°C for 30 min before the addition of cytokines.
Hydroxyproline Assay
Hydroxyproline, which is directly proportional to type I collagen content, was measured by spectrophotometry (20). Briefly, the collagen gels were transferred to microcentrifuge tubes and centrifuged (2,000 × g, 10 min), and the supernatant was removed. The gel was then solubilized by heating in ddH2O (50 μl/gel). Samples (20 μl) mixed with 30 μl 3.3 N NaOH were hydrolyzed by autoclaving at 120°C for 20 min. The hydrolyzed samples were oxidized with 0.056 M Chloramine-T (in 50% n-propanol and acetate/citric acid buffer) for 20 min at room temperature, and then reacted with Ehrlich's reagent (1 M p-dimethylaminobenzaldehyde in n-propanol/perchloric acid [2:1 vol/vol]) at 65°C for 20 min. The absorbance was measured at 540 nm with an BenchMark Microplate Reader and Microplate Manager III software (Bio-Rad, Hercules, CA). N-acetyl-hydroxyproline (Cat#: 441562; Sigma, St. Louis, MO) was used as standard and the absolute amount of hydroxyproline in each gel (μg/gel) was obtained. Data were compared to control gel and expressed as % of the full amount of OH-proline expected (about 35 μg) based on the collagen used to form the gels and the amino acid composition of collagen (about 10% OH-proline).
Gelatin Zymography
To investigate the activity of gelatinases (MMP-2 and -9), gelatin zymography was performed. The conditioned media (500 μl per culture condition) were concentrated 10-fold by precipitation with cold ethanol and dissolved in distilled H2O (50 μl per sample). The same amount of medium from each condition was used. This makes it possible to compare the absolute amounts of MMPs in the various cultures. Gelatin zymography was performed by a modification of previously published procedures (17). The samples were dissolved in 2× electrophoresis sample buffer without 2-mercaptoethanol and analyzed in 10% SDS-PAGE containing 0.1% gelatin under nonreducing conditions. After electrophoresis, the gels were soaked in 2.5% (vol/vol) Triton-X 100 and gently shaken at room temperature for 1 h. The gels then were incubated in metalloproteinase activation buffer (50mM Tris-HCl, pH7.5, containing 5 mM CaCl2 and 1μ M ZnCl2) for 18 h at 37°C and stained with 0.4% Comassie blue and destained with destaining buffer. The gels then were dried between dialysis membranes (cellophane sheets; Amersham, Piscataway, NJ). Zones of proteolysis appeared as clear bands against a blue background.
Tissue Inhibitor of Metalloproteinase-1 Assay
Tissue inhibitor of metalloproteinase (TIMP)-1 in the media in which gels were floated was determined by enzyme-linked immunosorbent assay (ELISA). To do this, ELISA plates were coated with monoclonal anti–TIMP-1 antibody (R&D Systems, Inc., Minneapolis, MN) at 4°C overnight. After three washings (5 min each), standards and samples were added and incubated at room temperature for 2 h. After another three washings, bound antigen was detected after adding biotinylated anti-human TIMP-1 antibody (R&D Systems, Inc., Minneapolis, MN) for 1 h at room temperature. Horseradish peroxidase (HRP)-streptavidin (1:20,000 dilution) was then added and incubated at room temperature for 1h. Bound HRP was then detected with 3,3′,5,5′-tetramethylbenzidine (TMB). The reaction was stopped with 1M H2SO4 and the product was quantified at 450 nm in a Benchmark Microplate Reader (Bio-Rad, Hercules, CA).
Immunoblot Analysis of Metalloproteinases
To further identify the production of MMPs, immunoblots were performed. The supernatants (5 ml per condition) from three-dimensional culture were precipitated with 50% (vol/vol) ethanol and resuspended in distilled H2O (50 μl per sample). As above, equal amounts of medium were used for each lane from each condition to permit comparison of the absolute amounts released. The resuspended samples were mixed with an equal volume of 2× sample buffer (0.5M Tris-HCl, pH6.8, 10% sodium dodecyl sulfate [SDS], 0.1% bromphenol blue, 20% glycerol). After heating for 3 min at 95°C, the samples were separated by electrophoresis by 10% SDS-PAGE, and the proteins were transferred to polyvinyl difluoride (PVDF) membranes (Bio-Rad). Membranes were blocked in 5% nonfat milk in PBS-Tween at room temperature for 1 h and then probed with mouse anti-human MMP-1 or MMP-3 mAb (Calbiochem, Cambridge, MA). Target proteins were subsequently detected using HRP-conjugated goat anti-mouse IgG (Rockland, Gilbertsville, PA) in conjunction with an enhanced chemiluminescence detection system (ECL; Amersham Biosciences UK Limited, Little Chalfont, Buckinghamshire, UK).
RNA Isolation and Complementary DNA Synthesis
Collagen gels were digested with collagenase (0.5 mg/ml in RNAse-free PBS) at 37°C for 1 h. Total RNA was then extracted from the cell pellets with acid guanidine monothiocyanate, and precipitated with isopropylalcohol. The extracted RNA was dissolved in TE buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA) and the total amount was quantified spectrophotometrically. One microgram of total RNA was treated with DNAse I following the manufacturer's instructions (Invitrogen) for 15 min at room temperature to remove possible contaminating genomic DNA. The reaction was then stopped with 25 mM EDTA by heating at 65°C for 10 min, followed by 95°C for 5 min. For cDNA synthesis, ∼ 400ng of DNAse-treated RNA was transcribed with cDNA transcription reagents (Applied Biosystems, Branchburg, NJ) by using random hexamers, and the cDNA was used for quantitative real-time PCR.
Quantitative Real-Time PCR
Gene expression was measured with the use of the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as described previously (21). Primers and TaqMan probes were designed using the Primer Express 1.0 (Applied Biosystems) software to amplify < 150 base pairs. Probes were labeled at the 5′ end with the reporter dye molecule 6-carboxy-fluorescein (FAM) (22) and at the 3′ end with the quencher dye molecule 6-carboxytetramethyl-rhodamine (TAMRA). The target gene and the housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) were simultaneously tested in duplicate or triplicate. Data were normalized to the amount of GAPDH and expressed as fold of control. Real-time PCRs of cDNA specimens were conducted in a total volume of 50 μl with 1× TaqMan Master Mix (Applied Biosystems), and primers at 300 nM and probes at 200 nM. Sequences used were as follows: MMP-1 (forward), 5′-CGG TTT TTC AAA GGG AAT AAG TAC T-3′; MMP-1 (reverse) 5′-TCA GAA AGA GCA GCA TCG ATA TG-3′; MMP-1 (probe), 6FAM-AAT GTG CTA CAC GGA TAC CCC AAG GAC A-TAMRA; MMP-9 (forward), 5′-AGA GAG GAG GAG GTG GTG TAA GC-3′; MMP-9 (reverse), 5′-TGA CAG GCA AGT GCT GAC TCA-3′; MMP-9 (probe), 6FAM-TT TCT CAT GCT GGT GCT GCC ACA CA-TAMRA; MMP-3 (forward), 5′-GAT ATA AAT GGC ATT CAG TCC CTC TAT–3′; MMP-3 (reverse), 5′-CAA AGG ACAAAG CAG GAT CAC A-3′; MMP-3 (probe), 6FAM-CCT CCC CCT GAC TCC CCT GAG ACC-TAMRA; TIMP-1 (forward), 5′-GAC CAA GAT GTA TAA AGG GTT CCA A-3′; TIMP-1 (reverse), 5′-GGT TGT GGG ACC TGT GGA AGT A-3′; TIMP-1 (probe), 6FAM-TCG TCT ACA CCC CCG CCA TGG A-TAMRA; GAPDH (forward), 5′-CCA GGA AAT GAG CTT GAG AAA GT-3′; GAPDH (reverse), 5′-CCC ACT CCT CCA CCT TTG AC-3′; GAPDH (probe), 6FAM-CGT TGA GGG CAA TGC CAG CCC-TAMRA.
Thermal cycle parameters included 2 min at 50°C, 10 min at 95°C, and 40 cycles involving denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min.
Statistical Analysis
Individual experiments included triplicate gels for all experimental conditions. Results were always confirmed by repeating each experiment on separate occasions at least three times. Statistical comparisons were made from all experiments, including both the within- and between-group variance. Pooled data are expressed as percentage or fold of control condition. Group data were analyzed by one-way ANOVA followed by Tukey test. Differences of paired data were assessed by Student's t test (P < 0.05 was considered significant).
RESULTS
The Effect of Thrombin and Cytokines on Collagen Gel Degradation
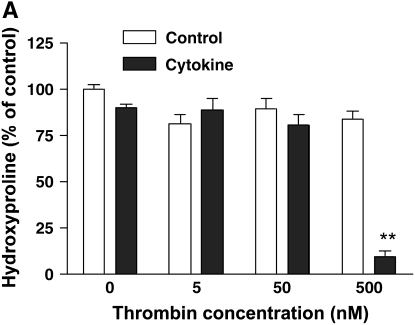
To determine if thrombin together with cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]) would induce collagen gel degradation, hydroxyproline content within the gels was determined (Figure 1A). No effect on gel degradation was observed when cells were exposed to either cytokines or thrombin (5 nM, 50 nM, 500 nM) alone (hydroxyproline content within gels was 80.7 ± 5.4% to 89.9 ± 1.8% of control, P > 0.05). In contrast, when added together with cytokines, thrombin (500 nM) together with IL-1β/TNF-α resulted in nearly complete degradation of the collagen populated with HFL-1 cells (hydroxyproline content was 9.1 ± 2.9% of control, P < 0.01). In the presence of thrombin inhibitor (hirudin), cytokines plus thrombin did not cause collagen degradation (Figure 1B). Similar results were observed in HBF-populated collagen gels (data not shown).
Figure 1.
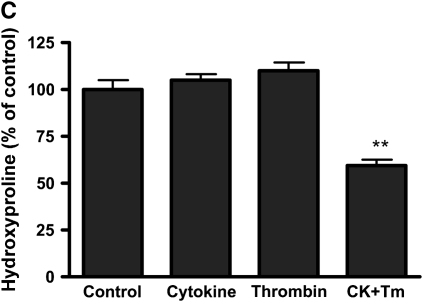
Effect of thrombin and cytokines on collagen gel degradation. (A) Cell-dependent effect. Gels containing HFL-1 fibroblasts (4.5 × 105/ml) were cast into serum-free DMEM containing different concentrations of thrombin (0 nM, 5 nM, 50 nM, and 500 nM) with or without cytokines (5 ng/ml TNF-α and 5 ng/ml IL-1β). The gels were cultured for 5 d and hydroxyproline content in the gels was determined. Vertical axis: hydroxyproline (% of Control). Horizontal axis: Thrombin (nM). Data presented are mean ± SEM for three separate experiments, each of which included triplicate gels for each condition. Open bars, SF-DMEM; filled bars, cytokines. The asterisks indicate P < 0.01 compared with control condition. (B) Effect of hirudin on collagen gel degradation. HFL-1 cells were cast into collagen gels and cultured for 5 d in the presence of cytokines (5 ng/ml of TNF-α and IL-1β) and thrombin (500 nM) with or without hirudin (5 U/ml). Gels were then harvested and hydroxyproline content was determined. Vertical axis: hydroxyproline (% of control). Horizontal axis: treatment. (C) Cell-independent effect. Gels containing HFL-1 fibroblasts (4.5 × 105/ml) were incubated with or without cytokines (5 ng/ml TNF-α and 5 ng/ml IL-1β) for 4 d. The conditioned medium was harvested. Gels without fibroblasts were cast and incubated in the conditioned medium with or without thrombin (500 nM). After 24 h, the hydroxyproline content in the gels was determined. Vertical axis: hydroxyproline content (% of Control). Horizontal axis: culture conditions. **P < 0.01 compared with control. CK, cytokines; Tm, thrombin.
To determine whether thrombin directly or indirectly activates MMPs, fibroblast-conditioned media were prepared by culturing the cells in three-dimensional collagen gels with or without cytokine stimulation. Collagen gels without cells were then incubated with the fibroblast-conditioned media in the presence or absence of thrombin, and hydroxyproline content was determined after 24 h of incubation. Fibroblast-conditioned media alone or thrombin was added to control-conditioned medium, but did not degrade collagen gels. However, when thrombin was added to cytokine-conditioned medium, collagen gels were degraded significantly (Figure 1C; hydroxyproline content was 58.7 ± 3.4% of control, P < 0.01), although the degradation was not as complete as that observed in the presence of cells.
Effect of Thrombin and Cytokines on Gelatinase Production and Activation
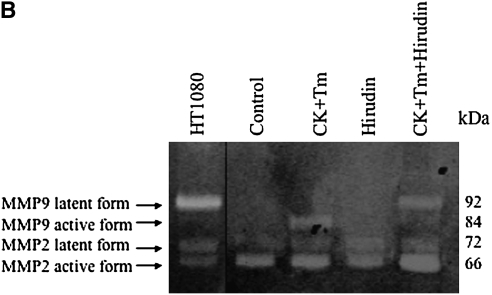
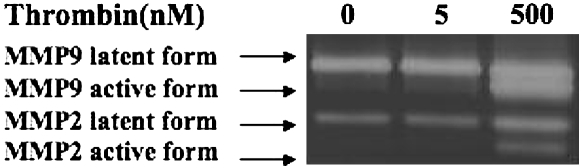
To determine if thrombin altered fibroblast production of collagen-degrading enzymes, several experiments were performed. First, zymography was performed to examine the effect of thrombin and cytokines on gelatinase (MMP-2 and MMP-9) production and activation (Figure 2). Under control conditions, both HFL-1 fibroblasts and adult bronchial fibroblasts cultured in three-dimensonal collagen gels released MMP-2 in both the latent and active forms, but no MMP-9 was detected. In response to stimulation by cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]), MMP-9 (latent form) and increased amounts of MMP-2 (both latent form and active form) were detected in HFL-1 cells (Figure 2A). In the presence of cytokines, thrombin concentration-dependently stimulated MMP-9 activation in HFL-1 cells (Figure 2A). Nearly all MMP-9 was present as the active form in cultures incubated with 500 nM thrombin in HFL-1 cells (Figure 2A), and this was completely blocked by hirudin (Figure 2B). Consistently, collagen degradation was completely blocked by hirudin in HFL-1 cell–populated gels (Figure 1B). Similar results were obtained with HBF (Figure 2C). Under control conditions, HBFs release MMP-2 but not MMP-9. In addition, HBF in three-dimensional collagen gel culture also produce other unidentified gelatinases corresponding in size to 45–50 kD (Figure 2C). In contrast to HFL-1 fibroblasts, both thrombin and cytokines alone stimulated MMP-9 production by HBFs, and also resulted in activated MMP-9. These effects were significantly augmented when both cytokines and thrombin were added together (Figure 2C).
Figure 2.
Effect of hirudin on thrombin-induced MMP activation in HFL-1 and HBF. (A) Thrombin-induced MMP activation in HFL-1 cells. HFL-1 cells were cast into collagen gels and cultured for 5 d in the presence of cytokines and thrombin. Media were harvested for gelatin zymography. (B) Effect of hirudin on MMP activation in the presence of cytokine and thrombin in HFL-1 cells. HFL-1 cells were cast into collagen gels and cultured for 5 d in the presence or absence of cytokines, thrombin, or hirudin. Media were harvested for gelatin zymography. (C) MMP production and activation in HBFs. HBFs were cast into collagen gels and cultured for 5 d in the presence of thrombin and/or cytokines. Media were harvested for gelatin zymography. CK, cytokines (IL-1β + TNF-α); Tm, thrombin. Supernatant from HT1080 cell monolayer culture was used as a positive control.
Effect of Thrombin and Cytokines on MMP-1 and MMP-3 Production and Activation
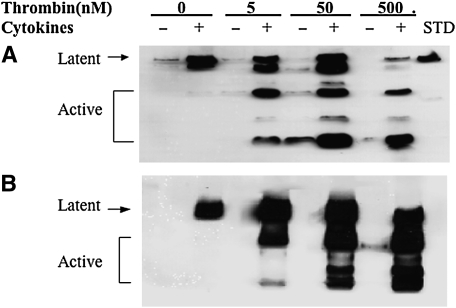
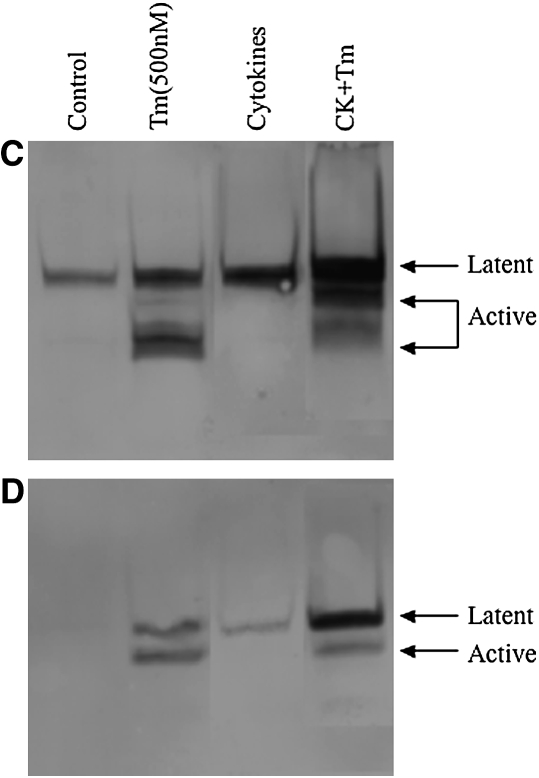
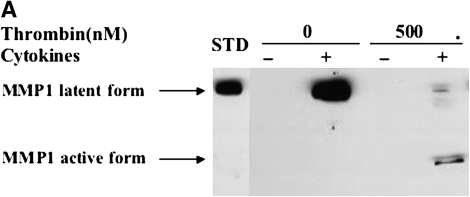
To determine the effect of thrombin and cytokines on MMP-1 and MMP-3 expression, immunoblotting was performed (Figure 3). Under control conditions, a small amount of MMP-1 in the latent form was produced by both HFL-1 or HBF cells (Figures 3A and 3C). Cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]) alone markedly induced MMP-1 release, and this was detected in the latent form. When thrombin was added together with cytokines, MMP-1 was subsequently converted to a lower molecular weight form corresponding to the active form of MMP-1. In the presence of cytokines, incubation with thrombin at the concentration of 500 nM resulted in most of the MMP-1 being present as the active form (Figures 3A and 3C).
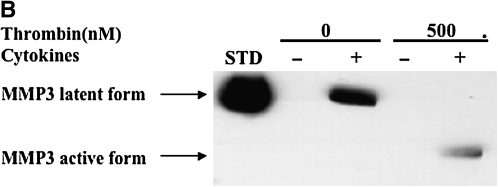
Figure 3.
Effect of thrombin and cytokines on MMP-1 and MMP-3 production and activation. Cultures of HFL-1 cell (A and B) or HBF (C and D) populated collagen gels were incubated with various concentrations of thrombin and cytokines for 5 d. Immunoblotting was used to identify MMP-1 (A and C) and MMP-3 (B and D). Arrows indicate MMP-1 or MMP-3 latent and active forms. STD: MMP-1 or MMP-3 standard.
MMP-3 was undetectable under control conditions (Figures 3B and 3D). Thrombin alone at concentrations of 5 nM and 50 nM had no effect on MMP-3 release by HFL-1 cells (Figure 3B). A higher concentration of thrombin (500 nM), however, stimulated HFL-1 cells to produce a small amount of MMP-3 in the lower molecular weight form corresponding to the active form (Figure 3B), while both latent and active MMP-3 were detected in HBF samples (Figure 3D). Cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]) induced readily detectable latent MMP-3, and in the presence of thrombin this was converted to the active form in a concentration-dependent manner (Figure 3B). Similar findings were observed in HBFs cultured in the three-dimensional collagen gels (Figure 3D).
Effect of Thrombin and Cytokines on TIMP-1 Release
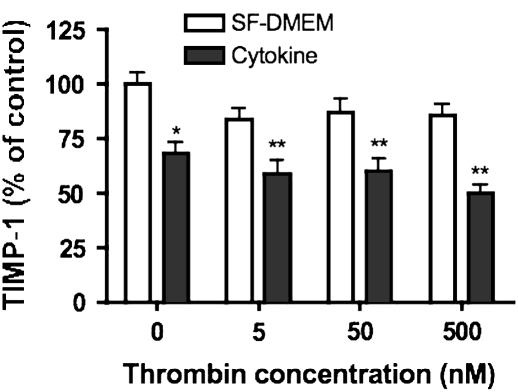
TIMP-1, an inhibitor of MMPs, was measured by ELISA (Figure 4). HFL-1 fibroblasts cultured in three-dimensional collagen gels released large amounts of TIMP-1. Thrombin added alone had no effect on TIMP-1 release. Cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]) inhibited TIMP-1 release (64.1 ± 6.9% of control condition, P < 0.05). Co-exposure to thrombin and cytokines further reduced TIMP-1. The TIMP-1 level was 60.1 ± 7.9%, 61.1 ± 8.3%, and 54.8 ± 5.0% of control (P < 0.01) when thrombin (5 nM, 50 nM, and 500 nM) and cytokines were added together.
Figure 4.
Effect of thrombin and cytokines on TIMP-1 production. Gels containing HFL-1 fibroblasts (4.5 × 105/ml) were cast into serum- free DMEM containing thrombin (0 nM, 5 nM, 50 nM, and 500 nM) with or without cytokines (5 ng/ml TNF-α and 5 ng/ml IL-1β). After 5 d of culture, ELISA was used to identify TIMP-1 in the culture medium. Vertical axis: TIMP-1 content (% of Control). Horizontal axis: Thrombin (nM). Data presented are mean± SEM for three separate experiments, each of which included triplicate gels for each condition. *P < 0.05, **P < 0.01 compared with control.
Effect of Thrombin and Cytokines on MMP-1, MMP-3, MMP-9, and TIMP-1 mRNA Production
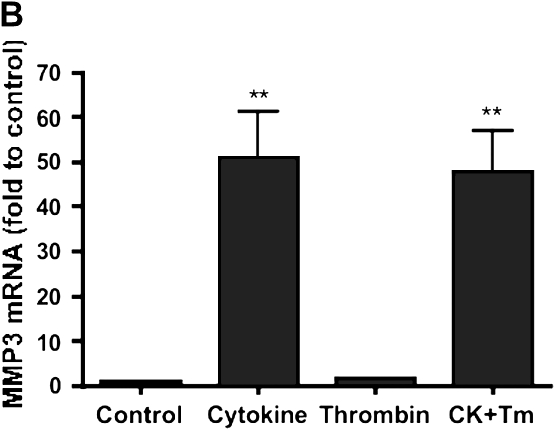
To determine whether the changes in MMP and TIMP release were associated with changes in gene expression, RT-PCR was performed. MMP-1 and MMP-3 mRNA were detected under control conditions. Cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]) significantly stimulated MMP-1 and MMP-3 mRNA levels (Figures 5A and 5B). MMP-1 and MMP-3 mRNA was 2.4- ±0.5- and 50- ±9-fold of control (P < 0.01) after stimulation by cytokines. Thrombin (500 nM) had no effect on MMP-1 and MMP-3 mRNA expression either alone or in combination with IL-1β/TNF-α (P > 0.05).
Figure 5.
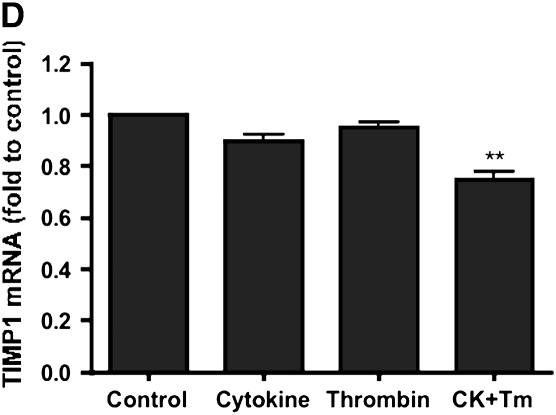
Effect of thrombin and cytokines on MMP-1 (A), MMP-3 (B), MMP-9 (C), and TIMP-1 (D) mRNA expression. HFL-1 fibroblasts cultured in three-dimensional gels were incubated with and without cytokines (5 ng/ml TNF-α and 5 ng/ml IL-1β) or thrombin (500 nM) for 6 h. MMP-1, MMP-3, MMP-9, and TIMP-1 mRNA were quantified by Taqman RT-PCR. Vertical axis: mRNA content (fold of Control). Horizontal axis: culture conditions. Data presented are mean ± SEM for three separate experiments, each of which included triplicate gels for each condition. CK, cytokines. **P < 0.01 compared with control condition, #P < 0.01 compared with CK group.
Little MMP-9 mRNA was detected under control conditions. Cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]) alone significantly stimulated MMP-9 mRNA expression (MMP-9 mRNA was 530- ± 270-fold of control, P < 0.01). Thrombin (500 nM) by itself had no effect on MMP-9 mRNA production, but further increased MMP-9 mRNA when added together with cytokines (Figure 5C). Co-exposure to thrombin (500 nM) and cytokines increased MMP-9 mRNA to 2,200- ± 400-fold of control (P < 0.01).
TIMP-1 mRNA expression was observed under control conditions. Both cytokines (TNF-α [5 ng/ml] and IL-1β [5 ng/ml]) and thrombin (500 nM) added alone resulted in a small decrease of the TIMP-1 mRNA, which was not statistically significant (88.3 ± 4.3% and 90.0 ± 5.0% of control, respectively, P > 0.05). TIMP-1 mRNA, however, was significantly inhibited by the combination of thrombin and cytokines to 74.9 ± 2.9% of control (P < 0.01) (Figure 5D).
The Direct Effect of Thrombin on MMP-1, MMP-2, MMP-3, and MMP-9 Activation
To further determine whether thrombin could directly activate MMPs, conditioned medium was incubated with thrombin (5 nM or 500 nM) and then analyzed for MMP activation. MMP-2 and MMP-9 were evaluated by zymography (Figure 6). In supernatant from HT1080 cell monolayer culture, both MMP-2 and MMP-9 were detected in latent form. Lower concentrations of thrombin (5 nM) had no effect on MMP-2 and MMP-9 activation. Thrombin at higher concentration (500 nM), however, significantly resulted in conversion of both MMP-2 and MMP-9 into lower molecular weight forms corresponding to the active MMPs. This contrasts with the effect of 5 nM thrombin added in the presence of fibroblasts, where activation of MMP-9 was readily observed (Figure 2A).
Figure 6.
Thrombin directly activates MMP-2 and MMP-9. Supernatant from HT1080 cell monolayer culture was exposed to thrombin (0 nM, 5 nM, and 500 nM) for 24 h, the media were harvested, and gelatin zymography was used to identify gelatinases. Arrows indicate the latent and active forms of MMP-2 and MMP-9.
MMP-1 and MMP-3 were analyzed by immunoblotting. Fibroblast-conditioned media were acquired by culturing HFL-1 cells in three-dimensional collagen gels with or without cytokine stimulation. In the control-conditioned medium, MMP-1 and MMP-3 were not detected, and there was no effect when the control-conditioned medium was incubated with thrombin. In the presence of cytokines, MMP-1 and MMP-3 were released in latent form, which could be readily detected. Even with relatively heavily loaded gels, however, no MMP-1 or MMP-3 in the lower molecular weight form was detected. In contrast, in the presence of thrombin (500 nM) lower molecular weight forms of both MMP-1 and MMP-3, corresponding to the active forms, were easily detected and represented most of the MMPs present after incubation (Figures 7A and 7B).
Figure 7.
Thrombin directly activates MMP-1 (A) and MMP-3 (B). HFL-1 fibroblasts cultured in three-dimensional collagen gels were stimulated with or without cytokines (5 ng/ml TNF-α and 5 ng/ml IL-1β). After 4 d, the conditioned media were exposed to thrombin (0 nM and 500 nM) for 24 h, the media were harvested, and immunoblotting was used to identify MMP-1 and MMP-3. Arrows indicate the latent and active form of MMP-1 or MMP-3. STD: MMP-1 or MMP-3 standard.
DISCUSSION
The current study demonstrates that thrombin together with the inflammatory mediators TNF-α and IL-1β synergistically induces collagen degradation within three-dimensional collagen gels populated by human fetal lung fibroblasts. This synergy occurs because the cytokines (TNF-α and IL-1β) induce the fibroblasts to produce and secrete matrix metalloproteases including MMP-1, -2, -3, and -9. These MMPs, however, are released in latent form. Thrombin, which has no effect on collagen degradation when added alone, results in conversion of the MMPs from their latent to their active forms, thus resulting in collagen degradation. Hirudin, which is an inhibitor of thrombin activity, blocks thrombin-induced MMP activation and collagen gel degradation, indicating that thrombin proteolytic activity is required. Similar effects were observed with human fetal lung fibroblasts and with fibroblasts isolated from adult human bronchial biopsies. Thrombin was able to directly activate the MMPs in the absence of fibroblasts. An indirect action of thrombin, mediated through the fibroblasts within the collagen lattice, however, is also likely, as thrombin added alone resulted in release and activation of MMP-1 and MMP-3. When added together with cytokines, these effects of thrombin were greater, and a synergistic increase in MMP-9 mRNA expression and a decrease in TIMP-1 mRNA was observed. Taken together, the results of this investigation indicate a role for thrombin in modulating tissue remodeling that occurs in an inflammatory milieu where the cytokines IL-1β and TNF-α may be released.
Extracellular matrix degradation plays an important role in tissue remodeling. The mechanisms that lead to ECM degradation, however, are incompletely defined. Among the proteases that can degrade ECM, MMPs are believed to play a prominent role (3, 4). This family of enzymes includes more than 25 members characterized by having zinc at the active site (5). The activity of MMPs is regulated at a number of levels. First, their production is regulated at the level of gene expression and, likely, by post-translational mechanisms. Second, most MMPs are released as latent precursors that are activated by proteolytic cleavage. Finally, activated MMPs can be inhibited by a family of endogenous inhibitors, the TIMPs. The extracellular activation of the MMPs is likely regulated through proteolytic cascades (10, 23). The current study suggests that thrombin may play a role in activating the MMPs.
Thrombin is a serine protease that cleaves fibrinogen to fibrin in the final steps of the clotting cascade (12). Activation of thrombin, therefore, is a key and highly regulated step in coagulation. Thrombin, however, has a number of other important biological activities. Thrombin is capable of cleaving the protease-activated receptors (PARs)-1, -3, and -4, which are present on the surface of many cell types (13, 14). Through these activities, thrombin is believed to participate in repair and remodeling responses by stimulating fibroblast migration, proliferation, and differentiation, and productions of extracellular matrix and growth factors (24–28). Consist with a role in the lung, increased levels of thrombin are reported in BAL fluid after allergen challenge in asthma, and are believed to contribute to the activity of BAL on fibroblasts (29). Thrombin may also modulate epithelial cell responses, including apoptosis (30) and induction of mucin gene expression (31). These multiple activities of thrombin are consistent with the concept that coagulation, repair, and remodeling are highly linked processes.
Thrombin has been reported to directly activate pro–MMP-2 in cultured vascular smooth muscle cells (9). In this previous report, however, thrombin activation of MMP-2 took place at the cell surface. The current report demonstrates that thrombin is capable of activating MMP-2 in the absence of fibroblasts. Thrombin was added to conditioned medium that contained several latent MMPs. After incubation with thrombin, active MMP-1, MMP-2, MMP-3, and MMP-9 were observed. Since MMP-3, when activated, is capable of activating MMP-2 and MMP-9 (32), the present study cannot distinguish whether thrombin is directly activating all the MMPs evaluated or whether it initiates a protease cascade. The current study does, however, establish that thrombin is capable of leading to MMP activation in the absence of cells.
A cell-dependent action of thrombin, however, is also supported by the current study. In this context, thrombin has been reported to be a potent stimulator of MMP-9 gene expression in human mesangial cells (33). The current study did not observe an effect of thrombin alone on MMP-9 expression in HFL-1 cells. However, the induction of MMP-9 mRNA expression that was induced by TNF-α and IL-1β was markedly potentiated by the addition of thrombin. This synergistic effect on MMP-9 appeared to be specific, as the induction of MMP-1 and MMP-3 mRNA, which was also observed with IL-1 β and TNF-α, was unaffected by the addition of thrombin. The data from the current study, therefore, suggest that thrombin can interact with the MMP system both directly in the extracellular milieu and indirectly through actions requiring cell-dependent processes.
HFL-1 cells are a widely used strain of human fetal lung fibroblasts. They are easily cultured in vitro, give reproducible results and, since they have been widely used, allow comparisons among various investigators. Both fetal and adult human lung fibroblasts can produce extracellular matrices (e.g., fibronectin and collagen), growth factors (e.g., TGF-β1), and can mediate collagen gel contraction (34–37). However, many phenotypic differences between fetal and adult human lung fibroblasts have been reported. For instance, fetal lung fibroblasts have a higher proliferation rate and are more sensitive to oxidative damage compared with adult lung fibroblasts (35–37). While the mechanisms for the differences between adult and fetal cells remain to be defined, in the current study, MMPs were produced and activated in both fetal and adult human lung fibroblasts in response to cytokine stimulation and thrombin activation. This suggests that these processes may be a general feature of lung fibroblasts. However, the effect of thrombin on MMP-9 appears to be cell type–dependent in that thrombin alone had no effect on MMP-9 expression by HFL-1 cells, but thrombin did stimulate release of MMP-9 from adult bronchial fibroblasts. This suggests that the response of fetal and adult cells to inflammation and injury, while generally similar, may differ in detail.
The cytokines IL-1β and TNF-α are believed to be “upstream” cytokines. Their release early in an inflammatory event is believed to initiate a cascade of complex inflammatory mediators . Modulation of this cascade by other mediators is believed to contribute to the variations in inflammation in different settings. Accumulating evidence suggests that cross-talk between inflammation and coagulation plays an important role in response to injury (38–40). In this context, TNF-α and IL-1β have been demonstrated to stimulate the release of MMPs from fibroblasts cultured in three-dimensional collagen gels (41), a result confirmed in the current study. The current study demonstrates that thrombin, which may be generated at the site of an inflammatory process, can interact with the MMPs produced and lead to active MMPs capable of mediating tissue degradation.
The activity of MMPs is also regulated by the presence of inhibitors. In this context, the current study evaluated TIMP-1, a major inhibitor of several MMPs. The cytokines IL-1β and TNF-α caused a small but significant reduction in TIMP-1 release. Thrombin further reduced TIMP-1 release. Whether the reductions observed are sufficient to shift the balance between MMP activity and inhibition was not determined. In an inflammatory milieu, where many stimuli that may be able to modulate TIMP release may be present, the ability of cytokines and thrombin to modulate TIMP expression could play a role.
Collagen degradation by thrombin, however, seems not solely dependent on its activation of MMPs. In this context, lower concentrations of thrombin (5 and 50 nM) activated MMPs without leading to collagen degradation. At the highest concentration of thrombin used (500 nM), which is still less than the thrombin concentrations used in in vitro and in vivo studies by other investigators (27, 42), collagen degradation was nearly complete. Although the in vivo concentrations of thrombin are not known, the activity observed in the present study was observed at concentrations substantially lower than used in other studies. The concentration–response curve was very steep, ranging from “no” to “essentially complete degradation over a single log dilution,” which corresponded to a single dilution with the methods used. It is possible that an even steeper concentration dependence would be observed with intermediate dilutions. The steep concentration–dependence curve contrasted markedly with the activation of MMPs observed with thrombin, which occurred concentration dependently over three logs of dilution. The mechanism remains to be defined. However, control of collagen degradation likely depends on complex interactions of many factors. The MMPs are known to interact in complex cascades. It is likely, in addition, that serine proteases other than thrombin play roles. Plasmin, for example, has been reported to activate several MMPs as has human neutrophil elastase (11). Cell surface proteases may also contribute. As these protease systems can activate each other and mutually inactivate their inhibitors, it is likely that the overall activity in an in vivo setting will behave as a complex network system. Such systems are often highly stable under many conditions, but then change rapidly when a critical threshold is reached. Many proteases are also regulated by the presence of inhibitors. A steep concentration–response curve could permit relative activity at a local region that is confined spatially, as diffusion reduces the initial concentration (43). The ability of thrombin to interact with MMPs directly and through interactions with fibroblasts may be key features of such a system.
Many diseases, including pulmonary fibrosis, pulmonary emphysema, chronic bronchitis, and asthma, are characterized by tissue inflammation and by alterations of tissue structure. The altered tissue structure likely results both from tissue damage and from active tissue remodeling. The current study demonstrates that the serine protease thrombin, which is likely activated in many settings in which tissue injury occurs, can interact with MMPs to lead to tissue degradation. MMPs, in turn, can be induced by cytokines present in the inflammatory milieu. The current study, therefore, supports the concept that tissue remodeling that characterizes many pathologic conditions present in the lung may result from the synergistic interactions of the inflammatory and coagulation cascades.
Funding for this project was provided by NIH grant 1R01 HL-6408804 and the Larson Endowment, University of Nebraska Medical Center.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0026OC on July 20, 2006
Conflict of Interest Statement: Q.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. X.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A.-M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.I.R. has participated as a speaker in scientific meeting and courses under the sponsorship of AstraZeneca and GlaxoSmithKline. He serves on advisory boards for Altana, AstraZeneca, Dey GlaxoSmithKline, and Inspire. He has conducted clinical trials for AstraZeneca, Centocor, GlaxoSmithKline, Pfizer, Roche, and Sanofi. He has served as a consultant for AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, and Roche. A patent is pending on the use of PDE4 inhibitors in repair; S.I.R. is a co-inventor of the patent owned by the University of Nebraska Medical Center.
References
- 1.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1990;3:507–511. [DOI] [PubMed] [Google Scholar]
- 2.Nagai A. Pathology and pathophysiology of chronic obstructive pulmonary disease. Intern Med 2002;41:265–269. [DOI] [PubMed] [Google Scholar]
- 3.Ferry G, Lonchampt M, Pennel L, de Nanteuil G, Canet E, Tucker GC. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett 1997;402:111–115. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 2003;28:12–24. [DOI] [PubMed] [Google Scholar]
- 5.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 1998;12:1075–1095. [PubMed] [Google Scholar]
- 6.Montano M, Beccerril C, Ruiz V, Ramos C, Sansores RH, Gonzalez-Avila G. Matrix metalloproteinases activity in COPD associated with wood smoke. Chest 2004;125:466–472. [DOI] [PubMed] [Google Scholar]
- 7.Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res 2001;2:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 2004;125:626–632. [DOI] [PubMed] [Google Scholar]
- 9.Galis ZS, Kranzhofer R, Fenton JW II, Libby P. Thrombin promotes activation of matrix metalloproteinase-2 produced by cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1997;17:483–489. [DOI] [PubMed] [Google Scholar]
- 10.Okada Y, Nakanishi I. Activation of matrix metalloproteinase 3 (stromelysin) and matrix metalloproteinase 2 (‘gelatinase’) by human neutrophil elastase and cathepsin G. FEBS Lett 1989;249:353–356. [DOI] [PubMed] [Google Scholar]
- 11.Pins GD, Collins-Pavao ME, Van De Water L, Yarmush ML, Morgan JR. Plasmin triggers rapid contraction and degradation of fibroblast-populated collagen lattices. J Invest Dermatol 2000;114:647–653. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Shavit R, Benezra M, Sabbah V, Bode W, Vlodavsky I. Thrombin as a multifunctional protein: induction of cell adhesion and proliferation. Am J Respir Cell Mol Biol 1992;6:123–130. [DOI] [PubMed] [Google Scholar]
- 13.Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol 2002;168:3577–3585. [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci USA 1999;96:11023–11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent GJ. No bit PARt for PAR-1. Am J Respir Cell Mol Biol 2005;33:213–215. [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol 2000;165:5788–5797. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, McCluskey K, Fujii K, Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol 1998;161:3071–3076. [PubMed] [Google Scholar]
- 18.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol 1972;54:626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three dimensional collagen gel matrix. In Vitro Cell Dev Biol 1996;32:427–433. [DOI] [PubMed] [Google Scholar]
- 20.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 1996;29:225–229. [DOI] [PubMed] [Google Scholar]
- 21.Wen FQ, Kohyama T, Liu X, Zhu YK, Wang H, Kim HJ, Kobayashi T, Abe S, Spurzem JR, Rennard SI. Interleukin-4– and interleukin-13–enhanced transforming growth factor-β2 production in cultured human bronchial epithelial cells is attenuated by interferon-γ. Am J Respir Cell Mol Biol 2002;26:484–490. [DOI] [PubMed] [Google Scholar]
- 22.Praet JP, Peretz A, Rozenberg S, Famaey JP, Bourdoux P. Risk of osteoporosis in men with chronic bronchitis. Osteoporos Int 1992;2:257–261. [DOI] [PubMed] [Google Scholar]
- 23.Okada Y, Watanabe S, Nakanishi I, Kishi J, Hayakawa T, Watorek W, Travis J, Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett 1988;229:157–160. [DOI] [PubMed] [Google Scholar]
- 24.Dawes KE, Gray AJ, Laurent GJ. Thrombin stimulates fibroblast chemotaxis and replication. Eur J Cell Biol 1993;61:126–130. [PubMed] [Google Scholar]
- 25.Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J Biol Chem 2001;276:45184–45192. [DOI] [PubMed] [Google Scholar]
- 26.Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude OP, Laurent GJ. Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. Biochem J 1998;333:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benezra M, Vlodavsky I, Ishai-Michaeli R, Neufeld G, Bar-Shavit R. Thrombin-induced release of active basic fibroblast growth factor-heparan sulfate complexes from subendothelial extracellular matrix. Blood 1993;81:3324–3331. [PubMed] [Google Scholar]
- 28.Herbert JM, Dupuy E, Laplace MC, Zini JM, Bar Shavit R, Tobelem G. Thrombin induces endothelial cell growth via both a proteolytic and a non-proteolytic pathway. Biochem J 1994;303:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terada M, Kelly EA, Jarjour NN. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am J Respir Crit Care Med 2004;169:373–377. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T, Moraes TJ, Vachon E, Ginzberg HH, Huang TT, Matthay MA, Hollenberg MD, Marshall J, McCulloch CA, Abreu MT, et al. Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells. Am J Respir Cell Mol Biol 2005;33:231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chokki M, Yamamura S, Eguchi H, Masegi T, Horiuchi H, Tanabe H, Kamimura T, Yasuoka S. Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. Am J Respir Cell Mol Biol 2004;30:470–478. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry 1990;29:10261–10270. [DOI] [PubMed] [Google Scholar]
- 33.Liu WH, Chen XM, Fu B. Thrombin stimulates MMP-9 mRNA expression through AP-1 pathway in human mesangial cells. Acta Pharmacol Sin 2000;21:641–645. [PubMed] [Google Scholar]
- 34.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest 2005;85:962–971. [DOI] [PubMed] [Google Scholar]
- 35.Forsyth NR, Evans AP, Shay JW, Wright WE. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell 2003;2:235–243. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Kohyama T, Wang H, Zhu YK, Wen FQ, Kim HJ, Romberger DJ, Rennard SI. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am J Physiol 2002;282:L1049–L1056. [DOI] [PubMed] [Google Scholar]
- 37.Liu XD, Umino T, Ertl R, Veys T, Skold CM, Takigawa K, Romberger DJ, Spurzem JR, Zhu YK, Kohyama T, et al. Persistence of TGF-beta1 induction of increased fibroblast contractility. In Vitro Cell Dev Biol Anim 2001;37:193–201. [DOI] [PubMed] [Google Scholar]
- 38.Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med 2005;15:254–259. [DOI] [PubMed] [Google Scholar]
- 39.Strukova S. Blood coagulation-dependent inflammation: coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci 2006;11:59–80. [DOI] [PubMed] [Google Scholar]
- 40.Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol 2005;166:1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu YK, Liu XD, Sköld CM, Wang H, Kohyama T, Wen FQ, Ertl RF, Rennard SI. Collaborative interactions between neutrophil elastase and metalloproteinases in extracelullar matrix degradation in three-dimensional collagen gels. Respir Res 2001;2:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obreja O, Rukwied R, Steinhoff M, Schmelz M. Neurogenic components of trypsin- and thrombin-induced inflammation in rat skin, in vivo. Exp Dermatol 2006;15:58–65. [DOI] [PubMed] [Google Scholar]
- 43.Campbell EJ, Campbell MA, Boukedes SS, Owen CA. Quantum proteolysis by neutrophils: implications for pulmonary emphysema in a1-antitrypsin deficiency. J Clin Invest 1999;104:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]