Abstract
Background
The authors tested the hypotheses that, compared with an overnight continuous femoral nerve block (cFNB), a 4-day ambulatory cFNB increases ambulation distance and decreases the time until three specific readiness-for-discharge criteria are met after tricompartment total knee arthroplasty.
Methods
Preoperatively, all patients received a cFNB (n = 50) and perineural ropivacaine 0.2% from surgery until the following morning, at which time they were randomly assigned to either continue perineural ropivacaine or switch to perineural normal saline. Primary endpoints included (1) time to attain three discharge criteria (adequate analgesia, independence from intravenous analgesics, and ambulation of at least 30 m) and (2) ambulatory distance in 6 min the afternoon after surgery. Patients were discharged with their cFNB and a portable infusion pump, and catheters were removed on postoperative day 4.
Results
Patients given 4 days of perineural ropivacaine attained all three discharge criteria in a median (25th–75th percentiles) of 25 (21–47) h, compared with 71 (46–89) h for those of the control group (estimated ratio, 0.47; 95% confidence interval, 0.32– 0.67; P <0.001). Patients assigned to receive ropivacaine ambulated a median of 32 (17–47) m the afternoon after surgery, compared with 26 (13–35) m for those receiving normal saline (estimated ratio, 1.21; 95% confidence interval, 0.71–1.85; P = 0.42).
Conclusions
Compared with an overnight cFNB, a 4-day ambulatory cFNB decreases the time to reach three important discharge criteria by an estimated 53% after tricompartment total knee arthroplasty. However, the extended infusion did not increase ambulation distance the afternoon after surgery. (ClinicalTrials.gov No. NCT00135889.)
Within the United States, pain control after total knee arthroplasty (TKA) is traditionally provided with a multimodal regimen of oral analgesics combined with intravenous opioids and/or epidural analgesia. A continuous femoral nerve block (cFNB) offers an alternative analgesic option. Unlike traditional intravenous opioid administration or epidural infusion, cFNB may be continued after discharge using a portable infusion pump.1 Consequently, ambulatory cFNB offers the potential of providing prolonged analgesia while simultaneously decreasing disability and hospitalization duration after TKA and other major hospital-based knee procedures. Within the United States, the average hospitalization duration after TKA decreased from nearly 11 days in 1990 to just over 4 days in 1996, but has remained relatively constant over the past decade.†††
There is one previous report of ambulatory cFNB after tricompartment TKA describing a small series of subjects who were discharged home the day after surgery with their perineural infusion continuing for over 4 days.2 However, that investigation did not include a control group, so the extent to which ambulatory cFNB influenced discharge-readiness remains unknown.
Two previous European studies found that after TKA or knee arthrolysis, using a 48- or 72-h hospital-based cFNB decreased rehabilitation center stays from 21 to 17 days and from 50 to 40 days, respectively.3,4 But as previously noted,5 these data did not suggest that hospital duration of stay is likely to be reduced with cFNB in the United States, where the average institutional stay of patients is already less than 1 week. In addition, the reason for the decreased stays in these investigations was primarily because of an accelerated ability to tolerate knee flexion—a critical discharge criterion for these patients remaining institutionalized for multiple weeks.3,4 In the United States, with a median hospitalization duration of fewer than 5 days, requiring the same degree of knee flexion as in the European studies is impractical. Therefore, a more important criterion for discharge in the United States is the ability to ambulate to allow postdischarge functioning. This essential endpoint—ambulation ability—remains uninvestigated in patients receiving a cFNB after TKA. Accelerated ambulatory ability may not be assumed from the previously published data on accelerated passive knee flexion: Unlike passive knee flexion, ambulation requires muscle strength, which is often decreased during continuous perineural local anesthetic infusion.6
Therefore, the primary objective of this dual-center, randomized, triple-masked (patients, investigators, and statisticians), placebo-controlled study was to test the hypothesis that, compared with an overnight cFNB, a 4-day ambulatory cFNB increases ambulation distance and shortens the time until three specific, predefined readiness-for-discharge criteria are met after tricompartment TKA. The three criteria were (1) adequate analgesia, (2) independence from intravenous opioids, and (3) sufficient ambulation. These criteria were chosen because failure to meet one or more of them accounts for the majority of hospitalization days at our institutions.
Materials and Methods
Enrollment
The institutional review board at each participating clinical center approved all study procedures (University of Florida, Gainesville, Florida; University of California San Diego, San Diego, California). Patients offered enrollment included adults (18–80 yr) scheduled to undergo primary, unilateral, tricompartment, cemented TKA via a 12- to 18-cm midline skin incision and parapatellar approach who desired a cFNB for postoperative analgesia. Exclusion criteria included a history of opioid dependence or current chronic analgesic therapy (daily use >4 weeks), allergy to study medications, known hepatic or renal insufficiency/disease, peripheral neuropathy, morbid obesity (body mass index >40 kg/m2), pregnancy, incarceration, or comorbidity that resulted in moderate or severe functional limitation. Participants provided written, informed consent, and because this was a multicenter trial, a data safety monitoring board (University of Florida) reviewed data and adverse events during enrollment.
Preoperative Management
Using a nerve stimulator (Stimuplex-DIG; B. Braun Medical, Bethlehem, PA) initially set at 1.2 mA, 0.1 ms, and 2 Hz, a femoral catheter (StimuCath; Arrow International, Reading, PA) was inserted using a technique similar to one previously described with a muscle contraction endpoint of the quadriceps at 0.20–0.40 mA via the placement needle.7,8 Forty milliliters mepivacaine, 1.5%, with epinephrine, 100 µg, was injected via the catheter with gentle aspiration every 3 ml. The femoral nerve block was evaluated 20 min later and considered successful when patients were unable to extend the knee and experienced a decreased sensation to cold of the skin in the femoral nerve distribution. Patients with a successful nerve block were retained in the study, and 10 ml ropivacaine, 0.5%, with epinephrine, 25 µg, was injected via the catheter.
Intraoperative Management
For the surgical procedure, patients received a standardized general anesthetic using sevoflurane, nitrous oxide, and oxygen. A 0.2% ropivacaine infusion was initiated via the femoral catheter with a basal rate of 8 ml/h, a patient-controlled bolus dose of 4 ml, and a lockout of 30 min. An intravenous hetastarch-based plasma volume expander (15 ml/kg) was administered before emergence.9 Fentanyl (25-µg increments) was administered when needed until the end of the procedure, at which time intravenous morphine sulfate was titrated for a respiratory rate of 12–14 breaths/min before emergence.
Postoperative Analgesics
All patients received a ropivacaine perineural infusion initiated in the operating room and continued until the morning after surgery, as well as 1 week of oral acetaminophen (975 mg every 6 h), a sustained-release oral opioid (OxyContin; Purdue Pharma, Stamford, CT; 10mg every 12 h), and either aspirin (650 mg daily) or celecoxib (200 mg every 12 h). Deep vein thrombosis prophylaxis was provided with either enoxaparin (40 mg daily, University of California San Diego) or the previously mentioned aspirin (University of Florida) beginning the morning after surgery and continued for 2 or 6 weeks, respectively. For breakthrough pain, patients were instructed to depress the bolus button on their infusion pump. Rescue opioid and route of administration were determined by pain severity using a numeric rating scale of 0–10, with 0 equal to no pain and 10 being the worst imaginable pain (table 1).10
Table 1.
Protocol for Rescue Analgesic Administration
| NRS | Analgesic | Route | Dose, mg | Administration |
|---|---|---|---|---|
| Postanesthesia care unit (recovery room) | ||||
| 1–2 | Oxycodone* | Oral | 5 | If patient desired |
| 3–4 | Oxycodone* | Oral | 10 | Every 30 min |
| 5–6 | Morphine | Intravenous | 2 | Every 10 min |
| Orthopedic ward | ||||
| 7–10 | Morphine | Intravenous | 4 | Every 10 min |
| <4 | Oxycodone* | Oral | 5 | If patient desired |
| 4–7 | Oxycodone* | Oral | 10 | Once |
| >7 | Morphine | Intravenous | 2–4 | Every 10 min until NRS <4 |
| Pain reassessed after 30 min | ||||
| <4 | Oxycodone* | Oral | 5 | If patient desired |
| 4–10 | Morphine | Intravenous | 2–4 | Every 10 min until NRS <4 |
Intravenous morphine (2 mg) was administered instead of oxycodone when oral intake was not tolerated.
NRS = pain on numeric rating scale (0–10, 0 = no pain and 10 = worst imaginable pain).
Randomization and Intervention
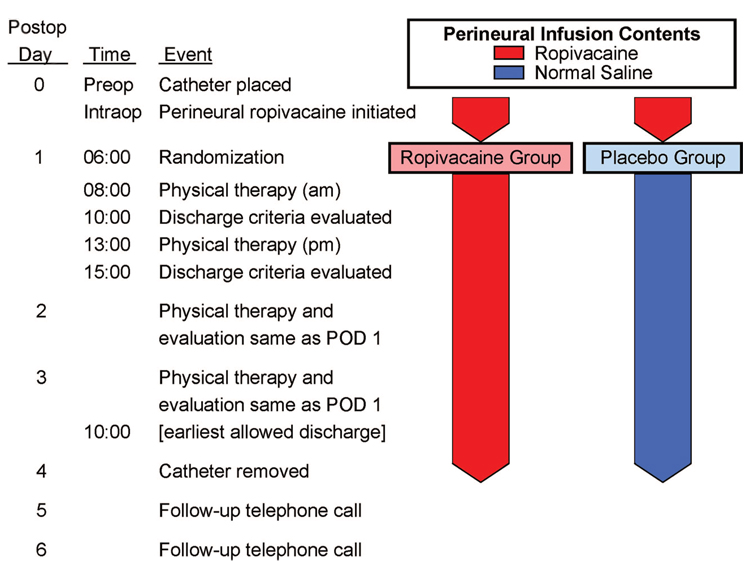
Patients were allocated to treatment after confirmation of a successful initial surgical block preoperatively. Patients were randomized to one of two groups—0.2% ropivacaine or normal saline (placebo)—stratified by institution using computer-generated tables by the investigational drug service of each participating center. Investigational drug service pharmacists prepared all perineural infusions. Investigators, patients, and all clinical staff were unaware of treatment group assignments. At 06:00 on postoperative day (POD) 1, each patient’s infusion pump, which contained 0.2% ropivacaine, was replaced with an infusion pump filled with study solution containing either additional 0.2% ropivacaine or normal saline (fig. 1).
Fig. 1.
Study design overview. POD = postoperative day.
Pain scores were recorded every 4 h (except when patients were sleeping) and when patients requested analgesics. Patients underwent physical therapy twice daily beginning the morning after surgery at approximately 08:00 and 13:00, and thereafter until discharge (fig. 1). If the physical therapist believed subject ambulation was limited because of quadriceps weakness, the perineural infusion was stopped for 1 h and then restarted at half the previous basal rate. At 18:00 on POD 2 (36 h after randomization), a portable infusion pump (Pain Pump 2 Blockaid; Stryker Instruments, Kalamazoo, MI) containing 400 ml of the same study solution (basal 5 ml/h regardless of previous basal rate, bolus 4 ml, lockout 60 min) replaced the previous infusion pump.
Primary Endpoints
Two hours after physical therapy sessions, each of the three discharge criteria were evaluated separately and scored as either “fulfilled” or “unfulfilled” by research nursing staff. The first primary endpoint was the time from surgical stop until all three of the criteria were fulfilled—without a reversion to unfulfilled status. For example, if a patient met all three criteria the morning of POD 1, subsequently met only two criteria later that afternoon, and again met all three criteria the following morning, the primary endpoint would be the number of hours from surgical stop until 10:00 on POD 2. The three specific readiness-for-discharge criteria were (1) adequate analgesia (numeric rating scale score <4), (2) independence from intravenous opioids in the previous 12 h, and (3) ambulation of at least 30 m without time limit.11
The second primary endpoint was the ambulatory distance during a Six-Minute Walking Test (6-MWT) the afternoon after surgery (7–8 h after randomization).12 The 6-MWT is used to measure the maximum distance that a patient can walk in 6 min on a 10-m level course.12 Patients were transported to the start of the course by wheelchair, assisted into a standing position, and instructed to walk from end to end at their own pace while attempting to cover as much ground as possible in 6 min using a four-leg walker. Because simple verbal encouragement may improve patient performance,13 therapists encouraged patients in a standardized manner using one of two phrases: “You’re doing well” or “Keep up the good work.” Patients were allowed to continue ambulating after the initial 6 min, and the total distance and reason(s) for ambulation cessation were recorded.
The secondary endpoint of passive knee flexion was measured with the patient in the supine position during each physical therapy session before the 6-MWT using a goniometer.
Hospital Discharge
Patients were discharged home or to a rehabilitation center (if they lacked a capable “caretaker” at home) with their portable infusion pump and perineural catheter in situ. Patients were discharged at the discretion of the orthopedic surgeons after meeting the three main discharge criteria, but never before 10:00 on POD 3. Patients and their caretakers were provided with verbal and written catheter/pump instructions, the telephone and pager numbers of an investigator available at all times, and prescriptions for their outpatient oral medications that did not differ from the oral analgesics provided in the hospital. Patients were telephoned in the evenings through POD 6 for data collection (appendix) and infusion oversight (e.g., appearance of the catheter site/ dressing).
In the evening of POD 4, patients’ caretakers removed the femoral catheters with physician instructions provided by telephone. Enoxaparin was administered in the mornings, whereas catheter removal occurred in the evening; the two events were separated by approximately 8–10 h. However, this temporal relation was by coincidence and not by design.
Statistical Analysis
The study was powered for the two primary endpoints. Based on a pilot study,2 the planning distribution for time-to-discharge readiness for the ropivacaine (placebo) group was as follows: 6 h, 71% (29%); 30 h, 14% (29%); 45 h, 14% (14%); and 54 h, 0% (29%). To ensure 80% power at P = 0.05 (two-sided) for the Wilcoxon rank sum test, we planned for 25 patients randomly assigned to each group on the basis of the formula of Shuster et al. 14 As for planning parameters for the 6-MWT on the afternoon of POD 1, the sample size was calculated on the basis of an SD of approximately 19mfor the pilot study,2 using the Wilcoxon rank sum test, where a study of 25 subjects per group would be sensitive to a difference of 0.83 SDs in medians, or approximately 16 m.
For consistency, all outcome variables (primary and secondary) were analyzed by the two-sided Wilcoxon rank sum test, which provides distribution-free P values and is highly robust against outliers (Statistical Analysis Software, Cary, NC). For descriptive purposes, Kaplan-Meier estimates were calculated and presented for several components of the primary endpoints. For convenient nomenclature, a two-sided P <0.05 was considered significant. Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints.15 Significant findings in secondary outcomes should be viewed as suggestive, requiring confirmation in a future trial before considering them as definitive.15
For the primary variables, effect sizes were estimated by the method of Hodges and Lehmann for a scale parameter.16 Essentially, this tests the null hypothesis that the distribution of an outcome variable under treatment A is the same as that of ρ times that of treatment B for every value of ρ. The values of ρ that cannot be rejected by a Wilcoxon rank sum test at two-sided P <0.05 form a 95% confidence interval for ρ. A value of ρ = 0.7, for example, is interpreted as the median (or any percentile) under treatment A is 70% of that of treatment B. For time to meeting discharge criteria, for example, this implies an expected 30% savings for A over B.
All patients receiving treatment were included in the analysis of time to reach the three discharge criteria. One patient, whose time to meet the three discharge criteria was known, withdrew before obtaining a 6-min walking distance on the afternoon of POD 1. Although this patient had to be excluded from the analysis of 6-min walking distance of the afternoon of POD 1, we revisited that analysis imputing a distance of zero for this patient. Taken together, the two analyses give the best approximation to intention-to-treat, the accepted standard for clinical trials.5
Results
During a 28-month period between January 2005 and April 2007, 51 patients enrolled and all had a perineural catheter successfully positioned per protocol (table 2). One subject exhibited no sensory or motor block 20 min after being given a local anesthetic bolus via the catheter, and therefore was not randomized per protocol. Among the remaining subjects (98%), 25 were randomly assigned to be switched from perineural ropivacaine to normal saline (placebo group) at 06:00 on POD 1, whereas 25 continued to receive perineural ropivacaine through POD 4 (ropivacaine group) (fig. 1).
Table 2.
Population Data, Perineural Catheter Details, and Surgical Information
| Ropivacaine Group (n = 25) |
Placebo Group (n = 25) |
|
|---|---|---|
| Age, yr | 66 (60–70) | 64 (60–69) |
| Sex, F/M | 14/11 | 15/10 |
| Height, cm | ||
| Male patients | 180 (175–188) | 179 (175–183) |
| Female patients | 163 (157–170) | 169 (158–170) |
| Weight, kg | ||
| Male patients | 108 (82–115) | 88 (75–119) |
| Female patients | 82 (73–87) | 73 (68–87) |
| Body mass index, kg/m2 | 31 (27–33) | 28 (24–32) |
| ASA physical status | II (II–II) | II (II–II) |
| Minimum needle current, mA | 0.28 (0.20–0.31) | 0.30 (0.24–0.31) |
| Intraoperative fentanyl (µg) | 250 (100–250) | 250 (175–250) |
| Intraoperative morphine, mg | 5 (2–10) | 5 (2–8) |
| Catheter insertion to randomization, h | 23 (21–23) | 23 (21–23) |
| Surgery duration, min | 107 (100–132) | 116 (112–134) |
| Tourniquet duration, min | 100 (96–112) | 108 (102–120) |
Values are reported as median (25th–75th percentiles).
ASA = American Society of Anesthesiologists.
Primary Endpoints
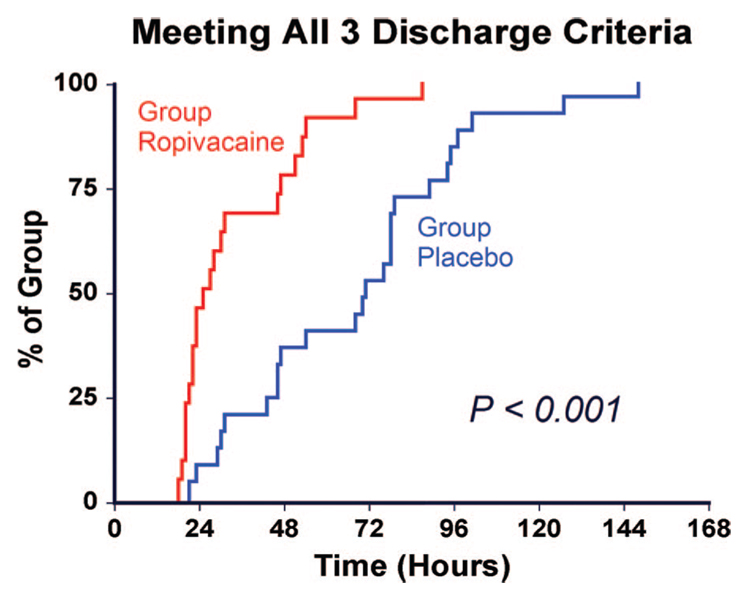
Patients receiving 4 days of perineural ropivacaine attained all three discharge criteria in a median (25th–75th percentiles) of 25 (21–47) h, compared with 71 (46–89) h for those of the control group (fig. 2; estimated Hodges-Lehmann ratio, 0.47; 95% confidence interval, 0.32–0.67; P <0.001). Patients randomly assigned to receive perineural ropivacaine ambulated a median of 32 (17– 47) m the afternoon after surgery, compared with 26 (13–35) m for those receiving perineural normal saline (fig. 3; estimated ratio, 1.21; 95% confidence interval, 0.71–1.85; P = 0.42).
Fig. 2.
Effect of femoral perineural ropivacaine infusion on the time to reach three important discharge criteria (adequate analgesia, independence from intravenous opioids, and the ability to ambulate at least 30 m) after tricompartment total knee arthroplasty. Data presented are Kaplan-Meier estimates of the cumulative percentages of patients meeting all three discharge criteria at each time point and subsequent time points. Data are for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4).
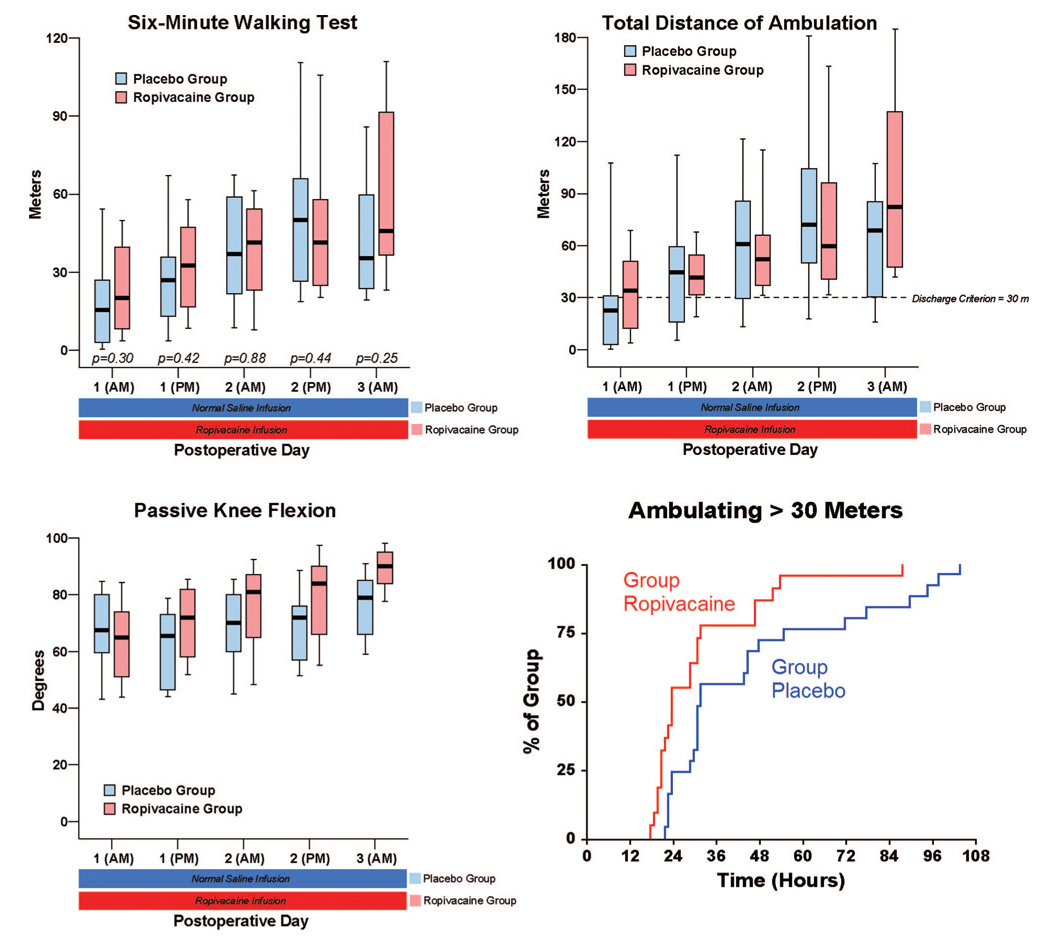
Fig. 3.
Effects of femoral perineural ropivacaine infusion on ambulation and passive knee flexion after tricompartment total knee arthroplasty. Kaplan-Meier estimates include the cumulative percentages of patients ambulating at least 30 m at each time point and subsequent time points. Other data are expressed as median (horizontal bar) with 25th–75th (box) and 10th–90th (whiskers) percentiles for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. P values are provided where statistical comparisons were applied.
Secondary Endpoints
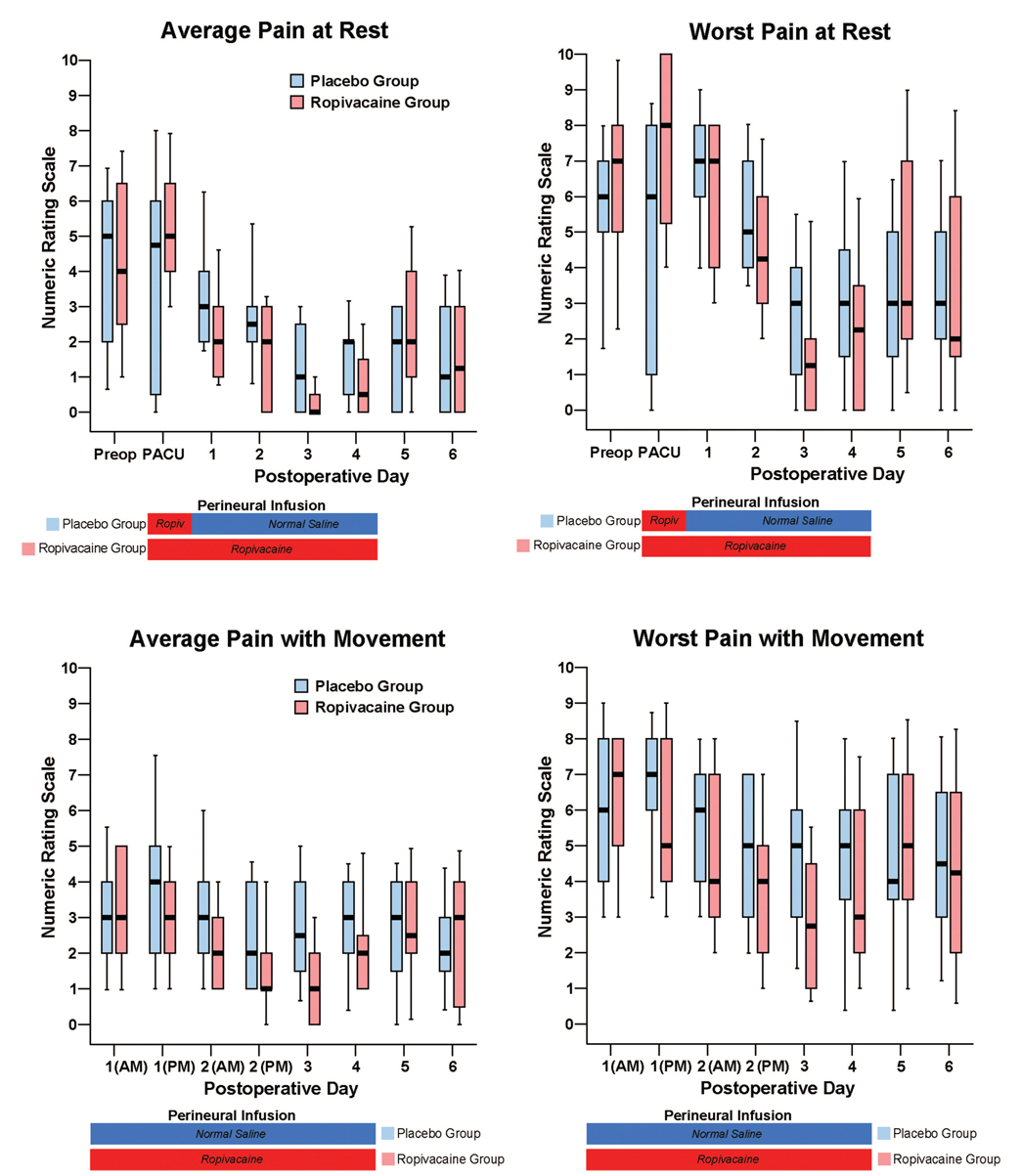
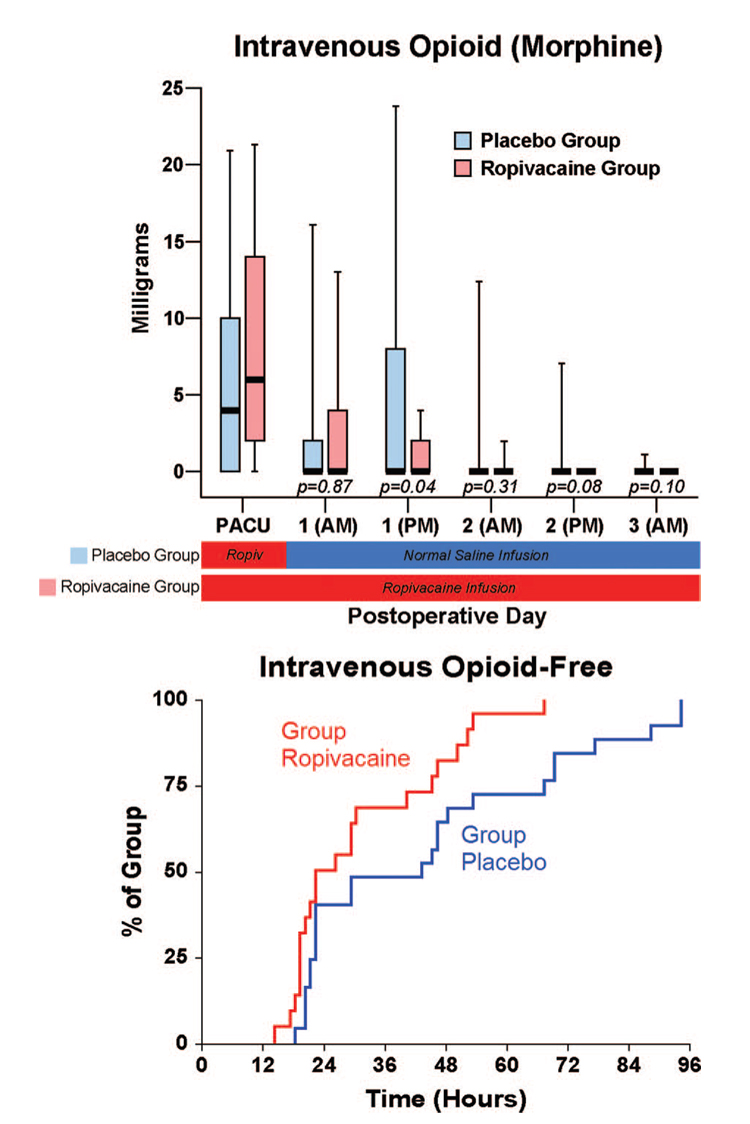
There were small differences overall in pain at rest between the treatment groups, but the dynamic pain scores diverged to a greater degree (fig. 4). While the differences in intravenous morphine requirements between treatment groups seem small as well (fig. 5), a higher percentage of patients receiving ropivacaine were intravenous-morphine free at each postrandomization time point (fig. 5). Similarly, there were only small differences in the total ambulatory distance between groups (fig. 4), but a higher percentage of patients receiving ropivacaine attained the discharge criteria of 30 m at each postrandomization time point (fig. 3).
Fig. 4.
Effects of femoral perineural ropivacaine infusion on postoperative pain after tricompartment total knee arthroplasty. Pain severity is indicated using a numeric rating scale of 0–10, with 0 equal to no pain and 10 being the worst imaginable pain. Data are expressed as median (horizontal bar) with 25th–75th (box) and 10th–90th (whiskers) percentiles for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. For this reason, no statistical comparisons were applied to the data of this figure. PACU = postanesthesia care unit.
Fig. 5.
Effects of femoral perineural ropivacaine infusion on intravenous morphine consumption after tricompartment total knee arthroplasty. Kaplan-Meier estimates include the cumulative percentages of morphine-free patients at each time point and subsequent time points. Other data are expressed as median (horizontal bar) with 25th–75th (box) and 10th–90th (whiskers) percentiles for patients randomly assigned to the ropivacaine group (perineural ropivacaine from surgery through postoperative day 4) or the placebo group (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. P values are presented where statistical comparisons were applied.
Both groups required similar doses of oral opioids for breakthrough pain (table 3). Ten subjects (43%) of the ropivacaine group had their basal ropivacaine infusion halved on POD 1 because of quadriceps weakness versus 3 subjects (12%) of the placebo group. One of the 10 subjects in the ropivacaine group required a second halving of her basal rate because of continued quadriceps weakness. Hospitalization duration was a mean (±SD) of 3.6 (±0.6) days in the ropivacaine group and 3.5 (±0.6) days in the placebo group (P = 0.74). Satisfaction with postoperative analgesia was scored 10.0 (10.0 – 10.0) in the ropivacaine group and 9.0 (7.5–10.0) in the placebo group (P = 0.002).
Table 3.
Oral Opioid Requirements and Sleep Disturbances
| Oral Opioid,* mg |
Difficulty Sleeping,† Subjects per Group |
At Least One Awakening,† Subjects per Group |
||||
|---|---|---|---|---|---|---|
| Postoperative Day/Night | Ropivacaine Group | Placebo Group | Ropivacaine Group | Placebo Group | Ropivacaine Group | Placebo Group |
| 0 | 0 (0–10) | 0 (0–5) | 5 | 1 | 4 | 4 |
| 1 | 25 (10–50) | 40 (25–60) | 0 | 6 | 1 | 8 |
| 2 | 20 (5–30) | 25 (20–45) | 0 | 2 | 0 | 2 |
| 3 | 0 (0–10) | 10 (0–20) | 0 | 5 | 2 | 4 |
| 4 | 5 (0–12.5) | 15 (5–20) | 6 | 8 | 6 | 10 |
| 5 | 25 (10–40) | 20 (10–35) | 4 | 7 | 7 | 8 |
| 6 | 20 (5–40) | 20 (10–40) | ‡ | ‡ | ‡ | ‡ |
Because each comparison dilutes all other P values, we restricted our analysis to 11 comparisons among secondary endpoints. For this reason, no statistical comparisons were applied to the data of this table.
Values are reported as median (25th–75th percentiles) for nonparametric data. Includes only immediate-release oxycodone provided for breakthrough pain for the previous 24 h as of 18:00 each day, with the exception of postoperative day 0, which includes only the postanesthesia care unit (recovery room). Excludes sustained-release oxycodone (10 mg) provided to all patients twice daily.
As a result of surgical pain.
Data not collected.
Protocol Violations and Adverse Events
From the ropivacaine group, two subjects requested study withdrawal on POD 0 before any study intervention, and an additional subject requested withdrawal on POD 1 after experiencing a myocardial infarction.‡‡‡
A 56-yr-old subject from the ropivacaine group had a pulmonary embolism on POD 3 but was discharged without sequelae after aggressive anticoagulation. One 74-yr-old subject from the ropivacaine group fell when walking into his house for the first time after being discharged the morning of POD 3. No injury occurred, but he was readmitted to the hospital for overnight observation. One subject from the ropivacaine group requested catheter removal the evening of POD 3 with the appearance of clear fluid leaking from her catheter site. Two subjects from the placebo group had their catheter inadvertently dislodged on POD 4. For purposes of analysis, each of these subjects was retained in his or her respective treatment group per the intention-to-treat principle.17
Discussion
This investigation provides evidence that, compared with an overnight cFNB, a 4-day cFNB decreases the time to reach three important discharge criteria by an estimated 53% after tricompartment TKA. Because the extended-duration cFNB may be provided on an ambulatory basis, prolonging the perineural infusion does not necessarily require prolonging hospitalization. This suggests that hospital duration of stay after TKA may be shortened in some cases while still providing the benefits of cFNB. Alternatively, for practices that currently discharge patients in fewer than 4 days, the benefits of cFNB may be extended after discharge with the use of ambulatory infusion.
Early Discharge
Our study protocol required patients to remain hospitalized until at least POD 3, even if they were discharge-ready earlier. There is a significant difference between discharge-readiness and actual discharge, as evidenced by similar duration of hospitalization in each group. A previous unblinded study that randomly assigned TKA patients to a single-injection ropivacaine FNB versus 48-h hospital-only ropivacaine cFNB within an established clinical pathway also reported similar hospitalization duration between treatment groups (3.8 vs. 3.9 days), even though patients with a cFNB had less pain and lower opioid requirements.18 A prospective observational investigation involving three Australian hospitals found that although 56% of patients were ready for home discharge on POD 7 after TKA, only 36% were actually released.19 These three studies suggest that decreasing hospital stay duration involves more than simply preparing patients to leave the hospital.
Additional factors, such as patient comorbidities,20 rehabilitation expectations,3,4 and hospital policy,19,21 may significantly influence hospitalization duration. For example, in the current study, many patients underwent their procedure on a Thursday. Even though all but one patient of the ropivacaine group were ready for discharge by POD 3—Sunday—there was an institutional bias against discharging patients home on the weekend, and so patients were usually discharged the subsequent Monday. Practitioners wishing to shorten hospitalization will thus need to overcome the very clinical pathways that were set up to facilitate— by standardizing— patient care.22
Considering that the protocol provided analgesics until pain was acceptably low (numeric rating scale score <4), it is not surprising that there is little difference in pain scores between the two treatment groups (with the exception of breakthrough pain during movement). In addition, the differences between treatment groups were not particularly dramatic for the other two discharge criteria we studied. However, when all three criteria were considered together—a primary endpoint of the study—providing a total of 4 days of perineural ropivacaine had a statistically and clinically significant impact on the time to reach the three criteria. Among the ropivacaine group patients, those who did not meet all three criteria usually both ambulated less than 30 m and required intravenous morphine. In contrast, those in the placebo group who did not meet all three criteria usually ambulated less than 30 m or required intravenous morphine. This difference explains why even though there was less than a 25%-point difference between groups in meeting the individual discharge criteria at each time point (fig. 3 and fig 5), there was a much larger difference when all three criteria were evaluated for the primary endpoint (fig. 2).
Ambulation
In contrast to discharge readiness, the results for our other primary endpoint—ambulation distance the afternoon after surgery as measured by the 6-MWT—were inconclusive (fig. 3). Multiple variables influence ambulatory ability in addition to the quality of analgesia,23 including preoperative quadriceps strength24; cardiovascular status25,26; physical characteristics, such as age, sex, height, and weight27; and general health status.28 And as it happened, the patients in the ropivacaine group tended toward a larger body mass index—and were therefore possibly more physically deconditioned—than their placebo group counterparts, which may have reduced their ambulatory ability.
Study Design
A unique aspect of this investigation was the analgesic regimen specifically tailored for outpatient management (table 1). This protocol was adopted to enable patients to first treat mild or moderate pain with an oral analgesic, followed by intravenous analgesics only when necessary, allowing evaluation of intravenous analgesic independence. An important component of the analgesic regimen was scheduled sustained-release oxycodone—an oral opioid—to provide the current standard of care at both of the enrolling institutions. Scheduled administration of this opioid probably decreased differences between the two treatment groups for multiple endpoints, which, although not compromising the study’s internal validity, complicates comparisons with previous investigations. However, internal validity is a strength of the current study design which provides data involving the association of cFNB and clinically relevant postoperative endpoints collected with a randomized, placebo-controlled protocol in which all participants were masked to treatment allocation.29 Previously published controlled investigations involving TKA provided cFNB for 48 –72 h exclusively in hospitalized patients and are limited by one or more methodologic issues, such as lack of adequately concealed treatment allocation (“blinding”),29 placebo-controls, inadequate or missing sample-size estimates, failure to specify a priori primary endpoint(s), and/or failure to provide treatment effect sizes.30,31
Study Limitations
The current study has its own set of limitations. Importantly, the control (“placebo”) group received an initial femoral nerve block followed by an overnight cFNB, and not simply a single-injection femoral nerve block and/or opioids as is common in many practices in the United States. In addition, although all subjects received sustained-release oral opioids, these may result in undesirable side effects, which were not assessed in this investigation. Although the postoperative questionnaire included validated measures such as the numeric rating scale for pain assessment,10 the instruments used to assess sleep quality and analgesia satisfaction have not been previously validated (appendix). This study also excluded patients who had taken opioids daily for more than the previous 4 weeks. Although precise figures are unavailable, undoubtedly a large percentage of patients undergoing TKA have received over a month of opioids daily, and whether the results of the current study remain applicable to this patient subset remains unknown.
Patient Safety
While this investigation suggests that the duration of hospitalization after TKA may be decreased with ambulatory cFNB, it does not define an appropriate subset of patients and incidence of complications associated with early discharge. Caution is warranted because, after TKA, the median times to myocardial infarction and pulmonary embolism are 1 and 4 days, respectively.32 One patient of the current study receiving perineural ropivacaine fell after discharge. Whether the cFNB was a contributing factor to this adverse event remains unknown because our study was not powered to detect such (presumably) rare complications.33,34
Two additional subjects had their catheter inadvertently dislodged on POD 4. Should a catheter dislocation or pump malfunction occur earlier during ambulatory cFNB, patients are at high risk of experiencing severe surgical pain unresponsive to oral opioids and requiring hospital readmission. It is for this reason that we required “caretakers” who could return patients to the hospital, if necessary. Related to this issue, patients with heart disease resulting in a moderate or severe functional limitation were excluded from participation out of concern that acute, severe pain could trigger an adverse cardiac event. There is also a report of clinically relevant hematoma after cFNB placement associated with low-molecular-weight heparin administration.35 Although the feasibility of converting tricompartment TKA into an overnight-stay procedure using ambulatory cFNB has been previously demonstrated,2 one small series of patients does not permit conclusions to be drawn regarding the relative safety of this practice. Therefore, additional study is required to define an appropriate subset of patients and assess the incidence of complications associated with earlier discharge after tricompartment TKA. Nonetheless, ambulatory cFNB may enable rapid hospital discharge to a skilled nursing facility or rehabilitation center where medical oversight would continue in case of an adverse event.35
Future Research
The fact that 43% of the ropivacaine group (vs. 12% of the placebo group) required a decrease in their basal infusion rate to enable ambulation suggests that an initial basal rate of 8 ml/h is too high for many patients when using a stimulating catheter and 0.2% ropivacaine. However, the optimal basal rate—or even whether an “optimal rate” exists—remains undetermined, and initially providing a lower rate may result in decreased cFNB benefits for a subset of patients. Similarly, the optimal perineural catheter design, combination of peripheral nerve blocks/catheters,36 local anesthetic type and concentration, 37 bolus dose volume and lockout duration, specific surgical procedures (e.g., unicompartmental TKA), relevant post-TKA endpoints, and many other details have all yet to be determined. Whether the potential benefits of providing 100 h of cFNB and ambulatory perineural infusion outweigh the potential risks must also be questioned and investigated.37 And, most importantly, additional study is required to define an appropriate subset of patients and assess the incidence of complications associated with earlier discharge after tricompartment TKA.
Acknowledgments
Supported by National Institutes of Health grant No. GM077026 from the National Institute of General Medical Sciences, Bethesda, Maryland; the Foundation of Anesthesia Education and Research, Rochester, Minnesota; National Institutes of Health grant Nos. RR00082 and RR000827 from the National Center for Research Resources, Bethesda, Maryland; the Departments of Anesthesiology, University of Florida, Gainesville, Florida, and University of California San Diego, San Diego, California; Stryker Instruments, Kalamazoo, Michigan; and Arrow International, Reading, Pennsylvania. Dr. Sessler is supported by National Institutes of Health grant No. GM061655 from the National Institute of General Medical Sciences, Bethesda, Maryland, and the Joseph Drown Foundation, Los Angeles, California. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities. Arrow International and Stryker Instruments provided funding and donated portable infusion pumps and perineural catheters for this investigation. These two companies had no input into any aspect of study conceptualization, design, and implementation; data collection, analysis, and interpretation; or manuscript preparation. Dr. Mariano conducts continuous peripheral nerve block workshops for Stryker Instruments. None of the other authors have any personal financial interest in this research.
The authors thank Beverly Morris, R.N., C.N.P., M.B.A. (Educator, Department of Nursing, University of California San Diego, San Diego, California); Patrick Olsen, R.N., B.S.N. (Nurse Manager), Claire Jenkins, R.N. (Assistant Nurse Manager), and Maureen Benetti, M.S., A.P.R.N., B.C. (Recruitment Director, Hillcrest Hospital, San Diego, California); Lisa Dacey, P.T., and Kerrie Olexa, P.T. (Physical Therapists, Department of Physical Therapy, Hillcrest Hospital); Rosalita Maldonado, B.S., and Marina Nekhendzy (Research Coordinators, Department of Anesthesiology, University of California San Diego); Jennifer Woodard, B.S. (Research Coordinator, Department of Anesthesiology, University of Florida, Gainesville, Florida); and the entire staffs of the Shands Hospital Regional Anesthesia Induction Area (Gainesville, Florida), University of Florida General Clinical Research Center (Gainesville, Florida), and Hillcrest Hospital Orthopedic Ward (San Diego, California) for invaluable assistance.
Appendix: Nightly Telephone Questionnaire
Pain Scores (Postoperative Days 3–6)
Please answer the following questions regarding your surgical pain in the last 24 h using a scale of 0–10, 0 being no pain at all and 10 being the worst pain you can imagine.
While resting in bed, what was the worst pain you have felt?
While resting in bed, what was the average pain you have felt?
While walking, what was the worst pain you have felt?
While walking, what was the average pain you have felt?
Opioid Use
(If Patient Was Discharged Postoperative Day 3)
Since you left the hospital, how many of your oxycodone tablets have you taken? These are the pills you take if your infusion pump does not decrease your pain enough.
(Postoperative Days 4–6)
Since we last spoke, how many of your oxycodone tablets have you taken? These are the pills you take if your infusion pump does not decrease your pain enough.
Sleep Quality (Postoperative Days 1–6)
Did you have difficulty sleeping last night because of pain?
Did you awaken last night because of pain?
If yes, then:
How many times did you awaken last night because of pain? (if ≥10 awakenings or complete insomnia, score = 10)
Satisfaction (Postoperative Day 4 Only)
What has been your satisfaction with your pain control following your surgery, using a scale of 0–10, 0 being very unsatisfied and 10 being very satisfied?
Footnotes
Abbreviated, preliminary results of this investigation were presented at the Annual Meeting of the American Society of Regional Anesthesia and Pain Medicine, Vancouver, British Columbia, Canada, April 20, 2007, and the Annual Meeting of the American Society of Anesthesiologists, San Francisco, California, October 14 and 17, 2007.
American Academy of Orthopaedic Surgeons Web site. http://www.aaos.org. Accessed May 10, 2007.
Myocardial infarction: A 66-yr-old man was noted at his preoperative orthopedic visit to have a systolic cardiac murmur. He was referred to an external cardiologist for further workup, and a subsequent external echocardiogram report included a 50% ejection fraction, otherwise without any major findings. The patient’s intraoperative course was unremarkable, but he experienced a myocardial infarction on POD 1. During cardiac catheterization, he was found to have not only severe stenosis of two cardiac vessels, but severe aortic stenosis as well. The patient requested study withdrawal.
References
- 1.Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: A review. Anesth Analg. 2005;100:1822–1833. doi: 10.1213/01.ANE.0000151719.26785.86. [DOI] [PubMed] [Google Scholar]
- 2.Ilfeld BM, Gearen PF, Enneking FK, Berry LF, Spadoni EH, George SZ, Vandenborne K. Total knee arthroplasty as an overnight-stay procedure using continuous femoral nerve blocks at home: A prospective feasibility study. Anesth Analg. 2006;102:87–90. doi: 10.1213/01.ane.0000189562.86969.9f. [DOI] [PubMed] [Google Scholar]
- 3.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Todd MM, Brown DL. Regional anesthesia and postoperative pain management: Long-term benefits from a short-term intervention. Anesthesiology. 1999;91:1–2. doi: 10.1097/00000542-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Borgeat A, Kalberer F, Jacob H, Ruetsch YA, Gerber C. Patient-controlled interscalene analgesia with ropivacaine 0.2% versus bupivacaine 0.15% after major open shoulder surgery: The effects on hand motor function. Anesth Analg. 2001;92:218–223. doi: 10.1097/00000539-200101000-00042. [DOI] [PubMed] [Google Scholar]
- 7.Ilfeld BM, Enneking FK. Perineural catheter placement for a continuous nerve block: A single operator technique. Reg Anesth Pain Med. 2003;28:154–155. doi: 10.1053/rapm.2003.50126. [DOI] [PubMed] [Google Scholar]
- 8.Ilfeld BM, Thannikary LJ, Morey TE, Vander Griend RA, Enneking FK. Popliteal sciatic perineural local anesthetic infusion: A comparison of three dosing regimens for postoperative analgesia. Anesthesiology. 2004;101:970–977. doi: 10.1097/00000542-200410000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Arellano R, Gan BS, Salpeter MJ, Yeo E, McCluskey S, Pinto R, Irish J, Ross DC, Doyle DJ, Parkin J, Brown D, Rotstein L, Witterick I, Matthews W, Yoo J, Neligan PC, Gullane P, Lampe H. A triple-blinded randomized trial comparing the hemostatic effects of large-dose 10% hydroxyethyl starch 264/0.45 versus 5% albumin during major reconstructive surgery. Anesth Analg. 2005;100:1846–1853. doi: 10.1213/01.ANE.0000152008.04333.53. [DOI] [PubMed] [Google Scholar]
- 10.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 11.Enloe LJ, Shields RK, Smith K, Leo K, Miller B. Total hip and knee replacement treatment programs: A report using consensus. J Orthop Sports Phys Ther. 1996;23:3–11. doi: 10.2519/jospt.1996.23.1.3. [DOI] [PubMed] [Google Scholar]
- 12.ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Pugsley SO, Sullivan MJ, Thompson PJ, Berman L, Jones NL, Fallen EL, Taylor DW. Effect of encouragement on walking test performance. Thorax. 1984;39:818–822. doi: 10.1136/thx.39.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shuster JJ, Chang M, Tian L. Design of group sequential trials with ordinal categorical data based on the Mann-Whitney-Wilcoxon test. Sequential Analysis. 2004;23:413–426. [Google Scholar]
- 15.Mariano ER, Ilfeld BM, Neal JM. “Going fishing”: The practice of reporting secondary outcomes as separate studies. Reg Anesth Pain Med. 2007;32:183–185. doi: 10.1016/j.rapm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Statist. 1963;34:598–611. [Google Scholar]
- 17.Todd MM. Clinical research manuscripts in anesthesiology. Anesthesiology. 2001;95:1051–1053. doi: 10.1097/00000542-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Salinas FV, Liu SS, Mulroy MF. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102:1234–1239. doi: 10.1213/01.ane.0000198675.20279.81. [DOI] [PubMed] [Google Scholar]
- 19.Oldmeadow LB, McBurney H, Robertson VJ. Hospital stay and discharge outcomes after knee arthroplasty: Implications for physiotherapy practice. Aust J Physiother. 2002;48:117–121. doi: 10.1016/s0004-9514(14)60205-1. [DOI] [PubMed] [Google Scholar]
- 20.Munin MC, Kwoh CK, Glynn N, Crossett L, Rubash HE. Predicting discharge outcome after elective hip and knee arthroplasty. Am J Phys Med Rehabil. 1995;74:294–301. doi: 10.1097/00002060-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Hughes K, Kuffner L, Dean B. Effect of weekend physical therapy treatment on postoperative length of stay following total hip and total knee arthroplasty. Physiother Can. 1993;45:245–249. [PubMed] [Google Scholar]
- 22.Kim S, Losina E, Solomon DH, Wright J, Katz JN. Effectiveness of clinical pathways for total knee and total hip arthroplasty: Literature review. J Arthroplasty. 2003;18:69–74. doi: 10.1054/arth.2003.50030. [DOI] [PubMed] [Google Scholar]
- 23.Carli F, Mayo N, Klubien K, Schricker T, Trudel J, Belliveau P. Epidural analgesia enhances functional exercise capacity and health-related quality of life after colonic surgery: Results of a randomized trial. Anesthesiology. 2002;97:540–549. doi: 10.1097/00000542-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32:1533–1539. [PubMed] [Google Scholar]
- 25.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt GH, Thompson PJ, Berman LB, Sullivan MJ, Townsend M, Jones NL, Pugsley SO. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;38:517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 27.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–274. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 28.Bautmans I, Lambert M, Mets T. The six-minute walk test in community dwelling elderly: Influence of health status. BMC Geriatr. 2004;4:6. doi: 10.1186/1471-2318-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 30.Houle TT. Statistical reporting for current and future readers. Anesthesiology. 2007;107:193–194. doi: 10.1097/01.anes.0000271870.76451.c9. [DOI] [PubMed] [Google Scholar]
- 31.Houle TT. Importance of effect sizes for the accumulation of knowledge. Anesthesiology. 2007;106:415–417. doi: 10.1097/00000542-200703000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96:1140–1146. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Klein SM, Nielsen KC, Greengrass RA, Warner DS, Martin A, Steele SM. Ambulatory discharge after long-acting peripheral nerve blockade: 2382 blocks with ropivacaine. Anesth Analg. 2002;94:65–70. doi: 10.1097/00000539-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Muraskin SI, Conrad B, Zheng N, Morey TE, Enneking FK. Falls associated with lower-extremity-nerve blocks: A pilot investigation of mechanisms. Reg Anesth Pain Med. 2007;32:67–72. doi: 10.1016/j.rapm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Bickler P, Brandes J, Lee M, Bozic K, Chesbro B, Claassen J. Bleeding complications from femoral and sciatic nerve catheters in patients receiving low molecular weight heparin. Anesth Analg. 2006;103:1036–1037. doi: 10.1213/01.ane.0000237230.40246.44. [DOI] [PubMed] [Google Scholar]
- 36.Morin AM, Kratz CD, Eberhart LH, Dinges G, Heider E, Schwarz N, Eisenhardt G, Geldner G, Wulf H. Postoperative analgesia and functional recovery after total-knee replacement: Comparison of a continuous posterior lumbar plexus (psoas compartment) block, a continuous femoral nerve block, and the combination of a continuous femoral and sciatic nerve block. Reg Anesth Pain Med. 2005;30:434–445. doi: 10.1016/j.rapm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Brodner G, Buerkle H, Van Aken H, Lambert R, Schweppe-Hartenauer ML, Wempe C, Gogarten W. Postoperative analgesia after knee surgery: A comparison of three different concentrations of ropivacaine for continuous femoral nerve blockade. Anesth Analg. 2007;105:256–262. doi: 10.1213/01.ane.0000265552.43299.2b. [DOI] [PubMed] [Google Scholar]