Abstract
Embryos that are homozygous for Splotch, a null allele of Pax3, have a severe neural crest cell (NCC) deficiency that generates a complex phenotype including spina bifida, exencephaly and cardiac outflow tract abnormalities. Contrary to the widely held perception that thymus aplasia or hypoplasia is a characteristic feature of Pax3Sp/Sp embryos, we find that thymic rudiments are larger and parathyroid rudiments are smaller in E11.5–12.5 Pax3Sp/Sp compared to Pax3+/+ embryos. The thymus originates from bilateral third pharyngeal pouch primordia containing endodermal progenitors of both thymus and parathyroid glands. Analyses of Foxn1 and Gcm2 expression revealed a dorsal shift in the border between parathyroid- and thymus-fated domains at E11.5, with no change in the overall cellularity or volume of each shared primordium. The border shift increases the allocation of third pouch progenitors to the thymus domain and correspondingly decreases allocation to the parathyroid domain. Initial patterning in the E10.5 pouch was normal suggesting that the observed change in the location of the organ domain interface arises during border refinement between E10.5 and E11.5. Given the well-characterized NCC defects in Splotch mutants, these findings implicate NCCs in regulating patterning of third pouch endoderm into thymus- versus parathyroid-specified domains, and suggest that organ size is determined in part by the number of progenitor cells specified to a given fate.
Keywords: thymus, parathyroid, neural crest cells, Pax3, pharyngeal pouch endoderm
INTRODUCTION
The thymus provides a unique and indispensable microenvironment for the generation of self-tolerant T cells that comprise an essential component of the adaptive immune response. The thymus and parathyroid glands originate in a bilateral fashion from endoderm of the third pharyngeal pouch (reviewed in (Anderson et al., 2006; Blackburn and Manley, 2004; Manley, 2000). Each third pouch initially forms a common primordium containing thymus- and parathyroid-specific domains. Although epithelial cells of the common primordia are morphologically indistinguishable, patterning of third pouch endoderm into organ-specific domains is apparent from the expression pattern of Foxn1 and Gcm2 transcription factors that are restricted to the thymus- and parathyroid-fated domains respectively (Gordon et al., 2001). In situ hybridization (ISH) analysis shows that Gcm2 expression is localized to the anterior and dorsal region of third pouch endoderm, whereas Foxn1 is expressed in ventral and posterior regions of the third pouch. After the initial patterning is established, the shared primordia detach from the pharyngeal endoderm at approximately embryonic day 12 (E12), shortly after which the thymus and parathyroid rudiments separate from each other. The bilateral thymic lobes undergo medial, ventral and caudal migration to reach their final position above the heart.
Epithelial-mesenchymal interactions are a common theme in the development of various organs including the thymus. NCCs migrate into the vicinity of the third pharyngeal pouches during formation of the common thymus/parathyroid primordia and are a major component of the condensing mesenchymal capsule that surrounds the thymus rudiment after it separates from the parathyroid (Jiang et al., 2000; LeLievre and Le Douarin, 1975). In vitro and in vivo studies have demonstrated that mesenchyme provides essential signals for thymus organogenesis. Removal of mesenchymal cells from intact or reaggregated fetal thymus organ cultures at stages after E12.5 arrests thymus development in vitro (Auerbach, 1960; Jenkinson et al., 2003). In vivo ablation of premigratory NCCs in chicks was reported to result in ectopic, hypoplastic or absent thymic lobes (Bockman, 1984).
Perithymic mesenchyme promotes proliferation of the fetal thymus epithelial rudiment resulting in expansion of intrathymic niches that support thymocyte development (Jenkinson et al., 2007). The NCC-derived mesenchymal capsule produces growth factors, Fgf7 and Fgf10 that activate the fibroblast growth factor receptor, FgfR2IIIb expressed on thymic epithelial cells (TECs) (Erickson et al., 2002; Jenkinson et al., 2003; Revest et al., 2001). Outgrowth of the thymic rudiment is arrested by E12.5 and severe thymus hypoplasia is apparent by E18.5 in embryos that express a truncated, inactive isoform of FgfR2IIIb (Revest et al., 2001). The reduced TEC proliferation observed in Fgf10 null embryos further substantiates the link between thymus outgrowth and Fgf signaling (Ohuchi et al., 2000; Revest et al., 2001). Additional factors that are implicated in thymus growth and development include IGF1 and IGF2 produced by PDGFRα positive fetal mesenchyme (Jenkinson et al., 2007), and BMP4 expressed in thymus domain endoderm and adjacent NC-derived mesenchyme (Bleul and Boehm, 2005; Patel et al., 2006; Tsai et al., 2003). These investigations largely address the role of NCCs in supporting growth of the thymic rudiment after E12.5; earlier roles are not yet well defined.
Pax3 is a transcription factor expressed by premigratory neural crest precursors and is required for the survival, proliferation and differentiation of NCCs (Conway et al., 2000; Epstein et al., 2000; Pani et al., 2002). The point mutation Splotch (Sp) is a null allele of Pax3 that abolishes protein function (Auerbach, 1954; Goulding et al., 1991). Homozygous Splotch embryos are a well-established genetic model of severe neural tube defects and NCC deficiency. Pax3Sp/Sp mutants have a complex phenotype characterized by numerous developmental defects including spina bifida, exencephaly, and cardiac outflow tract abnormalities resulting in embryonic lethality by about E13 (Conway et al., 2000; Epstein et al., 2000). In addition, ectopia and hypoplasia or aplasia of the thymus are considered to be characteristic features of Pax3Sp/Sp embryos (Auerbach, 1954; Conway et al., 1997; Franz, 1989; Machado et al., 2001). Recent reviews reflect a general perception that the thymus is frequently absent or small in Pax3Sp/Sp mutants (Anderson et al., 2006; Hollander et al., 2006). This assessment is consistent with, and indeed part of, the evidence supporting a requirement for NCCs in thymus organogenesis. However, our data demonstrate that thymic lobes in E12.0–E12.5 Pax3Sp/Sp embryos, although ectopic, are actually larger than those in Pax3+/+ littermates. This unexpected phenotype is not a consequence of enhanced proliferation or diminished apoptosis of epithelial cells in the thymus rudiment. Instead, ISH analysis of organ-specific markers demonstrates that the NCC defect in Pax3Sp/Sp embryos results in aberrant patterning of the thymus- and parathyroid-fated domains as well as disrupted boundary formation in the shared third pouch primordium. These findings suggest that NCCs are involved in patterning third pouch endoderm by regulating the spatial and temporal expression of transcription factors that influence thymus versus parathyroid fate specification of pouch endoderm.
MATERIALS AND METHODS
Mice
Pax3Sp/+ mice on a C57Bl6/J background were purchased from The Jackson Laboratory (Bar Harbor, ME) and crossed to generate Pax3Sp/Sp embryos and littermate controls. Noon of the day the vaginal plug was found was designated as embryonic day 0.5. Embryos were also staged by morphological criteria including somite number and eye and limb morphology.
In Situ Hybridization
Embryos were collected in PBS containing 0.1% Tween20 and fixed in 4% paraformaldehyde overnight. They were dehydrated in a series of 30%, 50%, 70%, 90%, and 100% ethanol and paraffin embedded. ISH was performed on slides using digoxygenin-labeled antisense riboprobes constructed from linearized plasmids containing cDNA templates for mRNA probe production, as previously described (Zamisch et al., 2005), except that the proteinase K digestion was omitted. The CrabP1 (cellular retinoic acid binding protein I), IL-7, Foxn1, Gcm2 and Bmp4 probes have been described (Alpdogan et al., 2006; Gordon et al., 2001; Leonard et al., 1995; Zamisch et al., 2005). Each ISH analysis was performed on at least three Pax3Sp/Sp mutants and two wildtype littermates. Sections were counterstained with nuclear fast red.
Three-dimensional reconstructions
Three-dimensional reconstructions were created by tracing digital images captured from serial sections of paraffin embedded tissue stained by H&E or ISH using Surf Driver (version 3.5.2) software (www.surfdriver.com). For E12.5 embryos, tracings of the trachea, esophagus and thyroid along with thymus lobes and parathyroids were used for consistent alignment. For E11.5 samples, pouch lumens were aligned. At least three embryos were analyzed at each developmental stage and one three-dimensional reconstruction was chosen for presentation.
Histochemistry and Immunohistochemistry
Sections were stained with hematoxylin and eosin (H&E) by standard techniques. For immunohistochemical staining, embryos were placed in OCT compound and 5μm sections were cut. Sections were fixed with acetone. Sections were stained with primary antibodies from the following sources: rabbit anti-mouse K5 (Covance Research; Richmond, CA); rat anti-K8 (Troma-1; Developmental Studies Hybridoma Bank; Iowa City, IA); rat anti-mouse major histocompatibility complex (MHC) class II (clone M5/114.15.2), rat anti-c-kit (clone ACK45), and rat anti-CD45 (30-F11) were purchased (clone MEC13.3) (BD Biosciences; San Diego, CA); mouse anti-pancytokeratin (clone C-11) (Sigma; Saint Louis, MO). MHC class II, PDGFRα and CD45 immunoreactivity was enhanced by biotinyl tyramide amplification (TSA kit; PerkinElmer Life Sciences; Boston, MA) and detected with streptavidin-fluorescein isothiocynate (FITC). Second step anti-immunoglobulin (anti-Ig) reagents included Texas Red (TxR) conjugated donkey anti-rabbit and donkey anti-rat Ig, as well as FITC conjugated donkey anti-mouse and donkey anti-rabbit Ig (Jackson ImmunoResearch Laboratories, West Grove, PA). Controls included slides incubated with species or isotype-matched mouse Ig. For costaining, sections were incubated simultaneously with primary Abs from different species. Microscopic analysis was performed with an Olympus ProVis AX70 microscope (Olympus, Melville, NY).
Apoptosis and Proliferation Assays
TUNEL staining was performed using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany). Frozen sections were fixed in 4% paraformaldehyde for 30 min at room temperature. The slides were rinsed in PBS and permeabilized with 0.1% Triton X 100/0.1% sodium citrate on ice for 2min. After an additional rinse with PBS, the slides were treated with solution A and B and incubated in the dark at 37°C according to manufacturers instructions. Proliferation was assessed by indirect immunofluorescence. Frozen sections from E11.5 Splotch and wildtype littermates were incubated with DAPI to detect nuclei and with rabbit anti-phosphohistone H3 (Upstate; Lake Placid, NY) followed by FITC-conjugated donkey anti-rabbit Ig (Jackson ImmunoResearch Laboratories, West Grove, PA) to detect mitotic cells (Hendzel et al., 1997).
RESULTS
Thymus lobes in Pax3Sp/Sp embryos are ectopic but not hypoplastic
After the common thymus-parathyroid primordia detach from the pharyngeal endoderm the developing thymus and parathyroid glands separate from each other while the thymic rudiments undergo ventral, medial and caudal migration toward the ventral pharynx. By E12.5, wildtype thymic lobes are located cranial to the heart and caudal to the laryngeo-tracheal groove (Fig. 1A). Consistent with earlier reports that NCC defects in Pax3Sp/Sp mice produce thymus aplasia or hypoplasia, we found no thymic tissue at a comparable position in Pax3Sp/Sp littermates (Fig. 1B). However, inspection of more anterior sections revealed surprisingly large Pax3Sp/Sp thymic lobes above the laryngeo-tracheal groove (anterior to the separation of the trachea and esophagus) (Fig. 1D). In contrast, only the anterior tip of the wildtype thymus was evident at a similar position (Fig. 1C).
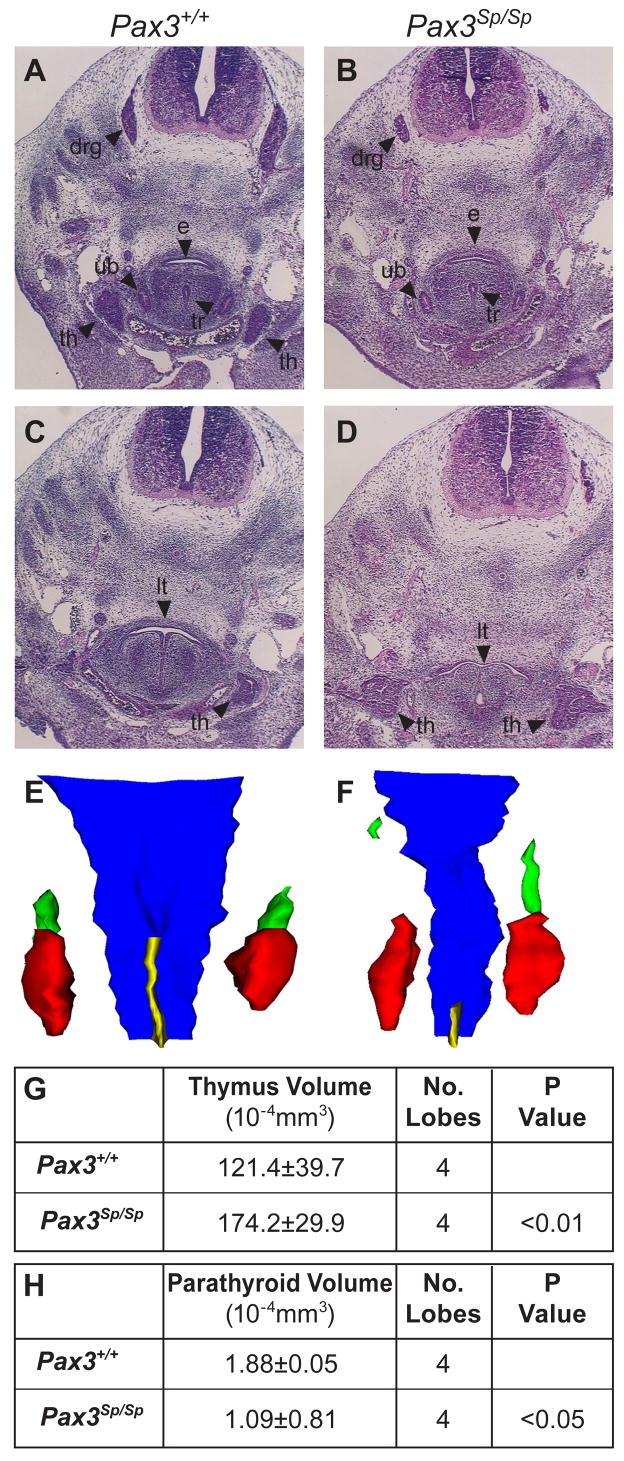
Figure 1. Thymus lobes in Pax3Sp/Sp embryos are large and ectopic.
(A–D) Transverse H&E stained sections from E12.5 embryos. Dorsal is up. Pax3+/+ thymus lobes are located cranial to the heart and posterior to the laryngeo-tracheal groove (A). Thymus lobes are not evident at a comparable position in the Pax3Sp/Sp embryo (B). Hypoplastic dorsal root ganglia are apparent in Pax3Sp/Sp embryos. Cranial sections, reveal large, ectopic thymus lobes in Pax3Sp/Sp mutant (D), whereas only the anterior tip of one lobe is evident in the wildtype littermate (C). 3-D reconstructions of Pax3+/+ (E) and Pax3Sp/Sp (F) pharyngeal regions showing thymus (red), parathyroid (green), esophagus (blue) and trachea (yellow). Average volume of Pax3+/+ and Pax3Sp/Sp thymic (G) and parathyroid lobes (H). Abbreviations are drg, dorsal root ganglia; e, esophagus; tr, trachea ; th, thymus; lt, laryngeo-tracheal groove; ub, ultimobranchial bodies.
To better visualize the relative dimensions and position of thymus lobes in E12.5 Pax3Sp/Sp and Pax3+/+ littermates, we generated 3-D reconstructions of the pharyngeal region using serial transverse sections (Fig. 1E, F). The results confirm that caudal migration of Pax3Sp/Sp thymus primordia is defective compared to their wildtype counterparts. We also found that, consistent with the larger size of mutant thymus lobes observed in histological sections, there is a significant increase in the volume of Pax3Sp/Sp thymus lobes compared to Pax3+/+ controls (Fig. 1G). Conversely, the Pax3Sp/Sp parathyroid primordia were smaller in volume than those of wildtype littermates (Fig. 1H). These results demonstrate that contrary to expectations, thymus aplasia or hypoplasia is not a typical consequence of the NCC defect in Splotch mutants.
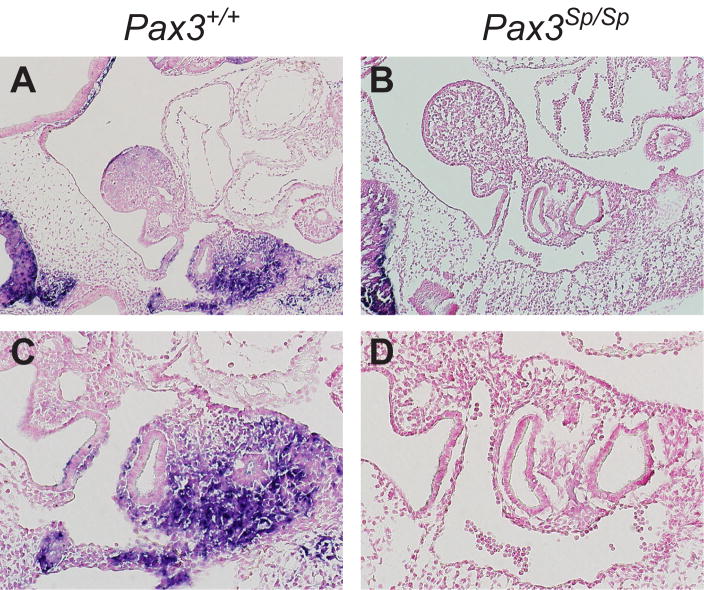
During normal ontogeny, the third pouch-derived thymus-parathyroid shared primordia detach from the pharynx at around E12. Inspection of H&E stained transverse sections showed that separation of these shared primordia from pharyngeal endoderm was delayed in four of six Pax3Sp/Sp embryos but none of six Pax3+/+ littermates (compare Fig. 2 B, D with Fig. 2A, C). To determine whether delayed detachment from the pharynx was specific to the third pouch-derived shared primordia or reflected a general pharyngeal pouch defect, we examined the ultimobranchial bodies, which are derived from the fourth pharyngeal pouch and normally detach from the pharynx prior to detachment of the thymus lobes. The normal detachment of ultimobranchial bodies observed in Splotch mutant embryos (Fig. 2E, F) demonstrates that the separation defect is restricted to the third pouch-derived thymus-parathyroid primordia. These results suggest that delayed separation from pharyngeal endoderm likely contributes to the ectopic position of Pax3Sp/Sp thymus lobes.
Figure 2. Delayed detachment phenotype is specific to the third pouch.
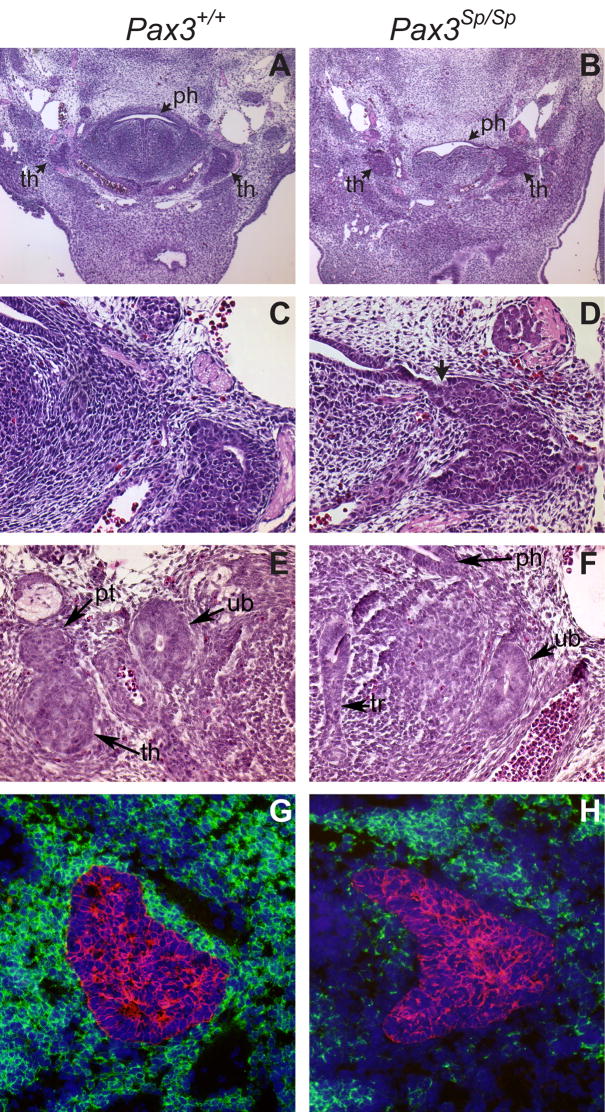
Transverse H&E stained sections from (A,C,E) Pax3+/+ and (B,D,F) Pax3Sp/Sp E12.5 embryos. Panels C and D are higher magnification (20X) images of A and B (5X). The arrow in D shows attachment of Pax3Sp/Sp thymus rudiment to the pharynx. Normal ultimobranchial body detachment occurs in Pax3+/+ and Pax3Sp/Sp embryos (E,F). Abbreviations are thymus (th), parathyroid (pt), pharynx (ph) and ultimobranchial body (ub). Transverse frozen sections from (G) Pax3+/+ and (H) Pax3Sp/Sp E12.5 embryos were stained with anti-PDGFRα (green), anti-pancytokeratin (red) and DAPI (blue).
Perithymic NCC localization and capsule formation are deficient in Pax3Sp/Sp embryos
The fetal thymus rudiment is encompassed by a NC-derived mesenchymal capsule that provides essential signals to the developing thymic epithelial compartment (Auerbach, 1960; Foster et al., 2008; Jenkinson et al., 2007). Thymic sections were stained with anti-PDGFRα to detect the NC-derived mesenchymal capsule surrounding the cytokeratin positive thymus epithelial rudiment in E12.5 Pax3Sp/Sp and Pax3+/+ littermates. Figure 2G shows abundant PDGFRα positive mesenchymal cells encompassing the wildtype thymic epithelial rudiment. In contrast, there is a striking deficiency in NC-derived perithymic cells as shown by the paucity of PDGRFα expressing cells adjacent to the Pax3Sp/Sp thymic rudiment (Fig. 2H). However, the presence of DAPI stained nuclei indicates the presence of other cell types proximal to the Pax3Sp/Sp thymic epithelial rudiment. These results confirm that the NCC defect in these mutants directly affects the contribution of mesenchyme to the early thymic rudiment.
To compare the pattern of NCC migration in the vicinity of the third pouch in E10.5 Pax3Sp/Sp and Pax3+/+ embryos, we assessed expression of CrabP1, a marker for migrating NCCs ISH analysis showed that NCCs were abundant in the dorsal region of the third pouch in E10.5 Pax3+/+ embryos (Fig. 3A, C). In contrast, there was a notable reduction of NCCs in the vicinity of the third pharyngeal pouch in E10.5 Pax3Sp/Sp mutants (Fig. 3B,D). These data are consistent with a previous report showing a reduction in NCCs populating the pharyngeal arches and cardiac outflow tract of Pax3Sp/Sp embryos (Epstein et al., 2000). By E11.5, NCCs were predominantly localized in the ventral region of the third pouch in wildtype embryos indicating a dorsal to ventral axis of migration, but were still dramatically reduced in the mutants (data not shown).
Figure 3. NCC localization is disrupted at E10.5 in Pax3Sp/Sp embryos.
Sagittal sections from E10.5 Pax3+/+(A,C) and Pax3Sp/Sp (B,D) embryos were incubated with anti-sense riboprobe for CrabP1 to detect migrating NCC. (C and D) are higher magnification views of the third pouch region. Cranial is up and ventral is left. The clear space surrounding the pouches is an artifact of tissue processing. There is a paucity of CrabP1 expressing NCCs in pharyngeal arches and pouches of Pax3Sp/Sp embryos.
Differentiation of thymic epithelium is not impaired in Pax3Sp/Sp embryos
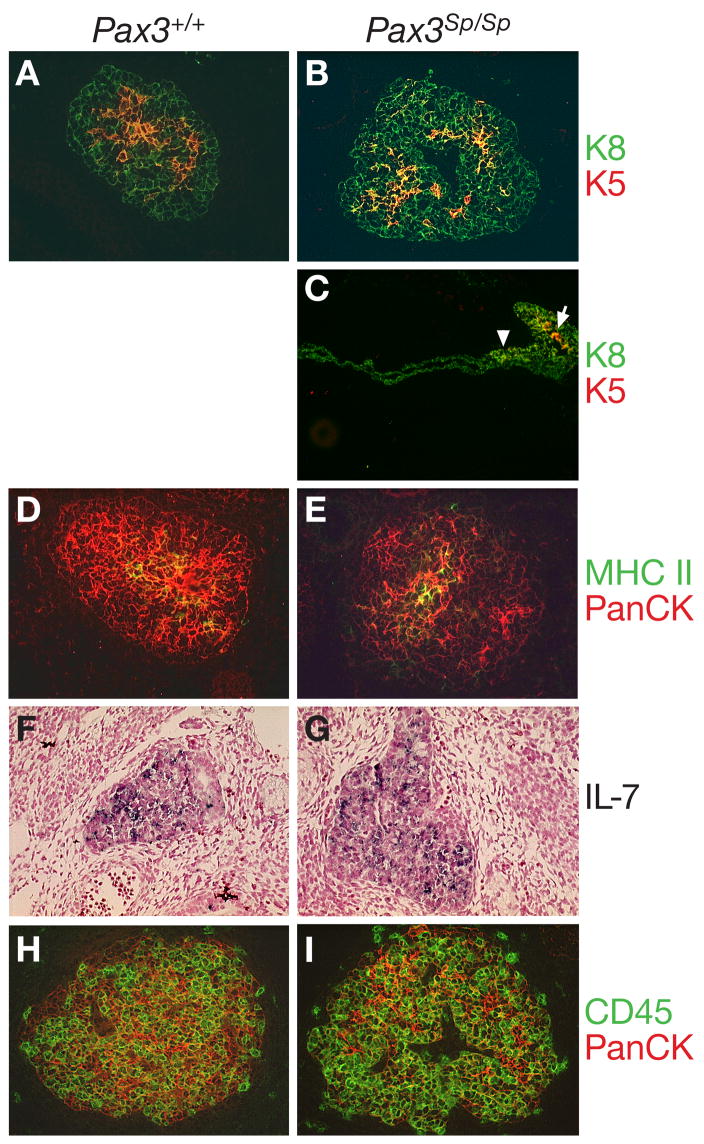
Since NC-derived mesenchyme has been implicated in TEC differentiation and function, we performed an immunohistochemical analysis to evaluate TEC differentiation in the ectopic thymic rudiment of Pax3Sp/Sp embryos. At E12.5 the thymic anlage consists predominantly of TECs that express cytokeratin 8 (K8), but not K5 as well as a central region of K8+K5+ TECs that contain progenitors of mature cortical and medullary TECs (Bennett et al., 2002; Gill et al., 2002) (Fig. 4A). Both K8+K5− and K8+K5+ TEC subsets were present in E12.5 Pax3Sp/Sp thymus lobes (Fig. 4B). The keratin expression pattern was correctly established even when the mutant thymic lobes failed to separate from the pharynx, indicating that separation from the pharyngeal endoderm is not required for TEC differentiation (Fig. 4C).
Figure 4. Epithelial differentiation is normal in E12.5 in Pax3Sp/Sp thymus lobes.
Transverse frozen sections from wild type (A) and Pax3Sp/Sp embryos (B,C) stained with anti-K5 (red) or anti-K8 (green). Note the presence of K8+K5+ epithelial cells in the center of the Pax3Sp/Sp rudiment (arrow) even when it remains attached to the pharyngeal endoderm (arrowhead). Frozen thymus sections form Pax3+/+ (D) and Pax3Sp/Sp (E) embryos stained with anti-MHC class II (green) and anti-pan-cytokeratin (red). ISH analysis of IL-7 expression in sagittal sections of E12.5 thymus from Pax3+/+ (F) and Pax3Sp/Sp (G) embryos. Frozen transverse sections from Pax3+/+ (H) and Pax3Sp/Sp (I) E12.5 embryos stained with anti-CD45 (green) and anti-pan-cytokeratin (red).
To obtain functional evidence of TEC differentiation, we examined MHC class II and interleukin-7 (IL-7) expression, as well as the timing of hematopoietic precursor cell (HPC) immigration. MHC class II expression is required for thymocyte selection and is normally initiated at approximately E12.5 (Shinohara and Honjo, 1997). MHC Class II expression was comparable in the Pax3Sp/Sp and Pax3+/+ fetal thymus lobes (Fig. 4D, E). IL-7 is a cytokine produced by TECs and required for thymocyte survival, proliferation and differentiation (Fry and Mackall, 2002). We previously found that IL-7 mRNA is highly expressed throughout wildtype thymic lobes at E12.5 (Zamisch et al., 2005). There was a comparable distribution of IL-7 expressing TECs in Pax3Sp/Sp and Pax3+/+ thymic rudiments (Fig. 4F, G). HPCs are recruited into the thymus beginning at approximately E11.5 in a process that depends on the production of chemokines such as CCL25 and CCL19 by TECs (Liu et al., 2006). A comparable influx of CD45 positive HPCs was observed in the thymic lobes of E12.0 Pax3Sp/Sp and Pax3+/+ embryos (Fig. 4H, I). Therefore, Pax3Sp/Sp TECs are capable of attracting HPC to migrate across the perithymic mesenchyme into the thymus rudiment. Similar results were obtained when thymocyte progenitors were detected by expression of c-kit confirming that Pax3Sp/Sp thymus lobes recruit HPCs (data not shown). Taken together, these results indicate that early TEC differentiation and function are intact in the early ectopic Pax3Sp/Sp thymus rudiment.
Proliferation and cellularity are comparable in third pharyngeal pouch endoderm of Pax3Sp/Sp and Pax3+/+ littermates
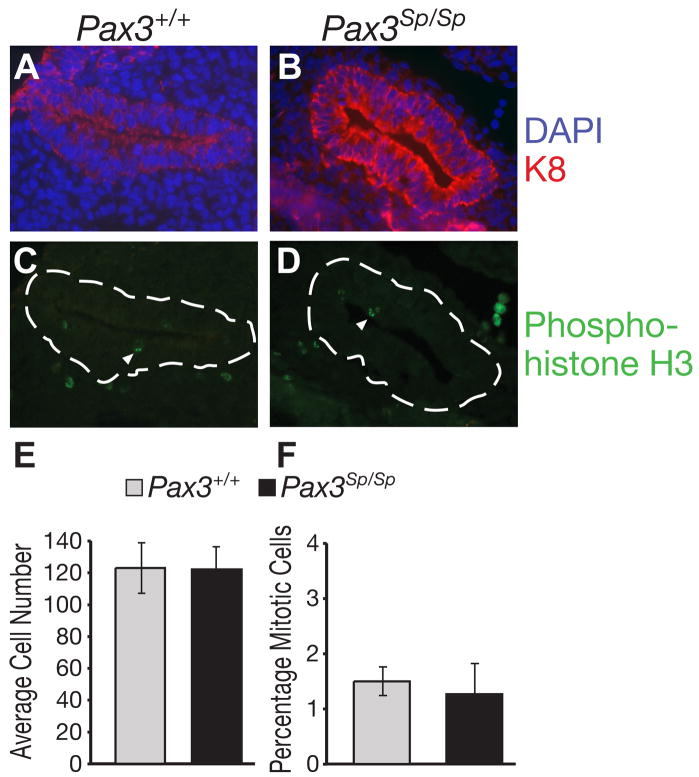
To investigate whether increased epithelial cell proliferation contributed to the unexpectedly large thymus lobes in Pax3Sp/Sp embryos, we determined the frequency of dividing cells in the shared thymus/parathyroid primordia of Pax3Sp/Sp and Pax3+/+ embryos using phosphorylated histone H3 as a marker of mitotic cells (Hendzel et al., 1997). Frozen sections from three Pax3Sp/Sp and three Pax3+/+ E11.5 embryos were co-stained with anti-K8 and DAPI to identify the third pouch and with anti-phosphorylated histone H3 (Fig. 5A–D). Approximately 1.5% of third pharyngeal pouch cells were in mitosis in both Pax3Sp/Sp and wildtype littermates (Fig. 5F). To determine if the increased size of Pax3Sp/Sp thymus lobes was associated with a decrease in apoptosis, the TUNEL assay was performed on E12.0 frozen thymus sections. At this stage there is little apoptosis in the thymus, and there was no difference in the frequency of apoptotic cells in Pax3Sp/Sp compared Pax3+/+ thymic rudiments (data not shown). Importantly, the average total number of cells in the combined thymus and parathyroid domains of the E11.5 littermates was not significantly different in Pax3Sp/Sp mutants compared to Pax3+/+ littermates (Fig. 5E). These data indicate that the Pax3Sp/Sp third pharyngeal pouch does not differ from wildtype in the frequency of proliferating or apoptotic cells or in the total size of the shared primordium.
Figure 5. Proliferation and cellularity in Pax3Sp/Sp common primordia.
Sagittal sections of E11.5 Pax3+/+(A, C) and Pax3Sp/Sp (B, D) embryos; cranial is up and dorsal is right. Staining for K8 (red) reveals epithelial cells in the common primordium; DAPI staining (blue) detects nuclei (A,B). Mitotic cells detected by costaining with anti-phosphohistone H3 antibody (green) (C,D). Average cell number (E) and percentage of mitotic cells (F) per section were determined by counting multiple sections from three Pax3+/+ and three Pax3Sp/Sp embryos.
Defects in the location and shape of the interface between thymus and parathyroid-fated domains in the common primordium of Pax3Sp/Sp embryos
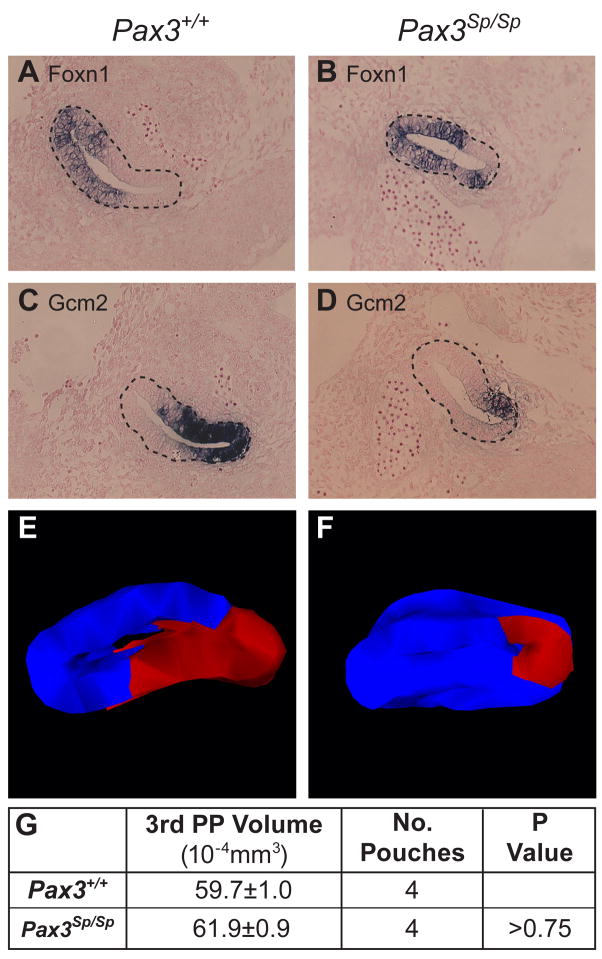
Although there is convincing evidence that NCCs produce growth factors required for outgrowth of the thymus rudiment after E12.5, it is not known if NCCs play an earlier role by influencing patterning of third pharyngeal pouch endoderm into thymus and parathyroid domains. To examine this issue, we analyzed Gcm2 and Foxn1 expression domains in the common thymus/parathyroid primordia of Pax3Sp/Sp embryos. ISH analysis was performed on serial sagittal sections from Pax3+/+ and Pax3Sp/Sp littermates at E11.5, the earliest stage at which the Foxn1 thymus-specific marker is expressed. At this developmental stage, the majority of cells in the common primordia express either Foxn1 or Gcm2 (compare A with Fig. 6C and Fig. 6B with Fig. 6D). Interestingly, we found an increase in the Foxn1-expressing thymus domain of the third pouch-derived primordium in Pax3Sp/Sp compared to Pax3+/+ littermates (Fig. 6A, B), with a corresponding decrease in the Gcm2 expressing parathyroid domain (Fig. 6C, D). Similar results were obtained in four Pax3Sp/Sp mutants and Pax3+/+ controls at this stage.
Figure 6. Thymus/parathyroid fate boundary is shifted in Pax3Sp/Sp third pharyngeal pouch.
Sagittal sections of E11.5 Pax3+/+(A,C) and Pax3Sp/Sp (B,D) embryos analyzed by ISH for FoxN1 (A,B) and Gcm2 (C,D). The third pouch is indicated by a dotted line. Cranial is up and ventral is to the left. 3-D reconstructions of FoxN1 (blue) and Gcm2 (red) expression domains (E,F). Average volume of Pax3+/+ and Pax3Sp/Sp third pharyngeal pouch (G).
To better visualize the relative proportions of the Foxn1 and Gcm2 expression domains in the common thymus/parathyroid primordia, three-dimensional images were reconstructed from the ISH data obtained on alternating serial sagittal sections from E11.5 Pax3Sp/Sp and Pax3+/+ littermates. These reconstructions show a marked dorsal shift in the location of the border between thymus and parathyroid fated domains in Pax3Sp/Sp common primordia resulting in a relative expansion of the Foxn1 expressing domain at the expense of the parathyroid domain (Fig. 6E, F). Foxn1 expressing cells occupied 71±5.0% (n=5) of the wildtype third pharyngeal pouch, but 78±2.8% (n=5) of Pax3 deficient pouch (p<0.003). Conversely, Gcm2 expressing cells occupied 28.8±5.2% of the wildtype pouch, but 21.2±2.8% of Pax3 deficient pouch (p<0.01). Importantly, we found that the common third pharyngeal pouches in wildtype and Pax3Sp/Sp mutants are comparable in volume (Fig. 6G). Thus, the altered border position reflects a relative increase in allocation of third pouch endodermal cells to a thymus fate resulting in a corresponding decrease in third pouch endodermal cells fated to become parathyroid.
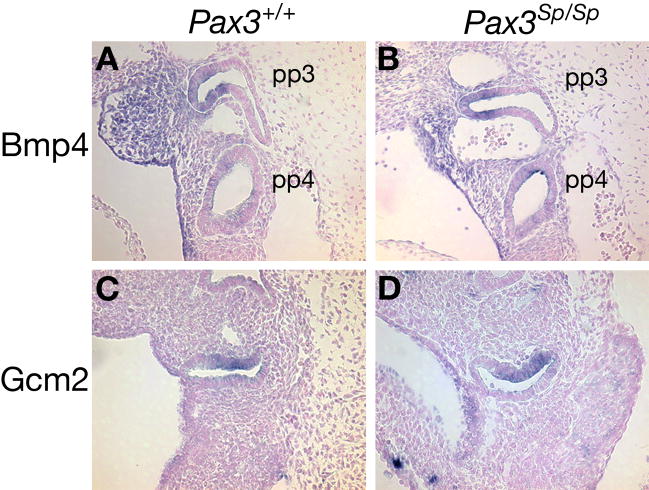
We also analyzed third pharyngeal pouch patterning in Pax3Sp/Sp embryos prior to the initial appearance of Foxn1 transcripts at E11.25. Gcm2 is expressed in a discrete domain of the third pouch endoderm of wildtype embryos at E10.5 (Gordon et al., 2001) (Fig. 7C). Although Foxn1 is not expressed at E10.5, the thymus domain is marked by the expression of Bmp4 at this stage (Alpdogan et al., 2006; Moore-Scott and Manley, 2005). In wildtype embryos at E10.5, Bmp4 expression is confined to the ventral posterior thymus domain (Fig. 7A). In contrast to the restricted Gcm2 and expanded Foxn1 domains at E11.5, both Gcm2 and Bmp4 expression in the third pharyngeal pouch of Pax3Sp/Sp mutants were similar to Pax3+/+ littermates at E10.5 (Fig. 7B, D). These results suggest that initial third pouch patterning is independent of NCC-derived signals.
Figure 7. Third pharyngeal pouch patterning is normal at E10.5 in Pax3Sp/Sp embryos.
Sagittal sections from Pax3+/+ (A,C) and Pax3Sp/Sp (B,D) embryos at E10.5 were stained by ISH with anti-sense riboprobe for Bmp4 (A,B) or Gcm2 (C,D) and counterstained with nuclear fast red. In all panels, ventral is left and cranial is up. pp3, third pharyngeal pouch; pp4, fourth pharyngeal pouch. Neither Bmp4 nor Gcm2 expression is changed in the mutants.
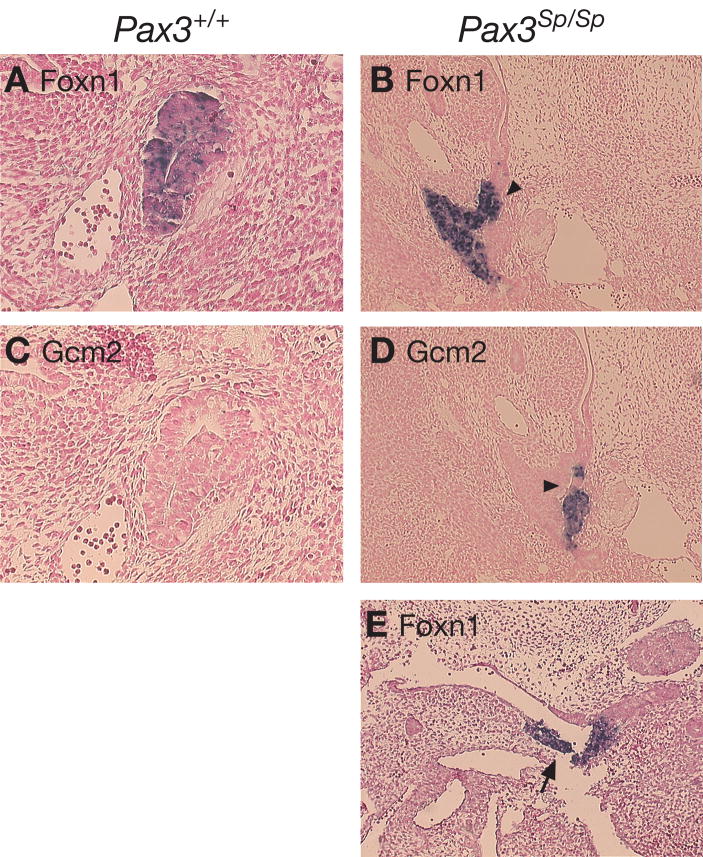
In addition to the altered location of the thymus/parathyroid interface at E11.5, abnormal arrangement of thymus- and parathyroid-specified domains at the boundary was frequently observed within Pax3Sp/Sp third pouch primordia. Whereas there is a well delineated, straight boundary between the thymus- and parathyroid-fated domains in the third pharyngeal pouch of Pax3+/+ embryos, the interface in Pax3Sp/Sp mutants is uneven. Figure 8 shows an example in which a portion of the Foxn1 expressing domain is interspersed with the Gcm2 expressing domain in the dorsal aspect of the pouch, rather than restricted to the ventral aspect of the primordium (Fig. 8B, D). Moreover, the Foxn1 expression domain was expanded well into the pharyngeal endoderm in some Pax3Sp/Sp mutants (Figure 8E). Foxn1 expression is never detected in non-pouch pharyngeal endoderm of wildtype embryos. Taken together, these data implicate NCC-derived signals in regulating the formation and/or maintenance of a discrete boundary between thymus- and parathyroid-fated domains in the shared third pouch primordia.
Figure 8. Delayed separation and mixing of thymus and parathyroid fate domains in E12 Pax3Sp/Sp embryos.
Transverse sections of E12.0 Pax3+/+ (A,C) and Pax3Sp/Sp (B,D,E) littermates analyzed for FoxN1 (A,B,E) and Gcm2 (C,D) expression by ISH. Ventral is down in (A–E). Arrowheads in B and D indicate mixing of FoxN1 and Gcm2 domains in Pax3Sp/Sp embryos. Note that the common primordium remains attached to the pharynx. (E) FoxN1 expression in Pax3Sp/Sp embryo extends into the pharyngeal endoderm exterior to the pouch.
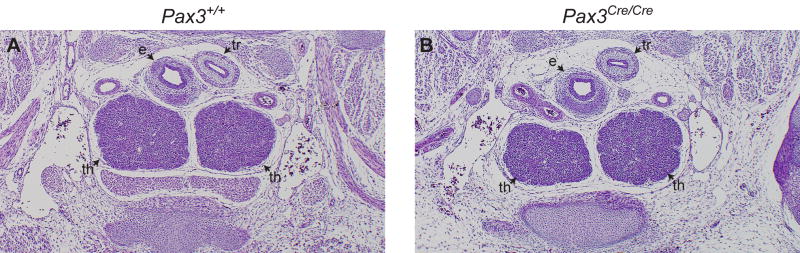
Thymus lobes in Pax3Cre/Cre embryos are ectopic but not hypoplastic
Since Pax3Sp/Sp embryos do not survive beyond E12.0–E12.5, it was not possible to directly determine the size and position of thymic lobes at later developmental stages. Therefore, we analyzed thymus development in Pax3Cre/Cre in which replacement of the first coding exon of Pax3 with a gene encoding Cre recombinase generates a null allele that fails to produce Pax3 protein (Engleka et al., 2005). Homozygous Pax3Cre/Cre embryos have severe neural tube and other NCC-associated defects, but survive several days longer than Pax3Sp/Sp embryos, perhaps due to the mixed genetic background in which modifier genes reduce the severity of cardiac defects. Similar to the Pax3Sp/Sp mutants, the E12.5 Pax3Cre/Cre thymus lobes are ectopic and larger than their wildtype littermates (data not shown). To determine whether thymus lobes become hypoplastic in older embryos, thymus development was assessed at E14.5. Interestingly, well developed thymus lobes are apparent in Pax3Cre/Cre embryos at this later developmental stage (Fig. 9A, B). Furthermore, analysis of 3D reconstructions revealed no significant difference in the volume of wildtype (435±15 10−4mm3; n=4) versus Pax3Cre/Cre (337±19 10−4mm3; n=4) thymus lobes at E14.5. This phenotype is discussed in more detail below.
Figure 9. Thymus lobes in E14.5 Pax3Cre/Cre embryos.
Transverse H&E stained sections from E14.5 Pax3+/+ (A) and Pax3Cre/Cre (B) embryos. Abbreviations are; e, esophagus; tr, trachea ; th, thymus.
DISCUSSION
Contrary to the generally held view that thymus aplasia or hypoplasia is a typical developmental anomaly in Splotch embryos, this report demonstrates that well-developed thymic lobes are present, but located in an ectopic anterior position. Given that the Splotch mouse is a well-characterized model of NC deficiency and that previous studies showed NC-derived mesenchyme is required for outgrowth of the E12.5 thymus rudiment (Erickson et al., 2002; Jenkinson et al., 2003; Jenkinson et al., 2007; Revest et al., 2001), it is not surprising that thymus development was assumed to be impaired in Pax3Sp/Sp embryos. However, prior studies did not examine the earliest stages of organogenesis when thymus- and parathyroid-fated endodermal cells occupy ventral and dorsal regions respectively of the third pharyngeal pouch primordia. The present report implicates NCCs in the previously unappreciated role of regulating the patterning of third pharyngeal pouch endoderm into thymus- and parathyroid-fated domains after pouch formation.
Although normal expression of organ-specific markers in third pouch primordia of E10.5 Pax3Sp/Sp mutants indicates that initial establishment of thymus- and parathyroid-fated domains is independent of NCC-derived signals, the final position of the border between thymus and parathyroid specific domains is shifted dorsally by E11.5, resulting in a relatively smaller Gcm2 expressing domain and a correspondingly larger Foxn1 expressing domain. This shift is not associated with changes in cell death or proliferation. Moreover, overall volumes of E11.5 third pouch-derived primordia were comparable in wildtype and Pax3Sp/Sp embryos. Taken together these findings indicate that more endodermal cells in the shared third pouch of Splotch mutants are specified to a thymus fate and fewer cells are specified to a parathyroid fate. This border shift accounts for the unexpectedly large thymus and smaller parathyroid rudiments observed in E12.5 Pax3Sp/Sp embryos. Moreover, the normal third pouch patterning observed in Pax3Sp/Sp embryos at an earlier developmental stage, E10.5, is consistent with our previous and current data showing that not all cells in the third pouch express Gcm2 or Bmp4 at this stage (Fig. 7 and (Gordon et al., 2001). This phenomenon results in an ‘unspecified’ central region at E10.5 that expresses neither organ-specific marker. Since all cells in the primordium express an organ-specific marker by E11.5, we propose that additional patterning events occur between E10.5–11.5, in which the precise location of the final boundary between the thymus and parathyroid domains is set. The current data strongly suggest that signals from NCCs are required for the final position of this boundary.
The notion that unique NCC-derived signals are not essential for thymus fate specification is supported by investigations showing the development of a functional thymus following transplantation of isolated pharyngeal endoderm obtained prior to NCC migration (Gordon et al., 2004; LeLievre and Le Douarin, 1975). Moreover, the NCC deficiency in Splotch mutant mice does not impair Foxn1 expression. Indeed, our results suggest that thymus fate specification is a NCC-independent process, since the presumptive thymus-specific domain as marked by Bmp4 expression is unaffected in the Pax3Sp/Sp mutants. During normal pouch patterning, Foxn1 is first expressed in the cells located at the distal end of the third pouch primordia and expands dorsally to the Gcm2 expression boundary (Gordon et al., 2001). This process appears to be operative in the Pax3Sp/Sp common primordia, resulting in a situation in which reduction of the Gcm2 expression domain in the Pax3Sp/Sp common primordia at E11.5 is compensated for by a relative expansion of the Foxn1 expression domain. This model could also explain the mixing of Gcm2 and Foxn1 expression domains that we frequently observe in Pax3Sp/Sp common primordia. Since NCC migration and function are not completely abrogated in Pax3Sp/Sp mutants, there is a small probability that any given NCC can migrate to a position proximal to the dorsal region of the common primordia and generate signals that affect gene expression patterns in the endoderm. However, since appropriate migration and function of Pax3Sp/Sp NCCs would be a stochastic process, nonconsecutive portions of dorsal endoderm would receive sufficient NCC-derived signals to affect patterning during the later boundary refinement phase, resulting in an apparent mixing of fate domains.
At first glance, our findings appear to be at odds with the notion that NCC deficiency results in thymus hypoplasia. A likely explanation for this paradox is that NCCs perform distinct functions during early patterning of the common primordia compared to later outgrowth of the thymic rudiment. Our evidence suggests that whereas NCC-derived signals do not play a direct role in initially committing third pouch endodermal progenitors to a thymus or parathyroid fate, they play an essential role in refining these domains between E10.5 and E11.5. It has been shown that by E12, perithymic mesenchyme produces growth factors IGF1 and IGF2 that are thought to induce growth of the early thymus rudiment (Jenkinson et al., 2007). Subsequently, at E13.5–E14, TECs upregulate FgfR2-IIIb expression and the mesenchymal capsule produces Fgf7 and Fgf10 to promote TEC proliferation (Jenkinson et al., 2003; Revest et al., 2001). Taken together, these data suggest that the functional role of NC-derived perithymic mesenchyme evolves throughout thymus organogenesis.
Although there is a severe reduction in NCC-derived mesenchyme in these mutants, mesoderm-derived mesenchyme remains. This raises the interesting possibility that some of the phenotypes seen could be due to the new proximity of mesoderm-derived factors to endoderm (a dominant effect), or could be mitigated by similar factors produced by both types of mesenchyme (a redundancy effect). One instance in which this issue may be important is at later stages in the Pax3Cre/Cre mice. It is not clear why the Pax3Cre/Cre thymus lobes do not maintain a comparatively larger volume at the later developmental stage. One possibility is that growth factors produced locally by mesodermal mesenchyme have limited ability to compensate for a deficiency in NC-derived growth factors. Alternatively, a physical reduction in the NCC mesenchyme capsule may provide inadequate signals for thymic epithelial cell growth, causing a slightly slower rate of growth for Splotch-derived stromal cells. While this reduction might result in a gradual thymic hypoplasia, the initially larger target size may mitigate the slower growth. In any case, the Pax3Cre/Cre model of NC deficiency confirms the Splotch data at E12.5 and shows that NC deficiency does not result in thymus hypoplasia in late gestation embryos.
Our results in Splotch mutants strongly suggest that NCC-derived signals are not essential for initial differentiation of TECs. The thymic epithelial rudiment in Pax3Sp/Sp embryos expresses several functional differentiation markers and supports the influx of HPCs. Distinct TEC subsets defined by keratin expression pattern develop in Pax3Sp/Sp mutants even when the thymic rudiment fails to detach from the pharyngeal endoderm. These observations are consistent with previous reports showing that sustained mesenchymal/TEC interactions are not required to support differentiation of immature TECs into functional cortical and medullary subsets (Jenkinson et al., 2003; Jenkinson et al., 2007). In contrast, a recent study reported impaired expression of MHC class II and other functional molecules in E13 embryos that are homozygous for the Patched deletion that disrupts the PDGFRα gene resulting in aberrant migration and increased apoptosis of NCCs (Itoi et al., 2007). The apparent discrepancy between the earlier report and our data may be due to the fact that the Patched deletion encompasses other relevant genes including c-kit (Nagle et al., 1994).
To our knowledge this is the first evidence implicating NCCs in patterning the third pouch endoderm into organ-specific domains. The results are reminiscent of our previous data showing that formation of the parathyroid-specific domain depends on signals from Shh produced by pharyngeal endoderm (Moore-Scott and Manley, 2005). It is possible that Shh acts in part through NCC mesenchyme to establish the parathyroid domain in the third pouch. Shh signaling to both the NCC and/or directly to the endoderm may be integrated to pattern the parathyroid domain in the third pouch endoderm. Separate NCC-derived signal(s) downstream of Shh would then act to set the parathyroid organ domain boundaries. Since NCCs are primarily located adjacent to the dorsal third pouch by E10.5 in both wildtype and mutant embryos, and Shh expression is restricted to the pharynx and excluded from the pouch itself, parathyroid fate is restricted to the dorsal domain. In Pax3Sp/Sp embryos, reduction or absence of NCC-derived signals could result in a shift in the final location of the parathyroid-thymus border within the pouch, resulting in a smaller Gcm2 expressing parathyroid domain. Thus, the NCC defect in Pax3Sp/Sp embryos ultimately results in an altered allocation of organ specific domains of the third pouch common primordia, with an increase in the thymus domain at the expense of the parathyroid domain.
The ectopic location of the thymic rudiments in Pax3Sp/Sp embryos likely accounts for the widely held, but erroneous perception that thymus hypoplasia or aplasia is a general phenotypic feature in Splotch mutants. Thymus ectopia can be caused either by delayed separation from the pharynx, or by a direct defect in migration, or both. The NCC deficiency in Pax3Sp/Sp embryos disrupts whatever mechanism is required for apoptosis and separation of the primordium from the pharynx at some frequency. Although we did not observe a decreased frequency of apoptotic cells within the thymic rudiment, the coordinated apoptosis required for this separation event occurs outside the primordium in a brief window at E12 (Gordon et al., 2004). Thus, the observed phenotype is likely due to changes in the timing or extent of apoptosis at the junction of the primordium and the pharynx in mutant embryos. Thymus ectopia could also result from a separate defect in migration of the separated thymus rudiment, which has also been associated with NCC defects.
Early investigations suggested that NCCs regulate segmentation and organization of pharyngeal endoderm into arches and pouches (reviewed in (Graham et al., 2004). However, neural crest ablation does not prevent pharyngeal arch development (Veitch et al., 1999). Since segmentation of pharyngeal endoderm is a general characteristic of chordates (Schaeffer, 1987), occurring prior to the evolutionary development of neural crest, perhaps it is not surprising that the pharyngeal endoderm has an intrinsic morphogenetic capacity. However, a role for NCCs in influencing the patterning of pouch endoderm into organ specific domains and in promotion of initial organogenesis remains possible. The data obtained using the NC deficient Splotch model implicates NCCs in a previously unrecognized role, that of final patterning third pharyngeal pouch endoderm into thymus- and parathyroid-fated domains. Although recent considerations of pharyngeal arch patterning have emphasized that pouch formation and anterior-posterior identity are established independently of NCCs, these findings refocus attention on the potential role of NCCs in regulating patterning of pharyngeal pouch endoderm.
Acknowledgments
We thank Francesca Vitelli and A. Baldini for advice and helpful discussions (Baylor College of Medicine). We thank Becky Brooks for assistance in manuscript preparation. This work was supported in part by grants as follows: NIEHS (ES07784), NCI (CA16672), NIH (HD056315) and by the Rothman/Stevenson fund to E.R.R. and as well as by NIH (HD035920) to N. R. M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, Suh D, Muriglan SJ, Boyd RL, van den Brink MR. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–60. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Jenkinson WE, Jones T, Parnell SM, Kinsella FA, White AJ, Pongrac’z JE, Rossi SW, Jenkinson EJ. Establishment and functioning of intrathymic microenvironments. Immunol Rev. 2006;209:10–27. doi: 10.1111/j.0105-2896.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- Auerbach R. Analysis of the developmental effects of a lethal mutation in the house mouse. J Exp Zool. 1954;127:305–329. [Google Scholar]
- Auerbach R. Morphogenetic interactions in the development of the mouse thymus gland. Dev Biol. 1960;2:271–384. doi: 10.1016/0012-1606(60)90009-9. [DOI] [PubMed] [Google Scholar]
- Bennett AR, Farley A, Blair NF, Gordon J, Sharp L, Blackburn CC. Identification and characterization of thymic epithelial progenitor cells. Immunity. 2002;16:803–814. doi: 10.1016/s1074-7613(02)00321-7. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4:278–89. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Boehm T. BMP signaling is required for normal thymus development. J Immunol. 2005;175:5213–21. doi: 10.4049/jimmunol.175.8.5213. [DOI] [PubMed] [Google Scholar]
- Bockman DEaKML. Dependence of thymic development on derivative of the neural crest. Science. 1984:223. doi: 10.1126/science.6606851. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM. Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the splotch (Sp(2H))/Pax3 mouse mutant. Cardiovasc Res. 2000;47:314–28. doi: 10.1016/s0008-6363(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development. 1997;124:505–14. doi: 10.1242/dev.124.2.505. [DOI] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, Lu MM, Thomas M, Liu E, Wessels A, Lo CW. Migration of cardiac neural crest cells in Splotch embryos. Development. 2000;127:1869–78. doi: 10.1242/dev.127.9.1869. [DOI] [PubMed] [Google Scholar]
- Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, Rubin JS, Rudensky A, Farr AG. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–78. doi: 10.1182/blood-2002-04-1036. [DOI] [PubMed] [Google Scholar]
- Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol. 2008;180:3183–9. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- Franz T. Persistent truncus arteriosus in the Splotch mutant mouse. Anat Embryol (Berl) 1989;180:457–64. doi: 10.1007/BF00305120. [DOI] [PubMed] [Google Scholar]
- Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- Gill J, Malin M, Hollander GA, Boyd R. Generation of a complete thymic microenvironment by MTS24(+) thymic epithelial cells. Nat Immunol. 2002;3:635–642. doi: 10.1038/ni812. [DOI] [PubMed] [Google Scholar]
- Gordon J, Bennett AR, Blackburn CC, Manley NR. Gcm2 and Foxn1 mark early parathyroid- and thymus-specific domains in the developing third pharyngeal pouch. Mech Dev. 2001;103:141–143. doi: 10.1016/s0925-4773(01)00333-1. [DOI] [PubMed] [Google Scholar]
- Gordon J, Wilson VA, Blair NF, Sheridan J, Farley A, Wilson L, Manley NR, Blackburn CC. Functional evidence for a single endodermal origin for the thymic epithelium. Nat Immunol. 2004;5:546–53. doi: 10.1038/ni1064. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. Embo J. 1991;10:1135–47. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Begbie J, McGonnell I. Significance of the cranial neural crest. Dev Dyn. 2004;229:5–13. doi: 10.1002/dvdy.10442. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–60. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hollander G, Gill J, Zuklys S, Iwanami N, Liu C, Takahama Y. Cellular and molecular events during early thymus development. Immunol Rev. 2006;209:28–46. doi: 10.1111/j.0105-2896.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- Itoi M, Tsukamoto N, Yoshida H, Amagai T. Mesenchymal cells are required for functional development of thymic epithelial cells. Int Immunol. 2007;19:953–64. doi: 10.1093/intimm/dxm060. [DOI] [PubMed] [Google Scholar]
- Jenkinson WE, Jenkinson EJ, Anderson G. Differential requirement for mesenchyme in the proliferation and maturation of thymic epithelial progenitors. J Exp Med. 2003;198:325–32. doi: 10.1084/jem.20022135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson WE, Rossi SW, Parnell SM, Jenkinson EJ, Anderson G. PDGFRalpha-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood. 2007;109:954–60. doi: 10.1182/blood-2006-05-023143. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–16. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- LeLievre CS, Le Douarin N. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morph. 1975;34:125–154. [PubMed] [Google Scholar]
- Leonard L, Horton C, Maden M, Pizzey JA. Anteriorization of CRABP-I expression by retinoic acid in the developing mouse central nervous system and its relationship to teratogenesis. Dev Biol. 1995;168:514–28. doi: 10.1006/dbio.1995.1099. [DOI] [PubMed] [Google Scholar]
- Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, Lipp M, Kondo S, Manley N, Takahama Y. Coordination between CCR7- and CCR9-mediated chemokine signals in pre-vascular fetal thymus colonization. Blood. 2006 doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- Machado AF, Martin LJ, Collins MD. Pax3 and the splotch mutations: structure, function, and relationship to teratogenesis, including gene-chemical interactions. Curr Pharm Des. 2001;7:751–85. doi: 10.2174/1381612013397726. [DOI] [PubMed] [Google Scholar]
- Manley NR. Thymus organogenesis and molecular mechanisms of thymic epithelial cell differentiation. Semin Immunol. 2000;12:421–428. doi: 10.1006/smim.2000.0263. [DOI] [PubMed] [Google Scholar]
- Moore-Scott BA, Manley NR. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol. 2005;278:323–35. doi: 10.1016/j.ydbio.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Nagle DL, Martin-DeLeon P, Hough RB, Bucan M. Structural analysis of chromosomal rearrangements associated with the developmental mutations Ph, W19H, and Rw on mouse chromosome 5. Proc Natl Acad Sci U S A. 1994;91:7237–41. doi: 10.1073/pnas.91.15.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–9. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002;16:676–80. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Gordon J, Mahbub F, Blackburn CC, Manley NR. Bmp4 and Noggin expression during early thymus and parathyroid organogenesis. Gene Expr Patterns. 2006;6:794–9. doi: 10.1016/j.modgep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Revest JM, Suniara RK, Kerr K, Owen JJ, Dickson C. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol. 2001;167:1954–1961. doi: 10.4049/jimmunol.167.4.1954. [DOI] [PubMed] [Google Scholar]
- Schaeffer B. Deuterostome Monophyly and Phylogeny. Evolutionary Biology. 1987;21:179–235. [Google Scholar]
- Shinohara T, Honjo T. Studies in vitro on the mechanism of the epithelial/mesenchymal interaction in the early fetal thymus. Eur J Immunol. 1997;27:522–529. doi: 10.1002/eji.1830270225. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Lee RA, Wu H. BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood. 2003;102:3947–53. doi: 10.1182/blood-2003-05-1657. [DOI] [PubMed] [Google Scholar]
- Veitch E, Begbie J, Schilling TF, Smith MM, Graham A. Pharyngeal arch patterning in the absence of neural crest. Curr Biol. 1999;9:1481–4. doi: 10.1016/s0960-9822(00)80118-9. [DOI] [PubMed] [Google Scholar]
- Zamisch M, Moore-Scott B, Su DM, Lucas PJ, Manley N, Richie ER. Ontogeny and regulation of IL-7-expressing thymic epithelial cells. J Immunol. 2005;174:60–7. doi: 10.4049/jimmunol.174.1.60. [DOI] [PubMed] [Google Scholar]