Abstract
Disruption of cerebral white matter has been proposed as an explanation for age-related cognitive declines. However, the role of specific regions in specific cognitive declines remains unclear. We used diffusion tensor imaging to examine the associations between regional microstructural integrity of the white matter and performance on age-sensitive cognitive tasks in a sample of healthy adults (N = 52, age 19–81 years). White matter integrity was assessed by fractional anisotropy (FA) and apparent diffusion coefficient (ADC) in multiple regions of interest (genu and splenium of corpus callosum, internal capsule limbs, prefrontal, temporal, superior/posterior parietal, occipital white matter) and related to processing speed, working memory, inhibition, task switching, and episodic memory. We found that age and regional white matter integrity differentially influenced cognitive performance. Age-related degradation in anterior brain areas was associated with decreased processing speed and poorer working memory, whereas reduced inhibition and greater task switching costs were linked to decline in posterior areas. Poorer episodic memory was associated with age-related differences in central white matter regions. The observed multiple dissociations among specific age-sensitive cognitive skills and their putative neuroanatomical substrates support the view that age-related cognitive declines are unlikely to stem from a single cause.
Keywords: aging, brain, MRI, diffusion tensor imaging, cognition, white matter, disconnection
1. Introduction
Decades of research in cognitive aging have established that cognitive performance declines with age, especially in processing speed, episodic memory, and executive functions (see Park & Schwarz, 2000; Verhaeghen et al., 1993 for reviews). However, the neural underpinnings of cognitive aging in healthy adults remain unclear and investigations of neuroanatomical correlates of cognitive performance have thus far yielded mixed results (see Raz, 2000; Raz & Rodrigue, 2006 for reviews).
Until recently, the in vivo search for brain correlates of cognitive aging focused on age-related expansion of cerebrospinal fluid cavities, local and global changes in parenchymal volume, and increase in the burden of white-matter hyperintensities (WMH), all relatively coarse morphometric indices of macrostructural integrity (reviewed in Raz & Kennedy, 2008). Development of Diffusion Tensor Imaging (DTI) (Pierpaoli & Basser, 1996) provided the means of investigating microstructural properties of the brain, as DTI is a magnetic resonance imaging (MRI) technique designed to measure the differences in constraints on water diffusion in various types of tissue. In short, compared to the random water diffusion in CSF, gray and especially white matter exhibit constrained diffusion due to tissues endowed with various molecular barriers, and thus DTI reports changes in the degree of constrains that occur in normal development and disease processes.
DTI data yield multiple indices of white matter integrity, among which the most frequently used are fractional anisotropy (FA) and apparent diffusion coefficient (ADC). FA, a dimensionless scalar (range 0–1), is a ratio of the likelihood of diffusion along the principal axis of the diffusion tensor to diffusion in orthogonal directions. It reflects the integrity of biological boundaries that constrain diffusion of the water molecules. ADC measures the rate of diffusivity and is sensitive to alterations in water diffusion in each canonical direction within a given volume unit (voxel). Further, in studies of aging, FA and ADC often show an inverse, but complementary relation as decreases in FA are accompanied by ADC increases in the same region (Sullivan & Pfefferbaum, 2006).
To date, multiple studies have examined age-related differences in diffusion properties of the white matter, both as a global brain compartment and within regions whose size and specificity varied considerably across studies. The preponderance of the extant literature shows widespread age-related declines in FA and elevations in diffusivity (Ardekani et al., 2007; Benedetti et al., 2006; Charlton et al., 2006; Charlton et al., 2007; Chen, et al., 2001; Chun, et al., 2000; Grieve et al., 2007; Lehmbeck et al., 2006; Nusbaum, et al., 2001; Rovaris et al., 2003; Shenkin et al., 2003). However, many studies reported regional variability in the age-related FA declines and ADC increases, thus suggesting that white matter vulnerability to aging may be selective and differential (Bhagat & Beaulieu, 2004; Chun et al., 2000; Deary et al., 2006; Furutani et al., 2005; Huang et al., 2006; Nusbaum et al., 2001; Pagani et al., 2008; Pfefferbaum et al., 2000; 2003; Salat et al., 2005; Shenken et al., 2003; 2005; Sullivan et al., 2001; Hugenschmidt et al., 2008; Zhang et al., 2005).
Among the studies that focused on regional diffusion properties of the cerebral white matter, most agree upon a decrease in FA and increase in ADC in the centrum semiovale, corona radiata, pericallosal frontal and parietal areas, and periventricular regions. Less consistent patterns of results have been found in other white matter regions, such as the limbs of the internal capsule and the splenium (Abe et al., 2002; Bhagat & Beaulieu, 2004; Furutani et al., 2005; Helenius et al., 2002; Huang et al., 2006; Hugenschmidt et al., 2008; Madden, et al, 2004; Nusbaum et al., 2001; Salat et al., 2005; Zhang et al., 2005). According to some studies, the effect of age is greater in the anterior than posterior regions of the brain (Ardekani et al., 2007; Head et al., 2004; Hugenschmidt et al., 2008; Kochunov et al., 2007; Madden et al., 2007; O’Sullivan et al., 2001; Pfefferbaum, et al., 2000; 2005; Salat et al., 2005; Sullivan et al., 2001; Sullivan & Pfefferbaum, 2006). However, age-related declines in the splenium of the corpus callosum have been reported as well (Abe et al., 2002; Bhagat & Beaulieu, 2004; Chepuri et al., 2002; Head et al., 2004; Ota et al., 2006; Pfefferbaum et al., 2000; 2005; Pfefferbaum & Sullivan, 2003; Salat et al., 2005; Sullivan, et al., 2006). Fiber-tracking analysis of DTI data showed that the most prominent age-related deterioration of the white matter is observed in association fibers (Stadlbauer et al., 2008), which connect the regions that are the last to complete myelination in the course of development (Flechsig, 1901). In fact, Raz (2000) reported a strong correspondence between the developmental order of myelination and the magnitude of volume decline with age. Regression of regional age effect measures and annualized shrinkage rates on Flechsig’s myelination precedence ranks revealed that those regions late to myelinate were those that demonstrated the strongest age-related shrinkage (inferior temporal, dorsolateral prefrontal, inferior parietal, orbitofrontal cortices). Myelination order accounts for 36% of the variance in age differences in regional cortical volumes, and 38% of the variance in regional cortical shrinkage in a five-year period (Raz & Kennedy, 2008).
Whereas the cumulative evidence of age-related differences in the cerebral white matter microstructure is compelling, little is known about their impact on cognitive abilities across the age span. The extant literature is quite sparse and it is not easy to discern a pattern in the discrepant findings, especially when regional indices of white matter integrity and specific cognitive processes vary. Increased ADC and reduced FA averaged over a large region such as centrum semiovale have been linked to poorer performance on a wide variety of cognitive tasks, whereas only sporadic correlations were observed between cognitive performance and DTI-derived indices of the parieto-occipital white matter (Shenkin et al. 2003; Deary et al., 2006). Sporadic associations have been observed between DTI-derived indices and performance on some executive tasks but not others and not in the same regions (O’Sullivan et al., 2001; Shenkin et al., 2005; Charlton et al., 2006; Grieve et al., 2007; Sullivan et al., 2006). Various measures of speed of processing, which has been shown to correlate with anisotropy in association tracts in young adults (Tuch et al. 2005), also correlate with better age-related white matter integrity in some samples (Bucur et al., 2007; Deary et al., 2006; Madden et al., 2004; Sullivan et al., 2006), but not others (Charlton et al., 2007; Grieve et al., 2007; Madden et al., 2007). In studies that combined measures of DTI with fMRI, fractional anisotropy accounted for some variance in age-differences in cognitive performance, including visual search (Madden et al., 2004), task switching (Gold et al., 2008), and memory (Bucur et al., 2007; Persson et al., 2006), but these associations are not consistently found.
There are many potential reasons for the discrepancies found in the extant literature. The studies vary widely in methods of measurement that include whole brain voxel-wise analyses, measures in very large regions of interest (ROI), or localized and specific, sometimes fiber-tract specific, regional measures. The selection of cognitive measures also varies widely across studies, and oftentimes is limited to single global indices amalgamating multiple cognitive domains. Differences in sample composition among studies can also contribute to variability in findings. Some samples contained only women, others were limited to men, some mixed left and right-handers, and frequently small extreme groups of young and old adults were compared. Finally, the definition of a healthy older adult also varies among the studies, with some of them not reporting screening for age-related conditions that are known to affect both brain anatomy and cognitive performance.
The purpose of this study was to examine the associations among multiple indices of age-sensitive cognitive skills (processing speed, episodic memory, working memory, inhibition, task switching) and specifically selected regional measures of white matter integrity. We aimed to overcome at least some of the outlined limitations by obtaining highly reliable manual measures of fractional anisotropy and apparent diffusivity in multiple specific regions of the cerebral white matter in a well-characterized healthy lifespan sample. Our goal was to test hypotheses about specificity of function-structure relationships by conducting multiple dissociation comparisons. We hypothesized that performance on the tests of executive functions would be positively associated with FA and negatively associated with ADC in white matter regions that underlie tertiary association areas (genu of corpus callosum, deep prefrontal white matter, superior/posterior parietal) but not primary sensory or secondary association cortices (splenium of corpus callosum, occipital white matter). We hypothesized that memory scores would be associated mainly with indices of white matter integrity in the temporal lobe association areas. Finally, we expected the indices of processing speed to correlate with FA and ADC measures in all white matter regions.
2. Method
2.1 Participants
Participants were paid healthy volunteers from the metropolitan Detroit area. They were recruited through media advertisements and flyers, and underwent health history screening via a health questionnaire as well as telephone and personal interviews. Persons who reported a history of cardiovascular (except controlled and uncomplicated essential hypertension), neurological or psychiatric conditions, head trauma with loss of consciousness for more than 5 min, thyroid problems, diabetes mellitus, and/or drug and alcohol problems were excluded from participation in the study. Persons with untreated hypertension established by blood pressure measurements on at least three occasions were also excluded from participation, as were any participants taking anti-seizure medication, anxiolytics, or antidepressants. Participants were screened for near, far, and color vision (Optec 2000 vision tester; Stereo Optical Co., Inc., Chicago, IL) and speech-range hearing (model MA27; Maico Diagnostics, Eden Prairie, MN) acuity. Participants were also screened for dementia and depression using Mini-Mental Status Examination (MMSE; Folstein, et al., 1975) with a cut-off of 26, and Geriatric Depression Questionnaire (CES-D; Radloff, 1977) with a cut-off of 15. All participants were consistent right-handers (> 75% on the Edinburgh Handedness Questionnaire; Oldfield, 1971), attained a minimum of high school education (mean 15.65 ± 2.36 years) and were native English speakers. All participants provided written informed consent and were debriefed in accord with university human investigations committee guidelines.
The total sample with complete DTI data consisted of 77 participants, and the age effects on white matter integrity in that sample are described in detail elsewhere (Kennedy & Raz, submitted). The current study reports on a subsample of 52 participants who had complete cognitive data. Sample demographic characteristics, by sex and for the total sample, are reported in Table 1.
Table 1.
Sample demographic information by sex and total sample: mean ± SD
| Group | N | Age | Edu | MMSE | CES-D | Systolic BP | Diastolic BP |
|---|---|---|---|---|---|---|---|
| Women | 33 | 48.97±19.69 | 15.39±2.28 | 28.76±1.03 | 3.94±3.29 | 122.42±14.40 | 73.51±8.39 |
| Men | 19 | 62.32±13.86 | 16.11±2.49 | 28.32±1.38 | 3.47±3.50 | 127.84±11.70 | 75.55±8.36 |
|
| |||||||
| Total | 52 | 53.85±18.79 | 15.65±2.36 | 28.60±1.18 | 3.77±3.34 | 124.40±13.61 | 74.25±8.36 |
Note. N = sample size; edu = years of education; MMSE = mini-mental status exam; CES-D = center for epidemiological study depression scale; BP = blood pressure mm Hg.
The 52 participants ranged in age from 19–81 years (mean 53.85 ± 18.79) and included 33 women and 19 men (a marginally significant difference in proportions: χ2 (1) = 3.77, p = .05). MMSE scores ranged from 26–30, with a mean of 28.60. None of the participants showed signs of depression on the CES-D, with scores ranging from 0–12 and a mean score of 3.77 ± 3.34. The women were younger than the men: 48.97 ± 19.69 vs. 62.32 ± 13.86 years, t(50) = 2.60, p = .01. The men and women did not differ significantly, however, in education level (t(50) = 1.05, ns), MMSE scores (t < 1, ns), or CES-D scores (t < 1, ns). Further, there were no sex differences in corrected near (mean 24/20) or far (mean 26/20) visual acuity, nor in systolic or diastolic blood pressure.
2.2 Materials
2.2.1 Cognitive Tests
2.2.2 Processing Speed
A verbal, Letter Comparison, and a nonverbal, Pattern Comparison (Salthouse & Meinz, 1995) test of processing speed were administered. These tests require participants to make rapid “same-different” judgments on either letter strings or line patterns containing three to nine line segments. Participants were given 30 sec per page (for two pages) to complete the items as quickly and as accurately as possible. The total number correct for both pages combined minus the number of incorrect items is the index of performance. Estimated reliability for letter comparison test is .77 and .87 for pattern comparison test (Salthouse & Meinz, 1995).
2.2.3 Executive Functioning
Working Memory
Size Judgment Span (SJS)
SJS (Cherry & Park, 1993) is a measure of nonverbal working memory in which the participant must hold a list of object names in mind while reorganizing the items in order of ascending physical size (e.g., tooth, violin, ship). Each set of trials increases by one item. Index of performance is total items correct. The estimated reliability of this task is .79 (Cherry & Park, 1993).
Listening Span (LS)
LS (Salthouse et al., 1990) is a measure of verbal working memory where the participant is required to simultaneously answer questions and hold the final word of each question in mind until the end of each trial and then report them in order. Each set of trials increases by one item. Index of performance is the absolute span, which is calculated by summing the number of correct items across blocks of trials on which the participant answered all of the items correctly, because it produces a wider range of scores than the other indices and is not particularly prone to capitalization on chance (Engle et al., 1992). The estimated reliability of this measure is .90 (Salthouse et al., 1990).
Two separate computerized n-back tests were administered as additional indices to assess the maintenance and storage of working memory (Hultsch et al., 1990; modeled after Dobbs & Rule, 1989). In one task, verbal n-back, the stimuli were single-digit numerals, whereas in the other, nonverbal n-back, nonverbal nonsense shapes were presented. The serial position of the item that the participants were asked to report varied from 1 to 3 (i.e. the task varied between 1-back, 2-back, and 3-back, presented in counterbalanced blocks). Indices of performance were response time (speed) and number of errors (accuracy). The estimated reliability for this task is .91 for the verbal and .88 for the nonverbal tests (Salthouse et al., 1996).
Inhibition
A paper version of the color Stroop task (Salthouse & Meinz, 1995; Stroop, 1935) was given as a measure of inhibition where the indices of performance are time to name the color of the ink in color compatible, color incompatible and neutral conditions over two trials. The interference score for this task is the response time in the incompatible condition minus the response time in the neutral condition. The estimated split-half reliability for this test is .72 (Salthouse & Meinz, 1995).
Cognitive Flexibility
A computerized version of the Wisconsin Card Sorting Test (WCST; Heaton et al., 1993) was administered to assess planning, inhibition, and perseveration of rules that guide the sorting of different cards by either color, form, or number. Number of perseverative errors was the index of performance. The test-retest reliability for perseverative errors is .83.
Task Switching
The ability to alternate between stimuli in one task and between two tasks was assessed by a computerized task-switching test (Salthouse et al., 1998). In these tests, the participants were asked to learn to associate certain keyboard keys with certain properties of digits. When these properties changed the participant had to respond with a different key. Participants were asked to switch between stimuli (left vs. right; single switch) or between tasks indicating whether a digit was more or less than 5, and whether it was odd or even (dual switch). Time (RT) and accuracy (number of errors) were the indices of performance on the test, and the difference between switch and non-switch trials served to assess the costs incurred by switching. The estimated split-half reliability for the right-left switch costs score is .71 and the estimated reliability for the more-odd switch costs score is .89 (Salthouse et al., 1998).
2.2.4 Episodic Memory
The California Verbal Learning Test (CVLT; Delis et al., 1987) served as a measure of free recall. In the CVLT, participants heard a list of 16 grocery items (List A) that fell into four categories and was presented 5 times. After each presentation trial the participant was instructed to recall as many of the items as possible in any order, but was not told that the items can be semantically organized. After the fifth trial a new grocery list was presented once (List B), followed by an immediately recall trial. Following that, the participant was asked to freely recall the first list that was presented five times (List A). Although CVLT produces several indices of performance, for this study only free recall of List B (generalized learning) and total List A were used.
Free recall was additionally assessed by Logical Memory subtest of the Wechsler Memory Scale – R (Wechsler, 1987). Participants heard a prose passage and were immediately asked to recall as much as possible. The procedure was repeated with a second short story. After a 30 minute delay, the participant is asked to recall (without cue) as much as possible from each story. The indices of performance on this test were the total number of story elements recalled from both stories. Split-half reliability is estimated to be .74 for immediate, and .75 for delayed presentation (Wechsler, 1987).
Memory for Names subtest (#1) of the Woodcock-Johnson Psychoeducational Battery – Revised (Woodcock & Johnson, 1989) was a measure of associative recognition memory. Participants viewed novel stimuli, pictures of “space creatures,” and were told the creatures’ names, novel nonsense stimuli as well. After the learning phase, participants were presented with images of the space creatures and asked to point to the creature named by the examiner. After a 30 minute delay, the space creatures were presented again, several to a page, and the participant was again required to point to the creature named by the experimenter. Indices of performance for immediate and delayed recognition were the total number of correctly reported name-picture associations. Estimated reliability for both the immediate and delayed presentation is .91 (Woodcock & Mather, 1989).
2.3 Blood Pressure Measures
Upon arrival to each cognitive session (and before testing began) each participant had his or her blood pressure measured using an analog mercury sphygmomanometer (Model 12–525; Country Technology, Gays Mills, WI) with a standard brachial cuff (Omron Professional) to obtain systolic and diastolic blood pressure. Participants were seated in a comfortable chair in a climate-controlled office. Blood pressure was sampled twice, once from each arm and averaged for each session. Current and prior hypertensive status and medication information was collected from a comprehensive health questionnaire completed before entrance to the study. Hypertension was operationally defined as systolic blood pressure greater than 140 mm Hg and diastolic pressure greater than 90 mm Hg (Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, 1997). Only participants who had controlled hypertension or were normotensive were allowed into the study.
2.4 MRI protocol
MR images were acquired on a 1.5 T Magnetom Sonata scanner (Siemens Medical Systems, Erlangen, Germany). Diffusion tensor imaging (DTI) data were acquired with a single shot echo-planar imaging (EPI) sequence acquired in the axial plane with 6 directions, b = 0 and 1000 mm2/sec, 10 averages, TE = 97 ms, TR = 5400 ms, acquisition matrix = 192 × 192, FOV = 345 mm, voxel size = 1.8 × 1.8 × 3 mm3. Duration of acquisition was 6.25 minutes.
2.4.1 DTI Processing
The DTI data were processed with the DTI module of Analyze software (BIR, Mayo Clinic, Rochester, MN, USA, http://www.analyzedirect.com/products/MRIDTI.asp). Each DTI scan was first binned into the baseline (b = 0) and six gradient encoded volumes (each containing 33 slices) using the Dicom Tool module and these seven separated scans were imported into the DTI module. After the diffusion gradient orientation information was entered for each volume, the data were thresholded to reduce extracerebral noise and the tensor was computed. FA and ADC maps were computed for each participant.
2.4.2. Region of Interest (ROI) Measurement
Images for manual tracing of Regions of Interest (ROIs) were displayed on a 21″ monitor and on a 21″ LCD digitizing tablet (Wacom Cintiq model 21UX; Wacom Inc., Vancouver, WA) and magnified ×2. Each ROI was traced manually with a stylus on the T2-weighted (b = 0) baseline image for each participant in native space and supplemented with simultaneous side-by-side views from the FA and FA color map images in the same native coordinate space to maximize neuroanatomic validity. The saved ROI was applied to the FA and ADC maps and mean and standard deviation FA and ADC were obtained within each ROI, for each participant separately on 3 slices bilaterally (except corpus callosum ROIs), and then averaged across the three slices.
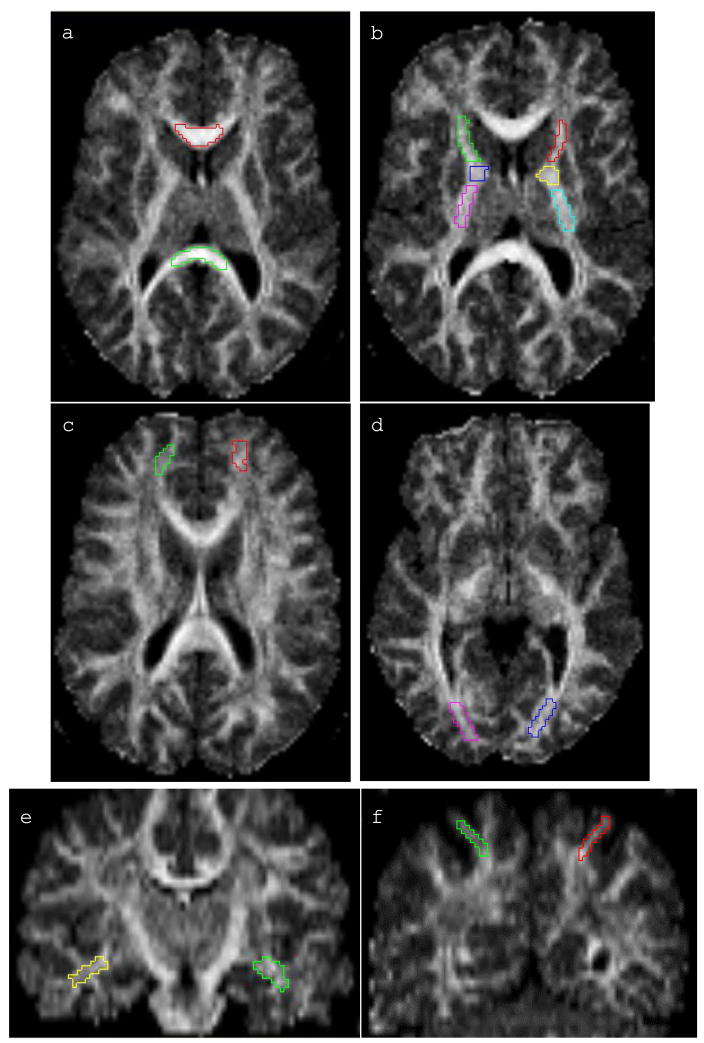
The ROIs were chosen based on the relevant literature with particular attention to long association tracts and the rules for placement were determined by neuroanatomical knowledge of the expert tracer with the aid of neuroanatomical atlases (primarily Duvernoy, 1999 and Mori et al., 2005). The ROIs were specifically drawn well-within the inner portions of the white matter regions to minimize the potential of partial voluming effects that can occur in the border voxels at the interface of gray/white and CSF/white boundaries. Regions measured for this study were the corpus callosum (genu and splenium), the internal capsule (anterior, genu, and posterior limbs), and subcortical association white matter samples from prefrontal, parietal, temporal and occipital regions, and were demarcated as described below. Examples of ROIs traced for DTI analyses are presented in Figure 1.
Figure 1. Illustration of ROIs used for DTI analyses.
White matter regions of interest ROIs manually drawn for obtaining FA and ADC values. (a) genu and splenium of corpus callosum, (b) bilateral anterior, genu, and posterior limbs of internal capsule, (c) bilateral superior prefrontal, (d) bilateral optic radiations, (e) bilateral temporal stem, (f) bilateral superior posterior parietal. Illustrations are displayed on the FA image.
Corpus callosum (CC)
The FA and ADC of the genu and the splenium were measured with a small ROI drawn on the genu and the splenium, medially-to-laterally on the axial plane, with reference to the sagittal and coronal planes using ortho review function of Analyze ROI module. Care was taken to exclude the major CSF regions (the ventricles), both visually and by monitoring the standard deviations. The genu and the splenium of the corpus callosum were measured on the same three slices in which both were optimally visible, which resulted in an area of the splenium that covered the entire tissue medially to laterally, and was more posterior and more inferior in location.
Internal Capsule (IC)
The FA and ADC of the anterior (ICa), genu (ICg), and posterior (ICp) limbs of the internal capsule were measured separately on the axial plane (bilaterally). Care was taken to remain within the white matter boundary and not include any pixels from the basal ganglia, thalamus or ventricles. The internal capsule was measured on the three slices in which all three limbs were optimally visible.
Prefrontal white matter
The FA and ADC of the prefrontal white matter were measured on the axial plane on three slices. The ROI was drawn on the white matter of the superior frontal gyrus with care taken to exclude any surrounding gray matter. The coronal and sagittal planes were used as references to guide placement. The selected slices were the three slices just ventral to the slice where the body of the corpus callosum became continuous. The white matter sampled was from prefrontal cortex, considerably anterior to the genu of the CC (to ensure prefrontal rather than motor or premotor regions were sampled), more medial than lateral (to capture pericallosal connectivity), and more superior than inferior to capture dorsal rather than orbital/ventral prefrontal cortex. This area reflects prefrontal association areas connecting with parietal association areas ostensibly via the superior fronto-occipital (SFO) and superior longitudinal (SLF) fasciculi.
Parietal white matter
The FA and ADC of the parietal lobe white matter was measured on the coronal plane from three consecutive slices beginning five slices posterior to the splenium. The superior posterior parietal area was located as superior to the posterior/splenial area of the corpus callosum and cingulate gyrus. The ROI was drawn in the widest portion of the posterior parietal white matter excluding somatosensory cortices, angular gyrus, and precuneus white matter to specifically gauge parietal association areas ostensibly connected with prefrontal association areas via the superior fronto-occipital (SFO) and superior longitudinal (SLF) fasciculi.
Temporal white matter
The FA and ADC of the temporal lobe white matter was measured on the coronal plane from three consecutive slices beginning after the anterior commissure and ending before the splenium of the corpus callosum, on the slices where the corticospinal tract descends into the brainstem and appears continuous. The temporal stem white matter was measured ventral to the superior temporal gyrus and the ROI was drawn down to the widest portion of the white matter before branching into the parahippocampal, fusiform, and inferior temporal branches to maximize temporal association areas (i.e., middle and inferior temporal), ostensibly within the uncinate fasciculus (UNC) which connects to (orbital) prefrontal cortex anteriorly and to the occipital lobes posteriorly via the inferior fronto-occipital (IFO) and inferior longitudinal (ILF) fasciculi.
Occipital white matter
The FA and ADC of the occipital lobe white matter was measured from the axial plane on three consecutive slices approximately between the first slice where the splenium is maximally formed to one slice inferior to the end of the putamen. The ROI was drawn on the white matter adjacent to the occipital horns of the lateral ventricles and below the splenium (the posterior forceps). A narrow rectangle was drawn to avoid inclusion of CSF or adjacent gray matter. This white matter ROI ostensibly includes fibers from the superior longitudinal (SLF) and inferior fronto-occipital (IFO) fasciculi and connects occipital with frontal and parietal and with temporal regions, respectively.
2.4.2.1 Reliability of ROI measurements
In order to ensure reliability of measurement, test-retest reliability was determined by one operator (KMK) by tracing eight participants’ images (for each ROI) on two separate occasions, two weeks apart. Reliability of the ROI measures (mean FA) in this study was assessed by an intraclass correlation formula, ICC(3) (Shrout & Fleiss, 1979). This is a conservative index of reliability that takes into account the order of the measured items and their values in each set, and is also sensitive to the differences between the two sets of measures. All region reliabilities (ICC 3) equaled or exceeded .90 and are listed in Table 2.
Table 2.
Reliability (intraclass correlation coefficients) for manual tracing of each brain region of interest (ROI)
| Region | ICC (3) |
|---|---|
| CC: genu | .90 |
| CC: splenium | .99 |
| ICa: anterior limb | .98 |
| ICg: genu | .90 |
| ICp: posterior limb | .96 |
| Prefrontal: superior prefrontal WM | .98 |
| Parietal: superior/posterior parietal WM | .97 |
| Temporal: temporal stem WM | .96 |
| Occipital: posterior forceps | .96 |
Note. CC – corpus callosum; IC – internal capsule; WM – white matter.
3. Results
The data for each theoretically defined cognitive domain were analyzed in a separate general linear model. In each model, age (centered at the sample mean) served as a continuous independent variable, sex was a categorical independent variable, and regional FA or ADC as continuous predictors. Because multiple measures of each cognitive construct were obtained, these test scores were used to form a multivariate dependent variable vector. To maximize statistical power, all nonsignificant interactions among the independent variables were removed from the models and reduced models were fitted to the data. Interactions with the within-subjects terms were adjusted by Huynh-Feldt epsilon to correct for violation of sphericity assumption of the repeated measures analysis. To ensure that the participants with controlled hypertension did not differ from the normotensives we examined each cognitive measure for differences related to hypertension diagnosis. That analysis revealed that no test scores differed between treated hypertensives and normotensives (all p’s > .05), and therefore, to conserve degrees of freedom, hypertension status was not entered into the models.
3.1 Age effects on white matter microstructure
Although the primary aim of the current study was to investigate effects of age-related differences in white matter microstructure on cognition, we first provide a summary of findings regarding the age effects on FA and ADC in this sample. A more detailed report on age-related effects on the larger sample drawn from the same population can be found elsewhere (Kennedy & Raz, submitted).
Because all ROIs except corpus callosum were measured bilaterally, left and right hemisphere measures were first compared. The only laterality differences that reached nominal significance were parietal FA (.43 on the left vs .46 on the right, t(51) = 2.51, p = .02), temporal ADC (.79 mm2/sec on the left vs .82 mm2/sec on the right, t(51) = 6.71, p < .001), and occipital ADC (.84 mm2/sec on the left vs .82 mm2/sec on the right, t(51) = 2.28, p = .03). However, given the number of regions involved, the magnitude of the differences, and the lack of a priori hypotheses regarding laterality effects, the lateral differences can be considered trivial and ROIs were collapsed across hemisphere. Descriptive statistics for regional FA and ADC (averaged across hemisphere) as well as their correlations with age are displayed in Table 3.
Table 3.
Descriptive statistics and correlations for diffusion tensor parameters by region of interest
| ROI | FA | ADC | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | CV | Mean ± SD | CV | rage,FA | rage,ADC | rFA,ADC | |
| CC genu | .77 ± .05 | .06 | .78 ± .006 | .05 | −.45*** | .36** | −.70*** |
| CC splenium | .81 ± .03 | .04 | .74 ± .005 | .05 | −.51*** | .43*** | −.46*** |
| IC ant limb | .50 ± .05 | .09 | .75 ± .004 | .04 | .17 | .24 | −.16 |
| IC genu | .56 ± .06 | .10 | .75 ± .004 | .04 | .12 | .30* | −.41** |
| IC post limb | .61 ± .03 | .05 | .74 ± .003 | .02 | −.51*** | .17 | −.30* |
| Frontal | .48 ± .04 | .09 | .77 ± .005 | .05 | −.60*** | .29* | −.50*** |
| Parietal | .45 ± .05 | .10 | .76 ± .005 | .04 | −.49*** | .34* | −.37** |
| Temporal | .46 ± .04 | .08 | .81 ± .003 | .04 | −.27* | .25 | −.38** |
| Occipital | .56 ± .06 | .11 | .83 ± .007 | .06 | −.59*** | .36** | −.28* |
Note: Mean ± SD standard deviation; CV – coefficient of variation = SD/mean. ADC mm2/sec was multiplied by 1000.
p < .05,
p < .01,
p < .001.
Fractional Anisotropy and Age
The analysis of general linear models with ROIs as a multivariate vector revealed a significant main effect of Age on FA, F(1, 48) = 18.41, p < .001. There was neither a main effect of Sex, nor a Sex × Age interaction (Fs < 1). We observed a significant within-subjects main effect of ROI, F(8, 384) = 432.30, p < .001 and a significant ROI × Age interaction, F(8, 384) = 4.95, p < .001, indicating that FA and age-related differences therein varied significantly across the examined regions. To decompose that interaction, we examined simple effects through univariate regressions. A summary of the regional associations with age is presented in Table 4. There were significant negative effects of Age on FA (older adults displayed lower FA) in all regions except the anterior and middle limbs of the internal capsule. However, the magnitude of age-FA associations varied across ROIs: the strongest age effects were observed in the prefrontal and occipital white matter, where age accounted for 35% and 36% of the variance, respectively (see Table 4).
Table 4.
Follow-up univariate analyses for the effects of age on regional FA
| ROI | Slope FA units/year | t | p | R2 |
|---|---|---|---|---|
| CC genu | −.001 | −3.56 | .0008 | .20 |
| CC splenium | −.0009 | −4.18 | .0001 | .26 |
| IC anterior limb | .0004 | 1.19 | ns | .03 |
| IC genu | .0004 | < 1 | ns | .02 |
| IC posterior limb | −.0009 | −4.16 | .0001 | .26 |
| Frontal | −.001 | −5.26 | <.00001 | .36 |
| Parietal | −.001 | −4.01 | .0002 | .24 |
| Temporal | −.0006 | −2.01 | .049 | .08 |
| Occipital | −.002 | −5.21 | <.00001 | .35 |
Note. CC = corpus callosum; IC = internal capsule. Slope is estimate of units of FA loss per year of calendar age. R2 = proportion of variance explained in regional FA by age.
Diffusivity and Age
The effects of age on regional diffusivity were examined in the same manner as above. There was a significant main effect of Age on ADC, F(1, 48) = 13.75, p = .001, and there was no significant main effect of Sex (F < 1). However, we observed a significant Sex × Age interaction, F(1, 48) = 4.14, p <.05. Examination of separate regressions for men and women showed that the effect of age on ADC was stronger in men (slope b = .0012 ± .0004 mm2/sec/year, t = 2.88, p = .01) than in women (slope b = .0003 ± .00016 mm2/sec/year, t = 2.10, p = .04), with no overlap between 95% confidence limits of the slopes. When an outlier (the oldest participant, an 81 year old man, with the highest average ADC) was temporarily removed from the analyses, the Sex × Age interaction became nonsignificant, suggesting that this case had undue influence. There was a significant main effect of ROI, F(8, 384) = 53.01, p < .001, and no significant interactions, indicating that ADC varied significantly across the ROIs regardless of age or sex. There was, however, a trend toward a ROI × Age interaction (p = .09).
3.2 Associations between white matter microstructural integrity and cognition
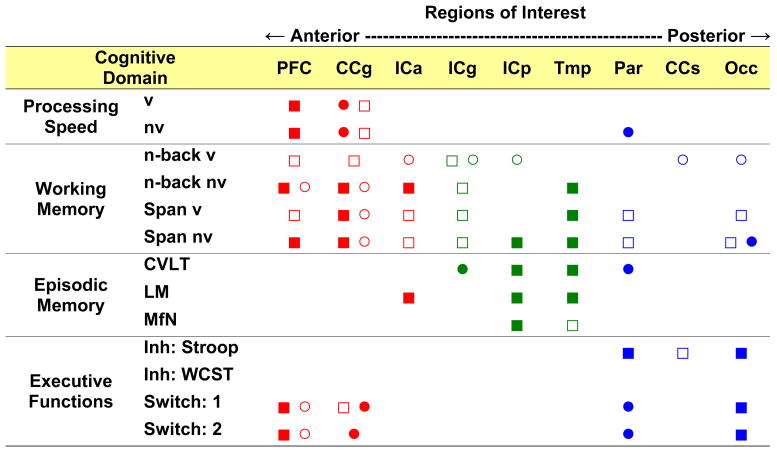
Due to the timed nature of many of the cognitive tests, skewed distributions required correction by natural logarithmic transformations (log[raw score +1]). In the less difficult conditions of the working memory task (e.g., for 1-back and 2-back) and in task switching, clear ceiling effects were observed in the distribution of errors. Therefore, for these conditions, only RT data were analyzed. Again, Sex was entered into each model and if non-significant, was removed to save power. All non-significant trends with FA and ADC are not mentioned in the text, but are noted in Figure 2 (denoted as open squares and circles).
Figure 2. Summary of structure-function dissociation findings: Regional white matter involvement and cognitive performance.
Figure 2 illustrates the regional pattern of white matter involvement in the various cognitive domains. ■ = FA; ● = ADC; □ and ○ = non-significant trend for FA and ADC, respectively (p < .06–.09). Speed of processing is related to the DTI-derived measures of primarily anterior (and fronto-parietal) regions; working memory is related to the measures in wide-spread, although largely anterior white matter; episodic memory is primarily associated with the measures of temporal and internal capsule white matter; inhibition is associated with posterior white matter measures and task switching depends on integrity of anterior and posterior regions.
PFC = superior frontal gyrus; CCg = corpus callosum genu; ICa = internal capsule anterior limb; ICg = internal capsule genu; ICp = internal capsule posterior limb; Tmp = temporal stem; Par = superior posterior parietal; Occ = occipital white matter; v = verbal; nv = nonverbal; Inh: Stroop = inhibition: Stroop interference; Inh: WCST = inhibition: Wisconsin Card Sort Test perseveration; Switch:1 = single switching cost; Switch: 2 = dual switching cost; CVLT = list free recall; LM = story free recall; MfN = name-picture association recognition.
3.2.1 Processing Speed (PS) and FA
Letter Comparison and Pattern Comparison tests formed the dependent variable, and Task was a two-level repeated measure factor (verbal, nonverbal). Total FA (i.e., the average of all ROIs) was a continuous predictor. Older adults performed substantially slower on the Processing Speed tasks, F(1,48) = 11.74, p < .001. A significant Age × Task interaction (F[1,48] = 6.68, p < .02) indicated, however, that age-related slowing on the nonverbal task (b = −0.21, 95%CI: −.28 to −.13, t = 5.28, p < .001) was greater than on the verbal task (b = −0.10, 95%CI: −.15 to −.05, t = 4.07, p < .001). There were no sex differences (F < 1). Total FA main effect was not significant (p = .12). Examination of zero-order correlations (after Dunn-Sidak correction for multiple comparisons) suggested significant relations between processing speed and regional FA (prefrontal, CCgenu, parietal) and these regions were included in linear models that contained all the predictors and interactions mentioned above.
Prefrontal white matter FA was a significant independent predictor of processing speed: higher prefrontal anisotropy was associated with faster processing across both tested domains, F(1, 47) = 5.42, p < .02. However, that effect was modified by a significant Task × Age × Frontal FA interaction, F(1, 47) = 4.54, p < .04. To decompose that interaction, separate post-hoc regressions were conducted on letter and pattern comparison tasks, with age and prefrontal FA as predictors. Age had a somewhat stronger effect on nonverbal (b = −.15, t = 3.13, p < .01) than verbal (b = −.07, t = 2.14, p < .05) processing speed, although the 95%CI overlapped. The association between prefrontal anisotropy and both tasks, however, was equipotent: r = .28, t = 2.07, p < .05 for nonverbal, and r = .30, t = 2.05, p < .05 for verbal processing speed. Examination of scatterplots (not shown) clarifies that middle-aged individuals with higher prefrontal FA evidenced the best performance, whereas the younger and older adults performed slower at a lower threshold of prefrontal FA.
Processing Speed and ADC
There was a significant main effect of CCgenu diffusivity, F(1,48) = 4.07, p < .05, indicating that higher diffusivity in the anterior CC was associated with slower processing speed. A significant Parietal ADC × Type interaction [F(1,48) = 7.88, p < .01] indicated that higher rate of diffusivity in the parietal ROI was associated with slower nonverbal (t = 2.83, p < .05), but not verbal (t < 1) processing speed.
3.2.2 Executive Functions: Working Memory (WM)
n-back verbal task and FA/ADC
The three levels of the verbal n-back test were entered into the GLM as a repeated measures multivariate vector (WM Load: 1-, 2-, or 3-back response time). There was a significant main effect of WM Load, [F(2, 96) = 4.10, p < .03] reflecting longer response times as the memory load increased. However, there was also a significant Age × WM Load interaction [F(2, 96) = 6.41, p = .005], indicating that the magnitude of WM Load effect on RT depended on age. Age was associated with prolongation of response under 1-back (r = .41, t = 3.16, p < .003) and 2-back (r = .30, t = 2.23, p < .04) but not 3-back (r = −.11, t < 1, ns) conditions. Variability of response times differed only slightly across the levels of task difficulty: CV = .03, .04, .05 for 1-, 2-, and 3-back, respectively. There were no sex differences (F < 1). No regional FA or ADC measures reached significance, but see Figure 2 for marginal effects.
n-back nonverbal task and FA/ADC
As in the previous model, response time was the dependent variable and the three levels of the nonverbal n-back test were entered as a repeated measure (WM Load). There was a significant main effect of age on nonverbal working memory response times, F(1, 48) = 10.50, p < .001. There were no effects of sex (Fs < 1) or Total FA (F = 1.50, p = .23). There was a significant main effect of WM Load, F(2, 96) = 2968, p < .001 with response times increasing with load.
In the models with regional FAs as predictors, there was a significant main effect of Frontal FA, F(1,47) = 4.23, p < .05, indicating that greater anisotropy in the prefrontal ROI was associated with better working memory. There was also a significant main effect of CC genu FA (F(1,47) = 4.67, p < .04) indicating higher anisotropy in this region was associated with better working memory. Additionally, there was a main effect of ICa FA (p = .02), but it was qualified by an Age × ICa FA interaction (F(1, 47) = 7.31, p < .01), which revealed that the ICa FA had a stronger association with nonverbal working memory in the young than in the middle and the old adults. A significant main effect of Temporal FA [F(1,480 = 8.24, p < .001] was qualified by a WM Load × Age × Temporal FA interaction, F(2,96) = 5.07, p = .02. Decomposition of this interaction indicated that for younger adults (but not middle or older) higher Temporal anisotropy was associated with faster response time on n-back 1 (r = −.60, p < .04), but not on trials 2 (r = −.19, ns) or 3 (r = −.28, ns). See Figure 2 for trends for ADC.
WM Span Tasks and FA
The two oral span tests (SJS and LSPAN) were analyzed together in a multivariate GLM (WM Type: verbal, nonverbal). There was a significant effect of Age on WM Span, F(1,49) = 5.27, p < .03, with older adults performing more poorly. There was also a main effect of WM Type, F(1,49) = 862.11, p < .001. There was a significant main effect of Total FA, F(1, 48) = 4.62, p < .04).
There was a significant main effect of CC genu FA on WM Span, F(1,48) = 4.17, p < .05. There was a significant Age × ICp FA interaction (p < .02), but also a significant Type × Age × ICp FA (F(1, 47) = 7.77, p < .008), which indicated that posterior limb of the IC was more strongly related to SJS in the young (r = .72, t = 3.30, p < .008) than the middle (r = .20, t < 1, ns) and older adults (r = −.29, t < 1, ns). ICp FA was not significantly related to LSPAN in any age group although the pattern of correlations was similar (r = .15, −.14, .07). There was a significant effect of Frontal FA, F(1,48) = 4.18, p < .05, and a significant WM Type × Frontal FA interaction, F(1,48) = 4.50, p < .04, which reflected the stronger association with SJS (nonverbal task; r = .41, t = 3.14, p < .003) than with LSPAN (verbal task; r = .24, t = 1.77, p < .08). A significant Age × Temporal FA interaction [F(1,47) = 5.09, p < .03] was qualified by a significant WM Type × Age × Temporal FA interaction, F(1,47) = 6.34, p < .02. Decomposition of this interaction indicated that only for the young was Temporal FA associated with the WM span tests (SJS r = .83, p < .001; LSPAN r = .79, p < .01). There was no significant relation between Temporal ROI anisotropy and WM span scores in the middle or older aged adults.
WM Span Tasks and ADC
A significant Type × Age × Occipital ADC interaction, F(1, 47) = 4.00, p = .05, indicated that Occipital ADC displayed the highest association with SJS in the young (r = −.59, t = 2.30, p < .05) compared to middle (r = .06, ns) and older (r = −.07, ns) adults. No significant association was found between Occipital ADC and LSPAN in any age group.
3.2.3. Inhibition/Cognitive Flexibility
Two tasks were used to measure cognitive flexibility or ability to inhibit a prepotent response or strategy in favor of a new one that is more adaptive to changes in task demands. The indices were log-transformed perseverative errors on WCST and log-transformed Stroop interference score (color incompatible minus color neutral). Cronbach’s alpha for these two measures was α = .52. These two scores were entered into a general linear model as a multivariate dependent variable (Task). There was a significant main effect of Age on the inhibition tasks, F(1,48) = 4.64, p < .04. There was a main effect of Task, F(1,48) = 24.91, p < .001.
FA/ADC
A significant Test × Parietal FA interaction (F(1, 47) = 4.14, p < .05) indicated that parietal FA was associated with Stroop task (r = −.36, t = 2.77, p < .008), but not with WCST (r = −.04, t < 1, ns). There was a significant Test × Occipital ADC (F(1, 47) = 4.22, p < .05) interaction, which indicated that occipital white matter diffusivity plays a greater role in Stroop task (r = .24) than WCST (r = −.09).
3.2.4. Task Switching
Log-transformed index of task-switching served as the dependent variable. It was computed as the log transformed switch costs (averaged switch trials RT minus averaged non-switch trials RT). Two switching tasks, the single switch (right/left task) and the dual switch (more/odd task) tasks were repeated measures. For three subjects a right/left switch cost could not be computed due to poor performance/outliers in response time, limiting these analyses to N = 49. Note that higher switch cost reflects poorer switching ability.
FA
There was no significant main effect of Age, suggesting that older adults did not have significantly higher switch costs than younger adults, F(1,46) = 1.91, p = .17. The main effect of Task was significant: F(1,46) = 164.60, p < .001. A significant main effect of Total FA on task switching (F(1, 45) = 4.89, p < .04) indicated that task switching costs depended on global white matter integrity.
A significant Test × Age × Frontal FA interaction (F(1, 44) = 4.09, p < .05) indicated that for the younger adults, Frontal FA was equally associated with both tasks (r = −.36 and −.34) whereas for the middle aged adults, the association with Frontal was higher for More/Odd (r = −.50 vs .03), but for the older adults Frontal FA was more strongly associated with the Right/Left task (r = −.37 vs .13). A significant main effect of Occipital FA was found for task switching, F(1,45) = 4.36, p < .05.
ADC
A significant Test × CCgenu ADC interaction was found, F(1, 45) = 5.63, p = .02, where CC genu ADC was more strongly associated with Right/Left (r = −.12) than More/Odd test (r < .01). A significant Test × Parietal ADC interaction was found, F(1,45) = 6.56, p = .01, where Parietal ADC was more strongly associated with Right/Left switch test (r = .21) than More/Odd switch test (r = −.07).
3.3. Episodic Memory
List recall and FA
For CVLT List B (generalized learning) there was no effect of Age (F < 1), but a main effect of Sex, with women performing better than men, F(1,48) = 7.62, p < .01. There was also a main effect of Total FA, F(1, 48) = 4.05, p < .05, indicating that global anisotropy was significantly related to learning. There were no significant interactions (Fs < 1). Age, Sex, and Total FA accounted for 31% of the variance in List B recall.
In regional analyses, we found a main effect of Temporal FA, where higher anisotropy in the temporal region was associated with better recall, F(1,48) = 4.39, p < .05. Age, Sex, and Temporal FA accounted for 30% of the variance in recall in this task.
ADC
There were main effects of ICg ADC, F(1,48) = 5.03, p < .03, and Parietal ADC, F(1,48) = 6.34, p =.01, where greater diffusivity in these regions was associated with poorer recall.
Similarly, for List A total recall, there was no effect of Age F(1, 48) = 1.38, ns, and women performed better than men, F(1, 48) = 5.02, p < .03. This model explained 26% of the variance in recall. In regional analyses, there was a marginal Sex × ICp FA interaction, F(1,47) = 3.95, p = .05, where this region was only significant for the women in predicting recall. There were no effects of regional FA on List A total recall.
Story Recall and FA/ADC
For Logical Memory immediate recall, there was no effect of age (F < 1), nor Sex (F < 1) nor Total FA, F(1, 48) = 2.21, p = .14. Regional analyses indicated there was a significant main effect of Temporal FA on immediate story recall, F(1,48) = 5.42, p < .03, where greater anisotropy in this region was associated with better recall for all adults. There was also a significant main effect of ICa FA, F(1,48) = 14.62, p < .001, and a significant main effect of ICp FA, F(1,48) = 4.32, p < .05, indicating that higher anisotropy in these portions of the IC are associated with better immediate memory recall. There were no effects of ADC.
For Logical Memory delayed recall, there were again no effects of Age (F = 1.19) or Sex (F < 1), or Total FA, F(1, 48) = 1.66, ns. In regional analyses, there was a significant main effect of ICa FA, indicating that greater anisotropy in the anterior limb of the IC is associated with better delayed recall, F(1,48) = 9.07, p < .01. There was also a significant Age × Temporal FA interaction, F(1,48) = 5.56, p < .03, which indicated that increased anisotropy in the temporal ROI was only associated with better delayed recall in the young (r = .66, p < .02) and middle (r = .58, p < .05) aged adults, but not in the older adults (r = −.09, ns).
Memory for Picture-Name Associations and FA/ADC
For immediate associative recognition on memory for names test, there was a significant effect of Age, F(1,48) = 6.84, p < .001, where older adults remembered fewer associate names than younger adults. This model explained 33% of the variance in immediate recognition. Regional analyses revealed a marginal effect of ICp FA, F(1,47) = 3.82, p = .056. There were no effects of ADC.
For delayed recognition on memory for names test, there was also a significant effect of Age, F(1,48) = 10.90, p = .001. There was no effect of Total FA (F < 1). Age, Sex, and Total FA explained 41% of variance in delayed associative recognition. Regional analyses found a nonsignificant trend for Temporal FA (p = .08) and no effects of ADC.
3.3. Summary
Figure 2 summarizes the observed associations between regional white matter microstructure and cognitive performance. Aging of the regional white matter, as indicated by FA and ADC, was linked to performance on all cognitive tests except WCST. The specific influential regions varied across tasks, and this differential involvement reflected both widespread connectivity from anterior to posterior regions, as well as more local circuitry. Specifically, age-related degradation in more anterior brain areas was associated with decreased processing speed and working memory, whereas decline in more posterior areas was associated with reduced inhibition and task switching. Poorer episodic memory was associated with age effects in more central white matter.
4. Discussion
The main finding in the present study is that in healthy adults, age-sensitive cognitive skills are differentially associated with regional white matter integrity. Whereas, in accord with previous reports, diffusion-based indices of white matter microstructure evidence broad age-related declines, their relation to cognitive performance (summarized in Figure 2) is quite complex.
Not surprisingly, one region-one task dissociations are rarely if ever observed. The ROIs in which white matter microstructure was measured in this study involve multiple neural circuits, and the tasks employed in this investigation rely on multiple cognitive processes. Nonetheless, the pattern of results can be interpreted in light of previous findings and theoretical expectations outlined in the introduction. Reduced integrity of the anterior cerebral regions that included prefrontal white matter and the callosal fibers that connect the anterior segments of the two hemispheres, along with parietal association white matter was associated with reduced speed of processing, a hallmark of cognitive aging (Birren, 1963; Salthouse, 1985). Processing speed was unrelated to integrity in the middle part of the brain. In contrast, all indices of episodic memory exhibited significant associations with the white matter anisotropy in the temporal and medial temporal regions. Executive functions that require at least some memory support, i.e., working memory span or capacity, also showed significant links to the middle cerebral white matter integrity. Notably, other executive functions that require little memory support but are focused on response selection and inhibition as well as management of conflicting task demands showed no temporal lobe involvement and evidenced significant correlations with the integrity of posterior brain regions.
Several more subtle dissociations were also observed. For instance, verbal processing speed depended on local anterior white matter integrity, whereas nonverbal processing speed depended on a fronto-parietal network (i.e., the superior longitudinal fasciculus or superior fronto-occipital fasciculus). Contrary to our hypothesis, speed-of-processing indices were not associated with global white matter deterioration but appeared to be linked to regional differences in microstructure that were similar to the putative substrates of executive functions (i.e., frontal-parietal association areas). Such specific association argues against the view of speed of processing as the index of generalized aging.
The literature on white matter substrates of age differences in processing speed is inconsistent. In older adults, reaction time measured on simple tasks is related to global indices of white matter integrity in some (Deary et al., 2006) but not other (Grieve et al., 2007; Charlton et al 2006; 2007) samples. On the other hand, links between regional anisotropy and visual detection speed (Madden et al., 2004) as well as the magnitude of speed influence on episodic retrieval (Bucur et al., 2007) have been reported. In the past, we have found a significant association between prefrontal (but not posterior) gray matter volume and speed of processing on a series of mental imagery tasks (Raz et al., 1999). Taken together, the results of these studies indicate that quick cognitive processing depends on the integrity of at least the prefrontal and anterior callosum fibers, and, as suggested by our present findings, also the fronto-parietal fibers, i.e. the networks that are considered the neural substrate of executive controlled processes. In classic theories of disconnection syndromes, a breakdown of transmission in the white matter connective fiber bundles disrupts or slows the mode of cognition that relies on the joined regions (see Catani & ffytche, 2005). Thus, our results support the idea that this slowing stems from degraded neural transmission along the axons of the aging brain.
We found age-related reductions in a widely distributed network of white matter connections to be associated with declines in working memory. These networks ranged from anterior (prefrontal, anterior callosum and internal capsule) to posterior (posterior internal capsule, temporal and occipital white matter) reflecting the importance of intact white matter across the brain for working memory performance. Such distributed support of working memory with age has been demonstrated previously with coarser regional measures (Charlton et al., 2006, 2007; Deary et al., 2006; Raz et al., 2007). The current finding bolsters the understanding that working memory, as assessed by the span and n-back tasks, is a multidimensional construct that reflects the state of a wide range of neural substrates encompassing most of the deep cerebral white matter.
The current finding of the association between reduced anisotropy of the posterior white matter (parietal, splenium, and occipital) and higher Stroop interference cost likely reflects the influence of distributed white matter systems such as the superior fronto-occipital fasciculus, superior longitudinal fasciculus, and inferior fronto-occipital fasciculus, which also have projections anterior to the prefrontal cortex. Age-related reduction in fiber integrity in these areas can have far-reaching effects in the brain. These results are also consistent with the literature on top-down modulation of posterior brain regions during inhibition and attention tasks (e.g., Erickson et al., 2008; Hopfinger et al, 2000) and with the notion that age-related alterations in top-down modulation or its substrates can influence multiple cognitive domains (Gazzaley & D’Esposito, 2007). A fiber-tracking study by Sullivan and her colleagues (2006) found that while regional segments of corpus callosal fiber properties did not predict Stroop interferences scores per se, they did predict word reading after controlling for age, predominantly in the posterior segment fibers in a sample of 10 older adults. The current findings are in accord with their posterior callosum findings and expand the regions reported in the literature to include the parietal and occipital white matter.
We observed an association between the costs of task switching and the integrity of a fronto-parietal network of white matter regions. Specifically, age-related reductions in prefrontal, anterior corpus callosum, superior/posterior parietal, and occipital white matter integrity were linked to higher switch costs. These results are consistent with functional studies that found that prefrontal and parietal activations support a diverse set of switching tasks (Wager et al., 2004) and a recent DTI and fMRI study found that decreased integrity of fronto-parietal white matter mediated age-related increases in switch costs (Gold et al., 2008). Diffusivity in anterior regions (O’Sullivan et al., 2001) and anisotropy in widespread cortical white matter (Grieve et al., 2007) has been associated with performance on the Trail making test, which requires alternation between numeric and letter stimuli, although in other samples (e.g., Charlton et al., 2006) no such associations were found.
In accord with other studies, we found no direct associations between regional or global indices of white matter integrity and perseveration on WCST (Charlton et al., 2007; O’Sullivan et al., 2001). On the other hand, in some samples, age differences in WCST performance have been related to white matter pathology (white matter hyperintensity burden) and prefrontal cortex volume (Gunning-Dixon & Raz, 2000; 2003; Head et al., 2002; Raz et al., 1998). That discrepancy across studies may reflect difference in sensitivity of various imaging approaches. It is possible that the type of perseverative deficits that are observed on WCST become apparent only when the white matter undergoes gross changes expressed in volume loss. The diffusion-based indices of white matter integrity may reveal deficits at an earlier stage and thus they do not show the associations with WCST performance. Taken together with the functional imaging literature (Colette & van der Linden, 2002), the current results indicate that age-related differences in executive functions do not dependent upon intact prefrontal white matter alone. Rather, they reflect the integrity of a widely distributed network of white matter connections, especially fronto-parietal networks, but also cerebellar connections. Because multiple executive functions do not show a clear “frontal” pattern of structure-function associations, a frequently used reification of executive functions with prefrontal regions is unwarranted (Buckner, 2004; Glisky et al., 1995; Greenwood, 2000; Stuss & Alexander, 2002; Tisserand & Jolles, 2003; West, 1996). Because longitudinal studies show that posterior association regions, e.g., inferior parietal lobule, are just as sensitive to aging as prefrontal regions (Raz et al., 2005), and because executive functions indeed depend on both prefrontal and posterior parietal cortices, the term “associative regional aging” may be more appropriate than “frontal aging” hypothesis.
Episodic memory showed a simpler pattern of regional microstructural associations than other functions. In this sample, age-related reductions in white matter microstructure in the internal capsule, temporal stem, and superior/parietal regions were associated with reduced performance on several memory tasks. These regions represent fibers in the uncinate fasciculus, which project anteriorly to the frontal cortex and posteriorly via the IFO and ILF to the occipital cortex. Parietal association cortex is connected via the SLF and SFO to frontal, temporal, and occipital cortex. Hence, intact white matter across a distributed network may underlie better memory in older adults. These findings are not directly comparable to the other studies that fail to find diffusion-based correlates of mnemonic performance (Deary et al., 2006; Shenkin et al., 2005; Grieve et al., 2007). In those studies, coarse sections of the white matter were sampled and the regions that we found associated with memory were not measured. In two studies of episodic memory, FA in the genu of the corpus callosum of older adults correlated with recruitment of the fMRI signal in the frontal regions (Persson et al., 2006) and reduced FA in the genu and pericallosal frontal regions was associated with speed of episodic retrieval (Bucur et al., 2007).
A strength of the current study is the use of manual ROI placement in native space for each participant, which allowed us to maximize neuroanatomical validity and to avoid the misregistration, segmentation, and smoothing errors that can occur in automated or semiautomated techniques (Jones et al., 2005), as well as other limitations of voxel-based methods that may be especially apparent in aging brains (Kennedy et al., 2008; Sullivan & Pfefferbaum, 2006; Tisserand et al., 2002). However, manual methods limit the choice of regions to a priori hypothesized selections, a trade-off we chose. Another potential limitation of this study is that although we were not interested in sex effects per se, because the women in this sample were on average younger than the men, sex differences are difficult to interpret when they occurred, especially in a relatively small sample, albeit larger than the median N = 38 participants in the extant studies. Further, given the large number of statistical trends found in the analyses, in larger samples additional structure-function associations may be revealed. The present study relied on legacy data we acquired with an older implementation of the DTI approach, and application of new advances in DTI methods may reveal findings that were missed in this study. Finally, the cross-sectional design implemented in this study limits the assessment of change to estimation based on age differences. True age-related changes can only be gauged by a longitudinal study, currently underway in our laboratory.
4.1. Conclusions
Our findings and those of others suggest that degradation of callosal fibers, both anterior and posterior, may reduce the likelihood of successful bilateral compensation in older adults (Cabeza, 2002; Dennis & Cabeza, 2008; Reuter-Lorenz, 2002; Tulving et al., 1994). We have also shown that white matter degradation along the major association pathways may lead to a sufficient disconnection that hampers transmission between cortical regions that provide neural support for different aspects of cognition. If maintenance of optimal cognitive performance in older adults depends upon compensatory “rerouting” of the information flow, then such a process is significantly jeopardized by reduced anisotropy and increased diffusivity in these regions. In sum, the current study demonstrates that both aging and degradation of the regional associative white matter exert independent additive effects on multiple domains of cognition, in support of a disconnection hypothesis of cognitive aging. The observed distinct and dissociable relations between structural characteristics of circumscribed brain regions and specific cognitive functions indicate that cognitive aging is unlikely to stem from a single factor.
Acknowledgments
This study was supported in part by grants R37 AG-011230 and T32 HS-013819 and by a Dissertation Award from the American Psychological Association. The study was conducted in partial fulfillment of requirements for the doctoral degree. Portions of this paper were presented at Society for Neuroscience Annual Meeting in November 2007 and Cognitive Aging Conference in April 2008.
Footnotes
Disclosure Statement
The authors have no actual or potential conflicts of interest associated with this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiology of Aging. 2002;23:433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magnetic Resonance in Imaging. 2007;25:154–167. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. Journal of Magnetic Resonance Imaging. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–539. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Birren JE. Research on the psychologic aspects of aging. Geriatrics. 1963;18:393–403. [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2008;7:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiology of Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Chen ZG, Li TQ, Hindmarsh T. Diffusion tensor trace mapping in normal adult brain using single-shot EPI technique. A methodological study of the aging brain. Acta Radiologica. 2001;42:447–458. doi: 10.1080/028418501127347160. [DOI] [PubMed] [Google Scholar]
- Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA. American Journal of Neuroradiology. 2002;23:803–808. [PMC free article] [PubMed] [Google Scholar]
- Cherry K, Park D. Individual differences and contextual variables influence spatial memory in younger and older adults. Psychology and Aging. 1993;8:517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- Chun T, Filippi CG, Zimmerman RD, Ulug AM. Diffusion changes in the aging human brain. American Journal of Neuroradiology. 2000;21:1078–1083. [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neuroscience and Biobehavioral Reviews. 2002;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test: Manual. San Antonio: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- Dennis N, Cabeza RC. Neuroimaging of Healthy Cognitive Aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3. Psychology Press; NY: 2008. pp. 1–54. [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply. 2. NY: Springer; 1999. [Google Scholar]
- Engle RW, Cantor J, Carullo JJ. Individual differences in working memory and comprehension: A test of four hypotheses. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:972–992. doi: 10.1037//0278-7393.18.5.972. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Kim JS, Sutton BP, Colcombe SJ, Kramer AF. Top-down attentional control in spatially coincident stimuli enhances activity in both task-relevant and task-irrelevant regions of cortex. Behavioral Brain Research. 2008 2008 Aug 29; doi: 10.1016/j.bbr.2008.08.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P. Developmental myelogenetic localisation of the cerebral cortex in the human subject. The Lancet. 1901 October;19:1027–1029. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Furutani K, Harada M, Minato M, Morita N, Nishitani H. Regional changes of fractional anisotropy with normal aging using statistical parametric mapping SPM. Journal of Medical Investigation. 2005;52:186–190. doi: 10.2152/jmi.52.186. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann NY Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.005. May 19 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society. 2000;6:705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. American Journal of Neuroradiology. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: the role of regional cortical shrinkage and cognitive resources. Psychology and Aging. 2002;17:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Girton LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Helenius J, Soinne L, Perkio J, Salonen O, Kangasmaki A, Kaste M, Carano RA, Aronen HJ, Tatlisumak T. Diffusion-weighted MR imaging in normal human brains in various age groups. American Journal of Neuroradiology. 2002;23:194–199. [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huang L, Ling XY, Liu SR. Diffusion tensor imaging on white matter in normal adults and elderly patients with hypertension. Chinese Medical Journal. 2006;119:1304–1307. [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cerebral Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychology and Aging. 1990;5:356–368. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Joint National Committee on Prevention Detection, Evaluation and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Archives of Internal Medicine. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-Related Differences in Regional Brain Volumes: A Comparison of Optimized Voxel-Based Morphometry to Manual Volumetry. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Lehmbeck JT, Brassen S, Weber-Fahr W, Braus DF. Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. Neuroreport. 2006;17:467–470. doi: 10.1097/01.wnr.0000209012.24341.7f. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiology of Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Pestscher LM, van Zijl PCM. MRI atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW. Regional and global changes in cerebral diffusion with normal aging. American Journal of Neuroradiology. 2001;22:136–142. [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical ‘disconnection’ as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pagani E, Agosta F, Rocca MA, Caputo D, Filippi M. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage. 2008;41:657–667. doi: 10.1016/j.neuroimage.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Park DC, Schwarz N. Cognitive Aging: A Primer. Philadelphia, PA: Psychology Press; 2000. [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magnetic Resonance in Medicine. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition - II. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Kennedy KM. A systems approach to age-related change: Neuroanatomical changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. New York, NY: Oxford University Press; 2008. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates, and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head DP, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural MRI. Neuropsychology. 1998;12:95–106. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Briggs SD, Marks W, Acker JD. Age-related deficits in generation and manipulation of mental images: II. The role of dorsolateral prefrontal cortex. Psychology and Aging. 1999;14:436–444. doi: 10.1037/0882-7974.14.3.436. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends in Cognitive Science. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Iannucci G, Cercignani M, Sormani MP, De Stefano N, Gerevini S, Comi G, Filippi M. Age-related changes in conventional, magnetization transfer, and diffusion-tensor MR imaging findings: study with whole-brain tissue histogram analysis. Radiology. 2003;227:731–738. doi: 10.1148/radiol.2273020721. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed of behavior and its implications for cognition. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 2. NY: Van Nostrand Reinhold Co; 1985. pp. 400–426. [Google Scholar]
- Salthouse TA, Fristoe N, McGuthry KE, Hambrick DZ. Relation of task switching to speed, age, and fluid intelligence. Psychology and Aging. 1998;13:445–461. doi: 10.1037/0882-7974.13.3.445. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. Journal of Gerontology: Psychological Sciences. 1996;51B:317–330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ. Aging, inhibition, working memory, and speed. Journal of Gerontology: Psychological Sciences. 1995;50B:297–306. doi: 10.1093/geronb/50b.6.p297. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Mitchell D, Skovronek E, Babcock R. Effects of adult age and working memory on reasoning and spatial abilities. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;15:507–516. doi: 10.1037//0278-7393.15.3.507. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, MacGillivray TJ, Deary IJ, Starr JM, Wardlaw JM. Childhood and current cognitive function in healthy 80-year-olds: a DT-MRI study. Neuroreport. 2003;14:345–349. doi: 10.1097/00001756-200303030-00010. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, Macgillivray TJ, Deary IJ, Starr JM, Rivers CS, Wardlaw JM. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovascular Disease. 2005;20:310–318. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing raters reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Age-related degradation in the central nervous system: assessment with diffusion-tensor imaging and quantitative fiber tracking. Radiology. 2008;247:179–188. doi: 10.1148/radiol.2471070707. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–1128. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goosens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journal of Gerontology: Psychological Sciences. 1993;48:157–171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-R Technical Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Manual for Woodcock-Johnson Psychoeducational Battery-Revised. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]
- Woodcock RW, Mather N. WJ-R tests of achievement: Examiner’s manual. In: Woodcock RW, Johnson MB, editors. Woodcock-Johnson psycho-educational battery-revised. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]
- Zhang YT, Zhang CY, Zhang J, Li W. Age-related changes of normal adult brain structure: analysed with diffusion tensor imaging. Chinese Medical Journal. 2005;118:1059–1065. [PubMed] [Google Scholar]