Abstract
The cytokine network in inflammatory bowel disease (IBD) is a complex, dynamic system that plays an important role in regulating mucosal innate and adaptive immune responses. While several studies have been done to evaluate immunomodulatory profiles in murine IBD, they have been limited to a relatively small number of cytokines that do not take into account its dependency of the interplay of multiple factors, and therefore the diagnostic potential of their cytokine profiles have been inconclusive. Herein we demonstrate a novel approach of comprehensive serum multiplex cytokine profiling to describe the modulation of 16 Th1, Th2, Th17 cytokines and chemokines in both acute and chronic murine models of DSS and TNBS-induced colitis. Distinctive disease-specific cytokine profiles were identified with significant correlations to disease activity and duration of disease. TNBS colitis exhibits heightened Th1-Th17 response (increased IL-12 and IL-17) as the disease becomes chronic. In contrast, DSS colitis switches from a Th1-Th17-mediated acute inflammation (increased TNFα, IL6, IL-17 and KC) to a predominant Th2-mediated inflammatory response (increase in IL-4 and IL-10 and concomitant decrease in TNFα, IL6, IL-17 and KC) in the chronic state. Profiles of multiple cytokines seen systemically were also validated locally in colonic mucosa. Moreover, advanced multivariate analyses identified discriminatory cytokine profiles that can be sufficiently used to distinguish unaffected controls from diseases, and one disease type from another. IL-6 and IL-12 stratified gender-associated disease activity in chronic colitis. Our studies provide insight into disease immunopathogenesis and illustrate the significant potential of utilizing multiplex cytokine profiles and bioinformatics as diagnostic tools in IBD.
Keywords: Cytokines, Multiplex ELISA, DSS Colitis, TNBS Colitis, Inflammatory Bowel Disease
INTRODUCTION
Inflammatory bowel disease (IBD) comprises two major forms of chronic inflammatory disorders of the gastrointestinal tract, Crohn’s disease (CD) and ulcerative colitis (UC), which are characterized by distinct clinical, histopathological, endoscopic and radiological features (1). However, the etiology and pathogenesis of these disorders have not yet been fully defined (2). Recent studies have indicated that a complex interplay of genetic, microbial and environmental factors culminates in sustained aberrant intestinal innate immunity which may be a central early mechanism, subsequently perpetuated by dysregulated adaptive immune responses (1, 3–6).
Given the broad variety of etiological factors and complex genetic heterogeneity of human IBD, much of our current understanding of IBD pathogenesis have come from the studies of various animal models (7). Although there are differences, they resemble several important immunological and histopathological aspects of human IBD. Therefore, murine models have become essential tools to investigate pathophysiological mechanisms and immunological processes underlying chronic mucosal inflammation, including DSS (UC-like) and TNBS (CD-like) colitis in IBD (7). Chemically induced murine models of intestinal inflammation are the most commonly used and best described, primarily because (a) the onset and duration of inflammation is immediate and controllable and (b) there are no artificial genetic deletion or manipulations that are usually not found in human IBD (7, 8). Furthermore, chemical murine models have been shown to be similar to human IBD in multiple aspects, including cytokine dysregulation (9). Therefore, they are useful tools to study mediators of the innate, adaptive and regulatory arms of the intestinal immune response (7, 8). It is important to note however that, while the similarity of chemical models to human IBD has been extensively described, there is uncertainty with regards to their equivalence to human UC or CD (10–12).
A cascade of inflammatory mediators, primarily cytokines, are key players of the innate and adaptive immune responses. They modulate important biological cellular functions that trigger downstream signaling pathways, and mediate immune cell proliferation and differentiation (13). Under normal conditions, the intestinal mucosa functions within a delicate balance of inflammatory cells where cytokine synthesis and cytokine-induced signal transduction pathways are tightly regulated by intricate feedback mechanisms and regulatory T cells (13, 14). In IBD, the pathophysiological dysregulated immunologic responses to commensal bacterial antigens is reflected by a dramatic shift/imbalance in the cytokine production profile at different stages of the disease process (5). This dysregulated imbalance is thought to be represented by a T-helper 1 (Th1) and T-helper 2 (Th2) polarization pattern in IBD, where CD has been thought to be a prototypic Th1 disorder {mediated by tumor necrosis factor (TNF)α, Interleukin(IL)-12, and Interferon (IFN)α}, while UC being primarily associated with Th2-type responses (mediated by IL-5 and with no IFNα predominance) (15). However, the polarization concept has also been challenged by studies indicating Th2 profiles (up-regulation of IL-5) in CD and Th1 profiles (TNF-α) in UC (16–18). It is also important to point out the recent increasing interest in the Th17 pathway, mediated by IL-23 and IL- 17, both of which are essential for the manifestation of chronic intestinal inflammation (19). Given the importance of the complex network of cytokine profiles and its current applications as targetedbiological agents, comprehensive analysis of cytokines are imperative to identify diagnostic and prognostic profiles.
The cytokine network in IBD is a complex and dynamic system in which cellular and humoral cytokines, chemokines, and growth factors regulate initiation and perpetuation of inflammation (14). To date, our understanding of the role of cytokines in IBD (in both human and animal models) has been largely limited to analyses of individual or small sets of cytokine(s). However, it is clear that functional redundancy, synergy, pleiotropy, and concomitant regulation result in a dynamic cytokine network dependent upon the complex interplay of multiple factors, rather than the isolated effects of single signaling molecules or pathways (13).
Herein, we perform the first comprehensive analysis of broad-spectrum of cytokines from both acute and chronic murine models of UC-like and CD-like IBD. These studies define specific cytokine profiles in the initiation and perpetuation of disease pathogenesis, demonstrating the utility of multiplex cytokine profiling as a sensitive and quantitative measure of disease activity/severity. These results also indicate that cytokine profiling can be used as diagnostic and prognostic tools in IBD, particularly cytokine-based targeted biological therapies in preclinical studies, human clinical trials, and for therapeutic strategies in IBD.
MATERIALS & METHODS
Animals
Male and female C57BL/6 and BALB/c mice (6–8 wk, 18–22 g) (Jackson Laboratories, Bar Harbor, ME) were group-housed at Johns Hopkins Animal Facility under controlled temperature (25°C) and photoperiods (12:12-hour light-dark cycle), and allowed unrestricted access to standard diet and tap water. Mice were allowed to acclimate to these conditions for at least 7 days before inclusion in experiments. For each group of experiments, mice were matched by age, sex, and body weight. Care and experimentation of mice were performed in accordance with institutional guidelines under protocols approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University.
Induction of Colitis
DSS Model
C57BL/6 mice were used for DSS colitis. Acute colitis was induced by feeding mice with 3% (wt/vol) dextran sodium sulfate (DSS) (molecular weight 40 kDa; ICN Biochemicals, Aurora, OH) dissolved in drinking water, which was fed ad libitum for seven days (10). For chronic DSS-induced colitis, mice were treated with 4 cycles of DSS (3%) for 7 days/cycle and 10 days of normal drinking water in between each cycle (10). Control C57BL/6 mice received the same drinking water without DSS (n=8 mice in each group).
TNBS Model
Since C57BL/6 are known to be relatively resistant to TNBS (2,4,6-trinitrobenzene sulfonic acid) colitis, the TNBS susceptible BALB/c strain were used for TNBS studies (20, 21). In the TNBS model, food (but not water) was withdrawn overnight for 12 hours, prior to hapten, TNBS (Sigma-Aldrich, St. Louis, MO) administration. Mice were lightly anesthetized using a modified open-drop exposure to a mixture of 20% v/v isoflurane in propylene glycol. 0.1 ml of TNBS (2 mg in 50% ethanol) was administered via an 18 gauge, 2.5 mm dia. feeding tube (Fine Science Tools, Inc. Foster City, CA) that was advanced through the rectum into the colon, until the tip was 4 cm proximal to the anus (20). To ensure retention of the haptenating agent within the entire colon and cecum, mice were held by tail in a vertical position for 30 seconds after the i.r. administration. Chronic TNBS colitis was developed by weekly enemas of 0.1 ml TNBS (2 mg in 50% ethanol) given once a week for a total of 5 wks (21, 22). Control BALB/c mice were administered 50% ethanol using the same technique. (n=8 mice in each group).
Evaluation of Colitis
Animal were observed twice daily for weight, water/food consumption, morbidity, stool consistency, piloerection, and the presence of gross blood in feces and at the anus. Disease activity index (DAI) was calculated by assigning well-established and validated scores for parameters that are somewhat analogous to the clinical presentation of human IBD, as per Cooper et al (23). The following parameters were used for calculation: (a) weight loss (0 point=none, 1 point=1–5% weight loss, 2 points=5–10% weight loss, 3 points=10–15% weight loss and 4 points- more than 15% weight loss), (b) stool consistency/diarrhoea (0 points =normal, 2 points= loose stools, 4 points=watery diarrhea), (c) bleeding (0 points= no bleeding, 2, slight bleeding, 4 points, gross bleeding {by ColoScreen occult blood test (Helena Laboratories, Beaumont, TX)}. The DAI was calculated as the total of these scores: the sum of weight loss, diarrhea, and bleeding, resulting in the total DAI score ranging from 0 (unaffected) to 12 (severe colitis). At day 7 following induction with DSS and TNBS, animals were sacrificed by CO2 overdose, rapidly dissected, and the entire colon was quickly removed and gently cleared of feces. Small segments of the colon taken for histopathology and immunohistochemistry (IHC) were fixed in 4% normal buffered formalin using Shandon Excelsior automated tissue processor (ThermoFisher Scientific, Waltham, MA), embedded in paraffin, sectioned at 4-um thickness with a paraffin microtome (ThermoFisher) and mounted on to microscope slides (Fisher Scientific, Pittsburgh, PA). These paraffin embedded slides were also used in immunhistochemistry (see below). Sections were stained with haematoxylin and eosin (Richard Allen Scientific, Kalamazoo, MI), and histological scores were blindly determined as per Obermeier et al (24). The following parameters were used for calculation: (a) Epithelial damage (0 point=none, 1 point=minimal loss of goblet cells, 2 points=extensive loss of goblet cells, 3 points=minimal loss of crypts and extensive loss of goblet cells, and 4 points=extensive loss of crypts) (b) Infiltration (0 point=none, 1 point= infiltrate around crypt bases, 2 points= infiltrate in muscularis mucosa, 3 points=extensive infiltrate in muscularis mucosa with edema, and 4 points=infiltration of submucosa). Histological activity index (HAI) was calculated as the sum of the epithelium and infiltration score, resulting in the total HAI score ranging from 0 (unaffected) to 8 (severe colitis) (24).
Isolation of colonic mucosa and extraction of proteins for western blot analysis
At 4°C, the mucosa was scraped from the colon, and samples were snap frozen, and stored at −80°C for remaining experiments. Fozen tissue samples were homogenized in homogenization buffer (50mM Tris-HCl, pH 7.2) containing Na3VO4 and a protease-inhibitor cocktail (Sigma-Aldrich) using a OmniTH homogenizer (OmniInternational, Marietta, GA). Following sonication, the homogenate was centrifuged at 2000×g for 5 mins. The resulting supernatants were collected as total mucosal proteins and protein concentrations were measured using the Bio-Rad Protein Assay (Bio-Rad Inc., Hercules, CA).
Myeloperoxidase Activity
The activity of the enzyme myeloperoxidase (MPO), a marker of polymorphonuclear neutrophil primary granules, was determined in colonic mucosa according to a previously described technique (25). Colonic mucosal scrapings were suspended in potassium phosphate buffer (pH 6.0) with hexadecyl trimethylammonium bromide buffer and a Sigma cocktail of protease inhibitors. Samples were then homogenized on ice with an OmniTH homogenizer, and sonicated. Following sonication, suspensions were centrifuged at 11000g for 15 minutes at 4°C, and supernatants were diluted in potassium phosphate buffer (pH 6.0) containing 0.167 mg O-dianisidine dihydrochloride (Sigma- Aldrich) and 0.0005% (vol/vol) H2O2. Changes in absorbance at 450 nm were recorded with a spectrophotometer every 30 seconds over 3 minutes. MPO was expressed in units per milligram of tissue, where 1 unit corresponds to the activity required to degrade 1 μmol H2O2/min/mL at 24°C.
Serum collection and Biometric Multiplex Cytokine Profiling
Blood was collected by cardiac puncture in endotoxin-free silicone coated tubes without additive. The blood samples were allowed to clot at room temperature for 30 min before centrifugation (2200×g, 4°C, 10 min) and the serum was collected and stored at −80°C until analyzed. A multiplex sandwich immunoassay from Bioplex protein array system (Bio-Rad Inc, Hercules, CA) which contains fluoroscence-labeled microspheres conjugated with monoclonal antibodies specific for 16 target cytokines was used (26–28). Serum samples were thawed and run in duplicates. Antibody-coupled beads were incubated with the serum sample (antigen) after which they were incubated with biotinylated secondary (detection) antibody before finally being incubated with streptavidin-phycoerythrin. A broad sensitivity range of standards (Invitrogen, Carlsbad, CA) ranging from 1.95 – 32000 pg/ml were used to help enable the quantitation of a wide dynamic range of cytokine concentrations while still providing high sensitivity. Bound molecules were then read by the Bio-Plex array reader which uses Luminex fluorescent-bead-based technology (Luminex, Austin, TX) with a flow-based dual laser detector with real time digital signal processing to facilitate the analysis of up to 100 different families of color-coded polystyrene beads and allow multiple measurements of the sample ensuing in the effective quantitation of cytokines (26–28). Analytes measured include IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-17, IFN-γ, TNF-α, Granulocyte-macrophage colony-stimulating factor (GM-CSF), Interferon-inducible protein (IP)-10, Keratinocyte-derived chemokine (KC), Monocyte chemoattractant protein (MCP)-1, Monokine induced by IFNγ (MIG), and Macrophage inflammatory protein (MIP)-1α (Source of antibodies: Invitrogen, Carlsbad, CA).
Discriminant Function Analysis
Discriminant Function Analysis (DFA) is a multivariate class distinction algorithm that allows one to construct a mathematical model of discrimination built in a stepwise manner. This analysis was used here to identify the cytokines that best discriminated between experimental colitis and controls, and was modeled as previously described (27, 29). Specifically, at each step, all variables are reviewed to determine which will maximally discriminate among groups. These variables are then included in a discriminative function, denoted a root, which is an equation consisting of a linear combination of cytokine changes used for the prediction of group membership. Variables will continue to be included in the model, as long as the respective F values for those variables are larger than the standard threshold (established by the analytical package Statistica, StatSoft, Tulsa, Oklahoma). The discriminant potential of the final equation from the forward stepwise DFA can then be observed in a simple multidimensional plot of the values of the roots obtained for each group. This multivariate approach identifies groups of analytes, the changes of which in levels can delineate profiles and create diagnostic patterns.
Antibodies used for mucosal cytokine analysis
Antibodies used include: IL-6 monoclonal antibody (mAb) from Transduction Labs (Lexington, KY), IL-23p19 polyclonal antibody (pAb) from Biolegend (SanDiego, CA), IL-17 pAb from Santa Cruz (Santa Cruz, CA), IFNγ pAb, IL-12p40 from Biosource Intl (Camarillo, CA), and Actin pAb from Sigma-Aldrich (St. Louis, MO).
SDS-PAGE and Western Blot analysis
Protein extraction and western blots were performed as previously described with minor modifications (30, 31). Briefly, proteins were separated on 12.5 % SDS polyacrylamide gels using Bio-Rad system apparatus and blotted onto nitrocellulose membranes. Nitrocellulose membranes were incubated in PBST-milk (PBS buffer containing 0.1 % Tween-20 and 5 % milk), and followed by primary antibodies (1h) for IL-6, IL-23p19, IL-17, IFNγ, IL-12p40 and Actin (diluted with PBST-milk). Blots were then washed with PBST 3 times (10 minutes each), and subsequently incubated (1h) with corresponding fluorescently labeled secondary IRDye antibodies (Rockland Immunochemicals, Inc., Gilbertsville, PA) diluted in PBST-milk. The blots were washed 3 times (10 minutes) and the fluorescence intensity of detected protein bands was quantified by the Odyssey system (LI-COR, Lincoln, NE).
Immunocytochemistry
Immunocytochemical detection was determined in 4 μM paraffin-embedded mounted slides (see evaluation of colitis), heat fixed and endogenous peroxidase blocked with 0.3% H2O2 (Sigma-Aldrich) in methanol (Fisher Scientific Co., Pittsburgh, PA). Paraffin-embedded slides were microwaved in 10 mM sodium citrate buffer, pH 6 (Sigma), at power level setting 9 (Panasonic Inverter Model oven) for 5 min and then cooled for 30 min prior to analysis. All sections were washed in PBS and preblocked with 5% normal goat serum (NGS) in phosphate buffered saline (PBS) for 30 min at room temperature. Mouse intestinal sections (n=5–10/intestine/animal) were incubated overnight (4°C) with antibodies to IL-6, IL-23/p19, and IFNγ were diluted in 5% NGS in PBS. Intestinal sections were then washed twice in PBS for 10 minutes and incubated with Alexa Fluor 488 (Invitrogen) goat anti-mouse IgG (IL-23/p19, IFNg) or Alexa Fluor 568 (Invitrogen) goat anti-mouse IgG (IL-6) for 1 hour at room temperature. Intestinal sections were rinsed in PBS and incubated with filtered 1% Sudan Black (Sigma-Aldrich) in 70% methanol to quench autofluorescence, rinsed and treated with Hoescht 33342 (Invitrogen) to stain nuclei blue. Following application of GelMount mounting media (Sigma), slides were sealed with nail polish and stored in the dark at 4°C until examined using an Zeiss 510 Meta confocal microscope (Zeiss, Maple Grove, MN).
Statistical analysis of cytokine profiles
Analyte concentrations were quantified by fitting using a calibration or standard curve. A 5- parameter logistic regression analysis was performed to derive an equation that allowed concentrations of unknown samples to be predicted. Statistical differences in measured values were assessed by a Mann Whitney U test. P values less than 0.05 were considered statistically significant.
RESULTS
Establishment of Acute and Chronic Experimental Colitis
Both DSS and TNBS are well-established animal models of mucosal inflammation that have been used for over two decades in the study of IBD pathogenesis and preclinical studies (7). Since C57BL/6 are highly susceptible to DSS colitis but are relatively resistant to TNBS colitis, C57BL/6 was used for DSS studies and the TNBS susceptible BALB/c strain were used for TNBS studies (21). Oral DSS administration for 7 days and intrarectal TNBS administration for 5 days induced acute colitis in C57BL/6 and BALB/c mice respectively. Disease progression in both models was characterized by weight loss, significant appearance of diarrhea/loose feces, and with visible fecal blood, resulting in significant DAI elevation (Fig 1A). Morphological examination of both DSS and TNBS colitis revealed significant reduction in colon length and loose bloody stools. In addition, TNBS colitis portrayed visible thickening of colon wall, and more diffuse colonic inflammation. Oral DSS administration in 4 cycles and weekly intrarectal TNBS administration for 5 weeks induced chronic colitis in C57BL/6 and BALB/c mice respectively. Chronic colitis was characterized by a marked reduction in colon length, gross bleeding, and ulceration in DSS models, with additional features of grossly thickened walls, and occasional adhesions and fibrosis in TNBS models, as evidenced by their significant DAI elevation (Fig 1A).
Figure 1. Administration of DSS and TNBS established acute and chronic experimental colitis.
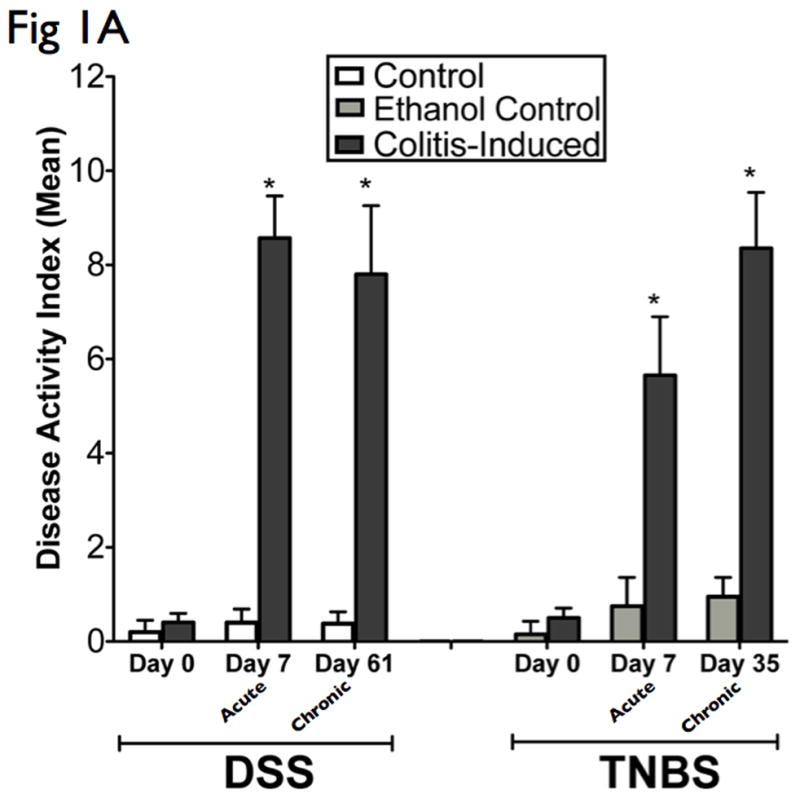

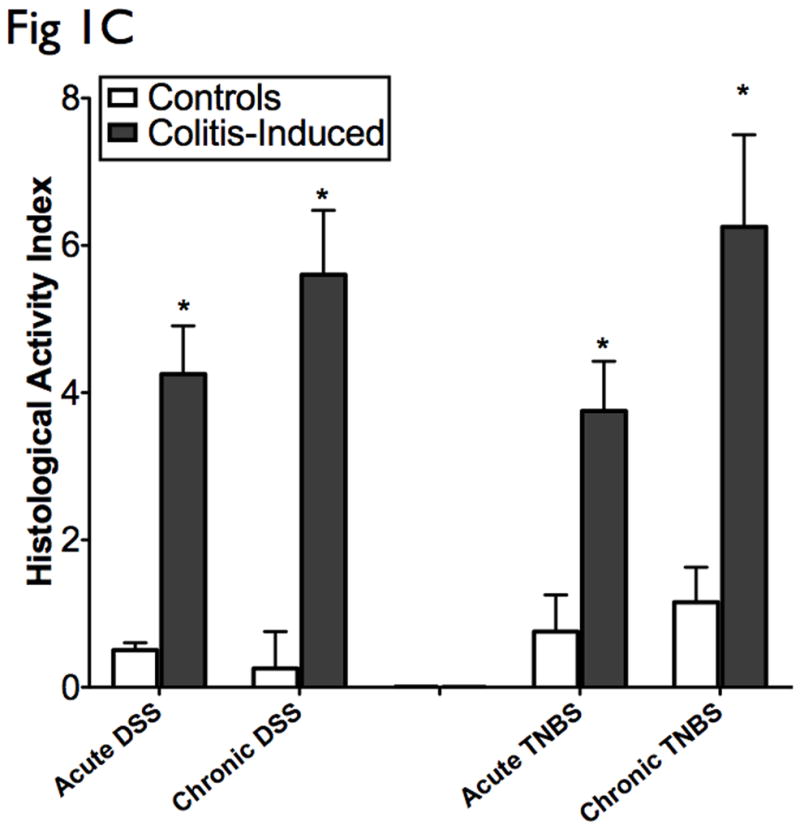

The results presented are representative of 8 mice from each group. (A) Clinical assessment of DSS and TNBS-induced colitis. DAI was scored from each mice for weight loss, stool consistency, and bleeding. (B) Histological analysis of acute and chronic DSS and TNBS colitis by H&E-stained colonic sections. (Structures: e, epithelial disruption; i, inflammatory infiltrate; m, lamina muscularis mucosae; s, submucosal edema; t, muscular thickening in lamina muscularis propria; magnification=20×). (C) For detailed histological analysis, colonic sections of each animal were scored in a blinded fashion as described in the Materials and Methods section. The resulting scores showed a significant increase in HAI after induction with colitis. (D) Myeloperoxidase activity in colonic mucosal scrapings were determined as described in the Materials and Methods section. Both acute and chronic DSS & TNBS had significant MPO activity compared with that in control animals. Mice induced with colitis demonstrated significant elevations in DAI, HAI and MPO, suggesting the effective establishment of acute and chronic experimental colitis.
Histologically, DSS acute colitis was characterized by focal crypt lesions, goblet cell loss and inflammatory cell infiltration at the areas of lesions, while in TNBS colitis was characterized by loss of architecture, and transmural immune cell infiltration extending through the mucosa and submucosa (Fig 1B). DSS chronic colitis was characterized by architectural derangements, epithelial necrosis, crypt abscesses, and diffuse lymphocytic infiltrate, while TNBS chronic colitis was characterized by generalized submucosal edema, mucosal necrosis and marked inflammatory infiltrate (Fig 1B). HAI was significantly elevated in the acute and chronic-induced DSS and TNBS murine models of colitis (Fig 1C). Neutrophil infiltration, as evidenced by increased MPO activity, was also significantly elevated in acute and chronic DSS and TNBS colitis. In general, higher MPO levels were observed in acute models when compared to chronic models, and with greater elevations in the DSS models when compared to the TNBS models (Fig 1D). As previously suggested (2, 8), the clinical and histological features of DSS colitis are “UC-like” (epithelial disruption, focal lesions and superficial inflammation), while those of TNBS colitis are “CD-like” (associated with transmural inflammation and edema).
Distinct cellular cytotoxic and chemotactic patterns in acute DSS & TNBS colitis
Cytokines are principal mediators of the innate and adaptive arms of the immune responses in mucosal inflammation. To analyze the influence of the cytokine patterns in acute colitis models, we performed multiplex serum cytokine profiling from acute murine models of DSS and TNBS colitis. The levels of 16 cytokines covering a broad-spectrum of immune and inflammatory mechanisms were measured in parallel following induction with colitis. Acute DSS colitis demonstrated a cytotoxic and chemotactic profile with significant elevated levels in IL-6, IL-17, TNF-α, and KC (p<0.05), when compared to controls (Fig 2A). Acute TNBS colitis also displayed a cytotoxic and chemotactic profile with significant elevated levels of IL-12, IL-17, IFN-γ, and MIP-1α (p<0.05), when compared to ethanol-treated controls (Fig 2A). IL-17 levels did not vary significantly different between DSS and TNBS acute colitis (Fig 2A). No significant differences were observed in levels of humoral cytokines, and other cellular cytokines and chemokines (Fig 2B). The cytokine profile of DSS and TNBS acute colitis is consistent with an acute inflammatory response in that it is characterized by a macrophage-derived cytokine profile, strong chemotactic pattern, and a polarized Th1-Th17 panel.
Figure 2. Distinct cellular cytotoxic and chemotactic patterns identified in acute experimental colitis.
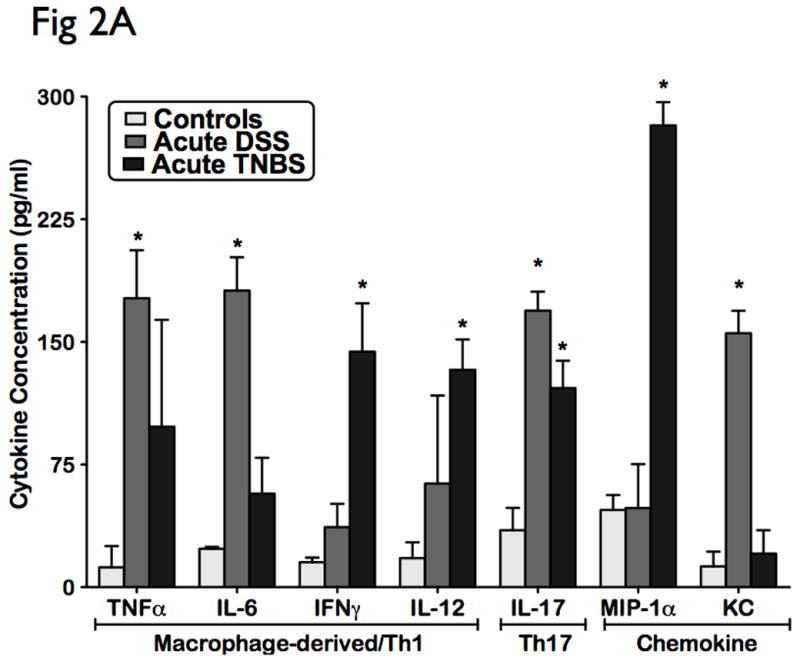

Levels of 16 cytokines were measured simultaneously using a biometric multiplex assay from serum of mice induced with acute DSS and TNBS-induced colitis. (A) Cytokine pattern in acute colitis is represented by a macrophage-derived (TNFa, IL-6, IL-12 IFNg), strong chemotactic pattern (MIP-1a, KC), and a polarized Th1-Th17 panel (IL-17, TNFa, IFNg). (B) No significant differences were observed in levels of humoral cytokines, and other cellular cytokines and chemokines. This suggest that the cytokine profile of DSS and TNBS acute colitis is consistent with an acute inflammatory response.
Distinct differential immune response in chronic DSS and TNBS colitis
In order to investigate the immunomodulatory profile during the regenerative, chronic phase of murine colitis, we analyzed the systemic cytokine patterns following chronic induction with DSS and TNBS. Interestingly, chronic DSS colitis displayed an enhanced pro-humoral cytokine bias with significant elevated levels of IL-6, IFN-γ, and IL-4, IL-10 (p<0.05), when compared to controls (Fig 3A). In contrast, chronic TNBS colitis portrayed a distinct cytotoxic and chemotactic cytokine bias including increases in IL-12, IL-17, and MIP-1α (p<0.05), when compared to ethanol-treated controls (Fig 3A).
Figure 3. Distinct differential immune response patterns identified in chronic experimental colitis.

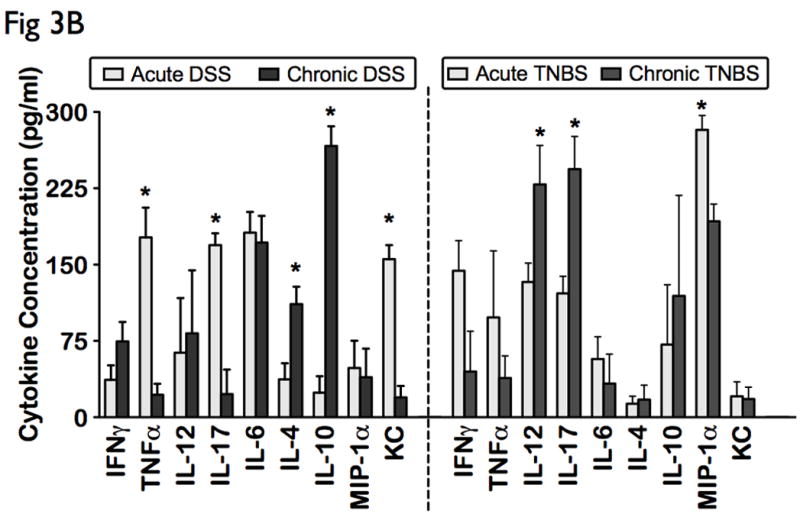

Levels of 16 cytokines were measured simultaneously using a biometric multiplex assay from serum of mice induced with chronic DSS and TNBS-induced colitis. (A) Cytokine profiles in chronic colitis is represented by an enhanced pro-humoral cytokine profile in chronic DSS (IL-6, IFN-γ, and IL-4, IL-10), and a distinct cytotoxic and chemotactic cytokine profile in chronic TNBS (IL-12, IL-17, and MIP-1α). (B) Acute inflammation in DSS colitis converts to a predominant Th2-mediated inflammatory response in the chronic state (lower levels of TNFα, IL-17 and KC, and elevated levels of IL-4, and IL-10), but chronic TNBS colitis is characterized by an enhanced Th1-Th17 immune response (higher IL-12 and IL-17 levels). (C) Levels of other cytokines, including chemokines did not change significantly between the models.
Compared to acute DSS colitis, chronic DSS colitis had significantly lower levels of TNFα, IL-17 and KC, and significantly elevated levels of IL-4, and IL-10, suggesting a Th2 dominant profile in chronic colitis. In contrast, chronic TNBS colitis had significantly higher IL-12 and IL-17 levels, when compared to acute TNBS colitis, suggesting that chronic colonic inflammation in TNBS is characterized by an enhanced Th1-Th17 immune response (Fig 3B, right panel). Levels of other cytokines, including chemokines did not change significantly between the models (Fig 3C). Our data therefore suggests that while TNBS colitis heightens the Th1-Th17 response as the disease becomes chronic, the Th1/Th17-mediated acute inflammation in DSS colitis converts to a predominant Th2-mediated inflammatory response in the chronic state.
Discriminative cytokine profiles identify novel disease-specific sex-stratification patterns
A DFA is distinct from the above analyses in that it is a class distinction modeling method that identifies sets of variables that best discriminate predefined groups. Results of DFA can be visualized on a multidimensional plot, with class discrimination power represented by distance among groups. Interestingly, all disease states were readily distinguished from controls (Fig 4A). Representative of their relative disease activity, chronic models mapped closer to controls than acute models. Additionally, while acute DSS and TNBS animals had somewhat overlapping profiles, chronic DSS and TNBS colitis were highly distinct, mirroring the clearer differentiation of disease activity in the chronic state (Fig 4A). Of the 16 cytokines, IL-4, IL-6, IL-12, IL-17 and IFN-γ were identified by DFA as having the highest power for class discrimination between the models, suggesting that these cytokines play a significant role in pathology within this cohort (Fig 4A). These results suggests that this is directly proportional to intergroup cytokine variation and intragroup heterogeneity in cytokine profiles and disease pathology (Fig 4A).
Figure 4. Multivariate analysis identifies novel subsets of discriminatory cytokines that can distinguish disease types and severity, and further identifiy disease-specific sex-stratification patterns.
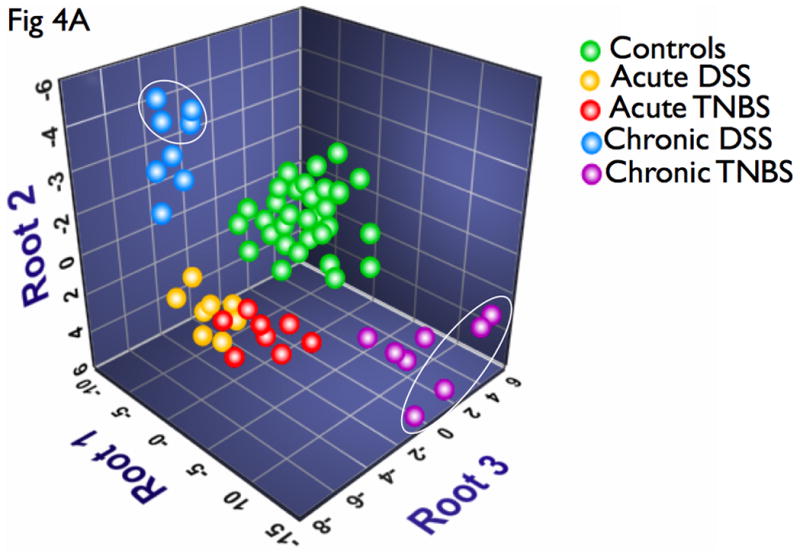
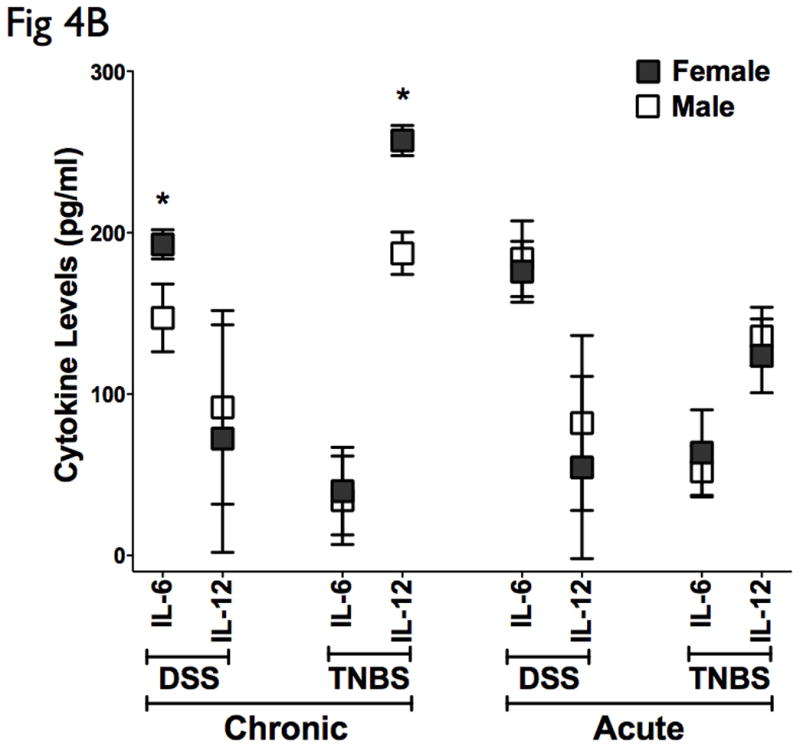
DFA was used to identify subset of cytokines whose expression values can be linearly combined in an equation, denoted a root, whose overall value is distinct for a given characterized group. (A) A graphical representation of the discriminatory potential of DFA. The variables identified by the DFA is plotted in three dimensions to visually represent the relative differences in cytokines among the distinct populations. Each ball represents an animal, and several clusters were mapped and had distinct relevance to the subgroups in context. One subgroup of the chronic DSS and TNBS colitis mapped further away from controls than the main group, marked by ovals, all of which were females. (B) Cytokine analysis of both the chronic DSS and TNBS colitis groups identified elevations in IL-6 and IL-12 in the females relative to males respectively, but not in acute colitis. There were no significant changes in CAI, HAI, and MPO between the genders (data not shown). These results suggest that the DFA is useful in evaluating relative disease activity, as more severe disease maps distal to unaffected controls.
Interestingly, there appeared to be a subgroup of the chronic DSS and TNBS colitis that mapped further away from controls than the main group (Fig 4A, marked by ovals), and all of these animals were female. A sex-stratified cytokine analysis of both the chronic DSS and TNBS colitis groups identified elevations in IL-6 and IL-12 in the females relative to males respectively (Fig 4B). Interestingly, however, CAI, HAI, and MPO did not reveal significant difference in disease activity between the genders (data not shown). Our results therefore have identified a select group of 5 distinct cytokines that can statistically discriminate between experimental colitis and controls, of which 2 cellular cytokines can stratify gender-associated ‘severe’ form of chronic colitis. The markers identified and the algorithm defined by DFA may be useful in evaluating relative disease activity, as more severe disease maps distal to unaffected controls.
Distinct cytokine profiles in tissues reflect and validate systemic cytokine levels
To determine whether the observed systemic cytokine profiles in acute and chronic experimental colitis correlated with that of local levels seen within tissue, immunoblots and immunofluorescence analysis of colon from DSS and TNBS mice were performed. Proteins were extracted from mucosa that was scraped from freshly excised colon, and samples were analyzed with SDS-PAGE Western-blots using primary antibodies for IL-6, IL-23p19, IL-12p40, IL-17, and IFNγ. As shown in Fig 5A&B, a) both acute and chronic DSS colitis had significantly higher IL-6 protein expression in the colon than TNBS colitis, b) both acute and chronic TNBS colitis had significantly higher IL-12p40 and IL-17 protein expression in the colon than DSS colitis and c) acute TNBS colitis had higher IFNγ than acute DSS colitis. All of these above show similar patterns of changes to those observed in the systemic levels, thereby validating the correlation of the systemic immune response to that observed in local tissue. In order to further explore the nature of Th17 immunity in acute and chronic colitis, we assessed the colonic tissue levels of IL-23p19, a master regulator in CD and the principal mediator driving Th17 differentiation (32). Both acute and chronic TNBS colitis had significantly higher IL- 23p19 (and IL-17) protein expression than DSS colitis (Fig 5A&B), further confirming the role of Th17-mediated inflammation in the TNBS model.
Figure 5. Distinct cytokine profiles in tissues reflect and validate systemic cytokine levels.

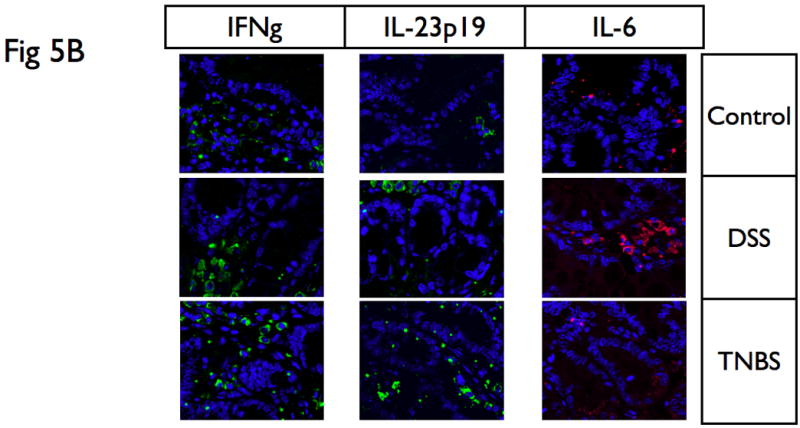
(A) Proteins were extracted from mucosa that was scraped from freshly excised colon, and samples were analyzed with SDS-PAGE Western-blots using primary antibodies for IL-6, IL-23p19, IL-12p40, IL-17, and IFNγ. While DSS colitis had significantly higher IL-6 protein expression in the colon than TNBS colitis, TNBS colitis had significantly higher IL-12p40, IL-23p19 and IL-17 protein expression in the colon than DSS colitis. In addition, acute TNBS colitis had higher IFNγ than acute DSS colitis. (B) Immunofluorescence analysis was performed on paraffin embedded tissue sections of DSS and TNBS acute colitis. IL-6 was significantly upregulated in DSS acute colitis, predominantly associated with lamina propria infiltrating mononuclear cells, while IFNγ and IL-23p19 were highly expressed in TNBS acute colitis. These suggest that cytokine profiles in tissues reflect that present within systemic levels. Actin was used as a loading control. The results presented are representative of 3 independent experiments.
To further validate and define the expression and localization of these immune mediators, we performed immunofluorescence analysis on paraffin embedded tissue sections of DSS and TNBS acute colitis. As shown in Fig 5C, IL-6 was significantly upregulated in DSS acute colitis, predominantly associated with lamina propria infiltrating mononuclear cells, while IFNγ and IL-23p19 were highly expressed in TNBS acute colitis, again predominantly present within infiltrating cells in the lamina propria, demonstrating the immune modulatory potential of inflammatory infiltrates, and finally, validating the observed systemic cytokine profiles in experimental colitis with that of local levels seen within tissue.
DISCUSSION
Apart from clinical and endoscopic indices, diagnosis and disease activity in IBD is currently assessed by conventional serological tests and laboratory indices, including but not limited to ASCA, ANCA, Cbir, ESR, CRP, etc (33, 34). However there is significant controversy regarding its sensitivity and/or specificity, and is recommended only as an adjunct to clinical indices rather than a credible replacement (35, 36). It is expected that additional biomarkers will be discovered as the applications of new technologies, such as that of advanced proteomics, becomes available (37).
In this era of biological agents, there is a decisive need for dependable means of quantitating inflammatory activity in IBD patients (38). As previously characterized (7, 8), murine chemical models of colitis have many similarities to human IBD, are characterized by cytokine dysregulation, have consistent colitis with a defined onset, and have become useful tools to study the innate and adaptive arms of the intestinal immune response in IBD. The mucosal immune system is the central effector of intestinal inflammation and injury, with cytokines playing a central role in modulating inflammation (1). Cytokines are key soluble immunoregulatory modulators of IBD pathology and are therefore a potential class of candidate biomarkers (13). However, studies to date have been limited to analyses of individual or small sets of cytokine(s) that do not take into account the influence of the complex cytokine network and its dependency of the interplay of multiple factors. Herein, we demonstrate that ‘multiplex cytokine profiling’ is a useful strategy to explore disease pathology, with the potential to characterize immune and inflammatory modulation in initiation and perpetuation of disease pathogenesis and subclassify groups and disease severity based on the immune response.
The serum cytokine profiles of both acute and chronic DSS colitis were readily distinguishable from those of unaffected controls. While acute DSS colitis was characterized by monokine (IL-6, TNF-α), Th17 (IL-17), and chemotactic (KC) profile, we see that it converts to a Th2-biased (IL-4, IL-10) inflammatory profile in chronic DSS colitis. The observed increased IL-6 and IFN-γ levels in chronic DSS colitis suggest that it acts synergistically to induce B-cell differentiation (39). DSS is a physical agent with an intrinsic capacity to disrupt the epithelial cell barrier, causing normal mucosal microfloral substances to activate mucosal macrophages, which in turn produce immunomodulatory cytokines (40). This macrophage-induced inflammation and tissue damage is accompanied by a cellular cytotoxic–mediated inflammatory response (macrophage/Th1/Th17 chemotactic profile) along the progression of colitis (7), the profile of which is similar to that previously observed in human IBD and suggests the relevance of these findings. Our data also suggests that the histological data supports the cytokine profile observed. However, following several cycles of DSS administration and regeneration over several weeks, as observed, a chronic inflammatory cell infiltrate becomes established in the mucosa, which may be responsible for the shift to a Th2-baised cytokine profile. Since UC has been predominantly attributed to be a Th2 mediated disease (15), our studies therefore imply that chronic DSS colitis is more representative of UC than acute DSS colitis, as suggested by other groups (41).
The TNBS acute and chronic colitis model enables the progressive study of the immune response to a hapten (TNBS), eventually leading to chronic colonic inflammation. While the systemic cytokine profile of acute TNBS colitis displayed a Th1 (IL-12, IFN-γ), Th17 (IL-17), and chemotactic (MIP-1α) profile, we see an enhanced Th1/Th17 intensity of response in the chronic model. This is in agreement with recent work that showed that peripheral Th17 CD4+ cells correlate with disease activity in chronic CD (42). We also confirmed the elevated Th1/Th17 systemic immune response at the mucosal level using antibodies to IL-12, IL-17 and IL-23p19, also suggesting the important role of IL-23 and Th17 differentiation in TNBS colitis. Our results are therefore consistent with recent hypothesis that IL-23 is a master regulator of colonic inflammation, as recently demonstrated in Crohn’s Disease (32, 43). Since TNBS is administered in ethanol, it may be that an ethanol-induced disturbance of epithelial barrier function precedes mucosal immune cell activation by a hapten-based antigenic stimulus (TNP epitope), which initiates and drives the IL-12 and IL-23 immune response observed herein. Consistent with the heightened Th1 response observed in the chronic TNBS colitis models, it has been proposed that TNBS colitis can drive IL-12 hyper-responsiveness, and subsequent Th1-based pathology (7, 44). Given that CD is widely recognized as a Th1 mediated disease, our cytokine profiles in TNBS colitis further support the relevance of the TNBS model to study CD-related immune responses.
Following an antigenic activation, distinct chemokines recruit specific immune cells characteristic for the inciting stimulus, and function as critical players in the regulation of the immune response (45). Our studies identified MIP-1α as the active chemokine participant in the signaling network that regulates the Th1/Th17 response in TNBS acute colitis, and perpetuates the chronic elevated Th1/Th17 response. This may explain why Pender et al. observed markedly worsened experimental IBD in mice, and enhanced Th1 immune response after systemic administration of MIP-1α (46). In addition, our profiles identified KC as the chemokine that drives the acute DSS Th1 response, which is consistent with the finding that MPO levels, a marker of neutrophil activity, is high in the colonic tissues in these animals, and is in agreement with prior hypothesis that KC may be an important chemokine that determines the severity of experimental colitis (47).
Among other important aspects of the results reported here was the fact that a forward stepwise DFA of cytokine data was able to effectively establish the diagnostic capability of a select group of 5 cytokines (IL-4, IL-6, IL-12, IL-17 and IFN-γ) that could best discriminate among experimental colitis and unaffected controls. The analytical potential of this multivariate algorithm was based on the finding that the dynamics of cytokine profiles in experimental colitis were modulated in a statistically interrelated disease-specific pattern from unaffected controls in proportion to the weighted level of cytokine activity and are therefore likely exist in a functional network that is specific for the DSS and TNBS colitis, suggesting its similarity to the complex interplay proposed in human IBD. Multivariate predictive algorithms potentially can enhance diagnostic power beyond single analytes. Recently these tools are proving to have clinical relevance in complex clinical states, and are increasingly improving the specificity, sensitivity, power and clinical relevance of laboratory testing (27). Specifically in human IBD, multivariate algorithms have been used to improve the accuracy of diagnostic testing (34).
The multivariate analysis also enabled the classification of an additional subgroup in both DSS and TNBS chronic colitis, that displayed significant elevated levels of 2 major cytokines, IL-6, and IL-12 respectively. It is interesting to note that this ‘severe cytokine profile’ subgroup were all females. We did not observe any difference in clinical or histopathological indices between the sexes. These data suggest that female mice may have increased inflammatory activity/severity that were not detectable by clinical or histopathological indices. To our knowledge, this is the first report of this kind in experimental colitis, and is consistent with recent reports that disease severity tends to be worse in female pediatric patients with Crohn’s disease (48). Hormonal differences between males and females have also been noticed with disease susceptibility to Th1/Th2 patterns in males and females (49). Further functional studies are necessary to help elucidate the pathophysiologic mechanisms of this finding.
Chemical murine models of IBD host an array of cytotoxic, humoral and chemokine responses, and a combination of immune complex, macrophage driven, and T cell-driven IBD occurs, characterized by inflammatory cell infiltration and tissue damage. Several of the current known pathogenic mechanisms of IBD, clinical research, and novel therapeutic strategies originated from the DSS and TNBS mucosal models of inflammation. However, the lack of adequate understanding of the complex cytokine network made it difficult to establish biologically mediated standards of diagnostic and prognostic variables to assess disease activity and therapeutic efficacy. Our studies of comprehensive multiplex cytokine profiling and multivariate analysis have identified distinct cytokines and dynamic patterns. The identified characteristic cytokine profiles can be used to better characterize murine pathology, and can be used as diagnostic biomarkers for differentiating between CD vs UC. These results therefore not only provide more insight into disease immunopathogenesis, but can also facilitate strategies for evaluating new potential therapeutic agents (based on targeted biological therapies) in preclinical studies.
It is however also important to note that multiplex cytokine assays have their limitations. Several new cytokines (such as IL-23) and analytes of interest are currently not yet available in multiplexed kits, and therefore, limits its utility. In addition, cytokine concentrations when measured by different commential kits show similar trends, but exhibit differences in absolute concentrations (50). The presence of interfering proteins such as heterophilic antibodies have been demonstrated to cause discrepancies among some samples tested between different kits (51). Careful optimisation and validation of multiplex cytokine assays prior to large-scale studies have therefore been widely-recommended (52). It may therefore be note-worthy here that our multiplex assays have also completed rigorous in-house pre-study validation for analytical sensitivity, specificity, freeze–thaw stability, accuracy, and precision. Despite these known limitations, several studies underscore the important utility of multiplex cytokine assays as diagnostic and prognostic tools in diverse autoimmune, inflammatory, infectious and malignant conditions (26, 27, 53–57). Since multiplex ELISAs are rapidly evolving and new (and more) specific antibodies suitable for this technology become available, new and important targets (including IL-23) are expected to be included in the multiplex assay (58, 59). Therefore, while the application of multiplex cytokine technology specifically in IBD research is still in its infancy, its potential is unlimited (60).
In conclusion, we have demonstrated dysregulated patterns of disease-specific cytokine profiles in experimental colitis with significant correlations to disease duration and disease activity/severity. Our findings support the long-standing hypothesis that experimental colitis and therefore, IBD is a complex immune-inflammatory disorder involving the dysregulation of distinct cellular, humoral, innate immunity. We now demonstrate that within this intricate complexity, there is a significant distinct systemic signature.
Acknowledgments
The authors were supported primarily by Broad Medical Research Program (IBD-0119R), NIH-NIDDK R21 DK077064, NIH Ruth L. Kirschstein National Research Service Award, NIH grants 5U19AI062629, P20RR15577, and in part by NIH/NIDDK KO1- DK62264, R24-DK64388 (The NIDDK-Hopkins Basic Research Digestive Diseases Development Core Center), and OCAST OARS Grants AR061-015, and AR081-006. The authors thank Jennifer Sipes for her assistance with the Johns Hopkins mouse core facility, Maxim Suhodrev-Saksonov and Anurag Bajaj for their assistance with the mouse models of colitis, Dr. Theodore Bayless for his valuable advice and suggestions on the study and manuscript, and Dr. Igor Dozmorov for his guidance on the bioinformatics and discriminant functional analysis.
The authors were supported primarily by Broad Medical Research Program (IBD-0119R), NIH-NIDDK R21 DK077064, NIH Ruth L. Kirschstein National Research Service Award, NIH grants 5U19AI062629, P20RR15577, and in part by NIH/NIDDK KO1- DK62264, R24-DK64388 (The NIDDK-Hopkins Basic Research Digestive Diseases Development Core Center), and OCAST OARS Grants AR061-015, and AR081-006
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn’s disease. Immunol Rev. 2005;206:277–295. doi: 10.1111/j.0105-2896.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 4.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 5.Pizarro TT, Cominelli F. Cytokine therapy for Crohn’s disease: advances in translational research. Annu Rev Med. 2007;58:433–444. doi: 10.1146/annurev.med.58.121205.100607. [DOI] [PubMed] [Google Scholar]
- 6.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 8.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 9.Mizoguchi A, Mizoguchi E. Inflammatory bowel disease, past, present and future: lessons from animal models. J Gastroenterol. 2008;43:1–17. doi: 10.1007/s00535-007-2111-3. [DOI] [PubMed] [Google Scholar]
- 10.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho FA, Barnich N, Sauvanet P, Darcha C, Gelot A, Darfeuille-Michaud A. Crohn’s disease- associated Escherichia coli LF82 aggravates colitis in injured mouse colon via signaling by flagellin. Inflamm Bowel Dis. 2008 doi: 10.1002/ibd.20423. [DOI] [PubMed] [Google Scholar]
- 12.Deger C, Erbil Y, Giris M, et al. The effect of glutamine on pancreatic damage in TNBS-induced colitis. Dig Dis Sci. 2006;51:1841–1846. doi: 10.1007/s10620-006-9189-y. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 15.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 16.Fort M, Lesley R, Davidson N, et al. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. J Immunol. 2001;166:2793–2800. doi: 10.4049/jimmunol.166.4.2793. [DOI] [PubMed] [Google Scholar]
- 17.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 18.Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820–2828. doi: 10.1111/j.1572-0241.2002.07029.x. [DOI] [PubMed] [Google Scholar]
- 19.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002 doi: 10.1002/0471142735.im1519s49. Chapter 15:Unit 15 19. [DOI] [PubMed] [Google Scholar]
- 21.te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:995–999. doi: 10.1097/01.mib.0000227817.54969.5e. [DOI] [PubMed] [Google Scholar]
- 22.Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2- mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides. J Clin Invest. 2005;115:3057–3071. doi: 10.1172/JCI24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 24.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin KW, Barrett KE. Mast cells are not essential to inflammation in murine model of colitis. Dig Dis Sci. 1994;39:513–525. doi: 10.1007/BF02088336. [DOI] [PubMed] [Google Scholar]
- 26.Alex P, Szodoray P, Arthur E, et al. Influence of intraarticular corticosteroid administration on serum cytokines in rheumatoid arthritis. Clin Rheumatol. 2007;26:845–848. doi: 10.1007/s10067-006-0419-7. [DOI] [PubMed] [Google Scholar]
- 27.Alex P, Szodoray P, Knowlton N, et al. Multiplex serum cytokine monitoring as a prognostic tool in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:584–592. [PubMed] [Google Scholar]
- 28.Szodoray P, Alex P, Chappell-Woodward CM, et al. Circulating cytokines in Norwegian patients with psoriatic arthritis determined by a multiplex cytokine array system. Rheumatology (Oxford) 2007;46:417–425. doi: 10.1093/rheumatology/kel306. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis JN, Dozmorov I, Jiang K, et al. Novel approaches to gene expression analysis of active polyarticular juvenile rheumatoid arthritis. Arthritis Res Ther. 2004;6:R15–R32. doi: 10.1186/ar1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Galli T, Leu S, et al. Na+-H+ exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol. 2001;537:537–552. doi: 10.1111/j.1469-7793.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Zhang H, Cheong A, et al. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol. 2004;556:791–804. doi: 10.1113/jphysiol.2004.060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neurath MF. IL-23: a master regulator in Crohn disease. Nat Med. 2007;13:26–28. doi: 10.1038/nm0107-26. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Conklin LS, Alex P. New Serological Biomarkers of Inflammatory Bowel Disease. World J Gastroenterol. doi: 10.3748/wjg.14.5115. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura RM, Barry M. Serologic markers in inflammatory bowel disease (IBD) MLO Med Lab Obs. 2001;33:8–15. quiz 16-9. [PubMed] [Google Scholar]
- 35.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 36.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alex P, Gucek M, Li X. Applications of proteomics in the study of inflammatory bowel diseases: Current status and future directions with available technologies. Inflamm Bowel Dis. doi: 10.1002/ibd.20652. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 40.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 41.Kullmann F, Messmann H, Alt M, et al. Clinical and histopathological features of dextran sulfate sodium induced acute and chronic colitis associated with dysplasia in rats. Int J Colorectal Dis. 2001;16:238–246. doi: 10.1007/s003840100311. [DOI] [PubMed] [Google Scholar]
- 42.Veny M, Closa D, Esteller M, Pique JMJPAS. Th17 and Th1 Effector Responses in Crohn’s Disease. Gastroenterology. 2008 DDW Oral Presentation. [Google Scholar]
- 43.Elson CO, Cong Y, Weaver CT, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 44.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm Bowel Dis. 2008 doi: 10.1002/ibd.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pender SL, Chance V, Whiting CV, et al. Systemic administration of the chemokine macrophage inflammatory protein 1alpha exacerbates inflammatory bowel disease in a mouse model. Gut. 2005;54:1114–1120. doi: 10.1136/gut.2004.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchiya T, Fukuda S, Hamada H, et al. Role of gamma delta T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 48.Gupta N, Bostrom AG, Kirschner BS, et al. Gender differences in presentation and course of disease in pediatric patients with Crohn disease. Pediatrics. 2007;120:e1418–25. doi: 10.1542/peds.2007-0905. [DOI] [PubMed] [Google Scholar]
- 49.Saluz HP, Jiricny J, Jost JP. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proc Natl Acad Sci U S A. 1986;83:7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JPJ. Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 51.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 52.Djoba Siawaya JF, Roberts T, Babb C, et al. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS ONE. 2008;3:e2535. doi: 10.1371/journal.pone.0002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dehqanzada ZA, Storrer CE, Hueman MT, et al. Assessing serum cytokine profiles in breast cancer patients receiving a HER2/neu vaccine using Luminex technology. Oncol Rep. 2007;17:687–694. [PubMed] [Google Scholar]
- 54.Funding M, Hansen TK, Gjedsted J, Ehlers N. Simultaneous quantification of 17 immune mediators in aqueous humour from patients with corneal rejection. Acta Ophthalmol Scand. 2006;84:759–765. doi: 10.1111/j.1600-0420.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann TK, Sonkoly E, Homey B, et al. Aberrant cytokine expression in serum of patients with adenoid cystic carcinoma and squamous cell carcinoma of the head and neck. Head Neck. 2007;29:472–478. doi: 10.1002/hed.20533. [DOI] [PubMed] [Google Scholar]
- 56.Kofoed K, Schneider UV, Scheel T, Andersen O, Eugen-Olsen J. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem. 2006;52:1284–1293. doi: 10.1373/clinchem.2006.067595. [DOI] [PubMed] [Google Scholar]
- 57.Seoane E, Resino S, Micheloud D, et al. Lipid and Apoprotein Profile in HIV-1-Infected Patients After CD4-Guided Treatment Interruption. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e31817bbc07. [DOI] [PubMed] [Google Scholar]
- 58.de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–191. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alex P, Zachos NC, Conklin LS, et al. Distinct cytokine patterns as effective indicators of disease activity and severity in IBD. Gastroenterology. 2008;134:A204–A204. [Google Scholar]


