Abstract
Objective
To investigate whether adiposity influences endothelial progenitor cell (EPC) number and colony-forming capacity.
Design
Cross-sectional study of normal weight, overweight and obese adult humans.
Subjects
Sixty-seven sedentary adults (age 45–65y): 25 normal weight (BMI ≤ 25 kg/m2; 12 males/13 females); 18 overweight (BMI = 25–29.9 kg/m2; 12 males/6 females); and 24 obese (BMI ≥ 30 kg/m2; 18 males/6 females). All subjects were non-smokers and free of overt cardiometabolic disease.
Measurements
Peripheral blood samples were collected and circulating EPC number was assessed by flow cytometry. Putative EPCs were defined as CD45−/CD34+/VEGFR-2+/CD133+ or CD45−/CD34+ cells. EPC colony-forming capacity was measured in vitro using a colony-forming unit assay.
Results
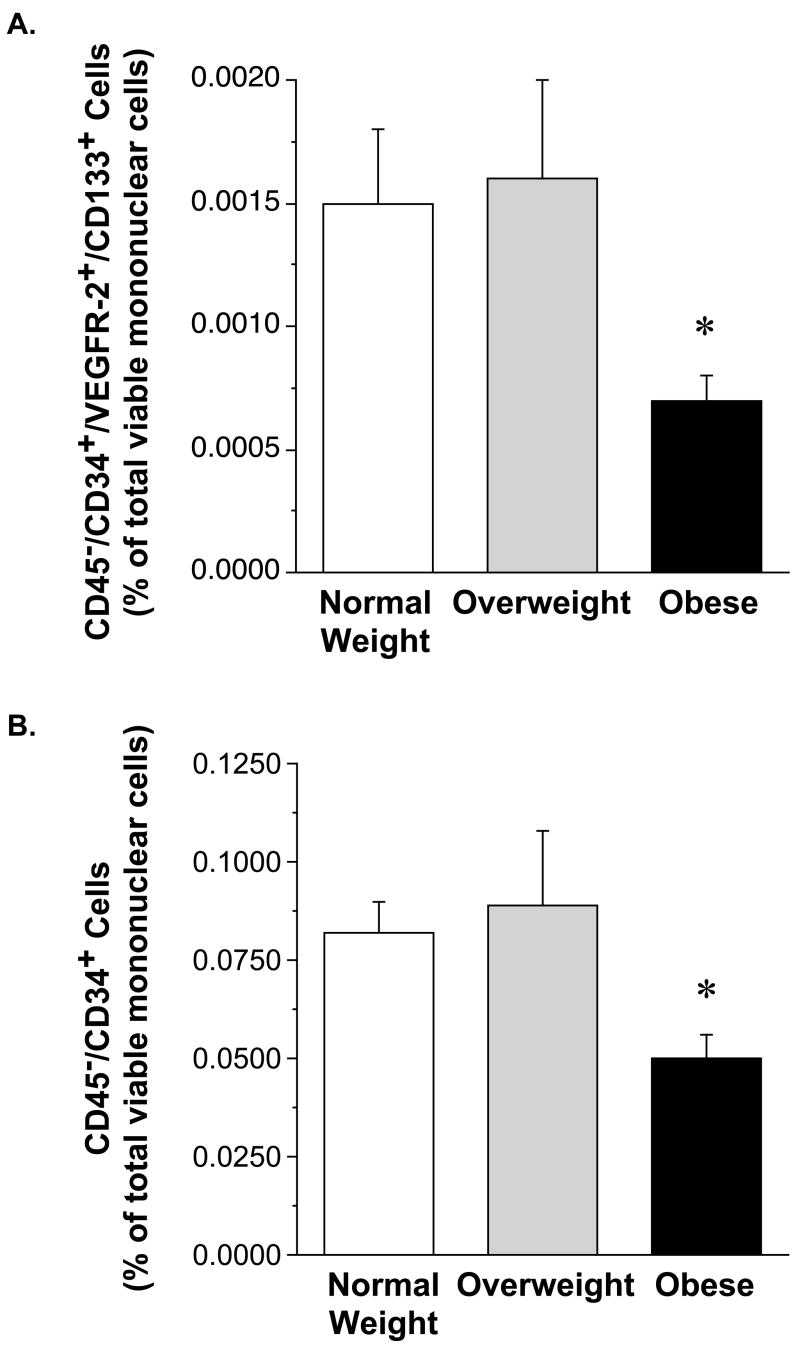
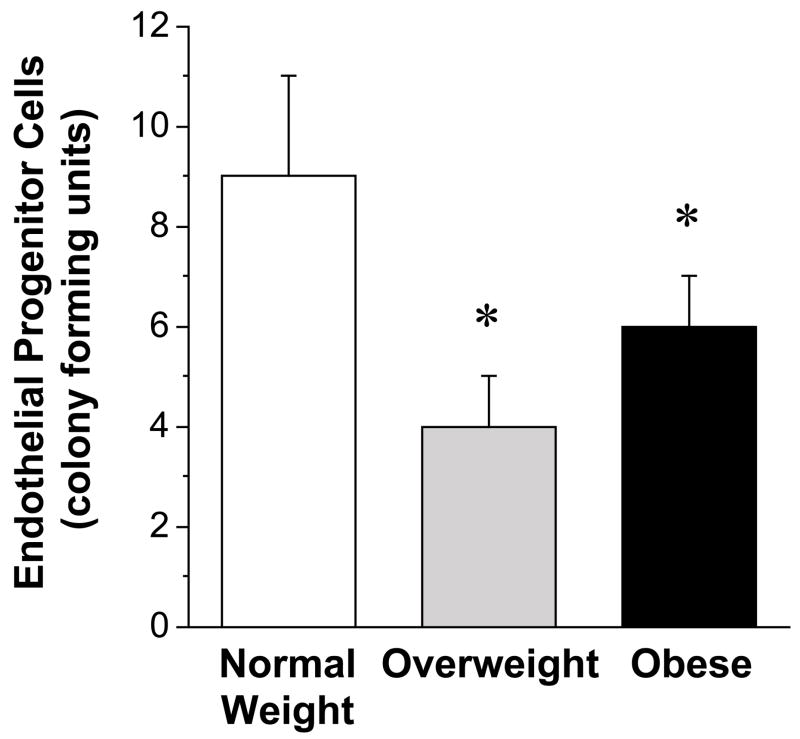
Number of circulating putative EPCs (either CD45−/CD34+/VEGFR-2+/CD133+ or CD45−/CD34+ cells) was lower (P<0.05) in obese (0.0007±0.0001%; 0.050±0.006%) compared with overweight (0.0016±0.0004%; 0.089±0.019%) and normal weight (0.0015±0.0003%; 0.082±0.008%) adults. There were no differences in EPC number between the overweight and normal weight groups. EPC colony-formation was significantly less in the obese (6±1) and overweight (4±1) compared with normal weight (9±2) adults.
Conclusion
These results indicate that: 1) the number of circulating EPCs is lower in obese compared with overweight and normal weight adults; and 2) EPC colony-forming capacity is blunted in overweight and obese adults compared with normal weight adults. Impairments in EPC number and function may contribute to adiposity-related cardiovascular risk.
Keywords: overweight, obesity, endothelial progenitor cells, colony-forming units
INTRODUCTION
Overweight and obesity are associated with increased rates of cardiovascular morbidity and mortality (1;2)
Endothelial damage and dysfunction is considered to be a major underlying mechanism for the heightened cardiovascular burden with increased adiposity. For example, alterations in endothelial function associated with overweight and obesity that precede and predispose to atherosclerosis and thrombosis include diminished endothelial vasodilator and fibrinolytic function (3;4). Although much attention has focused on factors that contribute to adiposity-related endothelial damage, such as inflammation and oxidative stress, recent studies indicate that endogenous endothelial repair and neovascularization processes also play an important role in vascular health and function.
It is now recognized that endothelial repair/regeneration is not only dependent upon the migration and proliferation of surrounding mature endothelial cells resident in the vascular wall, but also on the availability of circulating endothelial progenitor cells (EPCs) (5). Characterized in 1997 by Asahara and colleagues (6), EPCs possess the ability to proliferate, migrate, differentiate into mature endothelial cells and incorporate into preexisting and newly-forming blood vessels (6–9). Circulating EPCs have also generated interest as a novel biomarker of endothelial function and a prognostic indicator of cardiovascular morbidity and mortality. Two recent clinical studies reported that reduced levels of circulating EPCs independently predict atherosclerotic disease progression and death from cardiovascular causes in patients with established coronary artery disease (CAD) (10;11). Moreover, after adjusting for disease activity and risk factors, low numbers of circulating EPCs were associated with a four-fold increased risk of a future cardiovascular event (10). In addition, Kunz et al. (12) reported a strong inverse relation between EPC colony-forming capacity and CAD severity in individuals undergoing diagnostic cardiac catheterization, independent of traditional risk factors. Interestingly, the investigators noted that for every 10 EPC colony-forming unit increase, the likelihood of multivessel CAD declined by 20%. Numerical and functional deficits in circulating EPCs have also been linked to restenosis rates and impaired neovascularization after ischemic events (5;13).
Although reduced number and impaired function of EPCs have been linked to a number of pathologies associated with overweight/obesity, such as hypertension, hypercholesterolemia, diabetes and CAD (14–16), the influence of increased adiposity per se on circulating EPCs remains unclear. Accordingly, we tested the hypotheses that: 1) the number of circulating EPCs is lower in otherwise healthy overweight and obese compared with normal weight adults; and 2) EPC colony-forming capacity is also diminished in overweight and obese adults.
METHODS
Subjects
Sixty seven sedentary adults aged 45–65 years participated in the study: 25 normal weight (BMI >18.5 kg/m2 and <25 kg/m2; 12 male/13 female); 18 overweight (BMI ≥ 25 kg/m2 and ≤ 30 kg/m2; 12 M/6 F); and 24 obese (BMI ≥ 30 kg/m2; 18 M/6 F). All subjects were normotensive (arterial blood pressure ≤ 140/90 mmHg), non-smokers, non-medicated (including vitamins), and free of overt cardiovascular, metabolic, renal and hematologic disease, as assessed by medical history, resting and exercise electrocardiograms, and fasting blood chemistries. Subjects were excluded from the study if they exhibited plasma glucose >7.0 mmol/L; total cholesterol ≥ 6.0 mmol/L, LDL-cholesterol ≥ 4.5 mmol/L, triglycerides ≥ 2.5 mmol/L. All subjects were sedentary and had not performed regular physical exercise for at least 1 year before the beginning of the study. Female subjects were at least 1 year postmenopausal and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study. Prior to participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Body Composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual energy X-ray absorptiometry (Lunar Corp., Madison, WI, USA). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to published guidelines (17).
Maximal Oxygen Consumption (V̇ O2 max)
To assess aerobic fitness, subjects performed incremental treadmill exercise using a modified Balke protocol. Maximal oxygen consumption (V̇O2 max) was measured using on-line computer-assisted open circuit spirometry, as reported previously (18).
Metabolic Measurements
Fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques, as reported previously (3). Insulin resistance (HOMA-IR) was calculated according to the HOMA calculation: fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5 (19).
EPC Isolation and Characterization
Circulating mononuclear cells were isolated from peripheral blood sample by Ficoll density-gradient centrifugation (Histopaque 1077, Sigma Aldrich, St. Louis, MO, USA), washed and resuspended in growth medium (Medium 199, Gibco, Grand Island, NY, USA) supplemented with 20% fetal calf serum (Gibco), penicillin (100 U/mL, Gibco), and streptomycin (100 mg/mL, Gibco). Endothelial phenotype of these cells was confirmed by immunofluorescent staining for the uptake of DiI-ac-LDL (Biomedical Technologies Inc., Stoughton, MA, USA) and expression of von Willebrand factor (Dako, Glostrup, Denmark), VE-cadherin, CD31, and VEGFR-2 (Invitrogen, Carlsbad, CA, USA).
EPC Number
Circulating putative EPC number was determined by fluorescence-activated cell sorting (FACS) analysis following guidelines recommended by the International Society for Hematotherapy and Graft Engineering (20). Briefly, 2 × 106 cells were incubated at 4 °C for 30 minutes with monoclonal antibodies for PC7-conjugated CD45 (Beckman Coulter, Fullerton, CA, USA), FITC-conjugated CD34 (Beckman Coulter), PE-conjugated VEGFR-2 (R&D Systems, Minneapolis, MN, USA) and APC-conjugated CD133 (Miltenyi Biotech, Auburn, CA, USA). Non-viable cells were excluded with propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) and appropriate compensation controls were analyzed. Cells were gated for low expression of CD45, then CD34+ cells were analyzed for events double-positive for VEGFR-2 and CD133 and presented as a percent of total viable mononuclear cells. All samples were analyzed using a FC500 flow cytometer (Beckman Coulter) and the data analyzed by CXP software.
EPC Colony-Forming Assay
EPC colony-forming capacity was determined as described previously by our laboratory and others (16;21). Briefly, freshly isolated mononuclear cells were plated on 6-well plates coated with human fibronectin (BD Biosciences, San Jose, CA, USA) for 48 hours at 37 °C. Thereafter, 5 × 105 non-adherent cells from each subject were seeded onto 24-well fibronectin-coated plates (BD Biosciences). Growth medium was changed every 3 days, and the colony-forming units (CFUs) were counted in 4 random wells on day 7 by two independent investigators blinded to sample identification. Only CFUs consisting of multiple thin, flat cells emanating from a central cluster of rounded cells were counted.
Statistical Analysis
Group differences were determined by analysis of variance. Where indicated by a significant F value, Duncan’s post hoc test was performed to compare specific group means. Importantly, no main effects of gender, nor interactions of gender with BMI group, were found in any of the key outcome variables. Therefore, the data were combined and presented together. Relations between variables of interest were assessed by Pearson’s correlation coefficient and linear regression analysis. All data are expressed as means ± SE. Statistical significance was set a priori at P < 0.05.
RESULTS
Selected subject characteristics are presented in Table 1. All subjects were normotensive, normolipidemic, and normoglycemic. By design, body mass and body composition values were significantly higher (P<0.05) in the overweight and obese groups compared with the normal weight group. Although within clinically normal ranges, obese subjects demonstrated higher (P<0.05) resting systolic and diastolic blood pressure, and lower (P<0.05) HDL-cholesterol, than the normal weight controls. Obese subjects also had significantly higher plasma insulin concentrations and HOMA insulin resistance values compared with both the normal weight and overweight subjects. There were no differences amongst the groups in plasma concentrations of total cholesterol, LDL-cholesterol, triglycerides, and glucose.
Table 1.
Selected subject characteristics
| Variable | Normal Weight (N=25) | Overweight (N=18) | Obese (N=24) |
|---|---|---|---|
| Age (years) | 56±1 | 58±1 | 56±1 |
| Body mass (kg) | 70.3±2.1 | 80.1±2.4* | 97.5±2.8*† |
| BMI (kg/m2) | 23.4±0.4 | 27.6±0.2* | 32.9±0.5*† |
| Body fat (%) | 28.8±1.6 | 31.2±2.1 | 37.4±1.4*† |
| Waist circumference (cm) | 81.9±2.0 | 93.0±1.7* | 107.8±1.5*† |
| Systolic BP (mmHg) | 118±2 | 125±2 | 126±2* |
| Diastolic BP (mmHg) | 72±1 | 78±1* | 81±1* |
| V̇O2 max (L/min) | 2.2±0.1 | 2.7±0.2 | 2.6±0.1 |
| V̇O2 max (mL/kg/min) | 31.5±1.3 | 32.4±1.9 | 27.9±1.4 |
| Maximum heart rate (bpm) | 172±3 | 173±2 | 171±2 |
| RER at V̇O2 max | 1.19±0.01 | 1.14±0.01 | 1.16±0.02 |
| Treadmill Time (min) | 10.6±0.2 | 10.5±0.4 | 9.6±0.3 |
| Total Cholesterol (mmol/L) | 5.1±0.1 | 5.4±0.1 | 5.3±0.1 |
| HDL-Cholesterol (mmol/L) | 1.5±0.1 | 1.3±0.1 | 1.2±0.1* |
| LDL-Cholesterol (mmol/L) | 3.1±0.1 | 3.5±0.1 | 3.4±0.1 |
| Triglycerides (mmol/L) | 1.1±0.1 | 1.3±0.1 | 1.4±0.1 |
| Glucose (mmol/L) | 4.6±0.1 | 5.2±0.1 | 5.1±0.1 |
| Insulin (pmol/L) | 32.0±3.5 | 43.9±3.9 | 57.0±5.0* |
| HOMA IR | 1.2±0.1 | 1.7±0.2 | 2.2±0.2* |
BMI, Body Mass Index; BP, blood pressure; V̇O2 max, maximal oxygen consumption; RER, respiratory exchange ratio; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance.
Values are mean ± SEM.
P<0.05 vs. Normal Weight.
P<0.05 vs. Overweight.
The number of CD45−/CD34+/VEGFR-2+/CD133+ cells was ~50% lower (P<0.05) in the obese (0.0007 ± 0.0001 %) compared with the overweight (0.0016 ± 0.0004 %) and normal weight groups (0.0015 ± 0.0003 %) (Figure 1). Similarly, the number of CD45−/CD34+ cells was lower (P<0.05) in the obese (0.050±0.006%) compared with overweight (0.089±0.019%) and normal weight subjects (0.082±0.008%). There were no significant differences in either CD45−/CD34+/VEGFR-2+/CD133+ or CD45−/CD34+ cells between the overweight and normal-weight subjects. The capacity of EPCs to form colonies was lower (~40%; P<0.05) in both the overweight (4 ± 1) and obese (6 ± 1) groups versus the normal weight controls (9 ± 2) (Figure 2). There was no significant difference in EPC CFU number between the overweight and obese subjects.
Figure 1.
Circulating levels of CD45−/CD34+/VEGFR-2+/CD133+ and CD45−/CD34+ cells in normal weight, overweight, and obese adults.
Values are mean ± SE. *P< 0.05 vs. normal weight and overweight.
Figure 2.
Endothelial progenitor cell colony forming units for normal weight, overweight, and obese adults.
Values are mean ± SE. *P< 0.05 vs. normal weight
In the overall study population, there were significant inverse relations between BMI and the number of CD45−/CD34+/VEGFR-2+/CD133+ cells (r = −0.25), CD45−/CD34+ cells (r = −0.26) and EPC colony forming units (r = −0.24). Percent body fat was also negatively associated with the number of CD45−/CD34+ cells (r = −0.25, P<0.05). No other significant correlations were observed between either EPC number or colony-forming capacity and any other anthropometric, metabolic or hemodynamic variable.
DISCUSSION
The new findings of the present study are that: 1) the number of circulating EPCs is lower in obese compared with overweight and normal weight adults; and 2) EPC colony-forming capacity is blunted in overweight and obese adults compared with normal weight adults. To our knowledge, this is the first study to demonstrate adiposity-related impairments in EPC number and function in overweight and obese adults free of other cardiometabolic abnormalities.
Many of the cardiovascular complications associated with obesity are due, at least in part, to endothelial damage and/or dysfunction (22;23). Circulating EPCs are considered to be an important hemostatic mechanism for counteracting endothelial injury and the accelerated development of atherosclerosis associated with many cardiovascular risk factors, including obesity (24). For example, circulating EPC number is inversely related to the extent of carotid stenosis and lower extremity atherosclerosis in obese diabetic patients (25). Moreover, in patients with CAD, higher numbers of circulating EPCs have been linked to greater myocardial perfusion and vascularization (26;27) as well as overall better prognosis (10). To date, previous investigations evaluating the influence of adiposity on progenitor cell number have been hampered by design limitations involving the presence of multiple cardiovascular risk factors, overt disease and/or medication use, making it difficult to discern the impact of obesity per se on EPC number (28;29). In the present study, we demonstrate that the number of circulating putative EPCs, presented as CD45−/CD34+/VEGFR-2+/CD133+ cells as well as CD45−/CD34+ cells, is close to 50% lower in obese compared with overweight and normal weight adults. The absence of other established cardiometabolic risk factors in our study population suggests a primary negative influence of obesity on EPC number. From a clinical perspective, it is plausible that reduced EPC bioavailability may contribute to the increased incidence of endothelium-related vascular complications and reduced neovascularization with obesity (30–32). Interestingly, EPC number was not lower in the overweight compared with normal weight group. The number of CD45−/CD34+/VEGFR-2+/CD133+ and CD45−/CD34+ cells in the overweight adults was almost identical to that of the normal weight controls and, in turn, significantly higher than the obese adults. This finding was unexpected considering we have previously reported no differences in the degree of endothelial vasodilator and fibrinolytic dysfunction between overweight and obese adults similar to those studied herein (3;33). Nevertheless, greater number of EPCs in overweight adults may play a role in their lower risk of recurrent coronary events following acute myocardial infarction compared with obese adults of similar age (34).
The mechanisms responsible for the obesity-related reduction in circulating putative EPC number are not clear. Although we employed strict inclusion criteria in the present study, the obese adults did demonstrate higher, albeit in clinically normal ranges, levels of blood pressure, plasma insulin and insulin resistance. Thus, it is possible that the cumulative effects of these factors, along with other secondary consequences of excess body fatness, such as oxidative stress and inflammation, creates an environment resulting in higher consumption and/or exhaustion of EPC production. For example, the inflammatory cytokine C-reactive protein, although not measured in the present study, is often higher in obese compared with overweight and normal weight adults (35). C-reactive protein has been shown to promote apoptosis in EPCs in vitro (36), which could negatively impact circulating EPC number. Differences in body fat distribution between the overweight and obese subjects may also have contributed to our findings. Future studies are needed to determine whether differences in visceral and subcutaneous body fat depots influence EPC number.
There are conflicting reports in the literature regarding the appropriate defining criteria for the quantification of EPCs. Circulating EPCs are thought to be primarily derived from hematopoietic stem cells, however, non-hematopoietic mesenchymal stem cells and other monocytic cells from peripheral blood have also been shown to cross-differentiate into functionally active endothelial progenitors, or assume an endothelial-like phenotype (37;38). It has been suggested that the best strategy for enumeration is to isolate cells positive for both a hematopoietic stem cell surface membrane protein, such as CD34, and a marker of endothelial lineage, such as VEGFR-2, by flow cytometry (39). However, the antigen CD34 can also be weakly expressed in mature endothelial cells (40), that are also present in the circulation (41). As a result, it has been proposed that the immature hematopoietic stem cell marker CD133 also be used for enumeration (42;43). Peichev et al. demonstrated that circulating CD34+/VEGFR-2+/CD133+ cells differentiate into endothelial cell clusters in vitro and may also contribute to neoangiogenesis (40). In contrast, Case et al. (44) recently reported that CD34+/VEGFR-2+/CD133+ cells do not differentiate into endothelial cells, raising some doubt regarding their EPC designation. However, because the cells were cultured in isolation, devoid of interaction with other cell lines (a situation never encountered in vivo), it is possible that the culture conditions influenced the results. Of note, the same study also reported that CD34+ cells with low expression of the common leukocyte antigen CD45 do, give rise to proliferative endothelial cells even under the same restrictive culture conditions, demonstrating the endothelial potential of the CD45−/CD34+ cell populations. Recently, a statement from the EULAR Scleroderma Trials and Research Group recommended the use of CD34, VEGFR-2 and CD133 antigens to quantify circulating EPCs (45). In the present study, we believe, we employed antigenic profiles to identify putative EPCs (i.e. CD45−/CD34+ and CD45−/CD34+/VEGFR-2+/CD133+ cells) that encompass a cellular phenotype with confirmed endothelial differentiation capacity (44;46) and that, importantly, have been linked to cardiovascular disease risk and outcome (47–49). Given the lack of consensus in enumerating EPCs, our results must be viewed within the context of our antigenic profile. Other enumerating criteria, such as quantifying cells double positive for CD34 and VEGFR-2, may yield different results.
In contrast to circulating EPC number, the number of EPC colony-forming units was ~40% lower in both obese and overweight compared with normal weight adults. To our knowledge, this is the first study to demonstrate an adiposity-related reduction in EPC colony-forming capacity. Previous studies that have failed to find a relation between adiposity and EPC colony-forming units were either underpowered to detect a difference (16), or included a study population that was compromised by statin-use and documented CAD (50), factors which have been shown to influence EPC function (12;51). Once thought to be a surrogate marker of circulating EPC number, it is now generally accepted that the number of EPC colony-forming units does not correspond to the number of circulating EPCs measured by flow cytometry (52;53). The discrepancy in our results between EPC number (determined by FACS) and the number of colony-forming units further confirm the lack of association between these two measures. Nevertheless, clinical interest in EPC colony-forming units has increased due to the consistently observed relation between colony number and both endothelial function (16) and cardiovascular risk in healthy and diseased populations (12;21;54). These findings have prompted its use as a novel cardiovascular biomarker (11;55). Whether the number of EPC colony-forming units in overweight and obese adults is associated with endothelial dysfunction and adverse cardiovascular events remains to be determined.
The reasons for the reduced EPC colony-formation in overweight and obese compared with normal weight adults are not clear. Recent data indicate that the central clusters of cells that make up these colonies are comprised mainly of CD3+/CD31+/CXCR4+ T cells (56;57). Thus, it is possible that reduction in this subpopulation of T cells with adiposity is the underlying cause. However, in a currently ongoing study focused on these so-called “angiogenic T cells,” we have not observed a decline in their number in either overweight or obese adults compared with normal weight controls (unpublished observations). It is important to emphasize that the defining characteristic of an EPC colony-forming unit is the presence of spindle-shaped cells emanating from a central cluster of cells (16). These spindle-shaped cells, more so than the central colony clusters, have been shown to demonstrate immunologic and morphologic characteristics consistent with the identification of putative EPCs (58). It is possible that the same milieu of factors that negatively affect circulating EPC number in vivo may also impair their ability to contribute to cluster formation in vitro. Indeed, inflammatory and oxidative substances, such as CRP and oxidized LDL-cholesterol, have been shown to impair EPC function in vitro (59;60).
In conclusion, the results of the present study indicate that increased body fatness, independent of other traditional cardiovascular risk factors, adversely affects EPC biology. Obesity, but not overweight, is associated with lower levels of circulating EPCs compared with normal weight adults; whereas, both overweight and obesity are associated with blunted EPC colony-forming capacity. While the differential effects of overweight and obesity on circulating EPCs require more attention, diminished EPC bioavailability and impaired function may contribute to adiposity-related cardiovascular morbidity and mortality.
Acknowledgments
We would like to thank all of the subjects who participated in the study as well as Jeremy Stoner and Yoli Casas for their technical and administrative assistance. This study was supported by National Institutes of Health Awards HL076434, HL077450 and RR00051, American Heart Association Award 0555678Z and an American Diabetes Association Clinical Research Award.
References
- 1.Yan LL, Daviglus ML, Liu K, Stamler J, Wang R, Pirzada A, Garside DB, Dyer AR, Van HL, Liao Y, Fries JF, Greenland P. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295(2):190–198. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. American Heart Association, Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Van Guilder GP, Hoetzer GL, Smith DT, Irmiger HM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab. 2005;289(5):E807–E813. doi: 10.1152/ajpendo.00072.2005. [DOI] [PubMed] [Google Scholar]
- 4.Van Guilder GP, Hoetzer GL, Dengel DR, Stauffer BL, DeSouza CA. Impaired endothelium-dependent vasodilation in normotensive and normoglycemic obese adult humans. J Cardiovasc Pharmacol. 2006;47(2):310–313. doi: 10.1097/01.fjc.0000205097.29946.d3. [DOI] [PubMed] [Google Scholar]
- 5.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46(1):7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der ZR, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87(9):728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 9.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105(25):3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 11.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 12.Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, Goldschmidt-Clermont PJ, Dong C, Taylor DA, Peterson ED. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152(1):190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo Y, Imanishi T, Hayashi Y, Tomobuchi Y, Kubo T, Hano T, Akasaka T. The effect of senescence of endothelial progenitor cells on in-stent restenosis in patients undergoing coronary stenting. Intern Med. 2006;45(9):581–587. doi: 10.2169/internalmedicine.45.1663. [DOI] [PubMed] [Google Scholar]
- 14.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 15.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 16.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 17.Lohman T, Roche A, Mortorell R. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL: 1988. [Google Scholar]
- 18.DeSouza CA, Jones PP, Seals DR. Physical activity status and adverse age-related differences in coagulation and fibrinolytic factors in women. Arterioscler Thromb Vasc Biol. 1998;18(3):362–368. doi: 10.1161/01.atv.18.3.362. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 21.Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102(3):847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 22.Meyers MR, Gokce N. Endothelial dysfunction in obesity: etiological role in atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2007;14(5):365–369. doi: 10.1097/MED.0b013e3282be90a8. [DOI] [PubMed] [Google Scholar]
- 23.Shankar SS, Steinberg HO. Obesity and endothelial dysfunction. Semin Vasc Med. 2005;5(1):56–64. doi: 10.1055/s-2005-871742. [DOI] [PubMed] [Google Scholar]
- 24.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82(10):671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 25.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de KS, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26(9):2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 26.Lev EI, Kleiman NS, Birnbaum Y, Harris D, Korbling M, Estrov Z. Circulating endothelial progenitor cells and coronary collaterals in patients with non-ST segment elevation myocardial infarction. J Vasc Res. 2005;42(5):408–414. doi: 10.1159/000087370. [DOI] [PubMed] [Google Scholar]
- 27.Dobert N, Britten M, Assmus B, Berner U, Menzel C, Lehmann R, Hamscho N, Schachinger V, Dimmeler S, Zeiher AM, Grunwald F. Transplantation of progenitor cells after reperfused acute myocardial infarction: evaluation of perfusion and myocardial viability with FDG-PET and thallium SPECT. Eur J Nucl Med Mol Imaging. 2004;31(8):1146–1151. doi: 10.1007/s00259-004-1490-4. [DOI] [PubMed] [Google Scholar]
- 28.Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27(18):2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Ehmsen J, Braun D, Schneider T, Pfister R, Worm N, Wielckens K, Scheid C, Frommolt P, Flesch M. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J. 2008;29(12):1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- 30.McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105(23):2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz MB, Biyikoglu SF, Akin Y, Guray U, Kisacik HL, Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord. 2003;27(12):1541–1545. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]
- 32.Wee CC, Girotra S, Weinstein AR, Mittleman MA, Mukamal KJ. The relationship between obesity and atherosclerotic progression and prognosis among patients with coronary artery bypass grafts the effect of aggressive statin therapy. J Am Coll Cardiol. 2008;52(8):620–625. doi: 10.1016/j.jacc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Van Guilder GP, Stauffer BL, Greiner JJ, DeSouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol. 2008;294(4):H1685–H1692. doi: 10.1152/ajpheart.01281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rea TD, Heckbert SR, Kaplan RC, Psaty BM, Smith NL, Lemaitre RN, Lin D. Body mass index and the risk of recurrent coronary events following acute myocardial infarction. Am J Cardiol. 2001;88(5):467–472. doi: 10.1016/s0002-9149(01)01720-9. [DOI] [PubMed] [Google Scholar]
- 35.DeSouza CA, Van Guilder GP, Greiner JJ, Smith DT, Hoetzer GL, Stauffer BL. Basal endothelial nitric oxide release is preserved in overweight and obese adults. Obes Res. 2005;13(8):1303–1306. doi: 10.1038/oby.2005.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, Stewart DJ, Kutryk MJ. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109(17):2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 37.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109(3):337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 39.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197(2):496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 41.Sbarbati R, de BM, Marzilli M, Scarlattini M, Rossi G, van Mourik JA. Immunologic detection of endothelial cells in human whole blood. Blood. 1991;77(4):764–769. [PubMed] [Google Scholar]
- 42.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14(8):318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 44.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35(7):1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Distler JH, Allanore Y, Avouac J, Giacomelli R, Guiducci S, Moritz F, Akhmetshina A, Walker UA, Gabrielli A, Muller-Ladner U, Tyndall A, Matucci-Cerinic M, Distler O. EUSTAR statement and recommendations on endothelial precursor cells. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.091918. [DOI] [PubMed] [Google Scholar]
- 46.Timmermans F, Van HF, De SM, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27(7):1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 47.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24(8):1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 48.Diller GP, van ES, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bedard E, Gibbs JS, Bauersachs J, Hobbs AJ, Wilkins MR, Gatzoulis MA, Wharton J. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117(23):3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 49.Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;114(1 Suppl):I114–I119. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- 50.Hristov M, Fach C, Becker C, Heussen N, Liehn EA, Blindt R, Hanrath P, Weber C. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis. 2007;192(2):413–420. doi: 10.1016/j.atherosclerosis.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 51.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108(3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shantsila E, Watson T, Tse HF, Lip GY. Endothelial colony forming units: are they a reliable marker of endothelial progenitor cell numbers? Ann Med. 2007;39(6):474–479. doi: 10.1080/07853890701329283. [DOI] [PubMed] [Google Scholar]
- 53.Tura O, Barclay GR, Roddie H, Davies J, Turner ML. Absence of a relationship between immunophenotypic and colony enumeration analysis of endothelial progenitor cells in clinical haematopoietic cell sources. J Transl Med. 2007;5:37. doi: 10.1186/1479-5876-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill JM, Finkel T, Quyyumi AA. Endothelial progenitor cells and endothelial dysfunction. Vox Sang. 2004;87(Suppl 2):31–37. doi: 10.1111/j.1741-6892.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 55.Quyyumi AA. Circulating endothelial progenitor cells as novel biological determinants of vascular function and risk. Can J Cardiol. 2004;20(Suppl B):44B–48B. [PubMed] [Google Scholar]
- 56.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007 doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 57.Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, Kim TY, Kim JY, Kang HJ, Chae IH, Oh BH, Park YB, Kim HS. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116(15):1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 58.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 59.Ciulla MM, Giorgetti A, Silvestris I, Cortiana M, Montelatici E, Paliotti R, Annoni GA, Fiore AV, Giordano R, De MF, Magrini F, Rebulla P, Cortelezzi A, Lazzari L. Endothelial colony forming capacity is related to C-reactive protein levels in healthy subjects. Curr Neurovasc Res. 2006;3(2):99–106. doi: 10.2174/156720206776875876. [DOI] [PubMed] [Google Scholar]
- 60.Imanishi T, Hano T, Sawamura T, Nishio I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31(7):407–413. doi: 10.1111/j.1440-1681.2004.04022.x. [DOI] [PubMed] [Google Scholar]