Abstract
While it is known that the arachidonic acid metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) contributes to ischemic injury in the heart and brain, its role in kidney injury is unclear. Here we determined the effects on ischemia-reperfusion injury of the 20-HETE analogues, 20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid (5,14−20-HEDE), and N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14−20-HEDGE), and of the inhibitor of 20-HETE synthesis N-hydroxy-N-(4-butyl-2 methylphenyl) formamidine (HET0016). Using Sprague-Dawley rats we found that while treatment with the inhibitor exacerbated renal injury, infusion of both 5,14−20-HEDE and 5,14−20-HEDGE significantly attenuated injury when compared to vehicle or inhibitor-treated rats. Medullary blood flow, measured by laser-Doppler flowmetry, decreased to half of the baseline one hour after reperfusion in the control rats, but 5,14−20-HEDGE completely prevented this. Treatment of control animals with 5,14−20-HEDGE increased urine output and sodium excretion without altering their mean arterial pressure or glomerular filtration rate. Our results suggest that 20-HETE analogues protect the kidney from ischemia-reperfusion injury by inhibiting renal tubular sodium transport and preventing the post-ischemic fall in medullary blood flow. Analogues of 20-HETE may be useful in the treatment of acute ischemic kidney injury.
Keywords: 20-HETE, acute kidney injury, acute renal failure, HET0016, ischemia-reperfusion
Ischemic acute kidney injury contributes to kidney dysfunction and patient morbidity in a variety of clinical settings ranging from cardiovascular surgery to kidney transplantation.1 The renal injury following ischemia-reperfusion (I/R) results from multiple factors involving both changes in renal hemodynamics and injury to tubular epithelial cells.2 Acute ischemia leads to adenosine triphosphate depletion and initiates tubular cell injury. Following reperfusion, this injury is exacerbated by leukocyte activation, cytokine production, and an increase in reactive oxygen species.3 This may result in renal tubular epithelial cell necrosis or activation of apoptotic pathways in susceptible nephron segments.4 Alterations in renal hemodynamics following I/R have been well characterized and include loss of normal vascular reactivity and renal autoregulation, endothelial cell injury, red blood cell trapping, and impaired medullary blood flow (MBF).2,5-7 Compromised MBF in particular is thought to further ischemic injury because oxygen supply is inadequate to support the ongoing metabolic demands of tubular transport.2
Ischemic insults may activate membrane bound phospholipase A2 which stimulates the release of arachidonic acid (AA) from membrane phospholipids.8 Depending on the cell type, AA can then be metabolized into prostaglandins, leukotrienes, and 5-,12-, and 15-HETE by cyclooxygenases and lipoxygenases.9 The cytochrome P450 (CYP) system provides an additional pathway for AA metabolism. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a product of CYP ω-hydroxylase-mediated metabolism of AA and is produced in the kidney where it serves as an important regulator of vascular tone, renal blood flow (RBF), renal tubular sodium transport, and activates a number of intracellular signal transduction pathways involved in cell growth and survival.9,10 Recent studies have found that 20-HETE contributes to ischemic injury in the heart and brain and that inhibitors of the synthesis of 20-HETE reduce infarct size after reperfusion injury in both of these organs.10-13
Little is known about the function of 20-HETE in renal I/R injury. This is partially due to the lack of specific inhibitors of this pathway or molecular tools that can inhibit the CYP4A and −4F isoforms that produce 20-HETE in the kidney. At present, seven isoforms are known to produce renal 20-HETE which limits the utility of knockout or siRNA techniques that target a single isoform.9 However, the recent development of synthetic 20-HETE analogues has stimulated investigation into the function of 20-HETE in various disease models.14,15 This study examined the effects of stable 20-HETE analogues on the severity of experimental renal I/R injury in rats and explored their effect on renal sodium reabsorption and postischemic changes in renal hemodynamics.
RESULTS
Plasma concentrations of 20-HETE analogues following subcutaneous injection
Plasma concentrations of 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14−20-HEDE) and N-(20-hydroxyeicosa-5(Z),14(Z)-dienoyl)glycine (5,14−20-HEDGE) at 1, 4, 8, and 24 h after injection are presented in Figure 1. Peak blood levels were 366.9±31.46 and 253.6±39.87 ng/ml for 5,14−20-HEDE and 5,14−20-HEDGE, respectively (P = 0.11). The half-lives of the two analogues were similar and averaged approximately 4 h.
Figure 1. Chemical structures of 20-HETE analogues and plasma concentrations after subcutaneous injection.
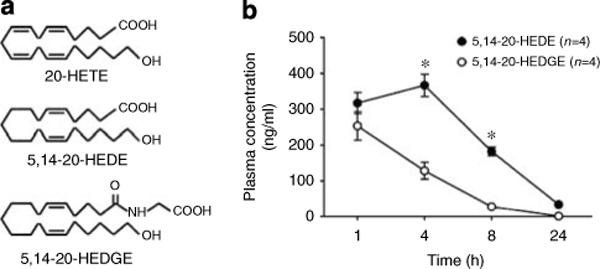
(a) Chemical structures of 20-HETE and the 20-HETE analogues 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14−20-HEDE) and N-(20-hydroxyeicosa-5(Z),14(Z)-dienoyl)glycine (5,14−20-HEDGE). (b) Time course of plasma levels of 20-HETE analogues following 10 mg/kg s.c. injection of 5,14−20-HEDE or 5,14−20-HEDGE in rats. *P < 0.001 vs the corresponding value in the 5,14−20-HEDGE-treated group.
Effect of 20-HETE on renal I/R injury
In vehicle-treated control rats, plasma creatinine increased from 0.5±0.05 to 2.8±0.3 mg/100 ml 24 h after ischemia (Figure 2). Blockade of the formation of 20-HETE by HET0016 exacerbated renal dysfunction as indicated by a significant increase in plasma creatinine levels. Pretreatment of the rats with the 20-HETE analogue, 5,14−20-HEDE (10 mg/kg), reduced plasma creatinine by approximately 50%. 5,14−20-HEDGE appears to be more efficacious in that a 10-fold lower dose (1 mg/kg) was able to almost completely prevent the rise in plasma creatinine 24 h following renal I/R injury. Furthermore, 5,14−20-HEDGE also mitigated renal I/R injury when administered after reperfusion (5,14−20-HEDGE group B).
Figure 2. Effect of 20-HETE analogues on renal dysfunction 24 h after renal I/R injury.
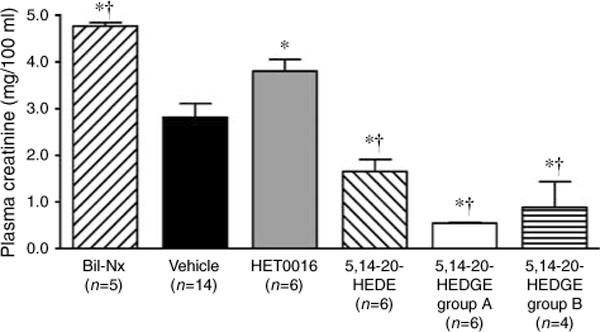
Rats were pretreated with vehicle; HET0016 (5 mg/kg s.c.), an inhibitor of the synthesis of 20-HETE; or the 20-HETE analogues 5,14−20-HEDE (10 mg/kg s.c.) or 5,14−20-HEDGE group A (1 mg/kg s.c.). 5,14−20-HEDGE group B received 5,14−20-HEDGE (1 mg/kg s.c.) 1 min after reperfusion. The bilateral nephrectomy group (Bil-Nx) served as an absolute renal failure control. Mean values ± s.e. are presented. *P < 0.05 vs vehicle control group. †P < 0.05 vs HET0016 group.
Effects of 20-HETE analogues on renal tubular injury and apoptosis following I/R
The histological appearance of tubular injury at the corticomedullary junction is presented in Figure 3. Fluorescence microscopy of the hematoxylin and eosin (H&E)-stained sections demonstrates distinct autofluorescence of necrotic tubules and tubular casts (Figures 3a and b). Diffuse tubular cell denudation, tubular cell necrosis, intratubular debris, and tubular casts were present in vehicle and HET0016-treated animals (Figure 3c). Administration of a 20-HETE analogue (5,14−20-HEDE or 5,14−20-HEDGE) resulted in less severe renal injury with only focal tubular necrosis or exfoliation of tubular cells (Figure 3c). The degree of renal injury was further quantified by morphometric analysis of autofluorescence in H&E-stained sections. Administration of a 20-HETE analogue (5,14−20-HEDE or 5,14−20-HEDGE) resulted in a marked reduction in the area occupied by necrotic tubular epithelium or cast material compared to vehicle- or HET0016-treated animals (Figure 3d). Furthermore, pretreatment with a 20-HETE analogue markedly reduced the number of apoptotic cells identified by terminal transferase dUTP nick-end labeling (TUNEL) staining compared to vehicle-treated controls (Figure 4).
Figure 3. Renal histology 24 h after renal I/R injury.
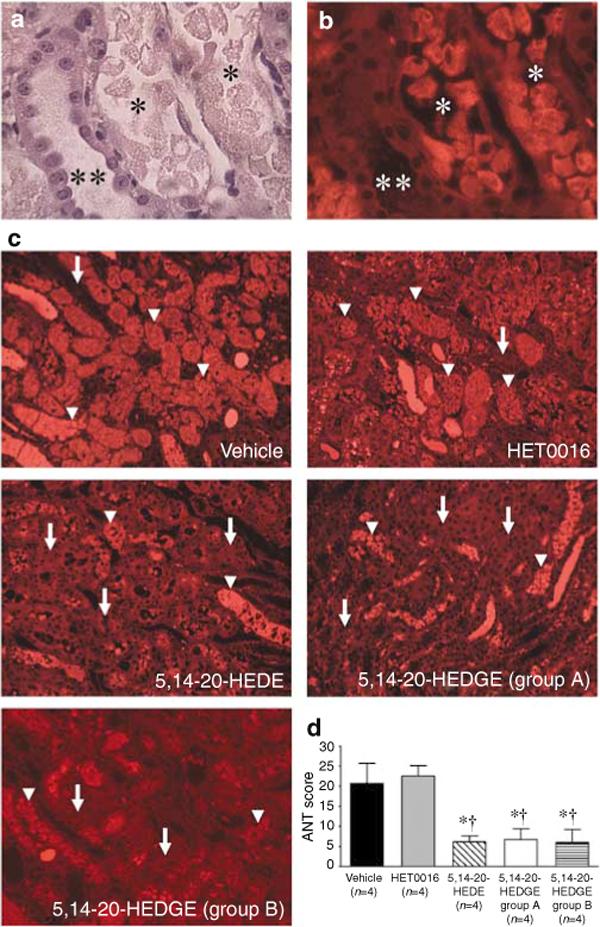
(a) Hematoxylin and eosin (H&E)-stained sections demonstrating intact (**) and necrotic (*) tubular epithelium following I/R injury. (b) Fluorescence microscopy of the same H&E-stained field. The necrotic tubules (*) exhibit marked autofluorescence compared to the intact tubule (**). Original magnification, × 400. (c) Fluorescence microscopy of representative H&E-stained cross-sections of the outer medulla is presented. Necrotic tubules (arrowheads) exhibit marked autofluorescence compared to intact tubules (arrows). Original magnification, × 100. (d) Tubular injury scores 24 h after renal I/R injury. Administration of the 20-HETE analogues, 5,14−20-HEDE or 5,14−20-HEDGE, resulted in less severe renal injury compared to vehicle controls or rats treated with HET0016. Mean values ± s.e. are presented. *P < 0.05 vs vehicle-treated group. †P < 0.05 vs HET0016.
Figure 4. Effect of the 20-HETE analogue, 5,14−20-HEDGE, on apoptosis 24 h after renal I/R injury.
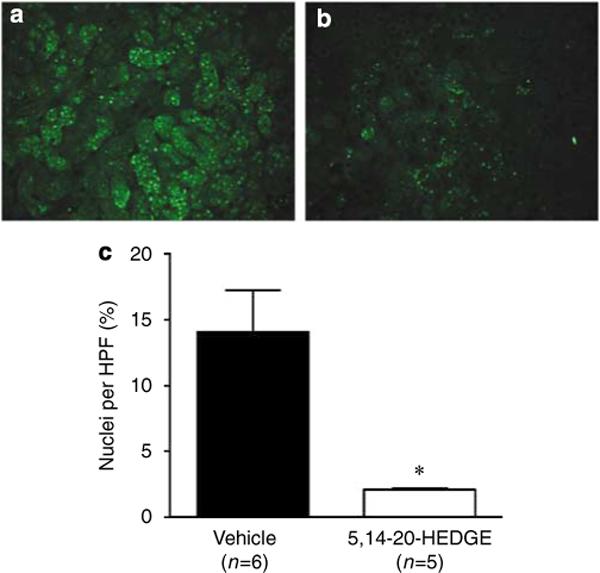
Apoptosis of renal tubular epithelial cells was assessed by TUNEL staining. (a) Vehicle treated. (b) 20-HETE analogue treated. (c) The number of apoptotic nuclei was significantly less in rats treated with the 20-HETE analogue, 5,14−20-HEDGE, when compared to vehicle-treated controls. Mean values ± s.e. are presented. *P < 0.05 vs vehicle-treated group.
Effect of the 20-HETE analogue, 5,14−20-HEDGE, on renal hemodynamics
Baseline cortical blood flow (CBF) and MBF were similar in the rats that received vehicle or 5,14−20-HEDGE. 5,14−20-HEDGE did not have a significant effect on CBF or MBF before the induction of renal ischemia. As expected, CBF and MBF fell by greater than 90% in both groups during the ischemic period and recovered after reperfusion. In vehicle-treated rats, MBF decreased to approximately 50% of baseline by 60 min following reperfusion and remained at that level throughout the remainder of the experiment (Figure 5b), which was equivalent to 3 h after reperfusion. In contrast, pretreatment of the rats with 5,14−20-HEDGE prevented the postischemic fall in MBF (Figure 5b). 5,14−20-HEDGE had no effect on postischemic CBF (Figure 5a) or mean arterial pressure (MAP) (Figure 5c) when compared to vehicle-treated rats.
Figure 5. Effect of the 20-HETE analogue, 5,14−20-HEDGE, on renal hemodynamics after renal I/R injury.
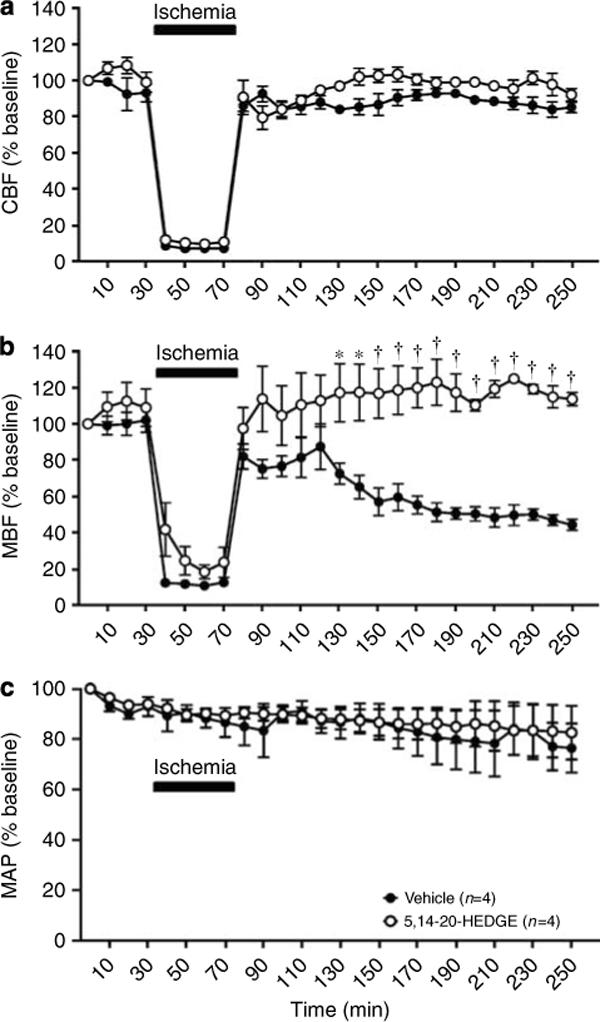
(a) The effect of 5,14−20-HEDGE (1 mg/kg s.c.) on cortical blood flow (CBF) in rats during and following renal I/R injury. (b) The effect of 5,14−20-HEDGE (1 mg/kg s.c.) on medullary blood flow (MBF) in rats during and following renal I/R injury. (c) The effect of 5,14−20-HEDGE (1 mg/kg s.c.) on mean arterial pressure (MAP) in rats during and following renal I/R injury. Values are represented as mean ± s.e. *P < 0.05 vs the corresponding value in the vehicle-treated group. †P < 0.001 vs the corresponding value in the vehicle-treated group.
In nonischemic rats, administration of 5,14−20-HEDGE did not significantly alter MAP, RBF, glomerular filtration rate (GFR), or urine potassium excretion when compared to control values (Figures 6 and 7). However, administration of 5,14−20-HEDGE was associated with significant increases in urine flow and urine sodium excretion compared to control values (Figure 7).
Figure 6. Effect of the 20-HETE analogue, 5,14−20-HEDGE, on renal hemodynamics in nonischemic rats.
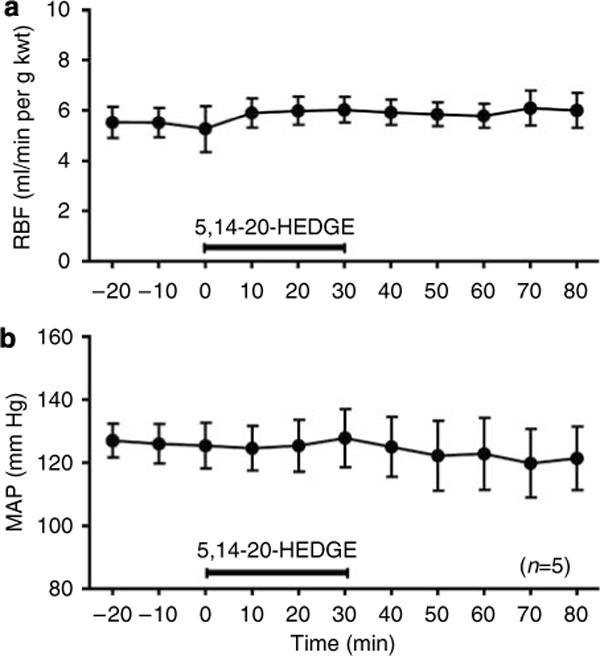
The effect of 5,14−20-HEDGE (1 mg/kg i.v.) on (a) renal blood flow (RBF) and (b) mean arterial pressure (MAP) in nonischemic rats. Mean values ± s.e. are presented.
Figure 7. Effect of the 20-HETE analogue, 5,14−20-HEDGE, on urine flow and urine sodium excretion in nonischemic rats.
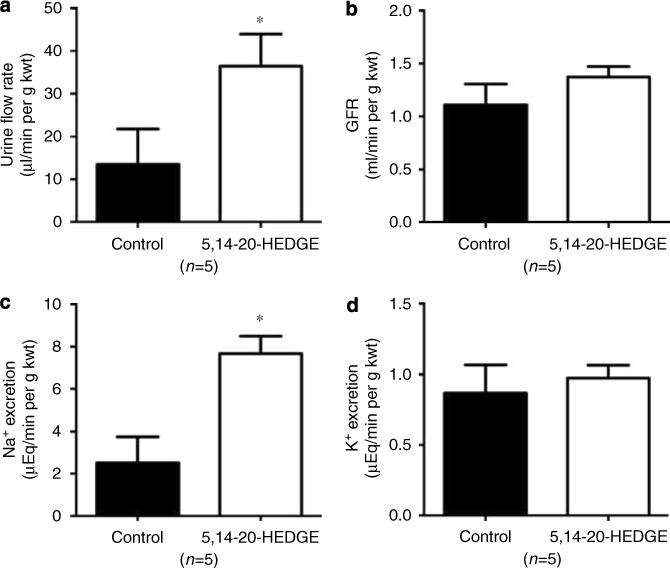
The effect of 5,14−20-HEDGE (1 mg/kg i.v.) on (a) urine flow rate, (b) glomerular filtration rate (GFR), (c) urine sodium excretion, and (d) urine potassium excretion in nonischemic rats is presented. Mean values ± s.e. are presented. *P < 0.05 vs control.
DISCUSSION
This study examined the function of 20-HETE in renal I/R injury. The results indicate that blockade of the formation of 20-HETE with HET0016 exacerbated renal dysfunction following I/R injury whereas treatment of rats with two analogues of 20-HETE mitigated renal dysfunction and tubular injury compared to control or HET0016-treated rats. Although the mechanism of action remains to be determined, this study indicates that the 20-HETE analogue, 5,14−20-HEDGE, promotes sodium excretion in the absence of changes in MAP, RBF, or GFR and it prevents the postischemic fall in MBF. Taken together, the present findings suggest that 20-HETE analogues may mitigate renal ischemia by inhibition of renal tubular sodium transport and by preserving postischemic MBF. Notably, 5,14−20-HEDGE was more efficacious than 5,14−20-HEDE even though it was administered at a 10-fold lower dose. The increased efficacy of 5,14−20-HEDGE might be related to the addition of the glycine group that may help the compound enter cells or prolong its action by protecting it from β-oxidation.
Previous studies have demonstrated a pathologic function for 20-HETE and CYP ω-hydroxylase in ischemic injury to the heart and brain. In this regard, selective inhibition of CYP ω-hydroxylase protects myocardium from ischemic injury and inhibition of 20-HETE production counteracts cerebral vasospasm and reduces infarct size in animal models of ischemic stroke.10-13 On the other hand, the function of CYPAA metabolites and 20-HETE in acute kidney injury has not been well characterized. Prior studies in the rat have shown that I/R results in a decrease in renal CYP4A2 ω-hydroxylase expression and microsomal conversion of AA to 20-HETE.16,17 This was suggested to be an adaptive response that might attenuate postischemic vasoconstriction as 20-HETE potentiates vasoconstriction of preglomerular vessels.17 However, these studies did not associate the reductions in CYP ω-hydroxylase activity with the severity of renal dysfunction or injury following ischemia. On the other hand, Portilla et al.18 demonstrated that pretreatment of rats with clofibrate to induce CYP4A expression and renal 20-HETE production, reduced renal dysfunction following I/R injury. These findings were the first to suggest a protective function of 20-HETE in renal I/R injury. However, clofibrate is also an activator of peroxisome proliferator-activated receptor-α that may afford protection from I/R by inhibiting neutrophil infiltration, cytokine production, and oxidative stress within the injured kidney.19 The present results are consistent with the findings of Portilla et al. and support a distinct protective function for 20-HETE in renal I/R and suggest that the decrease in renal CYP ω-hydroxylase activity and 20-HETE production following ischemia is maladaptive and contributes to renal injury and dysfunction.
The protective effect of 20-HETE analogues may result from more than one mechanism as administration of 5,14−20-HEDGE mitigated renal I/R when given before, or after, the ischemic interval. 20-HETE modulates vascular responsiveness to vasoconstrictors and administration of a 20-HETE analogue would be expected to reduce RBF and have a detrimental effect on renal I/R injury. However, the effects of 20-HETE may differ in the cortical and medullary circulation. In this regard, 20-HETE has been shown to increase renal MBF in a dose-dependent manner in rats.20 Furthermore, 20-HETE had a greater effect on medullary vasodilation than on cortical vasoconstriction.20 Several studies have established the importance of impaired MBF in the pathogenesis of renal I/R injury in the rat. For example, Vetterlein et al.7 utilized fluorescent tracers to assess postischemic blood flow and demonstrated a marked reduction in medullary plasma flow 1 h after of reperfusion. The present findings are consistent with prior studies that utilized LDF to assess postischemic MBF. Olof et al.21 found that MBF markedly decreased following 60 min of renal ischemia. Conesa et al.22 also demonstrated a decrease in MBF after renal ischemia. Furthermore, they demonstrated that N-acetyl-l-cysteine improved MBF after reperfusion and this directly correlated with preservation of renal function.22 In this context, we speculate that preservation of post-ischemic MBF is at least partially responsible for the protective effect of 20-HETE analogues demonstrated in this study.
Prevention of renal medullary hypoxia by inhibition of tubular sodium transport might also contribute to the renoprotective effect of 20-HETE analogues in I/R injury. In this study, administration of 5,14−20-HEDGE to rats resulted in a significant increase in urine sodium excretion without changing GFR or MAP, suggesting that it inhibits tubular reabsorption of sodium. These data are consistent with published reports demonstrating that inhibitors of 20-HETE synthesis blunt pressure natriuresis and promote sodium retention.23 Furthermore, 20-HETE has been shown to inhibit Na+ -K+ -ATPase and sodium transport in both the proximal tubule and medullary thick ascending loop of Henle.20,24,25 Thus, it is possible that 20-HETE analogues attenuate medullary hypoxia by decreasing oxygen demand during the ischemic period, thereby ameliorating renal dysfunction in a manner similar to other inhibitors of tubular electrolyte transport.26 For example, the selective dopamine receptor-1 agonist Fenoldopam increases sodium excretion similar to 20-HETE, but in contrast, is vasodilatory, decreasing systemic blood pressure while increasing RBF.27 Fenoldopam has been clinically tested for the prevention or treatment of acute kidney injury but the results have been inconclusive.28
This study did not explore and cannot exclude other mechanisms that may contribute to the renal protective actions of 20-HETE analogues in renal I/R injury. Administration of a 20-HETE analogue might be expected to mitigate inflammation following I/R as 20-HETE was recently shown to activate proliferator-activated receptor-α.29 Other pleiotropic actions of 20-HETE may include modulation of the inflammatory response to I/R by blocking platelet and neutrophil aggregation within the injured kidney as well mediating vasoprotection by stimulating mitogenesis and angiogenesis.9,30
CONCLUSION
In contrast to previous findings in the heart and brain, the results of this study indicate that 20-HETE may exert protective effects in the kidney following I/R injury. The protection afforded by 20-HETE analogues in renal I/R injury may be due to preservation of renal medullary oxygen supply by inhibition of tubular sodium reabsorption and prevention of the postischemic fall in MBF. Our finding that the 20-HETE analogue, 5,14−20-HEDGE, mitigated renal I/R injury when administered after the ischemic period greatly increases the clinical relevance of this study. Overall, these findings provide a basis for the development of 20-HETE analogues as novel preventative or therapeutic agents in ischemic acute kidney injury.
MATERIALS AND METHODS
General
Experiments were performed on 62 male Sprague–Dawley (SD) rats weighing 250−300 g that were purchased from Taconic Farms (Germantown, NY, USA). The rats were housed in an Animal Care Facility at the Medical College of Wisconsin, which is approved by the American Association for the Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
20-HETE analogues
The chemical structures of the 20-HETE analogues used in this study are shown in Figure 1. These analogues have improved stability in vitro compared to endogenous 20-HETE. The glycine substitution renders 5,14−20-HEDGE less susceptible to β-oxidation and the absence of the double bonds across the 8,9- and 11,12-carbons prevents its degradation by cyclooxygenase.31
Measurement of plasma levels of the 20-HETE analogues
Initial experiments were performed to assess the pharmacokinetics of the 20-HETE analogues. In these experiments, a chronic polyvinyl catheter was implanted into the jugular vein and exteriorized at the back of the neck of SD rats several days before the experiment. After a 3-day recovery period, the rats (n = 4 in each group) received an s.c. injection of either 5,14−20-HEDE (10 mg/kg) or 5,14−20-HEDGE (10 mg/kg) and blood samples were collected 1, 4, 8, and 24 h after administration. Plasma levels of 5,14−20-HEDE and 5,14−20-HEDGE were measured by liquid chromatography–mass spectrometry using an Applied Biosystems API 3000 LC/MS/MS (Foster City, CA, USA).
Renal ischemia-reperfusion injury model
This set of experiments was performed on six groups of rats. Group 1 underwent bilateral nephrectomy and served as an absolute renal failure control group. Rats in the following groups were pretreated with an s.c. injection of vehicle or one of three treatments, 30 min before induction of renal ischemia: group 2 received 1 ml/kg of the vehicle used for all experiments (11% sulfobutyl-β-cyclodextrin in 165 mm mannitol solution); group 3 received HET0016 (5 mg/kg), a selective inhibitor of the formation of 20-HETE;23 group 4 received the 20-HETE analogue, 5,14−20-HEDE (10 mg/kg); group 5 (5,14−20-HEDGE group A) received the 20-HETE analogue, 5,14−20-HEDGE (1 mg/kg).15,31 Group 6 (5,14−20-HEDGE group B) received 5,14−20-HEDGE (1 mg/kg, s.c.) 1 min after reperfusion.
The rats were anesthetized with ketamine (50 mg/kg, i.m.) and sodium pentobarbital (50 mg/kg, i.p) and were placed on a heated surgical table to maintain body temperature at 37 °C. A midline abdominal incision was made to expose the kidneys and the renal arteries and veins were bilaterally occluded for 30 min using microvascular clamps. The clamps were then removed, the abdominal incision was closed and the rats were allowed to recover for 24 h. Rats were then killed with sodium pentobarbital (100 mg/kg, i.p.), blood was collected from the aorta for measurement of plasma creatinine concentration using the Jaffe reaction and both kidneys were collected for histological analysis.
Analysis of the degree of tubular injury
The kidneys were fixed in 10% formalin and paraffin sections (3 μm) were prepared and stained with H&E. Eosin autofluorescence is intensified in necrotic tissue.32 The sections were examined using an Olympus BHT-2 (Olympus, Center Valley, PA, USA) epifluorescent microscope equipped with a 540-nm excitation filter and a 590-nm emission filter. For each specimen, five randomly chosen outer medullary fields were photographed using a digital color camera ( × 100 total magnification) and following background thresholding, the percentage of the area containing fluorescent necrotic tubular epithelium or cast material was quantified using Image-Pro Plus image analysis software (version 6.2; Media Cybernetics Inc., Bethesda, MD, USA). The severity of tubular injury (acute tubular necrosis score) for each specimen was expressed as the mean area containing autofluorescent necrotic tubular epithelium or cast material. TUNEL labeling was also performed to assess the degree of apoptosis following renal I/R injury (ApopTag Plus In Situ Apoptosis Fluorescein Detection Kit; Chemicon International, Temecula, CA, USA), following the manufacturer's protocol. Apoptotic nuclei were visualized at × 10 by fluorescence microscopy and quantified by Metamorph analysis (Metamorph Imaging Series Software; Molecular Devices Corporation, Dowington, PA, USA). A total of five fields were analyzed per section, with one section analyzed per animal. Data were expressed as the percentage of TUNEL-positive nuclei per total number of 4′6-diamidino-2-phenyl indole-stained cells.
Assessment of renal hemodynamics
These experiments were performed to determine if the renal-protective effect of the 20-HETE analogue, 5,14−20-HEDGE, on I/R injury was associated with changes in renal medullary hemodynamics. Rats were anesthetized with ketamine (50 mg/kg, i.m.) and inactin (100 mg/kg, i.p.; Sigma, St. Louis, MO, USA). Catheters were placed in the femoral artery for continuous measurement of arterial pressure and in the femoral vein for i.v. infusions. The left kidney was then exposed and placed in a kidney holder and a single-mode optical fiber was implanted 4 mm into the kidney for measurement of MBF by laser-Doppler flowmetry as previously described.33 CBF was measured by a second laser-Doppler probe held in static position by a micromanipulator 1 mm above the renal cortex. After surgery and a 30 min stabilization period MAP, CBF, and MBF were continuously recorded using WinDaq data acquisition software (DATAQ Instruments Inc., Akron, OH, USA) during a 5 min control period. Baseline MAP, MBF, and CBF were determined and then 5,14−20-HEDGE (1 mg/kg, s.c.) or vehicle (1 ml/kg, s.c.) were administered. At 30 min later the blood supply to the left kidney was occluded for 45 min. MAP, MBF, and CBF were recorded during ischemia and for 3 h following reperfusion.
Additional experiments were performed in nonischemic rats to determine the effect of 5,14−20-HEDGE on MAP, RBF, GFR, and urinary sodium and potassium excretion. Rats were anesthetized with ketamine (50 mg/kg, i.m.) and inactin (100 mg/kg, i.p.) and placed on a heated table to maintain body temperature at 37 °C. Catheters were placed in the femoral artery for continuous measurement arterial pressure and in the femoral vein for i.v. infusions. The left kidney was then exposed and a catheter was placed in the left ureter for collection of urine. A 2.0-mm diameter flow probe was placed around the left renal artery for measurement of RBF using a small animal blood flowmeter (Transonic System, Ithaca, NY, USA). Rats received an i.v. infusion of 0.9% NaCl solution containing 1% bovine serum albumin and 2% FITC-Inulin (Sigma) at 100 μl/min throughout the experiment. After surgery and a 30 min stabilization period, urine and plasma samples were collected during a 40 min control period. 5,14−20-HEDGE (1 mg/kg, i.v.) was then infused over 30 min. After a 30 min equilibration period, urine and plasma samples were recollected over a 40 min period. MAP and RBF were continuously recorded using WinDaq data acquisition software. GFR was determined by the clearance of FITC-Inulin.34
Statistical analysis
Mean values ± s.e. are presented. The significance of differences in mean values between groups was analyzed using a one-way analysis of variance, repeated measures analysis of variance with Dunns post hoc test, or paired t-test. A P-value <0.05 was considered to be statistically significant.
ACKNOWLEDGMENTS
This work was partially supported by Grants HL36279, HL29546, DK38226, and GM31278 from the National Institutes of Health; the Robert A. Welch Foundation; and a Mentored Clinical/Translational Research Award from the Clinical and Translational Science Institute at the Medical College of Wisconsin. We thank Averia Steinman for assistance with the mass spectrometry analysis and Denise McCabe and Christopher Chaber for assistance with the TUNEL analysis.
Footnotes
DISCLOSURE
Anna Zuk, Meghan McMullen, and Steve Ledbetter are employees of Genzyme Corporation. There is no financial interest to disclose.
REFERENCES
- 1.Lamiere N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 4.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 5.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Tada T, Brodsky SV, et al. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol. 2002;282:F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- 7.Vetterlein F, Petho A, Schmidt G. Distribution of capillary blood flow in rat kidney during postischemic renal failure. Am J Physiol. 1986;251:H510–H519. doi: 10.1152/ajpheart.1986.251.3.H510. [DOI] [PubMed] [Google Scholar]
- 8.Chien KR, Han A, Sen A, et al. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation- reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984;54:313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- 9.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 10.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 11.Gross ER, Nithipatikom K, Hsu AK, et al. Cytochrome P450 [omega]-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. J Mol Cell Cardiol. 2004;37:1245–1249. doi: 10.1016/j.yjmcc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Gross GJ, Falck JR, Gross ER, et al. Cytochrome P450 and arachidonic acid metabolites: Role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68:18–25. doi: 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Nithipatikom K, Gross ER, Endsley MP, et al. Inhibition of cytochrome P450{omega}-hydroxylase: a novel endogenous cardioprotective pathway. Circ Res. 2004;95:e65–e71. doi: 10.1161/01.RES.0000146277.62128.6f. [DOI] [PubMed] [Google Scholar]
- 14.Yu M, Cambj-Sapunar L, Kehl F, et al. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Tunctan B, Korkmaz B, Buharalioglu CK, et al. A 20-hydroxyeicosatetraenoic acid agonist, N-[20-hydroxyeicosa-5(Z), 14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated rats. Shock. 2008;30:329–335. doi: 10.1097/SHK.0b013e31816471c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura Y, Imaoka S, Gemba M, et al. Effects of ischemia-reperfusion on individual cytochrome P450 isoforms in the rat kidney. Life Sci. 1996;60:143–149. doi: 10.1016/s0024-3205(96)00604-2. [DOI] [PubMed] [Google Scholar]
- 17.Hercule H, Oyekan A. Renal cytochrome P450 oxygenases and preglomerular vascular response to arachidonic acid and endothelin-1 following ischemia/reperfusion. J Pharmacol Exp Ther. 2002;302:717–724. doi: 10.1124/jpet.302.2.717. [DOI] [PubMed] [Google Scholar]
- 18.Portilla D, Dai G, Peters JM, et al. Etomoxir-induced PPARalpha-modulated enzymes protect during acute renal failure. Am J Physiol Renal Physiol. 2000;278:F667–F675. doi: 10.1152/ajprenal.2000.278.4.F667. [DOI] [PubMed] [Google Scholar]
- 19.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyekan AO. Differential effects of 20-hydroxyeicosatetraenoic acid on intrarenal blood flow in the rat. J Pharmacol Exp Ther. 2005;313:1289–1295. doi: 10.1124/jpet.104.080218. [DOI] [PubMed] [Google Scholar]
- 21.Olof P, Hellberg A, Kallskog O, et al. Red cell trapping and postischemic renal blood flow. Differences between the cortex, outer and inner medulla. Kidney Int. 1991;40:625–631. doi: 10.1038/ki.1991.254. [DOI] [PubMed] [Google Scholar]
- 22.Conesa EL, Valero F, Nadal JC, et al. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R730–R737. doi: 10.1152/ajpregu.2001.281.3.R730. [DOI] [PubMed] [Google Scholar]
- 23.Williams JM, Sarkis A, Lopez B, et al. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 24.Ominato M, Satoh T, Katz AI. Regulation of Na-K-ATPase activity in the proximal tubule: role of the protein kinase C pathway and of eicosanoids. J Membr Biol. 1996;152:235–243. doi: 10.1007/s002329900101. [DOI] [PubMed] [Google Scholar]
- 25.Yu M, Lopez B, Dos Santos EA, et al. Effects of 20-HETE on Na+ transport and Na+-K+-ATPase activity in the thick ascending loop of Henle. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2400–R2405. doi: 10.1152/ajpregu.00791.2006. [DOI] [PubMed] [Google Scholar]
- 26.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 27.Singer I, Epstein M. Potential of dopamine A-1 agonists in the management of acute renal failure. Am J Kidney Dis. 1998;31:743–755. doi: 10.1016/s0272-6386(98)70043-5. [DOI] [PubMed] [Google Scholar]
- 28.Landoni G, Biondi-Zoccai GG, Tumlin JA, et al. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007;49:56–68. doi: 10.1053/j.ajkd.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Ng VY, Huang Y, Reddy LM, et al. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos. 2007;35:1126–1134. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 30.Chen P, Guo M, Wygle D, et al. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am J Pathol. 2005;166:615–624. doi: 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso-Galicia M, Falck JR, Reddy KM, et al. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol. 1999;277:F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 32.von Overbeck J, Saraga P, Gardiol D. An autofluorescence method for the diagnosis of early ischaemic myocardial lesions. A systematic study on 732 autopsies, including 182 cases of sudden death. Virchows Arch A Pathol Anat Histopathol. 1986;409:535–542. doi: 10.1007/BF00705423. [DOI] [PubMed] [Google Scholar]
- 33.Zou AP, Muirhead EE, Cowley AW, et al. Role of changes in renal hemodynamics and P-450 metabolites of arachidonic acid in the reversal of one-kidney, one clip hypertension. J Hypertens. 1995;13:557–566. doi: 10.1097/00004872-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol. 1999;276:F172–F177. doi: 10.1152/ajprenal.1999.276.1.F172. [DOI] [PubMed] [Google Scholar]


