Abstract
Melanin synthesis affects melanoma behavior and tumor responsiveness to therapy; therefore, we investigated metabolic changes in melanoma cells after induction of melanogenesis. Amelanotic and melanotic melanoma cells were labeled with 13C precursors and changes in their metabolism was analyzed by high-resolution magic angle spinning (HRMAS) nuclear magnetic resonance (NMR). HRMAS NMR demonstrated clear differences in the pattern of metabolic intermediates between amelanotic and melanotic cells. Although the exact nature of the metabolites requires further investigations, our comparative studies clearly show that induction of melanogenesis is associated with changes of glucose and sodium acetate metabolism, demonstrating HRMAS NMR as a powerful and noninvasive technique to investigate such process.
Keywords: HRMAS NMR, Melanogenesis, Melanoma, Metabolic changes, Stable isotope labeling
Melanogenesis plays an important role in the protection against damaging effects of solar radiation and in the organism adaptation to the changing environment, whereas melanogenic activity could serve as a cutaneous molecular sensor and transducer of noxious signals and regulator of local homeostasis [1–3]. Active melanogenesis can regulate behavior of melanoma cells [2], potentially affecting the outcome of therapy [4]. However, metabolic principles underlying this process in vivo are still poorly understood. High-resolution magic angle spinning (HRMAS)1 nuclear magnetic resonance (NMR) spectroscopy with stable isotope labeling is a non-destructive technique that can trace metabolites involved in multiple biological pathways [5–9]. By spinning at the magic angle (54.7°), the contribution from restriction in molecular motions, magnetic susceptibility, and chemical shift anisotropy to the NMR spectral broadening is reduced significantly [8,10]. Using HRMAS NMR and well-defined melanoma models in which melanogenesis can be induced by increased concentrations of a single factor, l-tyrosine [11,12], we investigated changes in cellular metabolism occurring after induction of melanogenesis in live cells.
Human SKMEL-188 and hamster AbC1 melanoma cells were cultured in Ham’s F10 medium (containing 10 µM l-tyrosine) supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD, USA) and 1% penicillin/streptomycin/amphotericin (Sigma–Aldrich, St. Louis, MO, USA). Melanin synthesis was induced by the addition of l-tyrosine to a final concentration of 400 µM, as described previously [11,12]. [U-13C]glucose, [2-13C] sodium acetate, and deuterium oxide (D2O) were obtained from Cambridge Isotope Laboratories (Andover, MA, USA). Labeling with [13C]glucose was done using [U-13C]glucose added to final concentration of 5 g/L (27.8 mM) for the final 24 h of cell culture before harvesting. For labeling with acetate, cells were incubated for 3 days with 5 mM [2-13C]acetate before harvesting.
HRMAS NMR experiments were performed on a Varian Inova 500-MHz spectrometer equipped with a 4-mm gHx NanoProbe (Varian NMR, Palo Alto, CA, USA). After harvesting, the cells were washed three times and resuspended in ice-cold PBS buffer made from D2O. Here 40 µl of cell suspension (~ 1 × 107 cells) being loaded into a glass rotor and sealed. The PBS/D2O buffer was prepared with the same composition of a normal PBS buffer (pH 7.2) except using D2O instead of water as solvent. Although we could not reliably measure the ‘‘pH” (actually pD) value for this buffer with a standard pH meter, because the difference in dissociation constant of water (pKw = 13.99) and D2O (pKw = 14.87) is small [13], the ‘‘pH” value of this PBS/D2O buffer will be very close to 7.2. Rotor speed was set to 2.5 kHz, and data were recorded at 295 K. Water-suppressed one-dimensional (1D) proton spectra were acquired using a rotor-synchronized Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence with a total delay of 40 ms. Two-dimensional (2D) 1H–13C heteronuclear single quantum correlation (HSQC) spectra were acquired using a rotor-synchronized pulse sequence with a recycle delay of 1 s and a total of 128 increments. For acetate-labeled black cells, 64 scans per increment were acquired due to weaker signals in these two samples (4 h acquisition for each sample). For acetate-labeled white cells and all glucose-labeled cells, 16 scans per increment were acquired due to their very strong signals (1 h acquisition for each sample). All data were transferred to a personal computer and processed with ACD Labs software (Toronto, Ontario, Canada). Peak intensities of selected peaks in 2D NMR spectra were labeled and further scaled based on number of scans for quantitative analysis among samples. Chemical shifts were referenced to the doublet of the methyl group in lactate at 1.33 ppm, and spectra assignments were performed based on literature reports [14].
1D proton NMR of melanoma cells in both normal media and 13C-enriched media were measured initially. These spectra strongly overlapped, thereby providing limited information on the metabolic changes associated with melanogenesis. The major changes in 1D analysis were decreased signal intensities corresponding to lactate for melanotic cells. Strong signal overlapping prevented unambiguous quantitative analysis. Therefore, we decided to employ a focused approach with 2D HSQC NMR to trace the metabolism of lipids and glucose by isotopic labeling with [2-13C]acetate and [U-13C]glucose, respectively. This approach allowed us to study these two important classes of metabolites in detail with greater confidence.
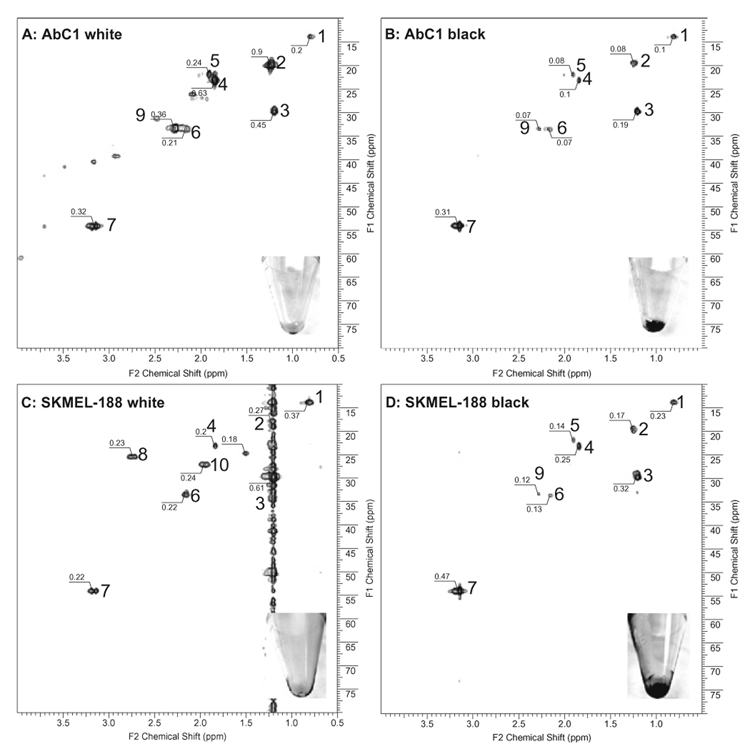
The insets in Fig. 1 show representative phenotypes of melanoma cells used for metabolic analyzes; for example, the cells were amelanotic when cultured in Ham’s F10 media (Figs. 1A and C: 16 scans, 128 increments), whereas the addition of 400 µM l-tyrosine resulted in production of melanin pigment (Fig. 1B and D: 64 scans, 128 increments). The absolute intensities for each peak are shown in the spectra. Note that peak intensities in Fig. 1B and D need to be scaled by a factor of 0.25 to have a valid comparison with Fig. 1A and C due to their greater number of scans (64 vs. 16). For example, the intensity of peak 1 in Fig. 1B should be 0.025 (apparent intensity of 0.1 times scale factor of 0.25) when we want to directly compare the same peak in Fig. 1A (intensity of 0.2). For AbC1 cells, all peak intensities in amelanotic cells are substantially higher than those in melanotic cells, ranging from 4-fold (peak 7) to more than 40-fold (peak 2). For SKMEL-188 cells, similar results are obtained, although the intensity differences were not as large as those for AbC1 cells. In addition, the number of detectable metabolites also decreased in melanotic cells (Fig. 1B and D) in comparison with amelanotic cells (Fig. 1A and C) labeled with [13C]acetate. The distributions of the metabolites were also different between melanotic and amelanotic cells for both cell lines. Due to the low natural abundance of 13C, only low-molecular-weight 13C-labeled metabolites incorporating the labeled acetate are expected to be detected in whole-cell HRMAS NMR under the experimental conditions. Therefore, two alternative explanations are offered. First, the overall metabolism associated with acetate transformation decreases during melanin synthesis, resulting in lower incorporation of labeled acetates. Alternatively, the metabolism involving acetates in melanin-synthesizing cells is greatly accelerated, leading to rapid incorporation of low-molecular-weight metabolites into macromolecules such as proteins and lipid components in cell membranes. Such macromolecules have highly restricted mobility and very low abundance expressed as very broad peaks with extremely low intensities in whole-cell NMR spectra. Because the melanin synthesis represents a cell differentiation program [2], we favor the second possibility. In fact, the comparison of detectable metabolites before (Fig. 1A) and after (Fig. 1B) melanin induction in AbC1 cells shows a clear decrease in concentration for acetate precursor (peak 4 in Fig. 1B), its initial metabolites (peaks 2 and 3), and mobile fatty acids (peaks 1, 6, 8, and 10) in melanotic cells. This suggested that a greater number of labeled small molecules were incorporated into macromolecules that cannot be detected. SKMEL-188 cells showed the same trend in the mobile fatty acids (peaks 1, 6, 8, and 10 in Fig. 1D) in which melanotic cells had a much lower intensity for these signals, indicating their further incorporation into macromolecules. There are also clearly detected differences in changes for both peak intensity and distribution after induction of melanogenesis between AbC1 and SKMEL-188 cells. This could be explained by intrinsic individual properties of these lines. Testing this hypothesis will require the detection of macromolecules using special media [15,16].
Fig. 1.
Proton–carbon HSQC HRMAS NMR of melanoma cells shows melanogenesis-associated alteration of metabolic pathways using labeled acetate. AbC1 amelanotic (A) and melanotic (B) cells and SKMEL-188 amelanotic (C) and melanotic (D) cells were incubated in [2-13C]sodium acetate-enriched media. Spectral assignment: terminal methyl group in fatty acids (1); methyl group in phosphate-related metabolites (2 and 3); methyl group of acetate precursor for labeling (4); methyl group in malonyl metabolites and/or choline-related metabolites (7); methyl groups in fatty acids (5, 6, 8, and 10). The absolute peak intensity is labeled in the spectra. Peak intensities of melanotic cells (64 scans for signal averaging) need to be scaled by 0.25 to directly compare with those in amelanotic cells (16 scans for signal averaging).
The HSQC spectra of both cell lines cultured in [U-13C]glucose-enriched media are shown in Fig. 2. Spectra of intact amelanotic cells (Fig. 2A and C) differ significantly from those of melanotic cells (Fig. 2B and D) for both hamster and human melanomas. We can clearly identify several classes of metabolites that suggest an association between metabolism and melanogenesis. First, there was clear scrambling of glucose labeling into lipids. The abundance of free mobile lipids is low in melanotic cells (Fig. 2B and D) as compared with amelanotic cells (Fig. 2A and C), indicating their incorporation into macromolecules, a result consistent with acetate-labeling studies.
Fig. 2.
HSQC spectra of melanoma cells labeled with [U-13C]glucose as a function of the phenotype. AbC1 amelanotic (A) and melanotic (B) cells and SKMEL-188 amelanotic (C) and melanotic (D) cells were incubated in [U-13C]glucose-enriched media. Square box: unknown peak tightly associated with melanogenesis. All spectra were acquired with 16 scans per increment. Therefore, absolute peak intensities (labeled for selective peaks) are directly comparable between spectra.
The second significant finding is that the detected glucose metabolites, especially glutamine and glutamate, have substantially higher intensity for amelanotic cells compared with that for melanotic cells. For example, the ratio of peak intensity for glutamine is 2.5 higher in amelanotic SKMEL-188 cells (Fig. 2C) than in melanotic SKMEL-188 cells (Fig. 2D). Furthermore, the concentration of lactate also decreases for melanotic cells. These data are consistent with increased metabolic activity as well as reliance on anaerobic glycolysis in the less differentiated and more malignant cancer cells [17]. Similarly, the energy-yielding metabolism of transplantable melanomas in hamsters, Bomirski melanotic melanomas, is more dependent on aerobic glycolysis in comparison with the more malignant amelanotic melanomas, which show a high degree of lactate production [18]. In addition, the induction of melanogenesis represents a process of cell differentiation requiring the production of complex macromolecules [2], and this may be the likely reason for the reduced intensity of detectable metabolites in melanotic cells because of the faster incorporation of small molecular metabolites into macromolecules. This process can also be complicated by the communication between pentose phosphate shunt and melanogenesis that uses NADPH (nicotinamide adenine dinucleotide phosphate, reduced form) as a cofactor during melanin synthesis [2,19,20]. Due to a lack of standards, we could not assign all of the peaks of interest, thereby restricting a thorough metabolic interpretation. For example, the intensity of the peak that has a proton chemical shift of 5.15 ppm (carbon chemical shift at 70 ppm) consistently decreases in both melanotic cells compared with their amelanotic partners (Fig. 2). Clearly, this peak is tightly associated with the melanogenesis. We attempted to study extracellular metabolites by examining various NMR spectra of conditioned media with inconclusive results, arising from the dominant glucose and tyrosine NMR peaks associated with their high concentrations. Nevertheless, the production of lactate (both 13C-labeled and nonlabeled) was clearly seen as a major extracellular metabolite (not shown), indicating high glycolysis (as expected) for malignant cells.
In conclusion, using HRMAS NMR and isotope labeling, we have shown for the first time significant alterations of both lipid- and energy-related metabolisms in live cells associated with melanin synthesis. The presented data also show that HRMAS NMR represents a powerful and noninvasive technique to study environmentally induced changes in melanoma cell metabolism in ex vivo conditions.
Acknowledgments
The work was supported by the Department of Pharmaceutical Sciences at the University of Tennessee Health Science Center and by National Institutes of Health/National Cancer Institute (NIH/NCI) grant 1R15CA125623 and in part by NIH grant AR052190. We thank Bob M. Moore II, Ph.D. for his editorial assistance.
Footnotes
Abbreviations used: HRMAS, high-resolution magic angle spinning; NMR, nuclear magnetic resonance; D2O, deuterium oxide; CPMG, Carr–Purcell–Meiboom–Gill; 1D, one-dimensional; 2D, two-dimensional; HSQC, heteronuclear single quantum correlation; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form).
References
- 1.Slominski A, Paus R, Schanderdorf D. Melanocytes as sensory and regulatory cells in the epidermis. J. Theor. Biol. 1993;164:103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 2.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 3.Pawelek JM, Chakraborty AK, Osber MP, Orlow SJ, Min KK, Rosenzweig KE, Bolognia JL. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment Cell Res. 1992;5:348–356. doi: 10.1111/j.1600-0749.1992.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Paus R, Mihm MC. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: selective review and hypothesis. Anticancer Res. 1998;18:3709–3715. [PubMed] [Google Scholar]
- 5.Keun HC, Beckonert O, Griffin JL, Richter C, Moskau D, Lindon JC, Nicholson JK. Cryogenic probe 13C NMR spectroscopy of urine for metabonomic studies. Anal. Chem. 2002;74:4588–4593. doi: 10.1021/ac025691r. [DOI] [PubMed] [Google Scholar]
- 6.Keshari KR, Zektzer AS, Swanson MG, Majumdar S, Lotz JC, Kurhanewicz J. Characterization of intervertebral disc degeneration by high-resolution magic angle spinning (HR-MAS) spectroscopy. Magn. Reson. Med. 2005;53:519–527. doi: 10.1002/mrm.20392. [DOI] [PubMed] [Google Scholar]
- 7.Bollard ME, Keun HC, Beckonert O, Ebbels TM, Antti H, Nicholls AW, Shockcor JP, Cantor GH, Stevens G, Lindon JC, Holmes E, Nicholson JK. Comparative metabonomics of differential hydrazine toxicity in the rat and mouse. Toxicol. Appl. Pharmacol. 2005;204:135–151. doi: 10.1016/j.taap.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Li W. Multidimensional HRMAS NMR: a platform for in vivo studies using intact bacterial cells. Analyst. 2006;131:777–781. doi: 10.1039/b605110c. [DOI] [PubMed] [Google Scholar]
- 9.Robertson DG. Metabonomics in toxicology: a review. Toxicol. Sci. 2005;85:809–822. doi: 10.1093/toxsci/kfi102. [DOI] [PubMed] [Google Scholar]
- 10.Weybright P, Millis K, Campbell N, Cory DG, Singer S. Gradient, high-resolution, magic angle spinning 1H nuclear magnetic resonance spectroscopy of intact cells. Magn. Reson. Med. 1998;39:337–345. doi: 10.1002/mrm.1910390302. [DOI] [PubMed] [Google Scholar]
- 11.Slominski A, Moellmann G, Kuklinska E, Bomirski A, Pawelek J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, l-tyrosine and l-dopa. J. Cell Sci. 1988;89:287–296. doi: 10.1242/jcs.89.3.287. [DOI] [PubMed] [Google Scholar]
- 12.Slominski A, Ermak G, Wortsman J. Modification of melanogenesis in cultured human melanoma cells. In Vitro Cell Dev. Biol. 1999;35:564–565. doi: 10.1007/s11626-999-0093-6. [DOI] [PubMed] [Google Scholar]
- 13.Lide DR, editor. CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- 14.Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal. Chem. 1995;67:793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- 15.Selenko P, Wagner G. Looking into live cells with in-cell NMR spectroscopy. J. Struct. Biol. 2007;158:244–253. doi: 10.1016/j.jsb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Serber Z, Straub W, Corsini L, Nomura AM, Shimba N, Craik CS, Ortiz P, de Montellano V, Dotsch V. Methyl groups as probes for proteins and complexes in in-cell NMR experiments. J. Am. Chem. Soc. 2004;126:7119–7125. doi: 10.1021/ja049977k. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Scislowski PW, Slominski A, Bomirski A. Biochemical characterization of three hamster melanoma variants: II. Glycolysis and oxygen consumption. Int. J. Biochem. 1984;16:327–331. doi: 10.1016/0020-711x(84)90107-1. [DOI] [PubMed] [Google Scholar]
- 19.Scislowski PW, Slominski A. The role of NADP-dependent dehydrogenases in hydroxylation of tyrosine in hamster melanoma. Neoplasma. 1983;30:239–243. [PubMed] [Google Scholar]
- 20.Scislowski PW, Slominski A, Bomirski A, Zydowo M. Metabolic characterization of three hamster melanoma variants. Neoplasma. 1985;32:593–598. [PubMed] [Google Scholar]