Abstract
SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) play a central role in regulating and facilitating vesicular traffic in eukaryotic cells. While SNAREs have been well characterized in other eukaryotes, little is known about their role in the unique protein trafficking pathways in Plasmodium falciparum. We have identified seven Qa-SNAREs in the P. falciparum genome and confirmed the gene structure of all seven, which in one case differs from the predicted structure in the database. Based on comprehensive sequence alignments we made predictions for the intracellular locations of all seven P. falciparum Qa-SNAREs, and confirmed the predicted location for one Qa-SNARE, PfStx1, which is most closely related to other eukaryotic plasma membrane Qa-SNAREs such as syntaxin 1. This is the first identified trafficking component localized proximal to the P. falciparum plasma membrane.
Plasmodium species are a diverse and extremely successful group of intracellular parasites which in humans cause 300–500 million cases and more than 1 million deaths from malaria each year [1]. The majority of human malaria mortality is caused by Plasmodium falciparum, which like all Plasmodium parasites has a complex life cycle involving both mosquito and human hosts. This complicated life cycle depends on a vast array of parasite-host interactions, and many of these interactions are controlled by the Plasmodium falciparum secretory pathway. For example during the intra-erythrocytic stage of P. falciparum development, which is the stage that causes all the symptoms and pathology of malaria, it is the secretory pathway that ingests hemoglobin from the erythrocyte cytosol to drive parasite growth and replication and it is the secretory pathways that traffics antigenically variable cytoadherence ligands to the erythrocyte plasma membrane to avoid the protective immune response.
Work on model organisms and cell lines has revealed a great deal about the molecular mechanisms of secretion, and both preliminary genome analysis and recent elegant mechanistic studies [2,3] have confirmed that many of these fundamental features of eukaryotic secretory pathways are conserved in P. falciparum. However, P. falciparum intra-erythrocytic stages also contain several unique organelles that are not readily classifiable into the classical eukaryotic secretory pathway. First, a food vacuole begins to form during the ring stage, where endocytosed hemoglobin is transported to and then metabolized [4,5]. Second, P. falciparum possesses an apicoplast, a non-photosynthetic plastid where fatty acids are synthesized and is unique to the phylum Apicomplexa [6,7]. Third, after invasion of the host erythrocyte, unique membrane-bound organelles called Maurer’s clefts develop outside of the parasites own plasma membrane in the erythrocyte cytosol and appear to play a role in trafficking P. falciparum proteins to the erythrocyte plasma membrane [8]. It is currently not known how any of these organelles intersect with the classical eukaryotic secretory pathway.
Organelles maintain a distinct identity because protein transport to them is a tightly controlled event. Proteins are transported between organelles by in membrane-bound vesicles and the direction and specificity of vesicle transport is governed in large part by two families of proteins, SNAREs and Rabs. Rabs are small GTPases of the Ras superfamily that cycle between the cytosol (GDP bound) and organelle membranes (GTP bound) and Rab effector proteins aid in vesicle tethering as well as specificity of vesicle fusion [reviewed in 9]. Eleven Rab gene homologues have been identified in P. falciparum [10]. SNAREs are a family of typically membrane bound proteins that are all characterized by a relatively conserved coiled-coil SNARE domain near the C-terminus [11, reviewed in 12] and can be functionally classified as v-SNARES, which are present on vesicles, or t-SNARES, which are present on a target organelle. Another nomenclature divides the SNAREs into Q-SNAREs and R-SNAREs according to the presence of a glutamine or arginine residue at the core of the SNARE domain. Q-SNAREs are further subdivided into Qa-, Qb-, and Qc-SNAREs with Qa-SNAREs having homology to syntaxin 1a, the neuronal plasma membrane protein used to define the t-SNARE class [13]. Membrane fusion occurs when 3 Q-SNARE domains and one R-SNARE domain come together to form tetrameric helical bundle complex that drives the fusion between the vesicle and target compartment [14]. SNAREs have been found in the genomes of all eukaryotes studied to date from the primitive single celled Giardia intestinalis to Homo sapiens [15] and the published genome of P. falciparum contains numerous sequences with homology to SNARE domains. A preliminary analysis of such sequences identified 18 members of the SNARE family in P. falciparum [16], and noted several unusual features of these sequences, but did not classify such sequences into Qa-, Qb-, Qc- and R-SNARE sub-families.
Because of their role in specifying the fidelity of vesicle fusion and presence primarily on organelles rather than vesicles, Qa-SNAREs are of particular interest as organelle markers. Given the presence of several organelles of unknown provenance in P. falciparum intraerythrocytic stages, we were particularly interested in identifying and localizing P. falciparum Qa-SNAREs. To identify P. falciparum Qa-SNAREs in particular, rather than all genes encoding SNARE domains, we performed a comprehensive BLAST search analysis by querying the P. falciparum genome with the complete set of Qa-SNARE sequences from all major eukaryotic model organisms: Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster and Homo sapiens. All significant hits (e value less than 0.05) were visually inspected for structural properties present in Qa-SNAREs from other species including size, lack of a signal sequence, a single transmembrane domain at the C-terminus, a coiled-coil region before the transmembrane domain with homology to the SNARE domain, and the presence of a second cluster of alpha helices at the N-terminus, the Habc domain that distinguishes syntaxin homologs from other SNARE domain containing proteins.
This initial approach identified seven P. falciparum Qa-SNAREs, which are listed in Table 1 (these genes are listed as PfStx for their homology to the founding member of the Qa-SNARE class, syntaxin which has the gene name HsStx1a). The P. falciparum SNARE IDs previously assigned in a non Qa-SNARE specific manner are also included in Table 1 for comparison [16]. RT-PCR confirmed that all seven genes are transcribed in blood stages (data not shown). Visual inspection of the sequence suggested that the exon/intron boundaries of two of the Qa-SNAREs, PfStx4 and PfStx6, appeared to be possibly misannotated, with both genes having a particularly AT-rich stretch near the 5’ end that could be introns rather than exons. We therefore cloned and sequenced the RT-PCR product for both genes. The PfStx4 cDNA sequence was identical to the gene product predicted in PlasmoDB, confirming that this gene does consist of a single exon. By contrast, the cDNA product for PfStx6 was smaller than the full-length genomic fragment (data not shown), confirming that PfStx6 is a multi-exon gene, However, the 5’ AT-rich region was missing from the PfStx6 RT-PCR product, indicating that the predicted exon/intron boundaries of this gene are indeed incorrect. We used a combination of RT-PCR and 5’-RACE to sequence the full-length PfStx6 transcript. This cDNA sequence showed that the PlasmoDB predicted PfStx6 product in fact included a non-coding region as part of the first predicted exon, resulting in the prediction of a second transmembrane domain in PfStx6, which would have been unique among all eukaryotic SNAREs [16]. The actual PfStx6 transcription start site begins 216bp down stream of the predicted start site, and our confirmed transcribed product includes several exon/intron boundaries that differ from the predicted product and encodes only one transmembrane domain near the C-terminus (GenBank Accession Number EF142857). This structure conforms much more closely to other eukaryotic Qa-SNAREs, including the presence of an N-terminal Habc domain, confirming PfStx6 as a member of the P. falciparum Qa-SNARE family.
Table 1. Plasmodium falciparum Qa-SNAREs.
Seven P. falciparum Qa-SNAREs were identified by searching the P. falciparum genome (www.plasmodb.org) using either t-BLASTn or Psi-BLAST programs using both the full length sequences and the more conserved C-terminal SNARE domain of Qa-SNAREs from the genomes of S. cerevisiae, D. melanogaster, C. elegans, and H. sapiens. P. falciparum Qa-SNAREs are referred to as Stx in keeping with the gene name of the original Qa-SNARE, syntaxin 1 (HsStx1). The accession numbers and closest known Qa-SNARE homologs for each PfStx gene are listed, as are the P. falciparum SNARE gene names previously assigned in a non-Qa specific manner [16]. A possible intracellular location of each P. falciparum Qa-SNARE is predicted based on their closest eukaryotic Qa-SNARE homologue.
|
P. falciparum Qa-SNAREs |
P. falciparum SNAREs [16] |
Closest Qa-SNARE homolog |
Predicted intracellular location |
|---|---|---|---|
| PfStx1 (PFB0480w) | PfSyn17 | HsSyn2, DmSyn1a | Plasma membrane |
| PfStx2 (PFL2070w) | PfSyn16 | HsSyn16, DmSyn16 | Trans-Golgi |
| PfStx3 (PFL0505c) | PfSyn2 | ScPep12, HsSyn7 | Endosome |
| PfStx4 (Pf14_0300) | PfSyn11 | CeSyn1c, CeSyn13 | Plasma membrane? |
| PfStx5 (MAL13P1.169) | PfSyn5 | HsSyn5, DmSyn5 | Cis-Golgi |
| PfStx6 (PF11_0052) | PfSyn13 | HsSyn7, ScSso1 | Food Vacuole, Plasma membrane? |
| PfStx7 (MAL13P1.365) | PfSyn18 | ScUfe1 | ER |
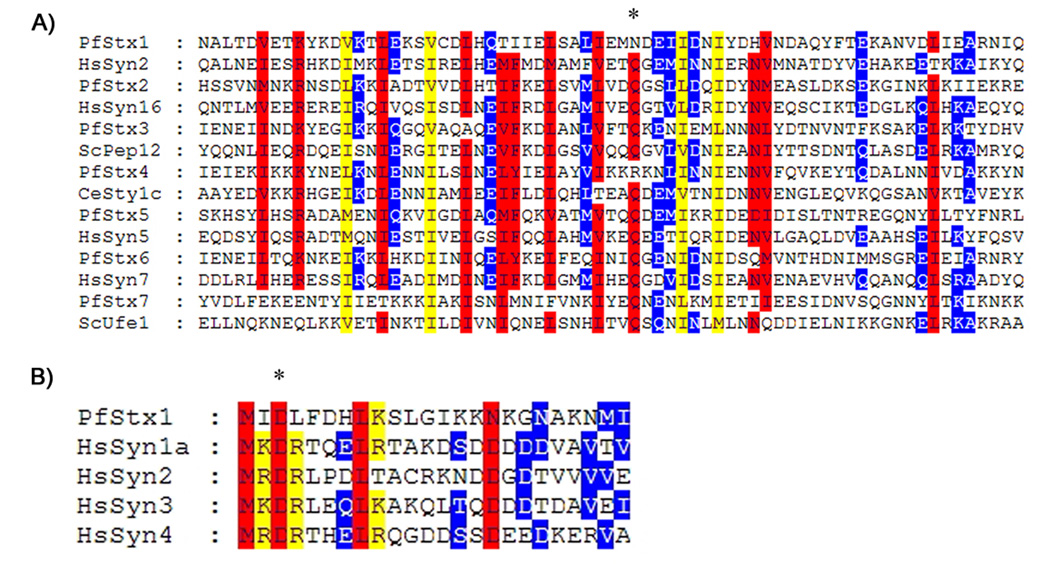
Having experimentally established the primary sequence of all seven P. falciparum Qa-SNAREs, we proceeded to sequence analysis. Figure 1a shows a CLUSTAL alignment of the seven P. falciparum Qa-SNAREs with their closest homolog among model organism Qa-SNAREs. Interestingly two of the P. falciparum Qa-SNAREs, PfStx1 and PfStx4, lack a conserved glutamate in the core of the SNARE domain (starred residue, Fig. 1a). However, the predicted plasma membrane Qa-SNARE sequences of several other protozoa, Encephalitozoon cuniculi and G. intestinalis, also lack this conserved glutamate indicating that it may not be universally conserved as once believed [17]. Because Qa-SNAREs that share the same intracellular location in different eukaryotes are often more closely conserved than are Qa-SNAREs in different locations in the same eukaryote, we were particularly interested in which Qa-SNAREs each Pf Qa-SNARE showed the highest homology to in their SNARE domain. In all seven cases, the SNARE domain of each P. falciparum Qa-SNARE was most closely related to one family of eukaryotic Qa-SNAREs (Fig 1a), allowing us to tentatively predict their intracellular location in P. falciparum parasites (Table 1).
Figure 1. Sequence alignment of PfStx proteins with other eukaryotic Qa-SNAREs.
A) Sequence alignment of the SNARE domain of all seven P. falciparum Qa-SNAREs and their closest Qa-SNARE homolog from other eukaryotes. Red indicates regions where there is 80% or more similarity, yellow indicates regions where there is 60% or more similarity, and blue indicates regions where there is 40% or more similarity. The conserved Q amino acid at the core of the SNARE domain is indicated by an asterisk. B) Alignment of the N-terminus of PfStx1 with the conserved N-terminal sequence of HsSyn1a, HsSyn2, HsSyn3, HsSyn4, all of which are plasma membrane Qa-SNAREs. The first 10 amino acids of HsSyn1a interact with the SM family protein Munc18 [18], and the D3 amino acid residue (asterisk) is essential for this interaction. Residues critical for the HsSyn1a-Munc18 interaction, including D3, are conserved in PfStx1.
PfStx1 was most closely related to plasma membrane Qa-SNAREs such as Syntaxin, down to the level of amino acid residues at the N-terminus (Fig 1b) that in Syntaxin interact with its binding partner, Munc-18 [18]. Published microarray data shows that PfStx1 transcription is up-regulated in schizonts, which would be expected for a plasma membrane Qa-SNARE given that schizogony involves the creation of multiple new plasma membranes [19]. We therefore chose to investigate PfStx1 as a test case for the utility of predicting Qa-SNARE intracellular location based on sequence alignments. To avoid issues of mislocalization due to epitope tagging or over-expression, both of which have been known to cause mistargeting of Qa-SNAREs in other eukaryotes, we generated PfStx1-specific antisera to follow endogenous PfStx1. The PfStx1 cytoplasmic domain was expressed as a hexa-his tagged recombinant protein in E. coli, purified using affinity chromatography, and used to generate polyclonal antiserum. Probing total protein extracts from P. falciparum schizont stage parasites revealed a single band at 37kDa, in keeping with the predicted size of PfStx1 (Fig 2a). PfStx1 was not detectable in ring stage parasites using this antibody (data not shown), presumably due to the low expression level in this stage established by microarray profiling [19].
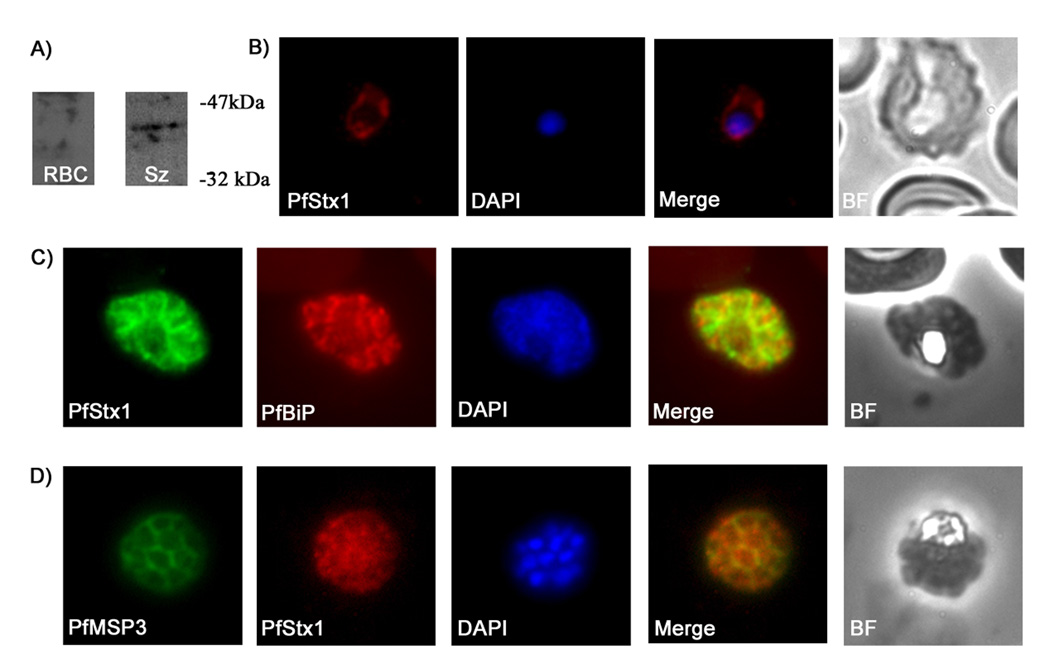
Figure 2. PfStx1 is localized proximal to the P. falciparum plasma membrane.
A) Western blot of P. falciparum protein extracts run on a 12% SDS gel and probed with anti-PfStx1 antiserum. Polyclonal anti-PfStx1 antiserum was raised against a his-tagged fragment of PfStx1. A partial sequence of PfStx1 (forward primer 5’-CGGGATCCGAATTAAGTATAGAATTAAAT-3’, reverse primer 5’-CGGGAATTCTTATCTAGCTTCTATTAAATCTAC-3’) was cloned into expression plasmid pRSETA (Invitrogen) and transformed into Escherichia coli BL21-DE3 pLysS. The resulting protein was expressed as a hexa-his fusion protein and was purified from E. coli pellets by resuspending the pellets in binding buffer (8M urea, 50mM TRIS pH 8.2, 100mM NaCl and 10mM imidazole) and passing through a Hi-Trap chelating HP column (Amersham Biosciences). Columns were washed 5 times with binding buffer and purified protein was eluted with increasing amounts of imidazole ranging from 30mM to 500mM. The purified protein was then used to immunize rabbits and rats (Cocalico Biological, Inc.). Lanes: RBC – total SDS extract of uninfected erythrocytes; Sz - total SDS extract from P. falciparum 3D7 schizont stage parasites. B–D) Immunofluorescence microscopy of fixed smears of P. falciparum parasites using anti-PfStx1 antiserum. Fixed air-dried smears of P. falciparum 3D7 were fixed in a 1:1 acetone methanol solution at −20°C for 30 minutes, washed with 1X PBS and then probed with the primary antisera. Secondary antisera (Molecular Probes) labeled with a fluorophore were used for visualization on an Olympus BX60 fluorescence microscope, and images were merged with Adobe Photoshop 5.0. In trophozoite stage parasites PfStx1 (red) has a plasma membrane like staining pattern and does not appear to be exported into the erythrocyte (B). In schizont stage parasites, PfSyn1 (green) again has a peripheral location that is distinct from the ER marker PfBiP (in red) (C). PfStx1 (red) colocalizes with PfMSP3 (in green), a plasma membrane marker in schizont stage parasites (D), confirming its predicted location proximal to the plasma membrane.
The anti-PfStx1 antiserum was then used in immunofluorescence experiments to determine the intracellular location of endogenous PfStx1. PfStx1 staining is visible at the periphery of early trophozoite stage parasites, consistent with a predicted plasma membrane location (Fig 2b). To confirm this, we performed co-staining in schizont stages. As expected, PfStx1 co-localized with PfMSP3 (Merozoite Surface Protein 3, a peripheral plasma membrane protein, Fig 2d) but is quite distinct from BiP, a marker for the P. falciparum endoplasmic reticulum (Fig 2c), as well as PfErd2, a marker for the cis-Golgi (data not shown). The experimentally established location of endogenous PfStx1, plasma membrane proximal, is therefore in agreement with the location predicted by sequence alignment with other eukaryotic Qa-SNAREs.
In conclusion, we have used RT-PCR and bioinformatics to annotate for the first time the Qa-SNARE family of P. falciparum, and established that PfStx1, which is most closely related to other plasma membrane Qa-SNARE, is localized proximal to the P. falciparum plasma membrane. This is only the second Qa-SNARE to be localized in P. falciparum and the first component of the P. falciparum plasma membrane vesicle trafficking machinery to be identified. Whether the Qa-SNARE predictions based on sequence alignment will hold true for the other P. falciparum Qa-SNAREs remains to be seen, but it should be pointed out that that the only other P. falciparum Qa-SNARE of known location, PfStx5, is in the cis-Golgi [16], which again conforms with the prediction based on sequence alignments (see Table 1). The prediction that PfStx2 is located in the trans-Golgi is perhaps the strongest, as the family of trans-Golgi Qa-SNAREs is one of the most conserved across the eukaryotic lineage. However, although the predicted locations of the other P. falciparum Qa-SNAREs are more tentative, given the interest in the mechanisms of protein trafficking to the food vacuole in particular, these predictions certainly provide obvious leads to follow, in this case PfStx3 and PfStx6.
Acknowledgments
Anti-PfBiP and PfErd2 antibodies were obtained through the MR4 (MRA19 and MRA-3, www.mr4.org), deposited by John Adams. Our thanks to Stephen Jordan for the anti-PfMSP3 antibody. This work was supported by the National Institute of Health research grant R03 AI064849.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Struck NS, Herrmann S, Schmuck-Barkmann I, de Souza Dias S, Haase S, Cabrera AL, Treeck M, Bruns C, Langer C, Cowman AF, Marti M, Spielmann T, Gilberger TW. Spatial dissection of the cis- and trans-Golgi compartments in the malaria parasite Plasmodium falciparum. Mol Microbiol. 2008;67:1320–1330. doi: 10.1111/j.1365-2958.2008.06125.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee MC, Moura PA, Miller EA, Fidock DA. Plasmodium falciparum Sec24 marks transitional ER that exports a model cargo via a diacidic motif. Mol Microbiol. 2008;68:1535–1546. doi: 10.1111/j.1365-2958.2008.06250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yayon A, Timberg R, Friedman S, Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J Protozool. 1984;31:367–372. doi: 10.1111/j.1550-7408.1984.tb02981.x. [DOI] [PubMed] [Google Scholar]
- 5.Rudzinska MA, Trager W, Bray RS. Pinocytotic uptake and the digestion of hemoglobin in malaria parasites. J Protozool. 1965;12:563–576. doi: 10.1111/j.1550-7408.1965.tb03256.x. [DOI] [PubMed] [Google Scholar]
- 6.Kohler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJ, Palmer JD, Roos DS. A plastid of probable green algal origin in Apicomplexan parasites. Science. 1997;275:1485–1489. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 7.McFadden GI, Reith ME, Munholland J, Lang-Unnasch N. Plastid in human parasites. Nature. 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 8.Kriek N, Tilley L, Horrocks P, Pinches R, Elford BC, Ferguson DJ, Lingelbach K, Newbold CI. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol Microbiol. 2003;50:1215–1227. doi: 10.1046/j.1365-2958.2003.03784.x. [DOI] [PubMed] [Google Scholar]
- 9.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quevillon E, Spielmann T, Brahimi K, Chattopadhyay D, Yeramian E, Langsley G. The Plasmodium falciparum family of Rab GTPases. Gene. 2003;306:13–25. doi: 10.1016/s0378-1119(03)00381-0. [DOI] [PubMed] [Google Scholar]
- 11.Sollner T, Whiteheart SE, Brunner M, Erdjument-Bromage E, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 12.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 13.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 15.Dacks JB, Doolittle WF. Novel syntaxin gene sequences from Giardia, Trypanosoma and algae: implications for the ancient evolution of the eukaryotic endomembrane system. J Cell Sci. 2002;115:1635–1642. doi: 10.1242/jcs.115.8.1635. [DOI] [PubMed] [Google Scholar]
- 16.Ayong L, Pagnotti G, Tobon AB, Chakrabarti D. Identification of Plasmodium falciparum family of SNAREs. Mol Biochem Parasitol. 2007;152:113–122. doi: 10.1016/j.molbiopara.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Dacks JB, Doolittle WF. Molecular and phylogenetic characterization of syntaxin genes from parasitic protozoa. Mol Biochem Parasitol. 2004;136:123–136. doi: 10.1016/j.molbiopara.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Khvotchev M, Dulubova I, Sun J, Dai H, Rizo J, Sudhof TC. Dual modes Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J NeuroSci. 2007;27:12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozdech Z, Llinás M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]