Abstract
Gadolinium (Gd) based contrast agents (GBCAs) in magnetic resonance imaging (MRI) are used in daily clinical practice and appear safe in most patients; however, nephrogenic systemic fibrosis (NSF) is a recently recognized severe complication associated with GBCAs. It affects primarily patients with renal disease, such as stage 4 or 5 chronic kidney disease (CKD; glomerular filtration rate <30 ml/min per 1.73 m2), acute kidney injury, or kidney and liver transplant recipients with kidney dysfunction. Contrast-enhanced MRI and computed tomography (CT) scans provide important clinical information and influence patient management. An alternative contrast agent is needed to obtain adequate imaging results while avoiding the risk of NSF in this vulnerable patient group. One potential alternative is ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles, which provide enhancement characteristics similar to GBCAs. We review our experience in approximately 150 patients on the potential benefits of the USPIOs ferumoxtran-10 and ferumoxytol. We focus on central nervous system (CNS) MRI but also review imaging of other vascular beds. Safety studies, including USPIO administration (ferumoxytol) as iron supplement therapy in CKD patients on and not on dialysis, suggest that decreased kidney function does not alter the safety profile. We conclude that for both CNS MR imaging and MR angiography, USPIO agents like ferumoxytol are a viable option for patients at risk for NSF.
Keywords: nephrogenic systemic fibrosis, magnetic resonance imaging, ferumoxytol, i.v. iron
The administration of gadolinium (Gd)-based contrast agents (GBCAs) in patients with kidney disease is a particular concern given the risk of nephrogenic systemic fibrosis (NSF). NSF is a severe systemic disorder that was recognized in 1997 in patients with kidney dysfunction. To avoid the development of NSF in patients with kidney disease, it is necessary to find a safer alternative contrast medium that provides comparable enhancement to GBCA.
Iron oxide nanoparticles are potential replacements for GBCA; they are an alternative blood-brain barrier (BBB) marker magnetic resonance (MR) contrast agent. These are promising compounds due to their T1 and T2 relaxation time shortening effect. Generally, larger molecules have a different biodistribution from the low-molecular weight GBCA. Therefore, superparamagnetic iron oxides (SPIOs, >50 nm diameter) with a short plasma half-life were found less optimal for vascular or central nervous system (CNS) imaging.1 Ultrasmall superparamagnetic iron oxides (USPIOs, <50 nm diameter) on the other hand can be used for contrast-enhanced MR imaging.2,3 (Table 1).
Table 1.
Superparamagnetic iron oxide MR contrast agents
| Short name | Generic namea | Trade name | Signalb | Status | Size (nm) | Bolus inj. | Developer |
|---|---|---|---|---|---|---|---|
| AMI-227 | Ferumoxtran-10 (USPIO) | Combidex/sinerem | Positive or negative | Development | 30 | NO | AMAG Pharmaceuticals Inc. (USA) Guerbet, SA (EU) |
| AMI-25 | Ferumoxides (SPIO) | Feridex/endorem | Negative | For sale | 100 | NO | AMAG Pharmaceuticals Inc. (USA) Guerbet, SA (EU) |
| Code 7228 | Ferumoxytol (USPIO) | Positive | Development | 30 | YES | AMAG Pharmaceuticals Inc. | |
| SH U 555 A | Ferucarbotran (SPIO) | Resovist/cliavist | Negative | For sale (EU, Australia, Japan)/development (USA) | 62 | YES | Bayer Schering Pharma AG |
| SH U 555 C | Ferucarbotran (USPIO)4 | Supravist | Positive | Development | 25 | YES | Bayer Schering Pharma AG |
| NC-100150 | PEG-feron (USPIO) | Clariscan | Positive | Development (discontinued) | 20 | YES | Amersham Health |
Inj., injection; SPIO, superparamagnetic iron oxides; USPIO, ultrasmall SPIO.
Short description.
High local concentrations and/or appropriate pulse sequence parameters, negative contrast can be achieved (e.g., first-track bolus); (modified from http://www.emrf.org/ and http://www.mr-tip.com/).
The coating compound and its surface charge are also major factors that influence the pharmacokinetics and pharmacodynamics. Consequently, there are major differences among the various USPIOs. For example, SH U 555C (Supravist) is a smaller molecule than ferumoxtran-10 (25 versus 30 nm), has a shorter half-life (6−8 versus 24−30 h), and is negatively charged, resulting in uptake by activated monocytes in vitro.5 Although SH U 555C was found to be taken up by circulating cells,6 neither ferumoxtran-10 nor ferumoxytol were found to do the same.7 SH U 555C enhancement was found to be independent from BBB breakdown, and has a more cellular, reticuloendothelial system-specific enhancement pattern than enhancement from GBCA.6
Ultrasmall superparamagnetic iron oxides, such as ferumoxytol and ferumoxtran-10, have shown excellent potential for brain tumor imaging similar to that achieved on conventional post-GBCA MRI8 because brain tumors often have an impaired BBB. Current clinical studies have investigated the newer agent, ferumoxytol, in subjects in whom contrast-enhanced imaging of CNS lesions may be a critical step in improving patient management. An advantage of ferumoxytol is that it has already been tested in patients with renal failure, unlike agents such as ferumoxtran-10 (USPIO), or the commercially available SPIO, AMI-25 (Feridex). Moreover, ferumoxytol can be safely given as a short intravenous bolus for MR angiography (MRA) and dynamic MR where it serves as a true blood pool agent. Although this article reviews clinical studies with ferumoxtran-10, it focuses on ferumoxytol as an alternative to GBCA in patients with kidney failure who require an MR contrast procedure (Table 2).
Table 2.
Ferumoxtran-10 (Combidex), Ferumoxytol, and Gd-DTPA (Magnevist)a
| Feature | Ferumoxtran-10 (Combidex) | Ferumoxytol | Gd-DTPA (Magnevist) |
|---|---|---|---|
| Basic element | Iron9 | Iron oxide | Gadolinium(III) |
| Molecular composition | Iron oxide coated with dextran9 | Iron oxide coated with semisynthetic carbohydrate | Gadolinium chelated with diethylenetriamine pentaacetic acid |
| r1 (mmol/s) at 0.47 T, 39 °C | 239 | 3810 | 3.4 |
| r2 (mmol/s) at 0.47 T, 39 °C | 539 | 8310 | 4 |
| Elimination plasma half-life | 24−30 h9 | 14 h11 | 1.6 h |
| Relative size of the particle | Around 30 nm9 | Around 30 nm11 | 0.357 nm |
| Permeability to intact BBB | Minimal | Minimal | Minimal |
| Clinical significance of the enhancing area | Imaging sites of BBB abnormality mainly in the presence of inflammatory cells | Imaging sites of BBB abnormality mainly in the presence of inflammatory cells | Detects abnormality in BBB and abnormal vascularity |
| Typical times to peak enhancement in brain tumors | 24−72 h1 | 24 h3 | 3.5−25 min |
| Signal change on T1-weighted sequence | High signal at low concentrations, low signal at higher concentrations12 | High signal at low concentrations, low signal at higher concentrations | High signal |
| Signal change on T2-weighted sequence | Low signal | Low signal | Slightly low signal |
| Distribution | Initially a blood pool agent and then intracellular in macrophages and reactive astrocytes | Initially a blood pool agent and then intracellular in macrophages and reactive astrocytes | Extracellular |
| Imaging dose | 2.6 mg of Fe/kg diluted in 100 ml (infusion) | 1−4 mg/kg | 0.2−0.6 ml/kg (0.1−0.3 mmol/kg) |
| Rate of administration | Infused over 30 min | Bolus | 10 ml/min or as bolus at 10 ml/15 s |
| Excretion | Stored with the body's iron reserve and used in hemopoesis | Stored with the body's iron reserve and used in hemopoesis | Renal |
BBB, blood-brain barrier.
The table shows the relevant features of the two contrast agents used in this study (modified from Murillo et al.13). There are differences in size, half-lives, clinical significance of the enhanced areas, distribution (plasma half-life), and side effects. GBCAs have excellent safety profiles in individuals with normal renal function and can be given in multiple doses. Ferumoxtran-10 is given slowly to avoid pseudoallergic reactions. Ferumoxytol can be administered as a bolus. A particle offerumoxtran-10 or ferumoxytol is almost 100 × larger than a GBCA.
CASE EXAMPLE
A 50-year-old male presented with a generalized seizure and loss of consciousness that occurred while at work. By the time he reached the emergency department he suffered from respiratory compromise and was placed on a ventilator. Subsequently he developed acute kidney injury requiring dialysis. His serum creatinine in the emergency department was 1.9 mg/100 ml and rapidly increased to 6 mg/100 ml. A noncontrast MRI scan demonstrated a brain lesion suspicious for tumor. The decision was made to do a stereotactic brain biopsy rather than a surgical resection due to the patient's compromised state. A GBCA was administered and the enhancing portion of the lesion was selected as the stereotactic target. Pathology was anaplastic astrocytoma grade III/IV. The patient fully recovered neurological and renal function and is receiving chemo and radiotherapy for a high-grade glioma (Figure 1).
Figure 1. Patient who presented to the Emergency Department comatose in acute renal failure with a large cerebral hemispheric mass.
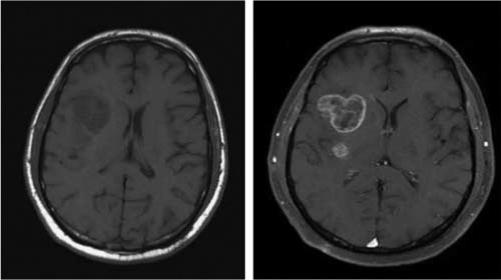
Axial T1-weighted MR scan without (left) and with (right) Gd 24 h post-admission when serum Cr was 6.0 mg/100 ml.
CAN FERUMOXYTOL HELP THIS PATIENT AND ANALOGOUS PATIENTS?
Nephrologist view
The association of NSF with GBCA was first reported in 2006, when five end-stage renal disease patients on hemodialysis who received GBCA developed NSF 2−4 weeks later. The risk in end-stage renal disease of developing NSF is estimated to be 2.4% in dialysis patients after any GBCA exposure, with a 4% prevalence in patients who received gadodiamide, an agent with a linear nonionic structure and relative ease of Gd dissociation from its chelate. Patients at risk include those with stage 4 or 5 chronic kidney disease (CKD; glomerular filtration rate <30 ml/min per 1.73 m2), acute kidney injury, or kidney and liver transplant recipients with kidney dysfunction.14,15 The earliest symptoms include pain and pruritus with clinical findings of edema, erythema, and warmth most commonly in the distal, then proximal lower extremities. Progressive muscle weakness and contractures can occur, along with cardiac, pulmonary, and scleral involvement.16
Given the complications of GBCA in patients at risk for NSF, other contrast agents are needed. USPIO nanoparticles have been used with excellent safety profiles in patients undergoing hemodialysis.17 If these agents were felt to be safe and gave equivalent, or superior radiological information, then USPIO nanoparticles would be an alternative to GBCAs for patients with kidney dysfunction requiring contrast-enhanced MRI.
As over 200 million patients have been exposed to GBCA,18 and <300 NSF cases have been diagnosed, the ∼1700 ferumoxytol patients to date are too small a number to accurately evaluate risk of other low-incidence complications. Although Gd chelates are considered safe, the exception is in patients with stage 4 or 5 CKD, where there is a slight but real risk of developing NSF. Thus, ferumoxytol is an interesting alternative contrast agent to avoid the small, but potentially fatal risk of NSF in this group of patients. As with any new drug, rare adverse events remain a risk for ferumoxytol.
MR physics and chemistry view
Although computed tomography has high spatial resolution, MRI is the imaging modality of choice when high-resolution anatomical detail with exceptional soft tissue contrast is demanded. Indeed, the ability of MRI techniques to generate exceptional contrast based on intrinsic tissue properties obviates the need for exogenous contrast media in many studies. Nevertheless, to increase lesion conspicuity, detect inflammation, improve vascular characterization, or demonstrate angiogenesis, MRI contrast agents are frequently used. It has been estimated that up to 40% of all MRI studies are now acquired using contrast media.
The five FDA-approved MRI contrast agents are based on the Gd(III) ion, which has excellent potency to catalyze the relaxation of the MRI water signal due to its seven unpaired electrons and favorable electronic relaxation times.19 To improve safety and pharmacokinetics, the Gd(III) ion must be chelated to an appropriate ligand, typically a low-molecular weight organic molecule. The archetypical ligand is DTPA5− (diethylenetriaminepentaacetate) that forms a very strong chelate with Gd(III). Tight binding of Gd by the chelate is important as both the free Gd and ligand are potentially toxic. In 1988, GdDTPA2− became the first FDA-approved MRI contrast agent. Since then four other low-molecular weight monomeric chelates of Gd(III) have gained FDA approval, each with unique biophysical properties. All approved GBCAs are administered intravenously, distribute into accessible extracellular spaces with a distribution half-life of ∼10 min, and are eliminated through the kidneys with a plasma half-life typically of ∼90 min in healthy human adults19 (Table 2). If kidney function is impaired, contrast agent plasma elimination can be markedly prolonged, with a half-life that may exceed 30 h in some individuals.20
Neuroradiology view
GBCA-enhanced MRI scans remain essential in numerous common clinical settings, including cerebritis, encephalitis, ventriculitis, demyelinating disease, subacute infarcts, leptomeningeal disease (infection and carcinomatous), characterization of primary and secondary CNS neoplasms, and for differentiating tumors from vascular malformations. In some cases, Gd enhancement will be the only diagnostic feature of the lesion, thus underscoring its importance. Correct imaging diagnosis frequently alters clinical management, and is often dependent on analysis of enhancement patterns.
Contrast agents are highly desirable for evaluating intracranial neoplasms, as in the current case example (Figure 1), for several reasons. These include:
Differentiating metastatic versus glial origin tumors. Metastatic disease in particular can be punctate or leptomeningeal in nature. Such lesions may only be identified with a contrast-enhanced MR study. Even contrast-enhanced computed tomography can miss these subtle types of tumor spread.
Enhancement usually suggests higher grade tumor, but is also useful in directing biopsy to the ‘highest yield’ site of tumor, thereby improving the likelihood of obtaining the highest grade portion of tumor for histologic analysis.
Contrast enhancement is useful on baseline studies to assist in future imaging follow-up because certain tumors would strongly be expected to enhance if recurrent, for example primitive neuroectodermal tumors or lymphoma. By contrast low-grade glial tumors may demonstrate recurrence by increasing T2 signal abnormality without enhancement, and new enhancement suggests the possibility of conversion of low-grade gliomas into a higher grade lesion such as glioblastoma multiforme.
Contrast enhancement patterns are occasionally helpful in clarifying cases of tumefactive multiple sclerosis that are better treated conservatively or diagnosed with confirmatory biopsy rather than attempting ‘gross total resection’ that may result in unnecessary deficits for patients.
Neurosurgical view
Enhanced preoperative MRI using conventional stereotactic and intraoperative MRI for brain biopsies and/or resection requires contrast. USPIO nanoparticles have a long plasma half-life and are trapped by reactive cells within the tumor. These trapped particles provide a method to demonstrate enhancing lesions without the artifact of repeat GBCA administration in the face of BBB and vascular injury due to surgery. USPIO particles represent a method to demonstrate enhancing intrinsic brain tumors without the drawbacks of intraoperative Gd enhancement. These lesions appear even on low-field strength intraoperative MRI.1,8,21 Ferumoxtran-10 and ferumoxytol, administered preoperatively, provides a stable imaging marker, even after surgical manipulation of the brain. However, it should be recognized that after crossing the impaired BBB, the distribution of iron oxides is fundamentally different from Gd chelates. Whereas USPIOs are taken up by phagocytic cells, Gd chelates distribute in the interstitial extracellular space (Figure 2). Furthermore, unlike SH U 555C, ferumoxytol is not taken up by circulating mononuclear cells, thereby making it more of a BBB marker than a marker for inflammation.6 However, after crossing the BBB, ferumoxytol is taken up by phagocytic cells.7
Figure 2. Schematic distribution comparing Gd versus USPIO crossing the BBB to tumor.
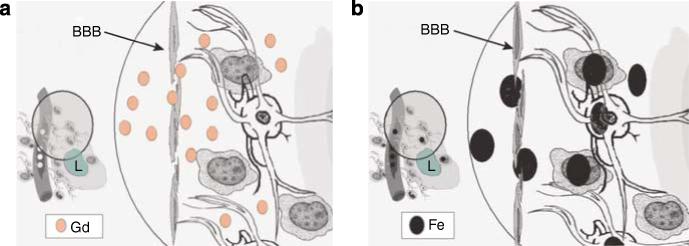
(a) Shows Gd chelates (abbreviated Gd in the figure) crossing from the intravascular compartment through a defective BBB (arrow) to the interstitial space. GBCA, which vary in propensity to release Gd, and thereby possibly risk of NSF (see text), remains in the extracellular space at the time of imaging (minutes after infusion). Macrophages, reactive astrocytes are shown around the lesion (L) but GBCA does not enter these cells. During its short half-life (less than 1 h), Gd rapidly crosses the BBB to produce a hyperintense signal on T1-weighted sequence on MR. In CKD, Gd-chelated contrast is not cleared rapidly and can be demonstrated in tissues.22 (b) Ferumoxtran-10 (Fe in black) and other ultra small iron oxides are much larger particles compared to GBCAs; these particles will slowly cross the defective BBB (arrow). Due to its prolonged half-life of 25−30 h particles of ferumoxtran-10 will progressively accumulate in the interstitial space where they will be trapped by reactive astrocytes and macrophages for days (up to 7 days). The intracellular metabolism of ferumoxtran-10 will take place in the lysosome of these cells where free iron will be released to the extracelullar space. Reactive astrocytes, macrophages, and other inflammatory cell are seen in different diseases affecting the CNS, among these stroke, neoplasm, and demyelinating processes. Figure modified from Murillo et al.13 (reproduced with permission of Expert Reviews Ltd).
Another imaging option is computed tomography scanning using iodinated contrast, but again, in patients with renal compromise, iodinated contrast has risk and computed tomography has less spatial resolution in soft tissue than MR. In our experience to date, all biopsies from tumors that enhance with USPIO have contained tumor cells.
SAFETY AND PRELIMINARY STUDIES WITH FERUMOXYTOL VERSUS OTHER IRON REPLACEMENT AND IMAGING AGENTS
Some iron oxide agents, such as Feridex and ferumoxtran-10, require slow infusion of relatively low doses to avoid adverse reactions, making them unsuitable for vascular applications, whereas ferumoxytol appears to have fewer systemic reactions and can be given as a bolus for MRA analysis of vasculature.23,11 Even so, with inflammatory lesions ferumoxtran-10 may have a slight advantage for anatomic imaging.
Ferumoxytol was developed as an intravenous iron-replacement therapy, specifically for anemic patients with CKD including those on dialysis. It has not yet been approved by the FDA for marketing but a New Drug Application was submitted in December 2007.
Following an infusion of iron in healthy individuals, dietary absorption of iron decreases, whereas in CKD patients, iron stores are depleted. In either case, attention to the potential of oxidative stress needs to be considered, although to date, no such toxicity has been seen in either normal individuals or CKD patients.
Over 1700 patients have been exposed to ferumoxytol in its clinical development program, and at least 1500 of these were patients with CKD in Phase 3 iron-replacement studies. To date, only one patient, (with a history of multiple drug allergies) has experienced an anaphylactoid reaction (hot flashes and itching, with no respiratory compromise) and severe hypotension a few minutes after receiving ferumoxytol. There have been no deaths that were considered to be related to ferumoxytol treatment.
The other intravenously administered iron-replacement therapies contain only paramagnetic iron, which would preclude safe administration in sufficient doses to provide adequate MR contrast.
Currently, there are no other iron oxide particles showing both safety in CKD, and in angiographic imaging. Although SH U 555A (Resovist) is approved in Europe, it is not approved in the United States, and furthermore has not been tested in CKD; this is also true for another USPIO called SH U 555C (Supravist).6,4 Mn-DPDP (Teslascan), a manganese contrast agent, is approved in the United States, although not marketed, and to our knowledge has not been evaluated in CKD. Thus, ferumoxytol is the only USPIO agent used for MRA and anatomic CNS imaging that to date has a safe track record in patients with kidney disease (Table 1).6,4,24
CLINICAL STUDIES
MR imaging with a nanoparticle magnetic resonance contrast agent
As compared with USPIO, MRI of the CNS carried out with Gd-containing contrast agents is based on low-molecular weight extracellular-chelated agents with a short half-life, giving rapid and transient imaging of brain and spinal cord permeability.13 USPIO nanoparticle MR contrast agents have shown excellent potential for imaging in the CNS. (Table 1) The agents consist of an iron oxide core with a variable coating that determines cellular uptake and biological half-life. In the CNS, ferumoxtran-10 has been most widely investigated in brain tumors.1 It is completely coated with native dextran, which protects against opsonization and endocytosis, and bestows a long plasma half-life of 24−30 h. On MR scans, the iron oxide agents demonstrate concentration related signal loss on T2- and T2*-weighted sequences. Lower concentrations of USPIO on T1-weighted sequences increase signal intensities, similar to that seen on Gd enhancement; higher concentrations of USPIO can cause profound signal loss on T1-weighted images due to extreme T2* shortening.12 In addition, iron oxide particles can be identified at the light and electron microscopic levels (Figure 3),1 allowing evaluation of their exact location within tissues,12 unlike other CNS MRI agents.
Figure 3. Nanoimaging: from bench to bedside.
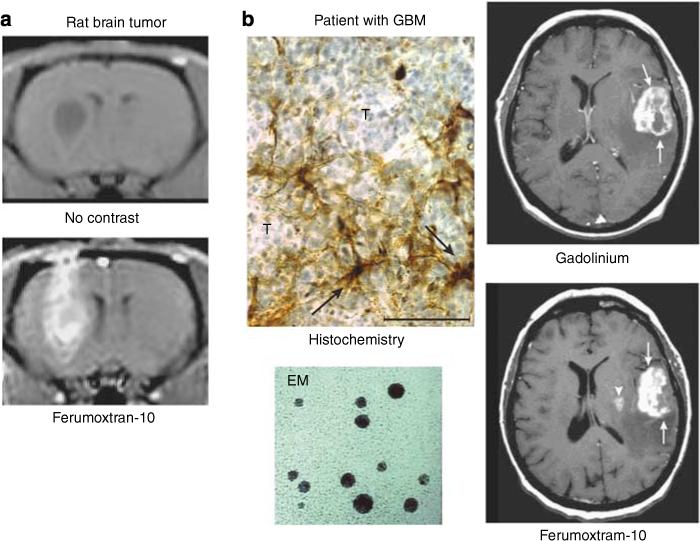
(a) Rat brain tumor before and after USPIO MR contrast. (b) Post-GBCA T1-weighted MRI shows a large left frontoparietal-enhancing tumor (arrows). At 24 h post–ferumoxtran, T1-weighted MRI shows intense enhancement in the left frontoparietal tumor (arrows) and a new lesion medial to the main tumor mass in the putamen (arrowhead). Cellular iron staining at the tumor–reactive brain interface shows iron uptake by the parenchymal cells with fibrillar processes (arrows) rather than by the round tumor cells (T). EM shows electron dense core of USPIO.1 (Reproduced from AJNR Am J Neuroradiol 2002; 23:510−519 with permission of Lippincott, Williams & Wilkins, 2008.)
Clinical trials using ferumoxtran-10 to image brain tumors
Ultrasmall superparamagnetic iron oxide agents have shown promise in clinical trials for tumor imaging, both systemically and in the brain. They can be useful in differentiating between malignant and benign lymph nodes, as well as in MRA and perfusion imaging of the brain, heart, and trunk vessels.23 In reactive brain lesions, the iron oxide agents may detect active inflammatory or demyelinating lesions6 after iron uptake in perivascular macrophages.25 More recently, several studies have looked at ferumoxtran-10-enhanced MR imaging of brain tumors. Early studies demonstrated the prolonged enhancement of brain tumors after USPIO administration when compared to standard Gd. Delayed MRI (24−48 h) following administration of ferumoxtran-10 has been reported to show additional areas of enhancement in brain tumors that were not seen with Gd (Figure 3),1 particularly in areas where reactive inflammatory cells were present.1,8 Prolonged enhancement of brain tumors (up to 7 days) in patients was associated with progressive accumulation of ferumoxtran-10 in reactive astrocytes and macrophages rather than tumor cells.1,8 In a subsequent study, ferumoxtran-10 was compared to Gd-enhanced MR to assess its potential in evaluation of a variety of CNS lesions.2 Ferumoxtran-10 showed different enhancement patterns in a variety of CNS inflammatory lesions in comparison to Gd (Figure 4). In particular, some cases of lymphomas and ischemic lesions demonstrated stronger enhancement (Figure 5).2 These observations and preclinical data suggest that ferumoxtran-10 enhanced MR, in addition to the usual Gd-enhanced MR, may identify those part(s) of the lesion that contain both reactive inflammatory changes and a BBB defect, thereby helping differentiate portions of a lesion with and without inflammatory reaction. This may have implications for targeting of diagnostic biopsies and the planning of surgical resections.
Figure 4. Patient with acute disseminated encephalomyelitis (ADEM).
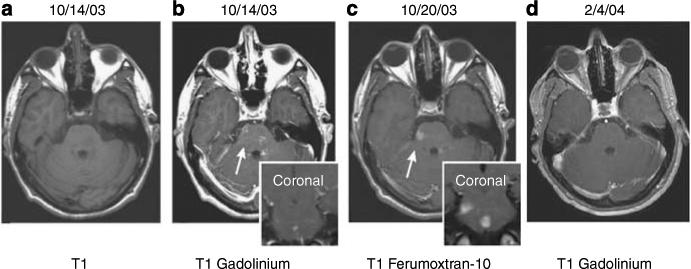
Axial T1-weighted MRI images without (a) and with (b) GBCA show faint, subtle enhancement in multiple brainstem lesions. At 6 days later (c) significant, more prominent, larger ferumoxtran-10 enhancement can be seen on the same site. At 3 months later (d), the lesions no longer enhance on T1-weighted images with GBCA.2 (Reproduced from AJNR Am J Neuroradiol 2005; 26:2290−2300 with permission of Lippincott, Williams & Wilkins, 2008).
Figure 5. Patient with Stroke.
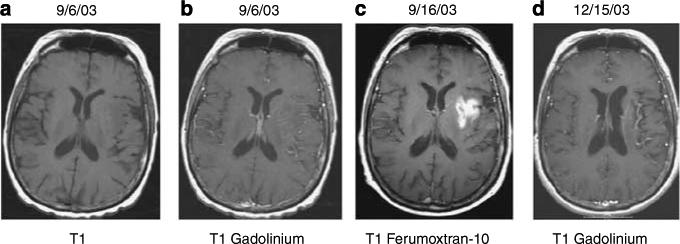
Axial T1-weighted MRI without (a) and with (b) GBCA show no significant enhancement in left insular cortex, basal ganglia, and frontoparietal lobe where DWI, T2, and FLAIR images showed high intensity (not shown) consistent with acute MCA infarction. At 10 days later, T1 images with ferumoxtran-10 (c) show significant enhancement in the same region. At 90 days later (d), axial T1-weighted image after GBCA shows no enhancement.2 (Reproduced from AJNR Am J Neuroradiol 2005; 26:2290−2300 with permission of Lippincott, Williams & Wilkins, 2008).
CNS imaging with ferumoxytol
The main difference between ferumoxytol and ferumoxtran-10 is the coating. Ferumoxytol is not dextran coated, and the modified carbohydrate design makes ferumoxytol better tolerated for bolus injections. Because of this modification and the fact that USPIO particles can serve as blood pool agents. Ferumoxytol may be used for MRA and perfusion of the brain and other organs. Ferumoxytol was supplied by AMAG Pharmaceuticals Inc. (Cambridge, MA, USA). Ferumoxytol can be used at early time points (seconds to minutes) to image the vasculature by MRA without the rapid extravasation into CNS lesions that limit MRA with Gd-based contrast agents (Table 2). USPIOs such as ferumoxytol, even in the presence of transfecting agents (unlike superparamagnetic iron oxides (SPIOs)) do not enter circulating phagocytic cells (Figure 6). These properties make ferumoxytol a promising agent in neurological diagnosis and neurosurgery in malignant gliomas (Figure 7).3 Ferumoxytol enhancement likely represents an inflammatory reaction with BBB leakiness, due to both a BBB abnormality and presence of phagocytic cells. As with ferumoxtran-10, the time to peak enhancement of ferumoxytol on T1-weighted MRI is typically around 24 h and lasts at least 72 h after administration. It may be useful in surgical interventions for malignant tumors in two ways. First, image guided neurosurgery or stereotactic biopsy may be better directed with the information provided by USPIOs such as ferumoxytol. Second, if gross resection is performed, the prolonged enhancement (up to 7 days) can aid intra- and postoperative assessment by MR during or after surgery without further administration of the contrast agent.21
Figure 6. Ferumoxides but not ferumoxtran-10 nor ferumoxytol (even in the presence of protamine sulfate) label circulating rat mononuclear cells after intravenous infusion.
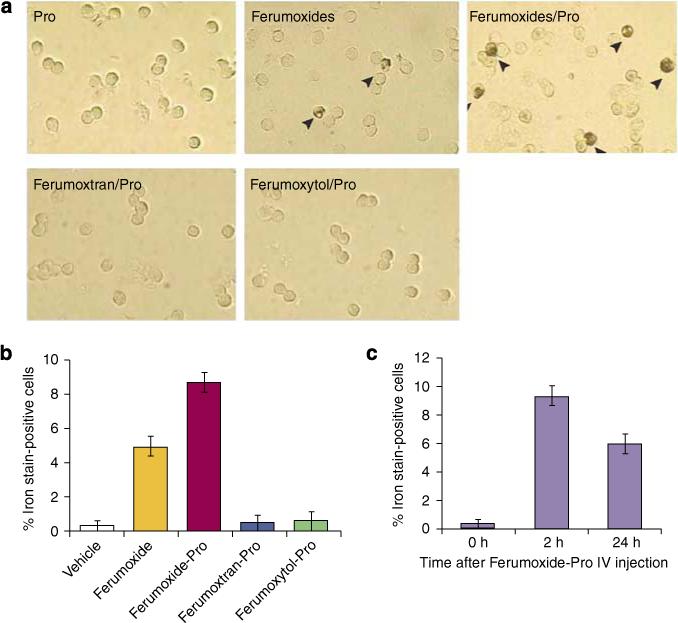
Female Long Evans rats received IV ferumoxides, ferumoxtran-10, or ferumoxytol (20 mg/kg) with or without preincubation with protamine sulfate (Pro) (2 mg/kg). (a) Mononuclear cells were isolated from peripheral circulation 2 h after injection and stained with DAB-enhanced Perl's blue. Iron-labeled cells with brown stain are indicated by arrowheads. (b) Percentage of iron-positive-stained cells in total mononuclear cell population. (c) Time course of ferumoxide-Pro in vivo iron-labeled rat mononuclear cells. Data are represented as mean ± s.e.m.7 (Used with permission from Am J Physiol Cell Physiol 2007; 293: C1698–C1708).
Figure 7. Gd versus ferumoxytol MR pre-operatively and intra-operatively in a glioma patient using a 3 T versus a 0.15 T magnet.
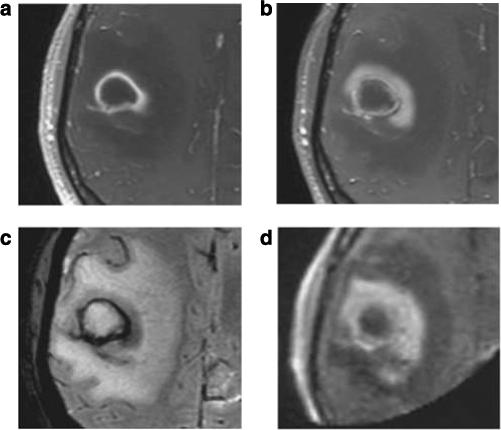
Glioma patient (a) T1-enhancement with GBCA. At 24 h after ferumoxytol infusion, (b) T1 enhancement, (c) T2*-weighted images. (d) Intraoperative T1-weighted 0.15 T MR 26 h after ferumoxytol injection.3 (Reproduced from Neurosurgery 2007; 60: 601−611; discussion 611−602, with permission of Lippincott, Williams & Wilkins, 2008).
Vascular enhancement with ferumoxytol for dynamic MR in the CNS
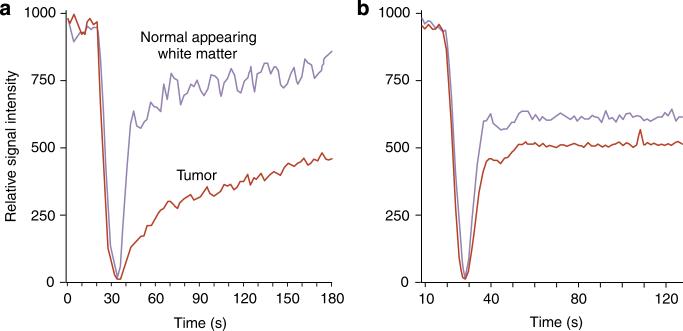
Although GBCA imaging relies on rapid crossing of the impaired BBB for anatomic imaging studies, it may be a limitation in performing dynamic studies of the vasculature in areas of abnormal BBB permeability. An agent that rapidly leaves the capillaries (Figure 8)3 may confound perfusion (blood volume and flow) measurements, whereas an agent like ferumoxytol, that leaves the vasculature slowly, provides more accurate measurements of perfusion in areas of leaky BBB at early time points as it then acts as a blood pool agent (Figure 9).26 Inflammation at the BBB is a fundamental component of CNS pathology in brain tumors as well as CNS injury from trauma or multiple sclerosis. Ferumoxytol does not leak out of blood vessels in the early phase after injection (i.e. is a blood pool agent) and therefore is excellent for dynamic MR perfusion and angiography.
Figure 8. Time of Flight Angiography in a patient with glioblastoma.
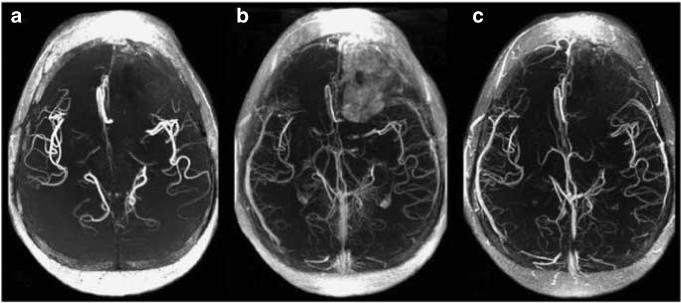
(a) Without contrast. (b) At 15 min after GBCA, more vessels are visible. There is an early leak into the left frontal tumor. (c) At 15 min post-ferumoxytol infusion, more vessels are visible, but there is no leak into the tumor.3 (Reproduced from Neurosurgery 2007; 60: 601−611; discussion 611−602 with permission of Lippincott, Williams & Wilkins, 2008).
Figure 9. Perfusion MR, time-intensity curves in a patient with pineal blastoma.
(a) Using GBCA, leakage of the contrast agent decreases the slope of the recovery curve in the tumor. (b) Ferumoxytol signal intensity curves show no leakage in the lesion.3 (Reproduced from Neurosurgery 2007; 60: 601−611; discussion 611−602 with permission of Lippincott, Williams & Wilkins, 2008).
Due to its prolonged half-life at later time points, ferumoxytol provides anatomic images of brain and also can be used for steady-state angiography (Figure 10).
Figure 10. Ferumoxytol susceptibility-weighted imaging at 7 T in human brain.
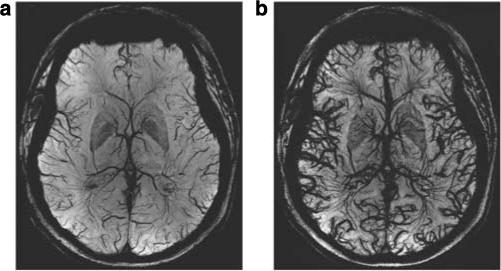
Vasculature MRI before ferumoxytol (a) and after 4 mg/kg ferumoxytol (b).
Vascular enhancement with ferumoxytol MRA outside CNS
First-pass and equilibrium phase MRA have been performed using ferumoxytol in 11 healthy volunteers, 6 stent graft patients,23 and 9 deep vein thrombosis patients. The examined vessels included carotid arteries, thoracic aorta, abdominal aorta, and peripheral arteries. First-pass MRA showed selective arterial enhancement, with both arterial and venous enhancement on delayed acquisitions. Selective venous enhancement could be obtained by subtraction of arterial phase images from equilibrium phase images. With ferumoxytol, arteries and veins can be selectively depicted in a single exam. Ferumoxytol may be very useful in assessing the aorta and peripheral arteries of renal patients who often have diabetes and/or hypertension with major arterial pathology. Figure 11 is a postrenal transplant MRA with ferumoxytol showing aortic aneurysm, iliac artery stent, transplanted kidney, and polycystic kidney all in same patient.27
Figure 11. MR abdominal angiograms (TR/TE: 3.5/1.3 ms) of a 60-year-old male acquired after injection of fourfold diluted ferumoxytol (4 mg/Fe/kg).
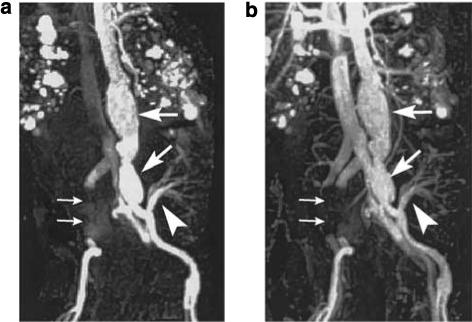
(a) First-pass image. (b) Equilibrium image. Aortic and left iliac aneurysms (large arrows) are demonstrated. The artery arising from the left iliac artery (arrowhead) supplies a transplanted kidney. The right iliac artery and vein cannot be seen due to metal artifact from right iliac aneurysm stent (small arrows). Note that in the first-pass image, the inferior vena cava is visible. Polycystic kidney disease is also demonstrated.27 (First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. (Reproduced from J Magn Reson Imaging 2005; 21: 46−52 with permission of Wiley-Liss, Inc., a subsidiary of Wiley Inc.)
CONCLUSION
Patients with kidney disease as defined above are at risk for NSF if given GBCAs. NSF is not common but can be severely debilitating and potentially fatal. USPIOs being developed for iron-replacement therapy primarily in patients with kidney disease, have excellent potential as MRI contrast agents. However, the distribution of USPIO and GBCA is different, with the former largely distributing into the reticuloendothelial system and blood pool, whereas the latter distributes into the extracellular space after a relatively short residence within the blood pool. To date, preclinical and clinical studies suggest excellent potential for USPIOs such as ferumoxytol as an alternative to GBCA in patients at risk for NSF, not only for anatomic imaging, but also for MRA and dynamic MRI.
ACKNOWLEDGMENTS
This research was supported by a Veteran's Administration Merit Review Grant and NIH grants NS33618, NS34608, and NS44687 from the National Institute of Neurological Disorders and Stroke to EAN and NIB1B EB007258 to WDR. AMAG Pharmaceuticals Inc. donated ferumoxtran-10 and ferumoxytol.
Footnotes
DISCLOSURE
The authors declared no competing interests.
REFERENCES
- 1.Varallyay P, Nesbit G, Muldoon LL, et al. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am J Neuroradiol. 2002;23:510–519. [PMC free article] [PubMed] [Google Scholar]
- 2.Manninger SP, Muldoon LL, Nesbit G, et al. An exploratory study of ferumoxtran-10 nanoparticles as a blood-brain barrier imaging agent targeting phagocytic cells in CNS inflammatory lesions. AJNR Am J Neuroradiol. 2005;26:2290–2300. [PMC free article] [PubMed] [Google Scholar]
- 3.Neuwelt EA, Varallyay CG, Manninger S, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601–611. doi: 10.1227/01.NEU.0000255350.71700.37. discussion 611–602. [DOI] [PubMed] [Google Scholar]
- 4.Tombach B, Reimer P, Bremer C, et al. First-pass and equilibrium-MRA of the aortoiliac region with a superparamagnetic iron oxide blood pool MR contrast agent (SH U 555 C): results of a human pilot study. NMR Biomed. 2004;17:500–506. doi: 10.1002/nbm.906. [DOI] [PubMed] [Google Scholar]
- 5.Metz S, Bonaterra G, Rudelius M, et al. Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. Eur Radiol. 2004;14:1851–1858. doi: 10.1007/s00330-004-2405-2. [DOI] [PubMed] [Google Scholar]
- 6.Vellinga MM, Oude Engberink RD, Seewann A, et al. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain. 2008;131:800–807. doi: 10.1093/brain/awn009. [DOI] [PubMed] [Google Scholar]
- 7.Wu YJ, Muldoon LL, Varallyay C, et al. In vivo leukocyte labeling with intravenous ferumoxides/protamine sulfate complex and in vitro characterization for cellular magnetic resonance imaging. Am J Physiol Cell Physiol. 2007;293:C1698–C1708. doi: 10.1152/ajpcell.00215.2007. [DOI] [PubMed] [Google Scholar]
- 8.Neuwelt EA, Varallyay P, Bago AG, et al. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol Appl Neurobiol. 2004;30:456–471. doi: 10.1111/j.1365-2990.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 9.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging. 1995;13:661–674. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs P, Shenouda M. A phase I pharmacokinetics and safety study of ferumoxytol, a new blood pool and macrophage specific superparamagnetic iron oxide MR contrast agent.. RSNA Scientific Assembly and Annual Meeting Program. Meeting Proceedings; Oak Brook: Illinois. 2003. [Google Scholar]
- 11.Landry R, Jacobs PM, Davis R, et al. Pharmacokinetic study of ferumoxytol: a new iron replacement therapy in normal subjects and hemodialysis patients. Am J Nephrol. 2005;25:400–410. doi: 10.1159/000087212. [DOI] [PubMed] [Google Scholar]
- 12.Neuwelt EA, Weissleder R, Nilaver G, et al. Delivery of virus-sized iron oxide particles to rodent CNS neurons. Neurosurgery. 1994;34:777–784. doi: 10.1227/00006123-199404000-00048. [DOI] [PubMed] [Google Scholar]
- 13.Murillo T, Sandquist C, Jacobs PM, et al. Imaging brain tumors with ferumoxtran-10, a nanoparticle magnetic resonance contrast agent. Therapy. 2005;2:871–882. [Google Scholar]
- 14.Swaminathan S, Shah SV. New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol. 2007;18:2636–2643. doi: 10.1681/ASN.2007060645. [DOI] [PubMed] [Google Scholar]
- 15.Willicombe M, Cunningham J. Nephrogenic systemic fibrosis: a sufficient reason to avoid gadolinium-based contrast in all patients with renal impairment? Semin Dial. 2008;21:140–141. doi: 10.1111/j.1525-139X.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan S, Horn TD, Pellowski D, et al. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med. 2007;357:720–722. doi: 10.1056/NEJMc070248. [DOI] [PubMed] [Google Scholar]
- 17.Besarab A, Coyne D, Bolton WK, et al. Ferumoxytol as an intravenous iron replacement therapy: safety results from two phase III studies in subject with chronic kidney diseases (CKD).. American Society of Nephrology 40th Annual Meeting and Scientific Exposition.; San Francisco. 2007.p. SU-PO805. [Google Scholar]
- 18.Bongartz G. Imaging in the time of NFD/NSF: do we have to change our routines concerning renal insufficiency? MAGMA. 2007;20:57–62. doi: 10.1007/s10334-007-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caravan P, Ellison JJ, McMurry TJ, et al. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 20.Grobner T, Prischl FC. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72:260–264. doi: 10.1038/sj.ki.5002338. [DOI] [PubMed] [Google Scholar]
- 21.Hunt MA, Bago AG, Neuwelt EA. Single-dose contrast agent for intraoperative MR imaging of intrinsic brain tumors by using ferumoxtran-10. AJNR Am J Neuroradiol. 2005;26:1084–1088. [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham JL, Thakral C. Tissue distribution and kinetics of gadolinium and nephrogenic systemic fibrosis. Eur J Radiol. 2008;66:200–207. doi: 10.1016/j.ejrad.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Ersoy H, Jacobs P, Kent CK, et al. Blood pool MR angiography of aortic stent-graft endoleak. AJR Am J Roentgenol. 2004;182:1181–1186. doi: 10.2214/ajr.182.5.1821181. [DOI] [PubMed] [Google Scholar]
- 24.Bremerich J, Bilecen D, Reimer P. MR angiography with blood pool contrast agents. Eur Radiol. 2007;17:3017–3024. doi: 10.1007/s00330-007-0712-0. [DOI] [PubMed] [Google Scholar]
- 25.Corot C, Petry KG, Trivedi R, et al. Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest Radiol. 2004;39:619–625. doi: 10.1097/01.rli.0000135980.08491.33. [DOI] [PubMed] [Google Scholar]
- 26.Akella NS, Twieg DB, Mikkelsen T, et al. Assessment of brain tumor angiogenesis inhibitors using perfusion magnetic resonance imaging: quality and analysis results of a phase I trial. J Magn Reson Imaging. 2004;20:913–922. doi: 10.1002/jmri.20202. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Tutton S, Vu AT, et al. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging. 2005;21:46–52. doi: 10.1002/jmri.20235. [DOI] [PubMed] [Google Scholar]