Summary
ATP-binding cassette (ABC) transporters utilize the energy from ATP hydrolysis to transport substances across the membrane. In recent years, crystal structures of several ABC transporters have become available. These structures show that both importers and exporters oscillate between two conformations: an inward-facing conformation with the substrate translocation pathway open to the cytoplasm and an outward-facing conformation with the translocation pathway facing the opposite side of the membrane. In this review, conformational differences found in the structures of homologous ABC transporters are analyzed to understand how alternating-access is achieved. It appears that rigid-body rotations of the transmembrane subunits, coinciding with the opening and closing of the nucleotide-binding subunits, couples ATP hydrolysis to substrate translocation.
Introduction
ATP binding cassette (ABC) transporters are ubiquitous integral membrane proteins that use the energy from ATP binding and hydrolysis to transport a diverse array of substrates across the membrane bilayer. ABC transporters contain two transmembrane domains or subunits (TMDs) that form the translocation pathway and two cytoplasmic nucleotide-binding domains or subunits (NBDs) that hydrolyze ATP (reviewed in [1]). ABC transporters diverged very early into two classes that correlate with the direction of substrate transport [2]. In importers, TMDs and NBDs reside on separate subunits while in exporters, TMDs are fused to NBDs. Importers, found in prokaryotes, contain additional periplasmic or cell-surface-associated binding proteins that bind substrates with high affinity and deliver them to the TMDs. Exporters recruit their substrates directly from the cytoplasm or lipid bilayer. Whereas the TMDs have low sequence similarity, the NBDs are highly conserved, each consisting of a RecA-like subdomain containing Walker A and B motifs and a helical subdomain containing the LSGGQ signature motif.
For more than two decades, genetic, biochemical and structural studies have provided a wealth of information valuable in understanding the molecular mechanism underlying ABC transport. These data have been summarized in several recent reviews [2–5]. Here we discuss the latest advances in the structural studies of full-length ABC transporters, focusing on conformational differences that may explain how substrates cross the membrane. Since the first high resolution structure of an ABC transporter, the E. coli vitamin B12 importer [6], was reported in 2002, crystal structures of five more importers and two exporters have been solved. In this review, we divide the structures into three categories, based on the architecture of the TMDs: small importers with a common five-helix core [7–10], large importers containing 20 membrane-spanning helices [6,11,12], and exporters [13–15]. The mechanism inferred from the small importers, where we have the most structural information, may well apply to other ABC transporters.
Small ABC importers
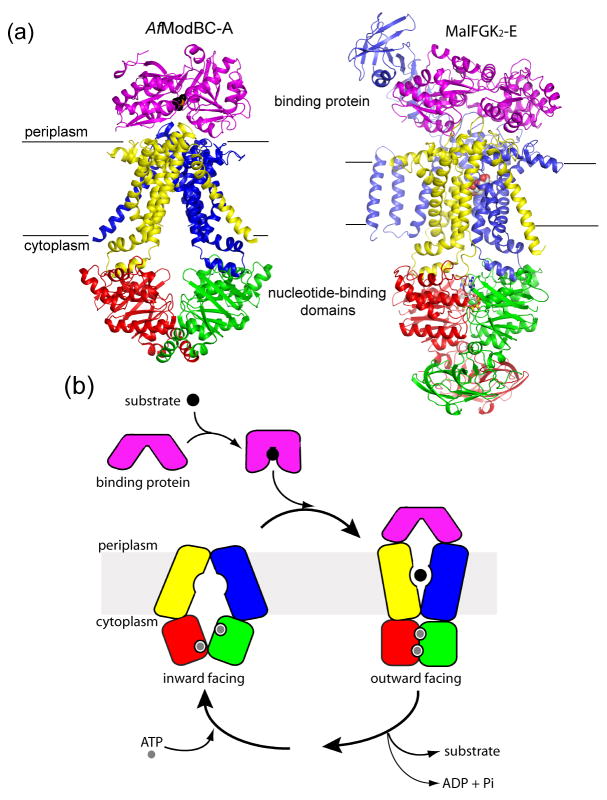
Two ABC importers, the molybdate/tungstate transporter from A. fulgidus (AfModBC-A) and the E. coli maltose transporter (MalFGK2-E), were crystallized in complex with their binding proteins [7,8]. The homodimer of the TM subunits (ModB) in the AfModBC-A structure [7] forms an inverted V-shaped cavity accessible to the cytoplasm (Figure 1A, left). The two ATPase ModC subunits are arranged in an open, nucleotide-free conformation, in which the Walker A motif of one subunit faces, but is separated, from the LSGGQ motif of the other. The binding protein ModA is in a closed conformation with substrate bound in a cleft between its two lobes and is docked onto the extracellular loops of ModB, placing the substrate directly above the closed entrance of the translocation pathway. In contrast, the two ATPase MalK subunits in the MalFGK2-E structure [8] form a closed dimer, with two ATP molecules bound along the interface in contact with residues from the Walker A/B motifs of one monomer and the LSGGQ motif of the opposite monomer (Figure 1A, right). The maltose binding protein (MBP or MalE), also docked at the periplasmic surface, is in an open conformation, contributing to the formation of a large, occluded cavity located at the interface of the TM subunits MalF and MalG that faces the periplasm (Figure 1A, right, see also Figure 2A). ATP hydrolysis is thought to occur in the closed NBD dimer conformation [16] and biochemical and biophysical evidence has linked the opening of MBP on one side of the membrane to the closure of the nucleotide-binding interface on the other side [17–19], providing an explanation for how MBP may stimulate the ATPase activity of the transporter when it binds.
Figure 1. Alternating-access in ABC importers.
A. Structures of the inward-facing molybdate/tungstate transporter AfModBC-A (left) and the outward-facing maltose transporter MalFGK2-E (right). For each, the binding protein is colored in purple, the transmembrane subunits are colored in blue and yellow, and the ATPase subunits are colored in red and green. Translocation substrates, tungstate and maltose, are shown in CPK format while ATP is shown in ball-and-stick format. B. A model for alternating-access in ABC importers, modified from [8].
Figure 2. Substrate binding sites defined in ABC importers.
A. Left, a slab view of the transmembrane cavity in the maltose transporter with the maltose substrate shown in CPK format. Residues of MBP that form the substrate-binding site in the open conformation are colored cyan. Right, a close-up view of the binding site with hydrogen bonds indicated by dashed lines. Figure modified from [8]. B. Ribbon representation of the methionine transporter (MetNI) and the molybdate/tungstate transporter (ModBC) in a “trans-inhibited” state. Substrates bound to the ATPase subunits are shown in CPK format.
Based on the structures of AfModBC-A and MalFGK2-E, we propose a model involving an alternating-access mechanism for ABC transporters that explains how transport is coupled to ATP hydrolysis (Figure 1B). In the inward-facing, resting state, the NBD dimer interface is held open by the TMDs. In the presence of ATP, interactions with the closed, substrate-loaded binding protein promote the progression from the resting state to the outward-facing, ATP hydrolysis transition state, in which the NBD dimer is closed for hydrolysis and the TMDs have reoriented to receive substrate from the binding protein. In the maltose uptake system, MBP binds more tightly to and thereby stabilizes the transition state conformation [17]. After ATP hydrolysis, the NBD dimer opens and the transporter reverts to the resting state concomitantly with exposure of the substrate to the cytoplasm. Given that the intracellular ATP concentration is ten times the Km of most ABC transporters [20], the nucleotide-binding sites are probably saturated with ATP in the resting state in vivo. We recently showed that closure of the NBD interface requires not only the binding of ATP, as seen with isolated NBDs [21,22], but also the binding of MBP to the intact transporter [19]. Consistently, in the absence of ATP the resting state of AfModBC-A was observed even though the binding protein was present [7].
The location of the substrates in AfModBC-A and MalFGK2-E elucidate the translocation pathway across the membrane. In the resting state structure of AfModBC-A, the substrate is bound to the closed binding protein [7]. In the outward-facing structure of MalFGK2-E [8], the substrate maltose is present at the base of the TM cavity, approximately halfway across the predicted lipid bilayer (Figure 2A). Although genetic data have suggested the presence of a substrate-binding site inside the TM subunits [23–26], the structure of MalFGK2-E provides the first picture of how TMDs interact with a substrate. In the maltose transporter, the translocation pathway is completely shielded from the membrane bilayer by TM helices of MalF and MalG and from the periplasm by the open, apo MBP. Maltose is bound in the cavity by ring stacking and hydrogen bonding interactions exclusively to MalF (Figure 2A).
In the most recent structures of the M. acetivorans molybdate/tungstate transporter (MaModBC) [9] and the E. coli methionine transporter (MetNI) [10], additional, unexpected substrate-binding sites were revealed in a domain C-terminal to the NBDs (Figure 2B). For both transporters, substrate was shown to inhibit the basal ATPase activity of the transporter, assayed in detergent, with half-maximal inhibition occurring at low micromolar concentrations. Compared to AfModBC-A, both MetNI and MaModBC show larger separations between their two NBDs coincident with a wider opening of the TMDs on the cytoplasmic side of the membrane (Figure 2B). These two structures are interpreted in the context of a trans-inhibition mechanism, whereby intracellular methionine inhibits the uptake of external methionine in a concentration dependent manner [27]. Binding of the substrate to the C-terminal extension of the ATPase subunit might stabilize these transporters in an inward-facing conformation, thereby preventing the transition to the outward-facing state [9,10].
A common feature of this group of importers is the TMD/NBD interface. Each NBD is bound to one TMD primarily through contacts of a short cytoplasmic helix of the TMD, “the coupling helix” [3], that lies approximately parallel to the membrane bilayer and docks in a surface cleft formed between the RecA-like and helical subdomains. Despite the differences in their sizes and conformational states, a common structural core consisting of five TM helices is found in the structures of ModB, MalF, MalG, and MetI [10]. The root mean square deviation (rmsd) between equivalent Cα positions among these structures is ~2.5 Å [10]. The fact that the TM cores of outward-facing MalF and MalG are super-imposable with the inward-facing cores of ModB and MetI suggests that transitions between the inward/outward- facing conformations is likely to involve rigid-body rotations of the core. The sequence similarity among these small importers is low, therefore, to understand the details of the conformational change leading to alternative-access, structures of both inward- and outward-facing states will have to be determined from a single or homologous ABC transporter.
Large ABC importers
The category of large ABC importers includes the E. coli vitamin B12 transporter (BtuCD) [6,11] and the homologous metal-chelate transporter from H. influenzae (HI1470/1) [12] (Figure 3). The functional unit of the vitamine B12 transporter consists of two copies each of the ATPase subunit (BtuD) and the TMD subunit (BtuC). Whereas BtuD exhibits a canonical NBD fold, the architecture of the TMD subunit is very different from the small importers; BtuC contains ten transmembrane helices that do not resemble the five-helix core of the smaller importers. The structures of BtuCD were determined both in the presence and absence of its binding protein BtuF. Structural comparison of BtuCD and BtuCD-F shows that the NBDs are essentially in the same open dimer configuration, with an rmsd of 0.5 Å in Cα positions. In addition, seven membrane-spanning helices (TM1-2, and 6-10) superimpose closely for the BtuC subunits in both structures. The major structural differences lie in a subdomain consisting of helices TM3/TM4/TM5/5a (the inner subdomain). In BtuCD, the two inner subdomains are symmetrical and lie in an outward-facing conformation [6]. In BtuCD-F, helices TM3-5a of the two subunits have different conformations, one similar to BtuCD (Figure 3, orange), the other shifts approximately 20 degrees (Figure 3, cyan). Because TM5 lies in the BtuC dimer interface, the rotation of the inner subdomain has a substantial impact on the translocation pathway. Consequently, the outward-facing substrate-translocation pathway in BtuCD is closed to both sides of the membrane in BtuCD-F. In HI1470/1, a homologous protein to BtuCD [12], the apo-NBDs are separated to a larger degree than in BtuCD and the TM cavity is closed to the periplasm and open to the cytoplasm (Figure 3). As noted by Rees and colleagues, reorientation of the translocation pathway between BtuCD and HI1470/I can be described as two rigid-body movements, rotations of the inner subdomains plus a ~9 degree twist of one TMD with respect to the other [12].
Figure 3. Structures of large importers revealing a flexible inner subdomain.
TM subunits are colored in blue and yellow with the inner subdomains (TM3/4/5/5a) highlighted in thicker ribbons (cyan and orange). The binding protein BtuF is colored purple and the ATPase subunits are colored in red and green.
The structures of BtuCD, BtuCD-F, and HI1470/1 provide clear evidence for the flexibility of the inner subdomain and its importance in mediating alternating-access. Similar to other ABC transporters, substrate translocation in BtuCD is coupled to ATP binding and hydrolysis [28], therefore it may seem counterintuitive to observe three different conformations in the absence of nucleotides. Since the inner subdomain makes no contacts and appears to move independently from the NBDs (Figure 3), a possible interpretation of the three structures is that in the resting state where the NBD dimer is open, the inner subdomain is flexible, with two different conformations captured in the crystal structures of BtuCD [6] and HI1470/1 [12]. In BtuCD-F, the position of the inner subdomain is influenced by the interactions with the binding protein, and thus exhibits an asymmetric conformation [11]. For the class of large importers, the alternating-access mechanism will likely involve rotations of two rigid-body groups per transmembrane subunit. The movement of the first rigid-body group (TM1-2, 6-10) would be coupled to the opening and closing of the NBDs while the second rigid-body group (TM3-5a or the inner subdomain) would move in response to the periplasmic binding protein. Modulation of the inner subdomain might be necessary to allow translocation of large substrates such as vitamin B12. It is tempting to suggest that, in the intermediate state where ATP molecules are bound at the closed NBD dimer interface, there will be an outward-facing cavity larger than that observed in the BtuCD structure [6], similar to that seen in MalFGK2-E [8]. Structures of these transporters in a nucleotide-bound/closed-NBD state will be necessary to fully elucidate the alternating-access mechanism.
ABC exporters
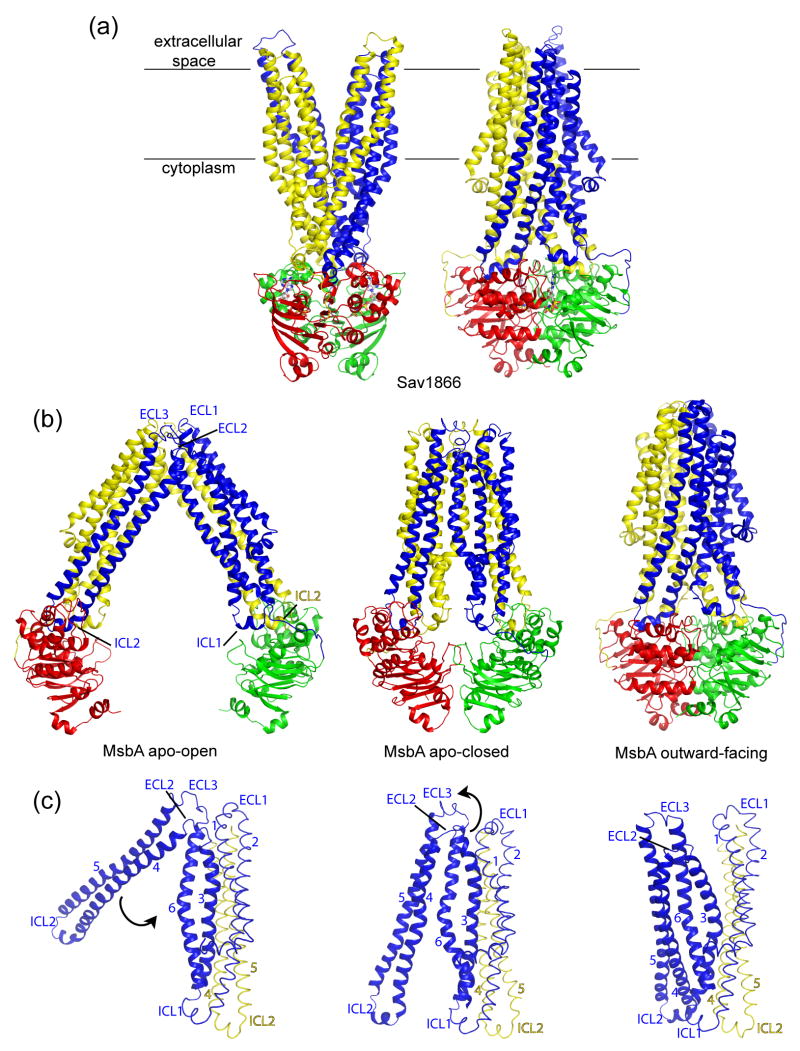
The structure of the multidrug transporter Sav1866 from S. aureus was the first high-resolution structure reported for an ABC exporter [13,14]. Sav1866 is a homodimer of half transporters, each subunit contains an N-terminal TMD with six helices and a C-terminal NBD with the canonical fold (Figure 4A). The NBDs of Sav1866 form a closed dimer with nucleotide bound and the TM helices split into two “wings” in the outer leaflet of the membrane, creating an outward-facing conformation (Figure 4A). Each wing consists of helices TM1-2 from one subunit and TM3-6 from the other subunit. The TM segments are connected by long intracellular loops (ICLs) that extend beyond the lipid bilayer into the cytoplasm. In contrast to the importers whose coupling helices contact a single NBD, each TMD of Sav1866 contains two intracellular coupling helices, one (ICL1) contacting the NBDs of both subunits, the other (ICL2) interacting solely with the NBD of the opposite subunit.
Figure 4. Structures of ABC exporters.
A. Structure of the outward-facing Sav1866. One subunit is colored in blue (TMD) and green (NBD) and the other subunit in yellow (TMD) and red (NBD). The bound nucleotide, AMP-PNP is shown in ball-and-stick format. B. Structures of MsbA in open inward- (left), closed inward- (middle) and outward-facing (right) conformations. The extracellular loops (ECL) and cytoplasmic coupling helices (ICL) are indicated. C. Transitions from the open inward- to outward facing conformations. For each state TM1-6 (blue) of one TMD and TM4-5 (yellow) of the other TMD are shown. Rotations of TM4-5 (blue) and TM3-6 (blue) necessary for the transitions are indicated by black arrows.
The recent structures of the lipid flippase MsbA, two nucleotide-bound and two apo forms have highlighted the dynamic nature of ABC exporters [15], as suggested by fluorescence and EPR studies [29,30]. The structures of MsbA co-crystallized with AMP-PNP or ADP/Vi are very similar to that of Sav1866, showing an outward-facing, intertwining conformation (Figure 4B). The two apo forms are both inward-facing with different degrees of separation of the two subunits. Two helices, TM4-5, cross over the dimer interface and associate with the opposing subunit. As a consequence, the coupling helix connecting TM4-5 (ICL2) maintains its interaction with the NBD of the other subunit in both apo and nucleotide-bound configurations while ICL1 breaks contact with the opposing NBD (Figure 4B). Transition from the open-apo form to the closed-apo form would involve a ~30 degree pivoting of the TM4-5 helices around a hinge formed by the extracellular loops ECL2 and ECL3, bringing the NBDs closer to each other [15] (Figure 4C). However, in the absence of nucleotide, the NBDs in the closed form are “misaligned”, in that the Walker A motif of one NBD sits opposite the Walker A motif of the second NBD, instead of the LSGGQ loop [15]. Formation of the canonical closed dimer from this closed-apo intermediate would require sliding of the two NBDs along the dimer interface, and would be coupled to a twisting motion in the TMDs that pulls TM3-TM6 away from TM1-TM2 of the same subunit to form the outward-facing conformation (Figure 4C).
Conclusions
In recent years, great advances have been made in determining structures of ABC transporters. The common lesson learned is that both importers and exporters adopt an outward-facing conformation in the ATP-hydrolysis transition state and an inward-facing conformation in the resting state. In keeping with their function, importers receive their substrates in the outward-facing state and release them in an inward-facing state, and it appears likely that exporters do the opposite, though an exporter has not yet been crystallized with substrate bound. Multiple conformations have been observed, demonstrating the conformational flexibility of ABC transporters. However, substantial biochemical analysis will be required to fully interpret these structures and to formulate models of substrate translocation.
Acknowledgments
Research in the Chen lab was supported by NIH grant (GM070515) and the Pew scholarship. Work in the Davidson lab was supported by NIH (GM49261) and the Welch Foundation. M.L.O is a postdoctoral fellow of the American Heart Association.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Biemans-Oldehinkel E, Doeven MK, Poolman B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 2006;580:1023–1035. doi: 10.1016/j.febslet.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 2.Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Dawson RJ, Hollenstein K, Locher KP. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol Microbiol. 2007;65:250–257. doi: 10.1111/j.1365-2958.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- 5.Davidson AL, Maloney PC. ABC transporters: how small machines do a big job. Trends Microbiol. 2007;15:448–455. doi: 10.1016/j.tim.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Locher KP, Lee AT, Rees DC. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 7.Hollenstein K, Frei DC, Locher KP. Structure of an ABC transporter in complex with its binding protein. Nature. 2007;446:213–216. doi: 10.1038/nature05626. [DOI] [PubMed] [Google Scholar]
- 8**.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. Crystal structure of the E. coli maltose transporter adopts an outward-facing conformation with a large occluded periplasm-facing, transmembrane cavity formed by the TMDs and capped by the maltose binding protein. Substrate is found at the base of this cavity and the NBDs are dimerized around bound ATP. [DOI] [PubMed] [Google Scholar]
- 9**.Gerber S, Comellas-Bigler M, Goetz BA, Locher KP. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science. 2008;321:246–250. doi: 10.1126/science.1156213. Structure of the M. acetivorans molybate/tungstate transporter shows that the C-terminal extension of the NBD binds substrate and locks the transporter in an inward-facing conformation, thereby preventing the dimerization of the NBDs and ATP hydrolysis. [DOI] [PubMed] [Google Scholar]
- 10**.Kadaba NS, Kaiser JT, Johnson E, Lee A, Rees DC. The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science. 2008;321:250–253. doi: 10.1126/science.1157987. Crystal structure of the E. coli methionine transporter is the smallest ABC transporter known so far. Each TMD has a five helix core similar to that of the ModBC and MalFGK2-E transporters. Similar to MaModBC, the NBDs contain a C-terminal extension that binds substrate and prevents ATP hydrolysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Hvorup RN, Goetz BA, Niederer M, Hollenstein K, Perozo E, Locher KP. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science. 2007;317:1387–1390. doi: 10.1126/science.1145950. Structure of the vitamin B12 importer bound to its binding protein BtuF reveals an asymmetric orientation of an inner TM subdomain that results in closure of the translocation pathway from either side of the membrane. [DOI] [PubMed] [Google Scholar]
- 12.Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. [DOI] [PubMed] [Google Scholar]
- 13.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 14.Dawson RJ, Locher KP. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 15**.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci U S A. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. Structures of MsbA with or without nucleotides reveal that MsbA undergoes large conformational changes in a transport cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fetsch EE, Davidson AL. Vanadate-catalyzed photocleavage of the signature motif of an ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2002;99:9685– 9690. doi: 10.1073/pnas.152204499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Sharma S, Quiocho FA, Davidson AL. Trapping the transition state of an ATP- binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc Natl Acad Sci U S A. 2001;98:1525–1530. doi: 10.1073/pnas.041542498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austermuhle MI, Hall JA, Klug CS, Davidson AL. Maltose-binding protein is open in the catalytic transition state for ATP hydrolysis during maltose transport. J Biol Chem. 2004;279:28243–28250. doi: 10.1074/jbc.M403508200. [DOI] [PubMed] [Google Scholar]
- 19*.Orelle C, Ayvaz T, Everly RM, Klug CS, Davidson AL. Both maltose-binding protein and ATP are required for nucleotide-binding domain closure in the intact maltose ABC transporter. Proc Natl Acad Sci U S A. 2008;105:12837–12842. doi: 10.1073/pnas.0803799105. Site-directed spin labeling electron paramagnetic spectroscopy is used to study conformational changes in the NBD dimer during the transport cycle. Both maltose binding protein and ATP are required to promote formation of the closed NBD dimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuhard J, Nygaard P. Purines and pyrimidines. In: Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Vol. 1 ASM Press; 1987. pp. 445–473. [Google Scholar]
- 21.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP- binding cassette dimer in an ABC transport cycle. Mol Cell. 2003;12:651–661. doi: 10.1016/j.molcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Ehrle R, Pick C, Ulrich R, Hofmann E, Ehrmann M. Characterization of transmembrane domains 6, 7, and 8 of MalF by mutational analysis. J Bacteriol. 1996;178:2255–2262. doi: 10.1128/jb.178.8.2255-2262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinke A, Grau S, Davidson A, Hofmann E, Ehrmann M. Characterization of transmembrane segments 3, 4, and 5 of MalF by mutational analysis. J Bacteriol. 2001;183:375–381. doi: 10.1128/JB.183.1.375-381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treptow NA, Shuman HA. Genetic evidence for substrate and periplasmic-binding- protein recognition by the MalF and MalG proteins, cytoplasmic membrane components of the Escherichia coli maltose transport system. J Bacteriol. 1985;163:654–660. doi: 10.1128/jb.163.2.654-660.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covitz KM, Panagiotidis CH, Hor LI, Reyes M, Treptow NA, Shuman HA. Mutations that alter the transmembrane signalling pathway in an ATP binding cassette (ABC) transporter. EMBO J. 1994;13:1752–1759. doi: 10.1002/j.1460-2075.1994.tb06439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadner RJ. Regulation of methionine transport activity in Escherichia coli. J Bacteriol. 1975;122:110–119. doi: 10.1128/jb.122.1.110-119.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng J, Ma J, Fan K, Wang W. The conformational coupling and translocation mechanism of vitamin B12 ATP-binding cassette transporter BtuCD. Biophys J. 2008;94:612–621. doi: 10.1529/biophysj.107.110734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong J, Yang G, McHaourab HS. Structural basis of energy transduction in the transport cycle of MsbA. Science. 2005;308:1023–1028. doi: 10.1126/science.1106592. [DOI] [PubMed] [Google Scholar]
- 30*.Borbat PP, Surendhran K, Bortolus M, Zou P, Freed JH, McHaourab HS. Conformational motion of the ABC transporter MsbA induced by ATP hydrolysis. PLoS Biol. 2007;5:e271. doi: 10.1371/journal.pbio.0050271. Motions of MsbA in a transport cycle are probed by pulse double electron-electron resonance and fluorescence homotransfer. Evidence for conformational changes upon substrate binding and ATP hydrolysis is presented. [DOI] [PMC free article] [PubMed] [Google Scholar]