Abstract
We previously reported that our novel compound 3β-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene (VN/124-1) is a potent CYP17 inhibitor/antiandrogen and strongly inhibits the formation and proliferation of human prostate cancer LAPC4 tumor xenografts in SCID mice. In this study, we report that VN/124-1 and other novel CYP17 inhibitors also cause down-regulation of AR protein expression in vitro and in vivo. This mechanism of action appears to contribute to their antitumor efficacy. We compared the in vivo antitumor efficacy of VN/124-1 with that of castration and a clinically used antiandrogen, casodex, and show that VN/124-1 is more potent than castration in LAPC4 xenograft model. Treatment with VN/124-1 (0.13 mmol/kg/twice daily) was also very effective in preventing the formation of LAPC4 tumors (6.94 vs. 2410.28 mm3 in control group). VN/124-1 (0.13 mmol/kg/twice daily) and VN/124-1 (0.13 mmol/kg/twice daily) + castration induced regression of LAPC4 tumor xenografts by 26.55% and 60.67%, respectively. Treatments with casodex (0.13 mmol/kg/twice daily) or castration caused significant tumor suppression compared with control. Furthermore, treatment with VN/124-1 caused marked down-regulation of AR protein expression, in contrast to treatments with casodex or castration that caused significant up-regulation of AR protein expression. The results suggest that VN/124-1 acts by several mechanisms (CPY17 inhibition, competitive inhibition and down-regulation of the androgen receptor). These actions contribute to inhibition of the formation of LAPC4 tumors and cause regression of growth of established tumors. VN/124-1 is more efficacious than castration in the LAPC4 xenograft model suggesting the compound has potential for the treatment of prostate cancer.
Keywords: CYP17 inhibitor/antiandrogen, prostate cancer prevention and inhibition
INTRODUCTION
Prostate cancer (PC) is the most prevalent cancer in men and the second leading cause of death in American men resulting in 218,890 new cases and 27,050 deaths per year from this disease [1]. Androgens play an important role in the development, growth, and progression of PC [2]. The testes produce most of the circulating testosterone (T), whereas approximately 10 % is synthesized by the adrenal glands. T is further converted in the prostate to the more potent androgen dihydrotestosterone (DHT) by the enzyme 5α-reductase [3]. Most PCs are initially dependent on androgens for their growth, and orchidectomy (either surgical or medical with a GnRH agonist) remains the standard of treatment. Although orchidectomy reduces androgen production by the testes, androgen synthesis in the adrenal glands is unaffected. Thus, orchidectomy combined with antiandrogens to block the action of adrenal androgens can be more effective and prolongs survival of PC patients [4].
The mechanisms through which androgen-dependent PC tumors survive and proliferate under androgen deprivation therapy (ADT) are not completely understood. However, it has been found that the androgen receptor (AR) is consistently expressed and active in multiple xenograft models of hormone refractory disease [5]. Amplified expression and increased sensitivity of AR in recurrent PC may be due to its increased stability, altered growth factor signaling, and mutations that broaden ligand specificity [6–9]. Additionally, reduction of AR expression in androgen sensitive and refractory models through the use of shRNA, or chemical means, have resulted in marked growth suppression of PC cells [10–13]. Further support for the role of AR and androgens in PC is the recent report of increased expression of genes of androgen converting enzymes and persistence of androgen regulated genes in androgen-independent PC [14–16]. These observations suggest that therapies that inhibit production of androgens and target multiple points in the AR signaling cascade could offer a more effective approach for prolonging remission of PC.
In the testes and adrenal glands, the last step in the biosynthesis of T involves two key sequential reactions, that are catalyzed by a single enzyme, the cytochrome P450 monooxygenase 17α-hydroxylase/17,20-lyase (CYP17) [17]. Ketoconazole, an antifungal agent and non-specific CYP450 inhibitor used with careful scheduling [18] can produce prolonged responses in otherwise hormone-refractory PC patients. Furthermore, ketoconazole was found to retain activity in advanced PC patients with progression despite flutamide withdrawal [19]. Although ketoconazole remains one of the most effective second line hormonal therapies for PC, its use is limited due to liver toxicity and other side effects. However, its antitumor efficacy suggests that more potent and selective inhibitors of CYP17 could provide useful agents for treating this disease [20].
We, and others, have reported a number of novel inhibitors of CYP17, and some have been shown to be strong inhibitors of testosterone production in rodent models [20–23]. Jarman and colleagues recently described the effects of their steroidal CYP17 inhibitor, abiraterone, in patients with PC [24, 25]. Some of our most effective CYP17 inhibitors possess additional activities, such as inhibition of 5α-reductase and/or are antiandrogens with potent antitumor efficacy [26–29].
In addition to being among the strongest CYP17 inhibitors known to date, the novel steroidal compounds VN/85-1, VN/87-1 and VN/108-1 were shown to reduce DHT stimulated LNCaP cell proliferation, and displace methyltrienolone (R1881), a synthetic androgen, from the mutated T877A AR at a 5 μM concentration [26]. VN/124-1 (Fig. 1) was found to be effective in vitro as well as in the LAPC4 xenograft model in male SCID mice [28]. In addition to inhibition of CYP17, VN/124-1 exhibited potent AR antagonism in binding studies and LNCaP luciferase transcription assays, as well as marked tumor growth suppression in LAPC4 xenografts [28]. In this report, we demonstrate that VN/124-1 and other novel CYP17 inhibitors cause down-regulation of AR protein expression in vitro and in vivo. This mechanism of action appears to contribute to their antitumor efficacy. We also compared the in vivo antitumor efficacy of VN/124-1 with that of castration and show that VN/124-1 is more potent than castration in human PC xenograft models.
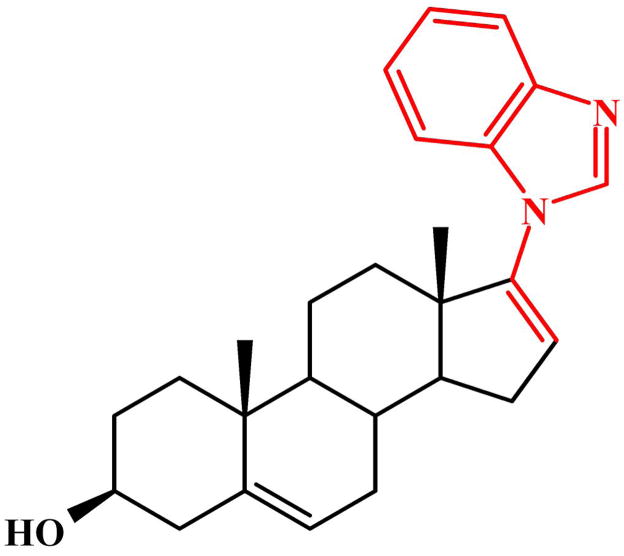
Fig. 1.
Chemical Structure of 3β-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene (VN/124-1)
MATERIAL AND METHODS
Casodex (Bicalutamide) was provided by Dr. E. Anderson from Astra Zeneca UK. The compounds VN/124-1, VN/125-1, VN/85-1, VN/87-1 and VN/108-1 were synthesized in our laboratory as described previously [26, 28]. AR antibody (SC-7305) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Tritiated methyltrienolone ([3H]R1881) was obtained from Perkin Elmer LAS (Waltham, Massachusetts).
Cell culture
LAPC4 cells were grown in IMEM supplemented with 15% FBS, 1% penicillin/streptomycin solution, and 10 nM DHT. LNCaP cells were maintained in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin solution. PC-3-AR cells were grown in the same media supplemented with 750μg/ml G418, for continued selection of the AR vector.
DNA Constructs and Transfections
The Probasin luciferase reporter construct ARR2-Luc was generated by insertion of the minimal probasin promoter ARR2, into the polyclonal linker region of PGL3-enhancer vector (Promega) as described previously [30]. The pRL-null (Promega) was used as the internal control. PC-3 cells stably transfected with the human wild-type AR (designated PC-3AR), and the T575A human AR mutation vector were kindly provided by Dr. Marco Marcelli (Baylor College of Medicine, Houston, TX; [31]. All transfections were carried out utilizing LipofectAMINE 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol.
Competitive binding assays
In order to determine if the CYP17 inhibitors interact with the AR, competitive binding assays were performed as described previously [28]. The ability of the test compounds (1 nM–10 μM) to displace [3H]R1881 (5.0 nM) from the AR was determined in LAPC4 cells (wild-type AR), PC3 cells transfected with wild-type AR (PC3-AR), LNCaP cells which express an endogenous AR with a mutation in the ligand binding domain (T877A), and PC3 cells transfected with an AR containing a mutation in the DNA binding domain (T575A).
Transcriptional activation - luciferase assay
LAPC4 and LNCaP cells were transferred to steroid-free medium 3 days before the start of the experiment, and plated at 1 × 105 cells/well in steroid-free medium. The cells were dual transfected with ARR2-Luc and the Renilla luciferase reporter vector pRL-null as described in DNA Constructs and Transfections. After a 24 hour incubation period at 37°C, the cells were incubated in fresh phenol-red free RPMI 1640 medium containing 5% charcoal-stripped FBS and treated with 10 nM DHT, ethanol vehicle and/or the selected compounds in triplicate. After an 18 hour treatment period, the cells were washed twice with ice-cold DPBS and assayed using the Dual Luciferase kit (Promega) according to the manufacturer’s protocol. Cells were lysed with 100 μl of luciferase lysing buffer, collected in a microcentrifuge tube, and pelleted by centrifugation. Supernatants (20 μl aliquots) were transferred to corresponding wells of opaque 96-well multiwell plates. Luciferase Assay Reagent was added to each well, and the light produced during the luciferase reaction was measured in a Victor 1420 scanning multi-well spectrophotometer (Wallac, Inc., Gaithersburg, MD). After measurement, Stop and Glo reagent (Promega) was added to quench the firefly luciferase signal and initiate the Renilla luciferase luminescence. Renilla luciferase luminescence was also measured in the Victor 1420. The results are presented as the fold induction, that is, the relative luciferase activity of the treated cells divided by that of the control, normalized to that of the Renilla.
AR Down-regulation and Degradation
In order to determine the ability of the test compounds to modulate AR protein levels, LAPC4 and LNCaP cells were treated with concentrations ranging from 1–15 μM for 24 hours. Cells were collected and lysates prepared. Equal amounts of total protein were analyzed for AR expression levels by western blot analysis. Equal amounts of total protein (50–100 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE 60 V, 3 hr) and transferred (90 V, 1 hr) to nitrocellulose membranes (Hybond ECL, Amersham). Immunodetections were performed using mouse monoclonal antibody against human AR (SC-7305 Santa Cruz Biotechnologies, Inc, Santa Cruz, CA). Immunoreactive bands were visualized using the enhanced chemiluminescence detection reagents (Amersham Corp., Arlington Heights, IL) according to the manufacturer’s instructions and quantitated by densitometry using ImageQuant 5.0 software.
For degradation studies, LNCaP cells were grown in serum free medium for three days, and treated with 10 μM cycloheximide alone, 15 μM VN/124-1 alone, or 10 μM cycloheximide + 15 μM VN/124-1 for 0, 2, 6, 12, and 24 hours. Cells were collected by centrifugation and the cell pellet was resuspended in chilled lysis buffer [0.1M Tris HCl, 0.5% TritonX-100, protease inhibitors (Complete™, Boehringer, Indianapolis, IN)] and sonicated for 20 seconds. The homogenates were transferred to Eppendorf tubes, incubated on ice for 30 minutes, and then spun at 14,000 rpm for 20 minutes. The supernatants were stored at −70°C. Western bolts were performed as above. Protein concentrations were determined with a Bio-Rad kit (BioRad, Hercules and CA).
Cell proliferation assay
To determine the effect of steroids and novel compounds on cell proliferation, each cell type was transferred into steroid-free medium three days prior to the start of the experiments (steroid-free medium consisted of phenol red free RPMI supplemented with 5% dextran-coated, charcoal treated serum and 1% penicillin/streptomycin solution). Growth studies were then performed by plating cells (1.5×104cells/well) in 24-well multiwell dishes (Corning, Inc. Corning, NY). After a 24 hour attachment period, the medium was aspirated and replaced with steroid-free medium containing vehicle or the indicated concentrations of androgens and novel compounds (1 nM – 10 μM). Control wells were treated with vehicle (ethanol). Casodex (bicalutamide) was used as a reference drug for comparison to a known anti-androgen. The medium was changed every three days and the numbers of viable cells were compared by MTT or XTT (LNCaP) assay on the seventh day.
For the MTT procedure, following incubation of cells for the above mentioned time, 0.5 mg/ml MTT was added to each well and incubated at 37°C for three hours. Following incubation, the medium was aspirated completely, with care taken not to disturb the formazan crystals. DMSO (500 μl) was used to solubilize these crystals. After slight shaking, the plates were read at 540 nM with a Victor 1420 scanning multi-well spectrophotometer. All results represent the average of a minimum of three wells. Additional control consisted of medium alone with no cells. XTT was performed essentially as MTT, with the deletion of the solubilization step and was preferred for the LNCaP cells that adhere poorly to the plates. A water soluble formazan was obtained using XTT, and the plates were read at 450 nM with the spectrophotometer.
In Vivo Antitumor Studies (LAPC4 Prostate Cancer Xenografts)
All animal studies were performed according to the guidelines and approval of the Animal Care Committee of the University Of Maryland School Of Medicine, Baltimore. Male severe combined immunodeficient (SCID) mice 4–6 weeks of age purchased from the National Cancer Institute, (Fredrick, MD) were housed in a pathogen-free environment under controlled conditions of light and humidity and allowed free access to food and water. Tumors were developed from LAPC4 cells inoculated subcutaneously (s.c.) into each mouse as previously described [28]. LAPC4 cells were grown in IMEM with 15% FBS plus 1% PS and 10 nm DHT until 80% confluent. Cells were scraped into DPBS, collected by centrifugation and resuspended in Matrigel (10 mg/mL) at 3 × 107 cells/mL. Mice were injected s.c. with 100 μL of the cell suspension at one site on each flank. Twice per week, the mice were weighed and tumors were measured with calipers for the duration of experiment. Tumor volumes were calculated by the formula: 4/3π × r1 2 × r2 (r1 < r2). Mice in the tumor formation prevention group (n = 5) were injected with VN/124-1 (0.13 mmol/kg/twice daily) in the vehicle (0.3% saline hydroxypropyl cellulose; HPC) from the day of inoculation for the duration of the experiments. The rest of the mice were monitored until tumors reached approximately 500 mm3, about 9 weeks after cell inoculation. Mice were assigned to five groups (five mice per group) for treatment so that there was no statistically significant difference in tumor volumes amongst the groups at the beginning of the treatment. The five groups were: control, castration, casodex (0.13 mmol/kg/twice daily), VN/124-1 (0.13 mmol/kg/twice daily), and VN/124-1 (0.13 mmol/kg/twice daily) + castration. Compounds (suspensions in 0.3% PHC) were given s.c. twice daily, 9 a.m. and 5 p.m. Mice were castrated under methoxyfluorane anesthesia. Control and castrated mice were treated with vehicle (HPC) only. At the end of the treatment period, the animals were sacrificed under flurothane anesthesia; tumors were excised, weighed and stored at −80°C. The liver and kidneys were also harvested and examined for any abnormalities. Animals were also monitored for general health status and signs of possible toxicity due to treatment.
Tumor Analysis
The protein extracts of the tumors from the above experiment were prepared by homogenizing the tissue in ice-cold DPBS containing protease inhibitors. Western blots were performed as described above.
Statistical Analysis
The total tumor volume of each group was compared using the log scale. Because of incomplete values, we used the quasi-t-test developed by Tan et al. [32] for comparison between two groups. We compared total tumor volume from day 63 to day 93, the total tumor volume from day 76 to day 93 (the treatment effect after two weeks), and at day 93. All computations were performed using S-PLUS. The treatment groups were compared with one another at 0.05 level of significance.
RESULTS
Competitive binding to wild type and mutant androgen receptors
LNCaP cells expressed a single class of high-affinity binding sites with Kd = 0.5 nM, and Bmax determined as 1.18 × 105 sites/cell. LAPC4 cells had a similar Kd of 0.4 nM with a Bmax of 6.1 × 104 sites/cell. Once the saturation concentration (5 nM) was determined, evaluation of the compounds previously tested at 5μM in LNCaP cells, VN/85-1, VN/87-1, VN/108-1 [33], was conducted over a full concentration range in both cell types. Casodex, an anti-androgen currently used as PC therapy, was included as a reference drug (Table 1). Abiraterone, a CYP17 inhibitor currently in clinical trials, was also tested.
Table 1. IC50 values for competitive AR binding.
Competitive inhibition of [3H]R1881 binding (LAPC4, PC3-AR, PC3-ART575A, and LNCaP cells)
| Compound | wild-type (IC50 nM) (LAPC4/PC3-AR) | T877A(IC50 nM) (LNCaP) | T575A (IC50 nM) (PC3-ART575A) |
|---|---|---|---|
| VN/85-1 | 341 | 1290 | 473 |
| VN/87-1 | 319 | 422 | NT |
| VN/108-1 | 868 | 831 | 1210 |
| VN/124-1 | 405 | 845 | 454 |
| VN/125-1 | 248 | 1240 | 383 |
| Abiraterone | - | - | NT |
| Casodex | 4300 | 971 | NT |
| Flutamide | 10985 | 11600 | NT |
NT= Not tested, - = less than 20 % inhibition at 10 μM
The ability of the test compounds to displace [3H]R1881 (5.0 nM) from the androgen receptor was determined in LAPC4 cells (wild-type AR), PC3 cells transfected with wild-type AR, LNCaP cells which express an endogenous AR with a mutation in the ligand binding domain (T877A), and PC3 cells transfected with an AR containing a mutation in the DNA binding domain (T575A). Cells were plated (2–3 × 105) in 24 well multiwell dishes in steroid-free medium and allowed to attach. The following day the medium was replaced with serum-free, steroid free RPMI supplemented with 0.1% BSA and containing [3H]R1881 (5 nM), test compounds (1 nM–10 μM), and 1μM triamcinolone acetonide to saturate progesterone and glucocorticoid receptors. Following a 2 hour incubation period at 37°C, cells were washed twice with ice-cold DPBS and solubilized in DPBS containing 0.5% SDS and 20% glycerol. Extracts were removed and cell associated radioactivity counted in a scintillation counter. The data was analyzed by nonlinear regression using Graphpad Prism software (GraphPad Software, Inc, San Diego, CA).
VN/85-1, VN/87-1, VN/124-1, and VN/125-1 had the highest affinity for the wild-type AR. In contrast abiraterone did not bind to the AR. There was no significant difference found between the wild-type AR in transfected PC3-AR cells and the wild-type AR expressed endogenously in LAPC4 cells (Table 1). VN/85-1, VN/124-1, and VN/125-1 had slightly greater ability to displace [3H]R1881 from the wild-type receptor than from the T877A mutant in LNCaP cells. The T877A AR contains a mutation in the ligand binding domain [34] which could account for the observed difference in binding. In contrast, VN/108-1 and VN/87-1 demonstrated nearly identical affinities for both receptor types. PC3-T575-AR cells express an AR with a mutation in the DNA binding domain [31]. Unlike the T877A AR, there was no apparent difference observed in VN/85-1, VN/124-1, or VN/125-1’s ability to displace [3H]R1881 from the T575A AR when compared with wild-type. Therefore additional compounds were not tested for AR affinity for this mutation.
Androgen Receptor Antagonism
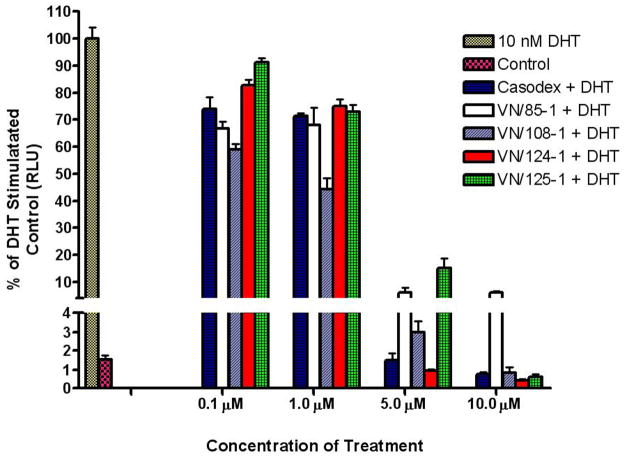
The compounds that showed strong binding affinity for the receptor were evaluated for antagonistic properties by the luciferase assay in LAPC4 and LNCaP cells transfected with the ARR2-Luc vector. These experiments were carried out against both receptor types, as there are reports of some wild-type AR antagonists, such as flutamide, functioning as T877A agonists [35, 36]. In both cell types, VN/124-1, VN/125-1 and VN/108-1 inhibited DHT induced transcriptional activation with similar potency as casodex. Casodex, VN/85-1, VN/124-1, and VN/125-1 at 10 μM concentration were all able to reduce WT and T877A AR-mediated transcriptional activation by 90–99% (Fig. 2). In LNCaP cells, VN/87-1 was the least effective of the compounds tested. When LNCaP-CYP17 cells were exposed to inhibitors in steroid free media, only VN/87-1 activated luciferase transcription, indicating that it is a partial agonist of the T877A AR, similar to flutamide. This compound was therefore excluded from further studies. None of the other compounds displayed agonistic properties in either cell line.
Fig 2.
Effect of compounds on DHT stimulated transcription. LAPC4 Cells were transfected with the ARR-2 reporter construct + the Renilla luciferase reporting vector pRL-null and treated with novel compounds for 18 hours in the presence of 10 nM DHT. Control represents baseline activity without androgen stimulation. Androgen stimulated luciferase activity (luminescence) was measured in a Victor 1420 plate reader. The results are presented as the fold induction, that is, the relative luciferase activity of the treated cells divided by that of the control, normalized to that of the Renilla.
AR Downregulation
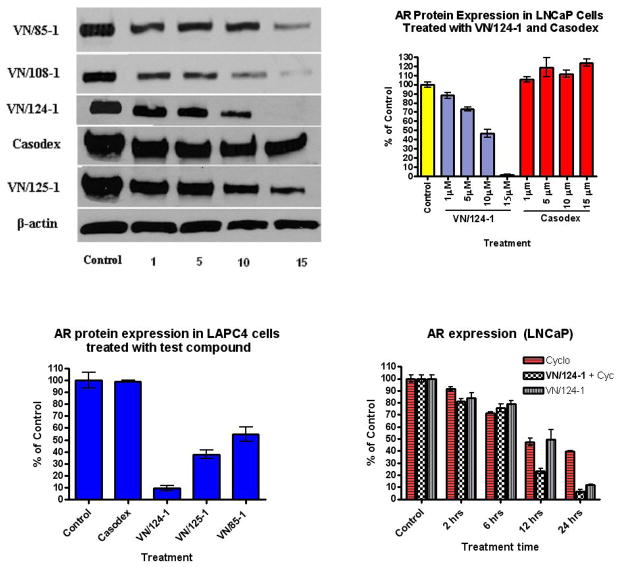
The compounds showed a dramatic down-regulation of wild-type and also mutated AR receptor. In LNCaP cells, nearly all of the test compounds induced a dose-dependent (1, 5, 10, 15 μM) decrease in AR levels whereas no change in total AR level was observed with casodex at these concentrations (Fig. 3A–B). VN/124-1 reduced expression by 50% at 10 μM, and displayed nearly complete suppression at 15 μM. VN/125-1, VN/85-1 and VN/108-1 were able to reduce AR protein expression by 65%, 70% and 90% respectively at a concentration of 15μM. In LAPC4 cells, VN/124-1 reduced expression by 89% at a 15 μM concentration. At the same concentration, VN/85-1 and VN/125-1 reduced expression by 50% and 66%, respectively (Fig. 3C).
Fig 3.
Western Blot Analysis of AR expression in vitro. Cells were treated with test compounds for 24 hours at the indicated concentrations (1–15 μM). Cell extracts were prepared and probed with anti AR and anti β-actin antibodies. (A) AR expression in LNCaP cells after 24 hr treatment with the indicated compounds. (B and C) Densitometry quantification of AR expression in LNCaP and LAPC4 Cells after treatment. (D) Densitometry quantification of AR expression in LAPC4 cells after treatment with 15 μM of the indicated compounds.
AR degradation
Although VN/124-1 was able to down-regulate the AR protein expression in a dose-dependent manner, it was still unclear whether the down-regulation was a result of decreased protein synthesis or increased degradation/AR destabilization. To determine protein degradation, de novo protein synthesis was inhibited using cyclohexamide and protein expression was measured at various time points.
Cycloheximide treatment alone reduced AR levels in a time dependent fashion, with 50% reduction observed at 12 hours and over 60% at 24 hours post treatment. VN/124-1 treatment did not alter AR degradation rate for the first 6 hours, however a rapid decline in AR level occurred between 6 and 12 hours post treatment, resulting in 50% less receptor than expressed at 6 hours, and 75% less than control. The observed difference between 12 and 24 hours followed a similar pattern in both cycloheximide and VN/124-1 groups, with only an additional decline of approximately 10% for each (Fig. 3D). These results suggest that VN/124-1 increases the degradation rate of the AR.
Inhibition of cell proliferation
The ability of the compounds to inhibit proliferation with and without DHT stimulation in LAPC4 and LNCaP cells was examined. In contrast to LNCaP cells, LAPC4 cells did not exhibit strong stimulation in response to DHT. This is in agreement with reports by other investigators [37]. As such, there was minimal difference between inhibition of DHT stimulated vs. non-stimulated LAPC4 cells for all test compounds, with IC50’s ranging from 1–7 μM (Table 2). VN/85-1 and VN/108-1 were able to reduce cell proliferation in a consistent dose-dependent manner, with potency equal to or greater than casodex. VN/124-1, and VN/125-1 were also highly effective, with IC50’s of 3.2 and 1.0 μM, as previously reported [28]. The time course to maximal effectiveness was similar among all test compounds, with onset of cell death being visually apparent no earlier than 48–72 hours post-treatment.
Table 2.
Effect of Novel Compounds on Cell Proliferation
| Compound | LNCaP IC50 (μM) | LAPC4 IC50 (μM) | ||
|---|---|---|---|---|
| DHT | + | − | + | − |
|
| ||||
| VN/85-1 | 3.7 | 1.9 | 4.2 | 3.4 |
| VN/87-1 | 4.8 | NT | NT | NT |
| VN/108-1 | 1.8 | 1.6 | 7 | 5 |
| VN/124-1 | 6 | 2.6 | 3.2 | 4 |
| VN/125-1 | 1.8 | 2.2 | 1.0 | 3 |
| Casodex | 8.6 | 4.6 | 10 | 9 |
| Flutamide | * | * | NT | NT |
LNCaP cells were seeded at 15,000 cells/well in 24 well multi-well plates, and LAPC4 cells were seeded at 15,000 cells/well. Cells were treated with the indicated concentration of compound in steroid free medium with or without 1 nM DHT (LNCaP), or 10 nM DHT (LAPC4) and allowed to grow for 7 days. The number of viable cells was compared by MTT assay (LAPC4) or XTT assay (LNCaP) on the 7th day.
NT = not tested, NI = less than 50% inhibition at 20 μM, * = stimulated proliferation
Previous results with VN/85-1, VN/87-1, and VN/108-1 have shown significant inhibition of LNCaP cell proliferation. All three compounds inhibited proliferation by 40–60%, and inhibited DHT stimulated proliferation at concentrations up to 5 μM [33]. Further evaluation of these compounds in DHT stimulated LNCaP cells, over a broader concentration range of (0.01–100 μM), indicated IC50 values of 1.8, 4.6 and 3.7 μM for VN/108-1, VN/85-1, and VN/87-1 respectively. To our knowledge, LNCaP cells do not express CYP17, or express very minimal amounts, as CYP17 activity is undetectable in LNCaP cells by our acetic acid releasing assay system (AARA). Therefore LNCaP viability assays do not completely represent the extent of our novel compounds’ potential effectiveness, as under physiological conditions there would be the added effect of decreased androgen production. The fact that our compounds were equally effective against both cell lines indicates increased clinical potential, as some anti-androgens such as flutamide have agonistic properties for the mutant AR as occurs in LNCaP cells.
VN/124-1 causes growth inhibition in LAPC4 xenograft model
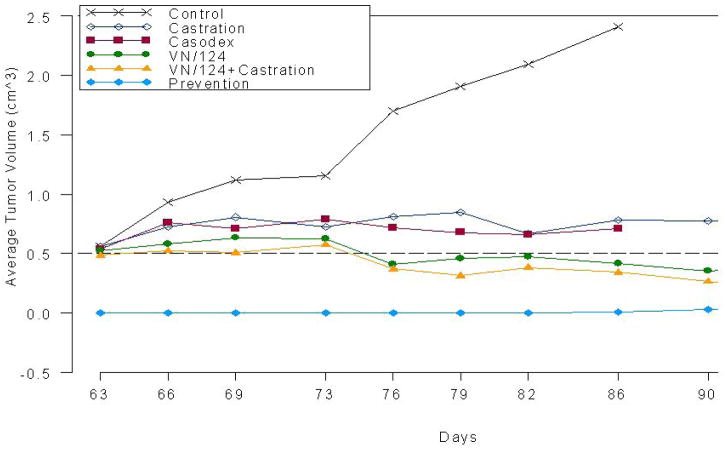
We determined the effects of VN/124-1 on prevention of LAPC4 tumor xenograft formation and also the effect of VN/124-1, VN/124-1 + castration, castration or casodex on tumor growth in vivo. LAPC4 cells were injected s.c. into SCID mice and one group of mice (n = 5; tumor prevention group) was treated with VN/124-1 (0.13 mmol/kg/twice daily) for 93 days starting the day after inoculation with LAPC4 cells. Approximately 9 weeks after inoculation tumors had formed in the other mice (approx 300 mm3), and these animals were assigned to five treatment groups: control (vehicle), castration, casodex (0.13 mmol/kg/twice daily), VN/124-1 (0.13 mmol/kg/twice daily) and castration plus VN/124-1 (0.13 mmol/kg/twice daily). It should be noted that experiments with the control and casodex groups were terminated on day 86 because of large tumors and drug shortage, respectively (Fig. 4). Treatment with VN/124-1 was very effective in preventing the formation of LAPC4 tumors (6.94 vs. 2410.28 mm3 in control group on day 86 (p<0.001)). Total tumor volume in the control mice increased by 4.3-fold over 3 weeks of treatment when the mice were sacrificed because of the large tumors.
Fig. 4.
Effects of VN/124-1, casodex, and castration on the prevention and growth of LAPC4 human prostate xenografts in male SCID mice. Mice bearing LAPC4 human prostate tumors were grouped and treatment started 63 days after cell inoculation except for the “prevention” group. In this group, treatment was begun with VN-124-1 on the day of cell inoculation. Treatments with both casodex and VN/124-1 were given at a dosage of 0.13 mmol/kg/twice daily. Control mice (vehicle treated mice were sacrificed after 86 days because of large tumors and mice treated with casodex were sacrified due to insufficient drug. Tumors of all treated groups were significantly different from control and the “prevention” group was also significantly different from all treated groups. *VN/124-1 alone and VN/124-1 plus castration were significantly different from castration and from casodex using multivariative analysis.
The tumors in the prevention group were significantly smaller than the tumors in control group and in all treatment groups (p < 0.0001). Compared to control groups with the total tumor volume from day 76 to day 93 (effect after two weeks of treatment), all treatments were significantly effective after two weeks except for treatment with casodex (p = 0.075). There were no significantly differences between treatment groups overall. Comparing the total tumor volumes at day 93, there were significant differences among all groups but there were no significant differences between castration and VN/124-1 groups (p = 0.33) and between the VN/124-1 group and the VN/124-1 + castration group (p = 0.059). However, we observed that in the castration and VN/124-1 groups, there was significant variation in tumor volumes due to reduced growth of tumors on either the left or right flanks of the mice. This resulted in large variations in the total tumor volumes for each group. However, when we compared the two groups (i.e. castration vs. VN/124-1) based on multivariate statistical analysis (allowing for differences in left tumor and right tumor) using F-test, there were significant differences between the tumor volumes of castration and VN/124-1 groups from day 76 to day 93 (p = 0.031) and at day 93 (p = 0.047). Furthermore, examination of the changes in average tumor volumes in all groups clearly shows the effects of the various treatments on tumor growth.
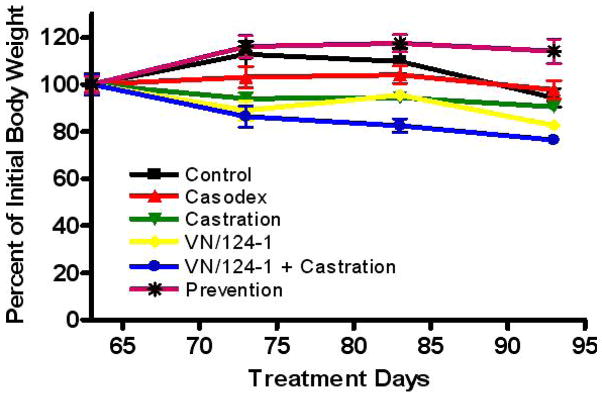
No significant changes in animal body weights were observed in all treatment groups (Fig. 5), suggesting that the treatments did not produce general toxicity in the mice.
Fig. 5.
Percent change in mouse body weight over treatment duration in LAPC4 human prostate cancer xenografts in male SCID mice.
Effects of treatments on androgen receptor expression in vivo
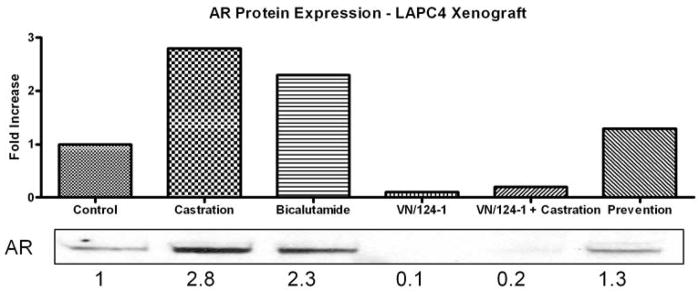
The striking difference in AR down-regulation between VN/124-1 and the other compounds, some of which had similar or better in vitro anti-proliferation, anti-androgen and lyase inhibition profiles, correlated with VN/124-1’s increased activity in reducing LAPC4 tumor xenograft growth. Therefore, VN/124-1 treated LAPC4 tumor xenografts were analyzed for AR expression to determine if VN/124-1 maintained its potent down-regulation properties in vivo (Fig. 6). Analysis of tumors revealed that treatments with VN/124-1 or VN/124-1 + castration caused marked reduction in AR protein of 10- and 5-fold, respectively. In contrast, treatment with bicalutamide or castration caused significant AR protein up-regulation of 2.3- and 2.8-fold, respectively. Treatment with VN/124-1 in the tumor-formation prevention study group caused a slight up-regulation (1.3-fold) of AR protein expression.
Fig. 6.
Western immunobloting analysis of whole cell lysates from LAPC4 tumors following various treatments.
Discussion
We have previously reported that some of our CYP17 inhibitors act as anti-androgens against the LNCaP AR [15, 27, 38]. As the test compounds are structurally similar to DHT, the most potent natural ligand for the AR, it seemed likely that the new compounds would also interact with the AR. The affinity of the compounds to the AR, was assessed in competitive binding studies carried out using the synthetic ligand methyltrienolone with the wild-type and two mutant forms of the AR. To determine if the compounds are agonists or antagonists, luciferase assays were performed in the presence and absence of DHT. The binding affinities of the compounds were strongest for the wild-type AR, with IC50’s ranging from 248 nM (VN/85-1). Although the strongest affinity was 10-fold weaker than DHT (22 nM) in the same system, it is still significantly stronger than the clinically used antiandrogens casodex (4.3 μM). Abiraterone, a CYP17 inhibitor now in clinical trials [17] did not bind to the AR. In LNCaP cells (T877A mutation), VN/85-1, VN/124-1, and VN/125-1 had lower affinities, whereas VN/108-1, VN/87-1 and flutamide had affinities equivalent to those for the wild type AR. Casodex displayed a much stronger affinity for the T877A AR (971 nM), which was approximately the same as VN/124-1 (845 nM) and VN/108-1 (831 nM). Conversely, all of the compounds tested against the T575A mutant, which has a mutation in the DNA binding domain [34], displayed affinities equivalent to the wild-type AR. As the T575A mutation is within the DNA binding domain [31], it is expected that the binding affinity would remain unchanged. The difference between wild-type AR and T877A AR is not unexpected, as the mutation in the latter confers broadened ligand specificity and obviously could have some effect on binding properties. Mutations in the AR’s ligand binding domain have been shown to alter the affinity of ligands and anti-androgens. Bohl et al. reported a two-fold higher affinity of casodex for W741L, a LBD AR mutant, as compared to the wild-type AR [39]. This is similar to our results with casodex and the T877A mutant. Interestingly, a few of our steroidal compounds exhibited a reduced affinity for the mutant T877A AR, in contrast to casodex’s increased affinity.
Recent evidence indicates that in the majority of PC cases, even in chemotherapy resistant disease, the AR is still expressed and required for growth [40–43]. It has also been shown that the AR can be activated by co-factors and other mechanisms independent of androgen levels [44–46]. In addition, it has been demonstrated that over-expression of AR in a castration-resistant xenograft model is consistent with observations in human clinical specimens, and over-expression of AR promotes the transition from a hormone-dependent xenograft to a castration-resistant xenograft [5, 47]. These observations suggest that directly targeting the AR and reducing AR levels to below a critical threshold may be a more effective approach to treatment than current antiandrogens.
In contrast to casodex, the antiandrogen currently used clinically, our novel compounds VN/85-1, VN/108-1, VN/125-1 and VN/124-1 were all able to greatly reduce AR levels. This effect was observed in both LAPC4 cells and LNCaP cells, with overall AR levels decreased by 60% or more. In both cell lines, VN/124-1 was significantly more potent than the other compounds, with nearly complete reduction of AR expression at 15 μM in LNCaP cells, and 89% in LAPC4 cells. Analysis of LAPC4 tumor samples from xenografts revealed that VN/124-1 also reduced AR in vivo, with a marked decrease in AR as compared to castration and control tumors. Results of analyzing tumor samples from our former study of VN/124-1 in xenografts [28] that were not previously reported showed similar reduction in AR, confirming this mechanism of action of VN/124-1 in vivo (Data not shown). Although VN/85-1 and VN/125-1 had similar or better characteristics than VN/124-1 in terms of inhibiting the CYP17 and reducing androgen modulated transcription, they were much less effective in vivo. However, these results could be explained in part by the greater effect of VN/124-1 on reducing AR levels in vitro and in vivo. Additional in vitro evidence supports this view, as reduction of AR expression produced a more pronounced effect on AR-induced transcription and cell growth than androgen deprivation in two androgen-insensitive PC cell lines, LNCaP-C42B4 and CWR22Rv1 [10].
The mechanism of AR down-regulation could occur through increased degradation or reduced protein synthesis. For our lead compound VN/124-1, AR degradation patterns were examined to determine whether AR stability was being affected. Destabilization of the AR has been shown in steroid depleted conditions, with half-life reduced from approximately six hours to three hours [48]. By using cycloheximide to inhibit new protein synthesis, and measuring the rate of degradation, it was possible to determine if VN/124-1 caused additional degradation beyond that normally observed under androgen deprivation. There was reduction of 50% in AR levels in the VN/124-1 treatment group versus control cells 6 hrs post-treatment. AR levels continued to decline over 24 hours, with an additional 10% reduction over control evident at 12 and 24 hours post treatment. This data indicates that VN/124-1’s down-regulation of the AR level is at least partly due to increased AR degradation. However, it should be noted that androgens have been shown to increase AR synthesis as well [48]. Therefore the possibility of an additional effect on modulating the rate of AR expression cannot be ruled out. Consequently, we are currently investigating the effects of VN/124-1 on AR mRNA expression. Also, the mechanism by which degradation occurs is still unknown. AR degradation has been shown to proceed through two proteolytic pathways. One relies on proteosomal degradation and occurs both in the absence (ligand-independent) or presence (ligand-dependent) of the hormone [reviewed by [49]]. The second engages PTEN and caspase-3 activity [50]. Interestingly, VN/124-1 is able to reduce AR levels in the presence and absence of androgens. Therefore, as long as the AR is functional, VN/124-1 may inhibit PC cell growth via AR down-regulation regardless of androgen-dependent or castration-resistant status.
In the LAPC4 xenograft, VN/124-1 is a more potent agent in reducing tumor growth than other compounds (VN/85, VN/87, and VN108) and is more effective than castration and casodex. VN/124-1 plus castration was also significantly better than castration alone or casodex. VN/124-1 was most effective at preventing the formation of LAPC4 tumor xenografts suggesting its potential as a chemopreventive agent. We show that unlike treatment with casodex or castration, which caused significant AR protein up-regulation, treatment with VN/124-1 markedly reduced AR protein levels both in vivo and in vitro. This additional property may account for the superiority of VN/124-1 in vivo compared to other more potent CYP17 inhibitors such as VN/85-1.
In summary, we have determined that VN/124-1 possesses several anti-cancer properties that target the AR. These include: (1) CYP17 inhibition to block the synthesis of androgens from all sources thus reducing the AR ligand, (2) direct AR antagonism, and (3) AR down-regulation. The combinations of these three important activities in a single entity (VN/124-1) have potential utility in the treatment of PC.
Acknowledgments
This work was supported by a grant from US National Institutes of Health (NIH), grant No: CA-27440 to AMHB, and we are grateful for the generous support. LAPC4 human PC cells were kindly provide by Dr Charles Sawyer of UCLA School of Medicine.
Abbreviations
- AR
androgen receptor
- PC
prostate cancer
- FBS
fetal bovine serum
- CYP17
17α-hydroxylase/17,20-lyase cytochrome P450
- DHT
dihydrotestosterone
- ADT
androgen deprivation therapy
- [3H]R1881
tritiated methyltrienolone
Footnotes
[VN/124-1 has been licensed to Tokai Pharmaceutical Inc., Boston, Mass, USA for further development for the treatment of prostate cancer.]
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD. Physiological basis of endocrine therapy for prostatic cancer. Urol Clin North Am. 1991;18:1–13. [PubMed] [Google Scholar]
- 3.Bruchovsky N, Wilson JD. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243(8):2012–21. [PubMed] [Google Scholar]
- 4.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumstein BA, Davis MA, Goodman PJ. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Eng J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 6.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332(21):1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 7.Tilley WD, Bentel JM, Aspinall JO, Hall RE, Horsfall DJ. Evidence for a novel mechanism of androgen resistance in the human prostate cancer cell line, PC-3. Steroids. 1995;60(1):180–6. doi: 10.1016/0039-128x(94)00031-7. [DOI] [PubMed] [Google Scholar]
- 8.Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res. 1996;2(2):277–85. [PubMed] [Google Scholar]
- 9.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9(4):401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 10.Li TH, Zhao H, Peng Y, Beliakoff J, Brooks JD, Sun Z. A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res. 2007;35(8):2767–76. doi: 10.1093/nar/gkm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan X, Li T, Wang H, Zhang T, Barua M, Borgesi RA, et al. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol. 2006;169(2):682–96. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha TL, Qiu L, Chen CT, Wen Y, Hung MC. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65(6):2287–95. doi: 10.1158/0008-5472.CAN-04-3250. [DOI] [PubMed] [Google Scholar]
- 13.Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, et al. Down-regulation of androgen receptor by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66(20):10064–72. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 14.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Nishiyama T, Hara N, Yamana K, Takahashi K, Labrie F. Importance of the intracrine metabolism of adrenal androgens in androgen-dependent prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(3):301–6. doi: 10.1038/sj.pcan.4500956. [DOI] [PubMed] [Google Scholar]
- 16.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 17.Hall PF. Cytochrome P-450 C21scc: one enzyme with two actions: hydroxylase and lyase. J Steroid Biochem Mol Biol. 1991;40(4–6):527–32. doi: 10.1016/0960-0760(91)90272-7. [DOI] [PubMed] [Google Scholar]
- 18.Trachtenberg J, Halpern N, Pont A. Ketoconazole: a novel and rapid treatment for advanced prostatic cancer. J Urol. 1983;130(1):152–3. doi: 10.1016/s0022-5347(17)51007-1. [DOI] [PubMed] [Google Scholar]
- 19.Small EJ, Baron AD, Fippin L, Apodaca D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157(4):1204–7. [PubMed] [Google Scholar]
- 20.Njar VC, Brodie AM. Inhibitors of 17alpha-hydroxylase/17,20-lyase (CYP17): potential agents for the treatment of prostate cancer. Curr Pharm Des. 1999;5(3):163–80. [PubMed] [Google Scholar]
- 21.Hartmann RW, Ehmer PB, Haidar S, Hector M, Jose J, Klein CD, et al. Inhibition of CYP 17, a new strategy for the treatment of prostate cancer. Arch Pharm (Weinheim) 2002;335(4):119–28. doi: 10.1002/1521-4184(200204)335:4<119::AID-ARDP119>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Hakki T, Bernhardt R. CYP17- and CYP11B-dependent steroid hydroxylases as drug development targets. Pharmacol Ther. 2006;111(1):27–52. doi: 10.1016/j.pharmthera.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Leroux F. Inhibition of p450 17 as a new strategy for the treatment of prostate cancer. Curr Med Chem. 2005;12(14):1623–9. doi: 10.2174/0929867054367185. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90(12):2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96(9):1241–6. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 26.Njar VC, Kato K, Nnane IP, Grigoryev DN, Long BJ, Brodie AM. Novel 17-azolyl steroids, potent inhibitors of human cytochrome 17 alpha-hydroxylase-C17,20-lyase (P450(17) alpha): potential agents for the treatment of prostate cancer. J Med Chem. 1998;41(6):902–12. doi: 10.1021/jm970568r. [DOI] [PubMed] [Google Scholar]
- 27.Long BJ, Grigoryev DN, Nnane IP, Liu Y, Ling YZ, Brodie AM. Antiandrogenic effects of novel androgen synthesis inhibitors on hormone-dependent prostate cancer. Cancer Res. 2000;60(23):6630–40. [PubMed] [Google Scholar]
- 28.Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48(8):2972–84. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 29.Handratta VD, Jelovac D, Long BJ, Kataria R, Nnane IP, Njar VC, et al. Potent CYP17 inhibitors: improved syntheses, pharmacokinetics and anti-tumor activity in the LNCaP human prostate cancer model. J Steroid Biochem Mol Biol. 2004;92(3):155–65. doi: 10.1016/j.jsbmb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10(4):309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Marcelli M, Ittmann M, Mariani S, Sutherland R, Nigam R, Murthy L, et al. Androgen receptor mutations in prostate cancer. Cancer Res. 2000;60(4):944–9. [PubMed] [Google Scholar]
- 32.Tan M, Fang HB, Tian GL, Houghton PJ. Small-sample inference for incomplete longitudinal data with truncation and censoring in tumor xenograft models. Biometrics. 2002;58(3):612–20. doi: 10.1111/j.0006-341x.2002.00612.x. [DOI] [PubMed] [Google Scholar]
- 33.Grigoryev DN, Long BJ, Nnane IP, Njar VC, Liu Y, Brodie AM. Effects of new 17alpha-hydroxylase/C(17,20)-lyase inhibitors on LNCaP prostate cancer cell growth in vitro and in vivo. Br J Cancer. 1999;81(4):622–30. doi: 10.1038/sj.bjc.6690739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173(2):534–40. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 35.Culig Z, Hoffmann J, Erdel M, Eder IE, Hobisch A, Hittmair A, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81(2):242–51. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steketee K, Timmerman L, Ziel-van der Made AC, Doesburg P, Brinkmann AO, Trapman J. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int J Cancer. 2002;100(3):309–17. doi: 10.1002/ijc.10495. [DOI] [PubMed] [Google Scholar]
- 37.Thompson TA, Wilding G. Androgen antagonist activity by the antioxidant moiety of vitamin E, 2,2,5,7,8-pentamethyl-6-chromanol in human prostate carcinoma cells. Mol Cancer Ther. 2003;2(8):797–803. [PubMed] [Google Scholar]
- 38.Klus GT, Nakamura J, Li JS, Ling YZ, Son C, Kemppainen JA, et al. Growth inhibition of human prostate cells in vitro by novel inhibitors of androgen synthesis. Cancer Res. 1996;56(21):4956–64. [PubMed] [Google Scholar]
- 39.Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci U S A. 2005;102(17):6201–6. doi: 10.1073/pnas.0500381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 41.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93(22):1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 42.Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002;9(1):61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62(4):1008–13. [PubMed] [Google Scholar]
- 44.Yeh S, Kang HY, Miyamoto H, Nishimura K, Chang HC, Ting HJ, et al. Differential induction of androgen receptor transactivation by different androgen receptor coactivators in human prostate cancer DU145 cells. Endocrine. 1999;11(2):195–202. doi: 10.1385/endo:11:2:195. [DOI] [PubMed] [Google Scholar]
- 45.Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc Natl Acad Sci U S A. 1998;95(10):5527–32. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61(11):4315–9. [PubMed] [Google Scholar]
- 47.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61(9):3550–5. [PubMed] [Google Scholar]
- 48.Syms AJ, Norris JS, Panko WB, Smith RG. Mechanism of androgen-receptor augmentation. Analysis of receptor synthesis and degradation by the density-shift technique. J Biol Chem. 1985;260(1):455–61. [PubMed] [Google Scholar]
- 49.Jaworski T. Degradation and beyond: Control of androgen receptor activity by the proteasome system. Cell Mol Biol Lett. 2006;11(1):109–31. doi: 10.2478/s11658-006-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol. 2004;18(10):2409–23. doi: 10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]