Abstract
Background
Polyethylene glycol (PEG), an osmotic laxative, is a very potent inhibitor of colon cancer in rats. In a search for mechanisms, we tested the hypothesis that fecal bulking and moisture decreases colon carcinogenesis. We also looked for PEG effects on crypt cells in vivo.
Methods
Fischer 344 rats (N=272) were given an injection of the colon carcinogen azoxymethane. They were then randomized to a standard AIN76 diet containing one of 19 laxative agents (5% w/w in most cases): PEG 8000 and other PEG-like compounds, carboxymethylcellulose, polyvinylpyrrolidone, sodium polyacrylate, calcium polycarbophil, karaya gum, psyllium, mannitol, sorbitol, lactulose, propylene glycol, magnesium hydroxide, sodium phosphate, bisacodyl, docusate, and paraffin oil. Aberrant crypt foci (ACF) and fecal values were measured blindly after a 30-day treatment. Proliferation, apoptosis, and the removal of cells from crypts were studied in control and PEG-fed rats by various methods, including TUNEL and fluorescein dextran labeling.
Results
PEG 8000 reduced nine-fold the number of ACF in rats (p<0.001). The other PEGs and magnesium-hydroxide modestly suppressed ACF, but not the other laxatives. ACF number did not correlate with fecal weight or moisture. PEG doubled the apoptotic bodies per crypt (p<0.05), increased proliferation by 25–50% (p<0.05) and strikingly increased (>40-fold) a fecal marker of epitheliolysis in the gut (p<0.001). PEG normalized the percentage of fluorescein dextran labeled cells on the top of ACF (p<0.001).
Conclusions
Among laxatives, only PEG afforded potent chemoprevention. PEG protection was not due to increased fecal bulking, but likely to the elimination of cells from precancerous lesions.
Keywords: Acute Toxicity Tests; Animals; Azoxymethane; toxicity; Carcinogens; toxicity; Cathartics; pharmacology; therapeutic use; Colonic Neoplasms; chemically induced; prevention & control; Feces; chemistry; Polyethylene Glycols; pharmacology; therapeutic use; Precancerous Conditions; chemically induced; prevention & control; Random Allocation; Rats; Rats, Inbred F344
Keywords: aberrant-crypt-foci, ACF, chemoprevention, colorectal cancer, laxative, polyethylene-glycol, PEG, rat, toxicity, toxicology, prevention, preneoplastic, cancer, block polymer, polycarbophil, karaya gum, psyllium, mannitol, sorbitol, lactulose, propylene glycol, magnesium hydroxide, sodium phosphate, bisacodyl, docusate, paraffin oil, TUNEL, apoptosis, fecal bulk, fecal moisture, fluorescein dextran
Introduction
The prevention of colorectal cancer could employ chemopreventive agents, but known agents afford only modest protection. We have previously established that a diet supplemented with an osmotic laxative suppresses an early putative step in the development of colon cancer in rats (1), namely, the number of aberrant crypt foci (ACF) induced by an azoxymethane (AOM) injection (2). The laxative was polyethylene glycol 8000 (PEG), whose formula is H-(O-CH2-CH2)n-OH, with n=200. PEG and PEG-like block-polymer pluronic F68 are the most potent known suppressors of ACF in rats (3, 4). PEG also strikingly suppresses the occurrence of AOM-induced cancers in rats (5, 6). This AOM-rat model predicts the chemopreventive efficacy of dietary agents in humans (7). Last, a population-based study shows that PEG-based laxative users have a halved risk of colorectal tumors (8).
The mechanism by which PEG suppresses ACF and cancer is not known. Fecal weight and moisture are more than doubled in PEG-treated rats (1). Constipation, by contrast, is associated with increased risk of colon cancers in several case-control studies (9–11). We thus looked for the chemopreventive effect of several laxative agents (12). We tested seven large bulking polymers (MW>2000), six poorly absorbed osmotic agents (MW<400), a lubricating oil, two secretagogues, and three PEGs. Since many studies report the modest protective effect of wheat bran (13), and the promotion of carcinogenesis by anthranoid laxatives (14–16), we did not test them. In vitro studies suggest a second possible mechanism: PEG halts proliferation (17), induces apoptosis (18), and restores differentiation (19) of adenocarcinoma cells in culture. We thus sought a PEG effect on these functions in the colon of rats. A third hypothetical mechanism is that, like mucus, PEG might protect the mucosa (20). Indeed, cell membrane can reseal after being damaged by mechanical stress in the gut (21). Pluronic facilitates this repair process in vitro (22), We tested this hypothesis using fluorescein dextran (21).
Materials and Methods
General Design and Initiation of Carcinogenesis
Seven sequential experiments were conducted in rats. Five 30-day studies tested the effect of laxative agents on AOM-induced ACF. Another 30-day study tested the effect of PEG on cell proliferation and apoptosis. Unless otherwise stated we used PEG 8000. One 100-day study made use of a fluorescent marker to see whether PEG protects or kills crypt cells. A total number of 272 four-week old Fisher 344 rats were obtained from Iffa-Credo (Lyon, France). They were acclimatized to the animal colony for one week, housed by pairs in stainless steel wire drop-bottom cages, in a temperature of 22 ± 2°C and with 12h/12h light and dark cycles. AIN76 diet from UAR (Villemoisson, France) and tap water were provided ad libitum. Each rat was given one AOM i.p. injection to induce ACF (20 mg/kg in saline), and randomized 7 days later to receive treatment. All chemicals came from Sigma (St. Quentin, France), unless stated otherwise. Body weights, food and water intake were monitored weekly. Daily fecal output was measured on freeze-dried feces collected under each cage of two rats for 24 hours. Fecal humidity was measured on fresh pellets obtained directly at the anus of each rat (weighed before and after freeze-drying). Thirty and seven days (ACF studies), or 100–130 days (fluorescent marker study) after the AOM injection, the animals were sacrificed by carbon dioxide asphyxiation. Investigator with French official agreement #31–121 handled animals, and care was in accordance with the guidelines of the European Council.
Studies of Laxative Agents
The effect of dietary laxative agents on AOM-induced ACF was compared to the standard AIN76 diet (control), in five 30-day studies. They involved 32, 36 and 42 male, and 48 and 52 female rats (no gender effect was seen). In each study some rats were randomized to a control group, and to a group given dietary 5% (w/w) PEG 8000 (ICN, Orsay France). Laxative agents which were given to the other groups of rats were: 5% of calcium polycarbophil (brought by 7% Fibercon, Whitehall-Healthcare, Madison, NJ), carboxymethylcellulose (of medium viscosity, ICN), polyvinylpyrrolidone (MW 8000, Acros-Organics, Geel, Belgium), kaolin, karaya gum (MW 950,000), lactulose (MW 342), mannitol (MW 182), paraffin oil (Merck, Darmstadt, Germany), PEG 400, PEG bisphenyl A-diglycidylether (MW15,000, Aldrich, St. Quentin, France), propylene glycol (MW 76), psyllium (Vigan-lab., France), sorbitol (MW 182), and sodium polyacrylate (3%, MW 8000, Aldrich), sodium phosphate (3%, 1:1 mix of NaH2PO4,2H2O:Na2HPO4,7H2O), magnesium hydroxide (1%), dioctyl sulfosuccinate sodium (0.3%, docusate), bisacodyl (0.01%), and piroxicam, a chemopreventive non laxative agent (0.04%). The end-points were the number of ACF, fecal weight and moisture, fecal water osmolarity, and alkaline phosphatase (ALP) activity. A single observer scored all colons blindly for ACF, in a randomized order, by Bird’s procedure (2). Fecal water was obtained by adding distilled water to feces directly obtained from the anus of each rat (1.5 ml per 0.15 g of freeze-dried sample), and processed as previously described (1). Osmolarity of fecal water was measured in duplicate with freezing point depression (Roebling,; Osmomat, Gonotec; Berlin, Germany). ALP is released in the fecal stream when epithelial cells are lysed (23, 24). Intestinal ALP activity in fecal water was measured according to Lapre (24), using a specific kit with p-Nitrophenyl phosphate as a substrate, and L-phenylalanine as a specific inhibitor of the intestinal isoenzyme. That PEG did not modify ALP values was specifically checked in vitro.
Study of PEG effect on Cell Proliferation and Apoptosis
Sixteen male rats received an AOM injection seven days before being randomized to a control group given pure water, and a treated group given 5% PEG in the drinking water for 30 days. Sixty minutes before sacrifice each rat received an i.p. injection of 5-bromo-2′-deoxyuridine (BrdU, 50 mg/kg in NaCl 9g/l). A 1 cm2 piece of distal colon was processed for histology after fixation in buffered formalin (10%). Proliferative cells in the colonic crypts were visualized immuno-histochemically using an anti-BrdU antibody (25). Cells in mitosis and apoptotic bodies per crypt were counted on 3 μm sections stained by Feulgen-fast green. Apoptotic bodies were recognized based on morphological identification (26). Apoptotic cells per crypt were also counted after Tdt-mediated dUTP-biotin nick end labeling of fragmented DNA (Oncogene Research Products) (25). An investigator scored 20 (proliferation) and 60 (apoptosis) fully visible crypts per rat in a blinded manner.
Study of PEG Effect on Cell Resealing and Removal in Normal and Aberrant Crypts
Twelve rats received an AOM injection, ten rats did not. They were sacrificed 106–131 days after initiation. Fifty hours before sacrifice, food was removed from cages. Thirty hours before sacrifice, all rats were given 250 mg fluorescein dextran by gavage (1 ml, MW 4400) (21). Eighteen hours before sacrifice ten rats were allowed to drink water with 5% PEG, and twelve rats were given pure water (see Table I). Colons were prepared as they had been for ACF. Fluorescein-labeled cells, and nuclei (to estimate total number of cells) were then counted in 100 normal and 60 aberrant crypts at 400 × magnification with a fluorescence microscope (Axiolab, Zeiss).
Table I.
An 18-hour PEG-treatment normalizes the proportion of cells labeled by fluorescein dextran on top of aberrant crypts, 105–130 days after an injection of carcinogen AOM to rats and 30 h after a 250 mg fluorescein dextran gavage.
| Rat Treatment | No. of rats | No. of crypts scored | Cells seen per crypt | Labeled cells per crypt | Mean percent of labeled cells | |
|---|---|---|---|---|---|---|
| none | 6 | 620 | 14.8 | 0.84 | 5.4 | |
| Normal Crypts | ||||||
| PEG | 4 | 460 | 15.2 | 0.67 | 4.0* | |
| AOM | 6 | 600 | 14.9 | 1.02** | 6.6 | |
| AOM + PEG | 6 | 680 | 14.8 | 0.66 | 4.1 ** | |
| SEM | 0.1 | 0.04 | 0.25 | |||
| ACF Crypts | AOM | 6 | 350 | 42.4 | 5.64 | 12.5 |
| AOM + PEG | 6 | 405 | 38.1 ** | 2.91 ** | 7.0** | |
| SEM | 0.7 | 0.2 | 0.4 | |||
Notes to Table I. SEM: pooled standard error of the mean;
p<0.001;
p<0.01; Dunnet’s test (normal crypts, comparison with “none” group) or Student’s test (ACF crypts, comparison with “AOM” group).
Statistical Methods
Group means were compared by Student’s t test, or by Welch’s t test when variances were not equal, or by Mann-Whitney test when data were not normally distributed. To compare many treated groups to a single control group, the Dunnett’s test was used when Bartlett’s statistics showed non-significant differences among variances. A non-parametric Kruskal-Wallis was used in other cases. Standard deviations (SD) are reported in the text, and pooled standard error of the mean (SEM) in Table I. All P values quoted correspond to two-tailed test, and P value below 0.05 was considered significant.
Results
The Effect of 19 Laxative Agents on AOM-induced ACF, and on Fecal Values
Five independent short-term studies were conducted to test the hypothesis that laxative drugs can decrease the number of ACF during the post-initiation period. The results are shown on Figure 1, using the ACF values in control rats to normalize data across the five studies (full data not shown). PEG, unique among laxative agents, strikingly suppressed ACF. Compared to control rats, PEG-treated rats had a nine-fold reduction in the number of ACF and a 13-fold reduction in the number of large ACF (mean of 5 studies, all p<0.001). Other PEGs also reduced the number of ACF, but to a lower extent. PEG 400 was roughly half as potent as PEG 8000, as previously shown for PEG 3350 (6). Other laxatives did not reduce significantly the number of ACF (Figure 1) except magnesium hydroxide. In bisacodyl-fed rats all crypts looked aberrant and colons could not be scored for ACF, but small tumors were detected (N=0.8/rat). Rats treated with the non-laxative NSAID piroxicam had significantly less ACF than controls (p<0.001), but twice more ACF than PEG-treated rats.
Figure 1. Among 19 laxative agents, PEG only strongly suppressed carcinogenesis in AOM-initiated rats.
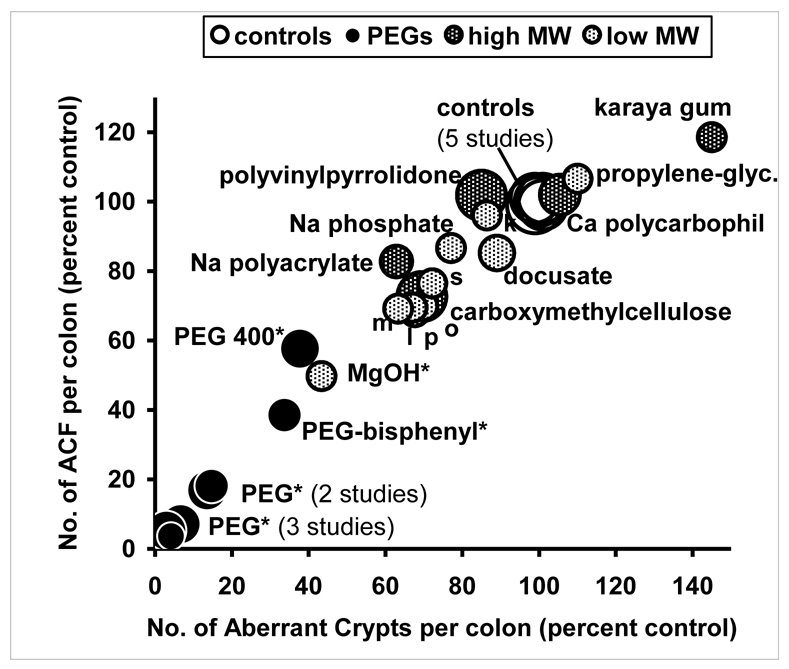
Fischer rats (N=210) were given an injection of azoxymethane. They were then randomized to a standard AIN76 diet containing one of 19 laxative agents (5% w/w in most cases). Aberrant crypt foci (ACF) were measured blindly after a 30-day treatment. Results from five sequential studies are shown after normalizing values to 100 for each control groups.
Area of circles matches groups’ size (large: 12 rats; small: 4 rats). Overlapping points were shifted verlapping to be more visible. Star indicates results different from control (Dunnet’s test). Unnamed small points: m: mannitol, l: lactulose, p: psyllium, o: paraffin oil, s: sorbitol, k: kaolin. Actual values of ACF (and of aberrant crypts) in the 5 control groups were 53 (119), 62 (139), 96 (200), 122 (264), 80 (172). Matching values in PEG-fed rats were 4 (8), 5 (8), 2 (6), 22 (39), 13 (23). Piroxicam and bisacodyl results are not shown on this figure (see text).
Laxative polymers of high MW, including PEGs, roughly doubled both the daily fecal output (dry weight) and the fecal moisture (Figure 2, full data not shown). The rats consumed and excreted about 1 g of PEG per day, which explains the increased fecal weight. Small MW agents had less effect on fecal values in rats, except magnesium hydroxide (doubled fecal moisture), kaolin and paraffin oil (doubled fecal dry weight). Karaya gum, carboxymethylcellulose, polyvinylpyrrolidone and PEG were thus potent laxatives in rats (Figure 2), but only PEG 8000 strongly reduced the ACF number (Figure 1). This suggests that the ACF inhibition by PEG 8000 was not due solely to its fecal bulking activity. Indeed, a modest inverse correlation was found between the ACF number in the colon of rats and fecal dry weight and fecal moisture. The correlation coefficients were −0.40 and −0.53 respectively (p<0.05, d.f. 25). However, after exclusion of PEG data, the ACF number did not correlate any longer with fecal dry weight or with fecal moisture.
Figure 2. High MW laxative agents increased the daily fecal excretion (dry weight) and the fecal moisture (percent water) in rats fed a purified diet for one month.
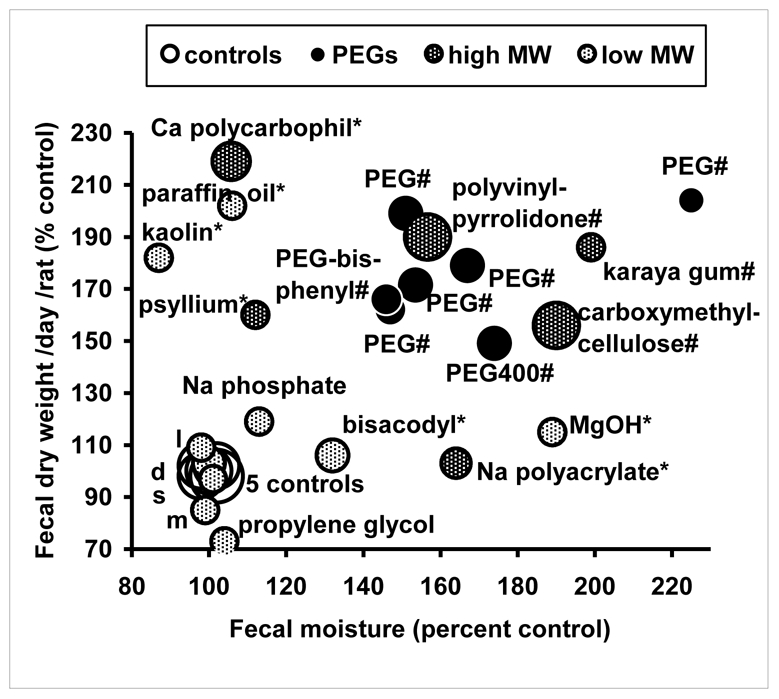
Design: see figure 1. *: different from control for fecal excretion or moisture; #: different from control for fecal excretion and moisture (Dunnet’s test). Unnamed small points (near controls): m: mannitol, s: sorbitol, d: docusate, l: lactulose. Actual values of fecal moisture given as % water (and fecal dry weight, g/d) in the 5 control groups were 30) (0.85), 45 (0.69), 41 (0.78), 45 (1.16), 44 (1.26). Matching values in PEG-fed rats were 68 (1.73), 68 (1.37), 69 (1.40), 66 (1.88), 68 (2.16).
In vitro data suggest that cytostatic effect of PEG on cell lines be due to PEG-induced osmotic pressure (17). Osmolarity of fecal water from all rats was thus measured, but it was not changed by PEG or by laxative treatments (p>0.05), and it did not correlate with ACF numbers (data not shown). These results show that PEG-prevention of carcinogenesis is not due to laxative effect, or to osmotic properties of PEG.
PEG Increased Crypt Cells Apoptosis and Proliferation
Apoptosis, an important prevention mechanism (25), was measured by two different techniques in 3 μm sections of colonic mucosa from control and PEG-treated rats. The number of apoptotic bodies was doubled in PEG-treated rats compared with controls. After a 30-day PEG-treatment of AOM-initiated rats, the number of apoptotic bodies per crypt scored on Feulgen-fast green stained sections increased from 0.05 ± 0.03 to 0.12 ± 0.04 (p=0.002). In a distinct experiment, the TUNEL labeled cells per crypt increased from 0.05 ± 0.02 in controls to 0.08 ± 0.03 in PEG-treated rats (p=0.04). In addition, PEG strikingly increased the ALP activity in feces (25-fold increase, per g of dry feces), and daily fecal excretion of ALP (44-fold increase, median of five studies). In one study, for instance, control and PEG-treated rats excreted 98 ± 34 and 4158 ± 1765 Sigma ALP Units/d respectively (p<0.001). Intestinal ALP activity in fecal water would reflect epitheliolysis (23, 24). Thus, PEG clearly enhanced apoptosis and epitheliolysis in the mucosa.
Crypt lengthening and cell proliferation are often associated with colon carcinogenesis (27). We thus speculated that PEG-prevention might be associated with crypt shortening and reduced cell proliferation. After a 30-day PEG-treatment in AOM-initiated rats, crypt height was reduced from 32.3 ± 2.6 cells in controls to 27.3 ± 2.9 in PEG-fed rats (p<0.01). But PEG surprisingly increased crypt cell proliferation. The BrdU labeled cells per crypt increased from 3.12 ± 0.58 in controls to 3.99 ± 0.63 in PEG-treated rats (p<0.01). Also, the number of mitoses per crypt increased from 0.35 ± 0.19 to 0.53 ± 0.11 (p=0.03). A duplicated study showed similar reduction of crypt length (p<0.01), and increased proliferation (p=0.04) after a 7-day PEG-treatment.
PEG Removed Cells on Top of Aberrant Crypts
Fluorescein dextran labeled cells were easily counted on top of normal and aberrant crypts (Figure 3). Twice more labeled cells were counted per AOM-initiated aberrant crypt cells, than per normal crypt cells (Table I, 12.5 and 5.4 cells respectively, p<0.001). According to McNeil, this might suggest that transformed cells on top of ACF are more fragile, or more exposed to mechanical stress, than normal crypt cells (21). After fluorescent labeling, an 18-hour PEG-treatment normalized the proportion of fluorescent cells in aberrant crypts: the percentage of labeled cells on ACF decreased from 12.5 to 7.0 % (p<0.001), which shows that PEG eliminated cells from ACF. Indeed, PEG-treatment slightly reduced total number of cells seen in ACF, from 42 to 38 cells per crypt (p<0.001). PEG-treatment also slightly decreased the percentage of labeled cells in normal crypts, from 5.4 to 4.0 (p<0.01).
Figure 3. Fluorescein dextran labeled cells on top of a four-crypt ACF.
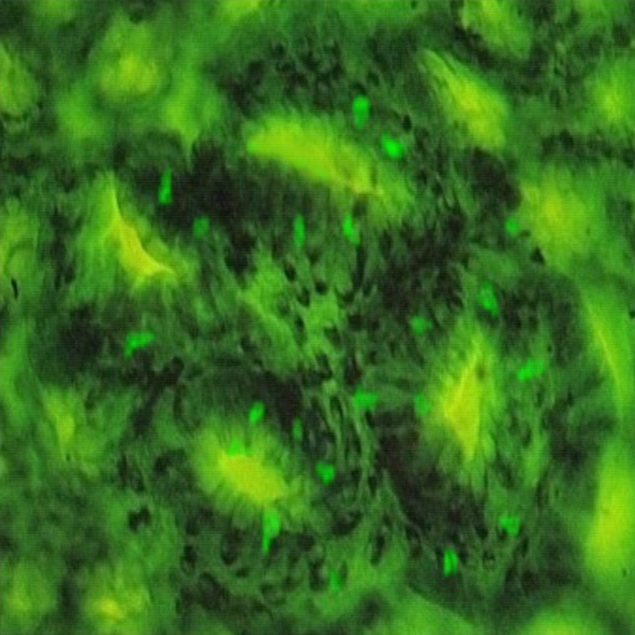
Fluorescence micrograph of colon mucosa (magnification × 400). The rat was sacrificed 130 d after an AOM injection, and 30 h after a 250 mg fluorescein dextran gavage (this rat did not receive PEG).
Discussion
This study demonstrates that (i) among 19 laxatives, only PEG 8000 and its analogs were potent chemopreventive agents against colon carcinogenesis in a rat model, (ii) the mechanism of PEG protection was not related to increased fecal bulking or water content, (iii) PEG induced proliferation, epitheliosyis, and apoptosis in colonic crypts, which confirms Roy’s findings (28), and (iv) PEG seems to remove damaged cells from the top of ACF.
The results show that a 30-day PEG-treatment reduced 9-fold the number of ACF, and 13-fold the number of large ACF (mean of 5 studies, all p<0.001). Previous studies have shown the following facts: Following a 105-day PEG-treatment rats have 20-times less ACF and 100 times less large ACF than controls (1). PEG is more potent than most known preventive agents in this rat model (3), and the effect is not limited to a specific carcinogen, since hydrazine, nitrosamine, and heterocyclic amine-induced ACF are suppressed (6). PEG effect is fast: a three-day PEG-treatment, one month after carcinogen injection, halves the number of ACF in rats (6).
The mechanism by which PEG can prevent carcinogenesis in rats is not known. Speculated mechanisms include (i) dilution of promoting compounds in the gut lumen due to PEG osmotic and bulking properties, (ii) protection of epithelia by PEG lubricating, coating, and sealing properties, (iii) elimination of cancer cells from the tumors by apoptosis induction. These three hypotheses are discussed here:
High-molecular weight PEGs are not absorbed from the gut of rats and bind water through hydrogen bonding (29). In PEG-fed rats, the fecal weight and moisture increased markedly (fig.2), and we have shown that the concentration of bile acids in fecal water is halved (1). However, the present study clearly shows that this bulking effect does not explain the anti-cancer properties of PEG. Indeed, some laxatives increased both fecal dry weight and fecal moisture as much as PEG, but they did not reduce ACF (e.g., karaya gum). Previous studies show that psyllium and wheat bran can double the fecal bulk, but only modestly suppress ACF (3). Magnesium hydroxide also showed some protective effect here. Magnesium is a known chemopreventive agent (30–32). It may act like calcium, unrelated with laxative properties (33, 34). Small tumors were detected in bisacodyl-fed rats only one month after AOM injection, thus confirming it is a strong tumor promoter (15). To conclude, because bulking does not cause chemoprevention, it should be possible to design PEG-like chemopreventive agents with no laxative effect.
Alternatively, PEG may protect the colonic mucosa by lubricating the fecal stream (29), by providing a mucin-like barrier and reducing inflammation (20), or by facilitating the membrane resealing (21), thus reducing the ACF over-proliferation. This hypothesis is supported by our in vitro data (17) and by the strong chemoprevention afforded by pluronic (4), a PEG-like agent that binds membranes and repairs cell membrane defects (22). In contrast, this study clearly shows that PEG increased proliferation, like aspirin and sulindac (35, 36). PEG also appears to increase the turn–over of cells on top of ACF crypts (table I), and greatly increased fecal ALP, a potential marker of epitheliolysis (23, 24). In addition, while a 0.05% PEG concentration protects cells in vitro (37), a 100-times higher concentration is needed to suppress ACF in vivo (5).
Lastly, according to in vitro results, PEG might eliminate transformed cells by apoptosis (17, 18). This would normalize or “erase” ACF, explaining the fast loss of ACF (6). Indeed, PEG-treatment doubled the number of apoptotic bodies in colonic crypts, as recently reported in ACF and in Min mice (28). This could be meaningful because a fish oil and pectin diet that increases apoptotic index by +25% decreases adenocarcinoma incidence by −50% in rats (25). In addition PEG strikingly increased epitheliolysis in the gut, increased proliferation but reduced crypt length, and halved the number of labeled cells on top of ACF (table I). Taken together, these facts suggest that PEG has an abrasive effect on the mucosa. How could abrasion be protective? Although it is highly controversial, the elimination of cells from the top of crypts might be enough to stop carcinogenesis, by preventing the top-down movement of transformed cells (38). This would explain why the inhibition was reversible in part when treatment was discontinued (6). Similarly, chemical peeling with PEG and salicylic acid strikingly suppresses skin tumor development in mice (39).
The present study is not definitive, and following questions might still be addressed: (i) Do non-protective laxatives induce apoptosis and epitheliolysis? (ii) We suggest that PEG removes cells from ACF, that often hold K-ras mutation. The DNA of shedded cells can be detected in stools (40). So, does PEG increase fecal flow of K-ras mutations? (iii) How could PEG induce apoptosis and epitheliolysis? Added into a culture medium, PEG increases the osmotic pressure and shrinks cancer cells (17). By contrast, in vivo, PEG drags water into the gut lumen, which in turn balances the osmotic pressure (29). Thus, PEG-effect is not due to osmotic pressure, but might be due to detergent properties. Indeed, PEG induces cell fusion in vitro, accumulates in cancer cells, and the optimal MW to enhance endocytosis and to suppress ACF is identical, i.e., 8000 (6, 41). Last, PEG-induced apoptosis may come from the modulation of transcription factors, e.g., induction of pro-apoptotic factor Par-4, and suppression of E-cadherin down-regulating factor SNAIL (18, 42).
To conclude, data suggest that PEG acts by eliminating cells from ACF. PEG is used as a mild laxative at a dose which matches the dose used in rats (43). A recent population-based study of 1165 colonoscopies in France shows that Forlax® users (a PEG 4000-based laxative) have a halved risk of colorectal tumors (8). High MW PEGs have no known toxicity, are not absorbed, not metabolized, and not fermented. We thus suggest PEG be tested in a clinical trial.
Acknowledgments
Studies were funded by Ligue-32, INRA and DGER, Ministry of Agriculture, France.
References
- 1.Corpet DE, Parnaud G. Polyethylene-glycol, a potent suppressor of azoxymethane-induced colonic aberrant crypt foci in rats. Carcinogenesis. 1999;20(5):915–918. doi: 10.1093/carcin/20.5.915. [DOI] [PubMed] [Google Scholar]
- 2.Bird RP. Observation and quantification of aberrant crypts in murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 3.Corpet DE, Tache S. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutrition and Cancer. 2002;43(1):1–21. doi: 10.1207/S15327914NC431_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parnaud G, Tache S, Peiffer G, Corpet DE. Pluronic F68 block polymer, a very potent suppressor of carcinogenesis in the colon of rats and mice. British Journal of Cancer. 2001;84(1):90–93. doi: 10.1054/bjoc.2000.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parnaud G, Tache S, Peiffer G, Corpet DE. Polyethylene-glycol suppresses colon cancer and causes dose-dependent regression of azoxymethane-induced aberrant crypt foci in rats. Cancer Research. 1999;59(20):5143–5147. [PubMed] [Google Scholar]
- 6.Corpet DE, Parnaud G, Delverdier M, Peiffer G, Tache S. Consistent and fast inhibition of colon carcinogenesis by polyethylene glycol in mice and rats given various carcinogens. Cancer Research. 2000;60(12):3160–3164. [PubMed] [Google Scholar]
- 7.Corpet DE, Pierre F. How good are Rodent Models of Carcinogenesis in Predicting Efficacy in Humans? Systematic Review and Meta-Analysis of Colon Tumour Chemoprevention in Rats, Mice and Men. European Journal of Cancer. 2005 doi: 10.1016/j.ejca.2005.06.006. in the press. [DOI] [PubMed] [Google Scholar]
- 8.Dorval ED, Viguier J, Bertrand P, Barbieux JP, Brondin B, Jankowski JM, et al. Prevention of colorectal adenomas by polyethylene glycol (PEG): a population-based study of 1165 colonoscopies in France. Proc AACR. 2003;44(2):174–979. [Google Scholar]
- 9.Sonnenberg A, Muller AD. Constipation and cathartics as risk factors of colorectal cancer - a meta-analysis. Pharmacology. 1993;47(Suppl 1):224–233. doi: 10.1159/000139862. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs EJ, White E. Constipation, laxative use, and colon cancer among middle-aged adults. Epidemiology. 1998;9(4):385–391. [PubMed] [Google Scholar]
- 11.Roberts MC, Millikan RC, Galanko JA, Martin C, Sandler RS. Constipation, laxative use, and colon cancer in a North Carolina population. Am J Gastroenterol. 2003;98(4):857–64. doi: 10.1111/j.1572-0241.2003.07386.x. [DOI] [PubMed] [Google Scholar]
- 12.Schiller LR. Review article: the therapy of constipation. Alimentary Pharmacology & Therapeutics. 2001;15(6):749–763. doi: 10.1046/j.1365-2036.2001.00982.x. [DOI] [PubMed] [Google Scholar]
- 13.Harris PJ, Ferguson LR. Dietary fibres may protect or enhance carcinogenesis. Mutation Research. 1999;443(1–2):95–110. doi: 10.1016/s1383-5742(99)00013-7. [DOI] [PubMed] [Google Scholar]
- 14.VanGorkom BAP, DeVries EGE, Karrenbeld A, Kleibeuker JH. Review article: anthranoid laxatives and their potential carcinogenic effects. Alimentary Pharmacology & Therapeutics. 1999;13(4):443–452. doi: 10.1046/j.1365-2036.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 15.Borrelli F, Mereto E, Capasso F, Orsi P, Sini D, Izzo AA, et al. Effect of bisacodyl and cascara on growth of aberrant crypt foci and malignant tumors in the rat colon. Life Sciences. 2001;69(16):1871–1877. doi: 10.1016/s0024-3205(01)01263-2. [DOI] [PubMed] [Google Scholar]
- 16.Mascolo N, Mereto E, Borrelli F, Orsi P, Sini D, Izzo AA, et al. Does senna extract promote growth of aberrant crypt foci and malignant tumors in rat colon? Digestive Diseases and Sciences. 1999;44(11):2226–2230. doi: 10.1023/a:1026696402212. [DOI] [PubMed] [Google Scholar]
- 17.Parnaud G, Corpet DE, Gametpayrastre L. Cytostatic effect of polyethylene glycol on human colonic adenocarcinoma cells. International Journal of Cancer. 2001;92(1):63–69. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1158>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Roy HK, Dibaise JK, Black J, Karolski WJ, Ratashak A, Ansari S. Polyethylene glycol induces apoptosis in HT-29 cells: potential mechanism for chemoprevention of colon cancer. Febs Letters. 2001;496(2–3):143–146. doi: 10.1016/s0014-5793(01)02420-6. [DOI] [PubMed] [Google Scholar]
- 19.Laboisse CL, Maoret JJ, Triadou N, Augeron C. Restoration by polyethylene glycol of characteristics of intestinal differentiation in subpopulations of the human colonic adenocarcinoma cell line HT29. Cancer Research. 1988;48:2498–2504. [PubMed] [Google Scholar]
- 20.Karlsson PC, Hughes R, Rafter JJ, Bruce WR. Polyethylene glycol reduces inflammation and aberrant crypt foci in carcinogen-initiated rats. Cancer Lett. 2005;223(2):203–9. doi: 10.1016/j.canlet.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 21.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96:1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 22.Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112:719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- 23.Lapre JA, Kleibeuker JH, Vandermeer R. Intestinal alkaline phosphatase activity in fecal water reflects epitheliolysis and is decreased by dietary calcium. Gastroenterology. 1991;100:A378. [Google Scholar]
- 24.Lapre JA, Devries HT, Termont DSML, Kleibeuker JH, Devries EGE, Vandermeer R. Mechanism of the protective effect of supplemental dietary calcium on cytolytic activity of fecal water. Cancer Research. 1993;53(2):248–253. [PubMed] [Google Scholar]
- 25.Chang WCL, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18(4):721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 26.Kerr JFR, Winterford CM, Harmon BV. Apoptosis Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Barnes CJ, Hardman WE, Cameron IL. Presence of well-differentiated distal, but not poorly differentiated proximal, rat colon carcinomas is correlated with increased cell proliferation in and lengthening of colon crypts. International Journal of Cancer. 1999;80(1):68–71. doi: 10.1002/(sici)1097-0215(19990105)80:1<68::aid-ijc14>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Roy HK, Gulizia J, DiBaise JK, Karolski WJ, Ansari S, Madugula M, et al. Polyethylene glycol inhibits intestinal neoplasia and induces epithelial apoptosis in Apc(min) mice. Cancer Letters. 2004;215(1):35–42. doi: 10.1016/j.canlet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Schiller LR, Emmett M, Santa Ana CA, Fordtran JS. Osmotic effects of polyethylene glycol. Gastroenterology. 1988;94(4):933–41. doi: 10.1016/0016-5085(88)90550-1. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T, Shinoda T, Yoshimi N, Niwa K, Iwata H, Mori H. Inhibitory effect of magnesium hydroxide on methylazoxymethanol acetate-induced large bowel carcinogenesis in male F344 rats. Carcinogenesis. 1989;10:613–616. doi: 10.1093/carcin/10.3.613. [DOI] [PubMed] [Google Scholar]
- 31.Mori H, Morishita Y, Mori Y, Yoshimi N, Sugie S, Tanaka T. Effect of Magnesium Hydroxide on Methylazoxymethanol Acetate-Induced Epithelial Proliferation in the Large Bowels of Rats. Cancer Letters. 1992;62(1):43–48. doi: 10.1016/0304-3835(92)90196-3. [DOI] [PubMed] [Google Scholar]
- 32.Mori H, Morishita Y, Shinoda T, Tanaka T. Preventive effects of magnesium hydroxide on carcinogen-induced large bowel carcinogenesis in rats. Basic Life Sci. 1993;61:111–118. doi: 10.1007/978-1-4615-2984-2_10. [DOI] [PubMed] [Google Scholar]
- 33.Wang A, Yoshimi N, Tanaka T, Mori H. The inhibitory effect of magnesium hydroxide on the bile acid-induced cell proliferation of colon epithelium in rats with comparison to the action of calcium lactate. Carcinogenesis. 1994;15(11):2661–2663. doi: 10.1093/carcin/15.11.2661. [DOI] [PubMed] [Google Scholar]
- 34.Yang CY, Chiu HF. Calcium and magnesium in drinking water and risk of death from rectal cancer. International Journal of Cancer. 1998;77(4):528–532. doi: 10.1002/(sici)1097-0215(19980812)77:4<528::aid-ijc9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Craven PA, Derubertis FR. Effects of Aspirin on 1,2-Dimethylhydrazine-Induced Colonic Carcinogenesis. Carcinogenesis. 1992;13(4):541–546. doi: 10.1093/carcin/13.4.541. [DOI] [PubMed] [Google Scholar]
- 36.Moorghen M, Orde M, Finney KJ, Appleton DR, Watson AJ. Sulindac enhances cell proliferation in DMH-treatment mouse colonic mucosa. Cell Prolif. 1998;31:59–70. doi: 10.1046/j.1365-2184.1998.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaels JD, Papoutsakis ET. Polyvinyl alcohol and polyethylene glycol as protectants against fluid-mechanical injury of freely-suspended animal cells (CRL 8018) J Biotechnology. 1991;19:241–257. doi: 10.1016/0168-1656(91)90062-z. [DOI] [PubMed] [Google Scholar]
- 38.Shih IM, Wang TL, Traverso G, Romans K, Hamilton SR, Ben-Sasson S, et al. Top-down morphogenesis of colorectal tumors. Proc Natl Acad Sci USA. 2001;98(5):2640–5. doi: 10.1073/pnas.051629398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dainichi T, Ueda S, Isoda M, Koga T, Kinukawa N, Nose Y, et al. Chemical peeling with salicylic acid in polyethylene glycol vehicle suppresses skin tumour development in hairless mice. British Journal of Dermatology. 2003;148(5):906–912. doi: 10.1046/j.1365-2133.2003.05282.x. [DOI] [PubMed] [Google Scholar]
- 40.Sidransky D, Tokino T, Hamilton SR, Kinzler KW, Levin B, Frost P, et al. Identification of ras Oncogene Mutations in the Stool of Patients with Curable Colorectal Tumors. Science. 1992;256(5053):102–105. doi: 10.1126/science.1566048. [DOI] [PubMed] [Google Scholar]
- 41.Ross PC, Hui SW. Polyethylene glycol enhances lipoplex-cell association and lipofection. Biochimica et Biophysica Acta - Biomembranes. 1999;1421(2):273–283. doi: 10.1016/s0005-2736(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 42.Wali WL, Koetier JL, Bissonnette M, Roy H. Polyethylene glycol (PEG) suppresses transcription factor SNAIL in AOM-induced aberrant crypt foci (ACF) and human colon cancer line HCT-116. Gastroenterology. 2003;124(5):A-604. [Google Scholar]
- 43.DiPalma JA, DeRidder PH, Orlando RC, Kolts BE, Cleveland MB. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Amer J Gastroenterol. 2000;95(2):446–450. doi: 10.1111/j.1572-0241.2000.01765.x. [DOI] [PubMed] [Google Scholar]


