Abstract
Molecular therapeutics is a recognized promising approach for melanoma, but relevant target genes remain elusive. We report that overload of the recently cloned H11/HspB8 induces apoptosis in 55% of examined melanoma cultures. Apoptosis was determined by activation of caspases-9 and -3 and TUNEL, and was not seen in normal melanocytes. It was associated with H11/HspB8 complexation with TAK1 and activation of TAK1 and p38MAPK. TAK1 was not bound, nor activated by the H11/HspB8 mutant W51C, which has dominant anti-apoptotic activity. β-catenin was phosphorylated by activated TAK1, inhibiting its nuclear accumulation and MITF and CDK2 expression. The dominant negative TAK1 mutant K63W inhibited β-catenin phosphorylation and caspase activation. The data indicate that H11/HspB8 overload causes melanoma growth arrest and apoptosis through TAK1 activation and suggest that H11/HspB8 is a promising molecular therapy target.
INTRODUCTION
Apoptosis is a tightly regulated irreversible process. It is primarily mediated by cysteine proteases (caspases), which are activated by the cleavage of inactive zymogens (procaspases) in a sequential cascade or by autocatalysis. In the intrinsic cascade, caspase-9 activates caspase-3, which is a main effector of apoptosis and it is responsible for the proteolytic cleavage of proteins required for cell survival (Aurelian, 2005). Apoptosis is a promising molecular therapy platform for melanoma, an aggressive and resistant cancer with increasing incidence and a growing lifetime risk (Fink et al., 2001; Margolin, 2004; Tsao and Sober, 2005). However, genes that reconnect the apoptotic cascade in melanoma remain elusive.
Transforming growth factor β activated kinase 1 (TAK1) is a member of the MAP3K family. It phosphorylates MKK-3,-4,-6 and-7, thereby activating the pro-apoptotic c-Jun N-terminal kinase (JNK) and p38MAPK cascades (Davis et al., 2000) and was implicated in prostate cancer apoptosis (Edlund et al., 2003). TAK1 also negatively impacts on the transcriptional activity of β-catenin (Ishitani et al., 1999), which is involved in melanoma development, through its target, Microphthalmia-associated transcription factor (MITF) (Widlund et al., 2002).
We first cloned the heat shock protein H11/HspB8 by screening an expression library with antibody to 13 residues within the PK fragment of the HSV-2 protein ICP10 (Smith et al., 2000) that also contains a degenerate α-crystallin motif (Chabaud et al., 2003). H11/HspB8 contains nuclear export sequences, does not translocate to the nucleus upon heat shock, and has protein kinase activity (Gober et al., 2003, 2005; Chowdary et al., 2004; Hase et al., 2005). Unlike other family members, which are overexpressed in tumor cells and exert cytoprotective activity (Ciocca and Calderwood, 2005), H11/HspB8 is silenced in melanoma (Sharma et al., 2006), where its overload triggers apoptosis (Gober et al., 2003). The studies described in this report were designed to determine the mechanism of apoptosis induced by H11/HspB8 in melanoma cells.
RESULTS
H11/HspB8 triggers apoptosis in melanoma, but not melanocytes
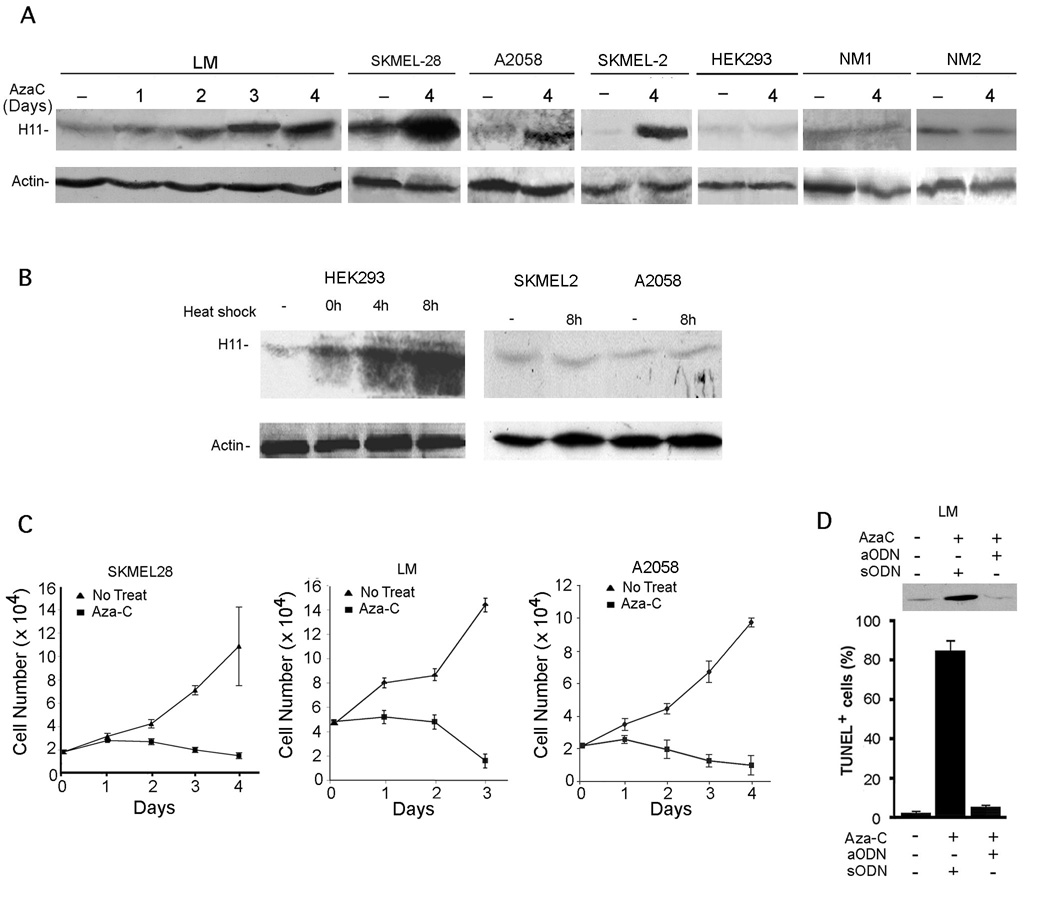
Because H11/HspB8 DNA is aberrantly methylated/silenced in 50–60% of melanoma but not melanocytes (Sharma et al., 2006), we wanted to know whether its upregulation triggers melanoma-specific apoptosis. Seven melanoma lines (SKMEL-2, SKMEL-28, A375, A2058, G361, HT144, SKMEL-31), 2 freshly isolated melanoma cultures (LM and MR), and 2 cultures of normal melanocytes (NM1 and NM2) were treated with Aza-C (2µM) and examined for H11/HspB8 expression and apoptosis. A time-dependent increase in H11/HspB8 levels was seen in SKMEL-2, SKMEL-28, A2058, A375 and LM cells, but not in normal melanocytes or HEK293 cells (Fig. 1A). Aza-C induced H11/HspB8 overload was accompanied by decreased melanoma cell viability (determined by trypan blue exclusion) (Fig. 1C) and apoptosis (determined by TUNEL), which was blocked by H11/HspB8 aODN, but not sODN (Fig. 1D). In HEK293 (not melanoma) cells, H11/HspB8 was upregulated by heat shock (Fig. 1B), but it did not induce apoptosis (data not shown).
Fig. 1. Aza-C induces H11/HspB8 overload and apoptosis in melanoma.
A. Cells treated or not with Aza-C (2µM) were immunoblotted with H11-181 or actin antibodies. B. Immunoblotting as in A of cells heat shocked (1h, 42.5°C) and allowed to recover (0–8hrs; 37°C). C. Melanoma cells were treated as in A and viable cells were counted. D. LM cells treated with Aza-C (2 µM; 4 days) and H11/HspB8 sODN or aODN (30µM) were immunoblotted with H11-181 antibody and assayed by TUNEL.
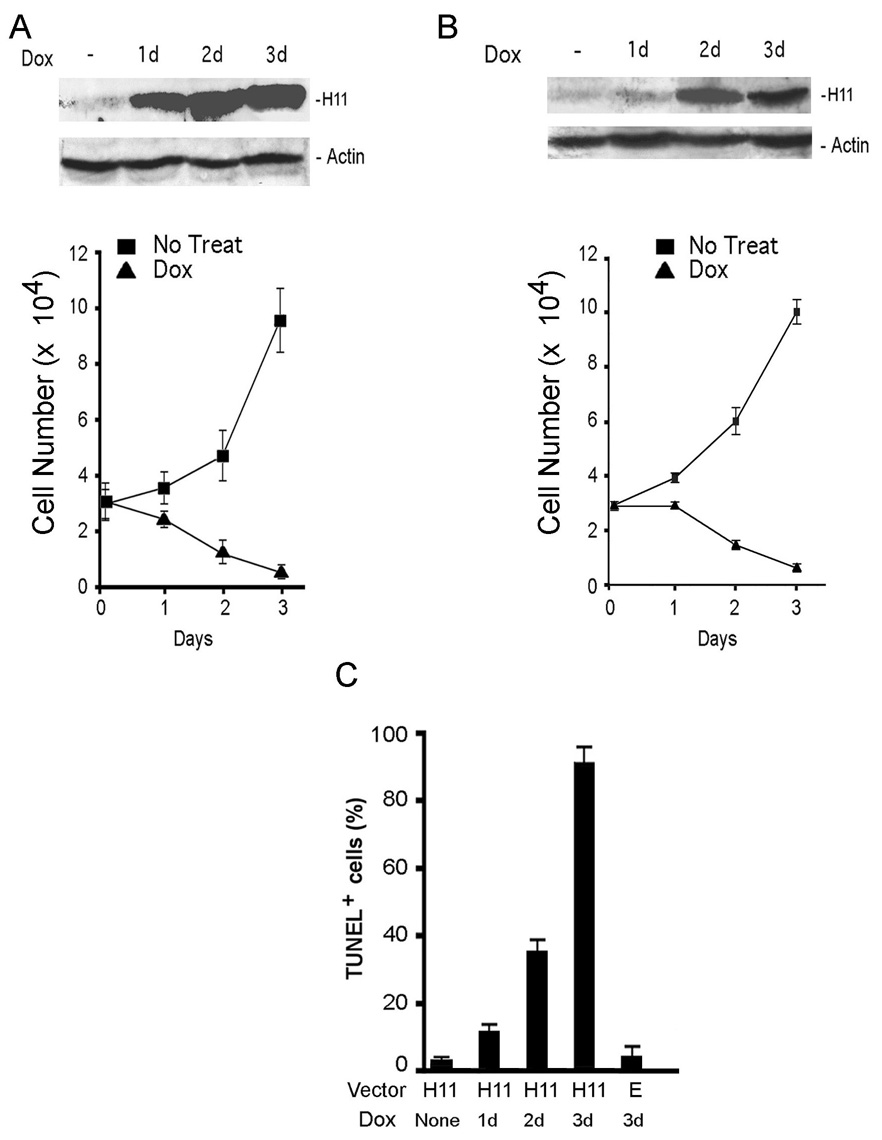
Dox (2µg/ml) treatment of melanoma cells stably transfected with Tet-regulated H11/HspB8 decreased viability and increased apoptosis (day 3; 70–87% TUNEL+ cells), associated with H11/HspB8 overload (Fig. 2). It did not alter the expression of Hsp70 and Hsp27 (Supplement Fig. 1), suggesting that they are not involved in H11/HspB8 induced apoptosis. Dox had no effect on untransfected cells or cells transfected with the empty vector (Fig. 2C).
Fig. 2. Dox induces apoptosis in H11/HspB8 transfected melanoma cells.
H11/HspB8 transfected SKMEL2 (A) and A2058 (B) cells treated with Dox (2µg/ml) were immunoblotted with H11-181 and actin antibodies and viable cells were counted. C. A2058 cells transfected with the Tet-regulated H11/HspB8 (H11) or empty (E) vectors were treated with Dox and assayed by TUNEL.
Dox-induced H11/HspB8 overload causes caspase activation
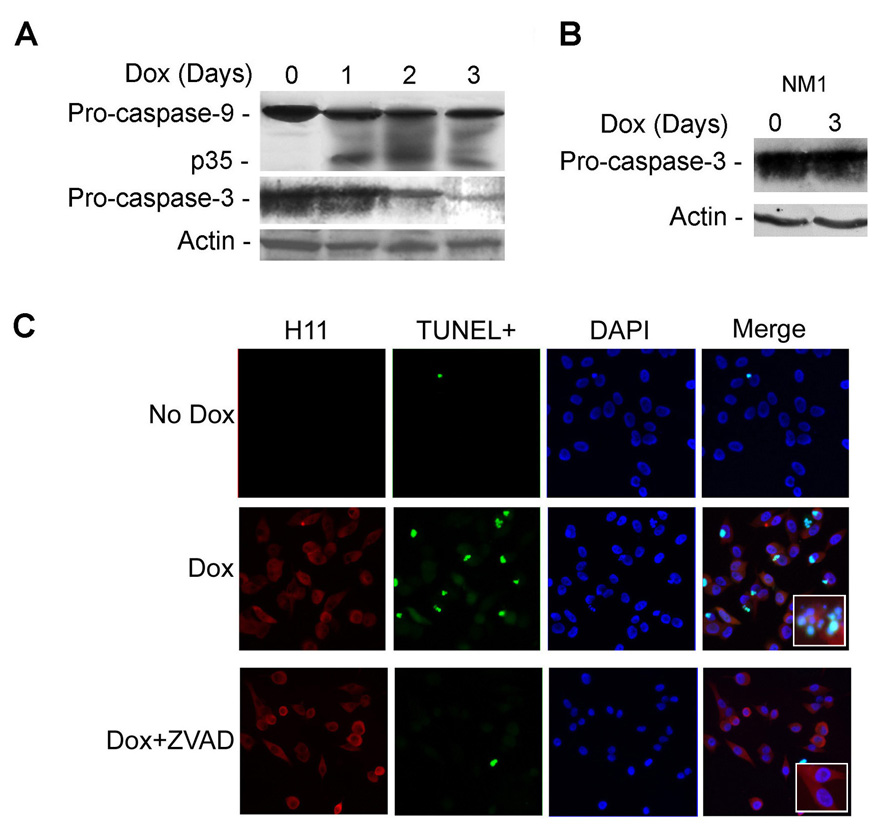
Extracts of H11/HspB8-transfected melanoma cultures treated or not with Dox, were immunoblotted with antibodies to caspase-9 or -3. The levels of procaspase-9 decreased on day 1 after Dox treatment, concomitant with the appearance of its p35 cleavage product and were followed by decreased levels of procaspase-3 (indicative of activation) (Fig. 3A). Caspase activation was specific for H11/HspB8 overload and was not seen in Dox-treated melanocytes (Fig. 3B). TUNEL co-localized with H11/HspB8 overload, as evidenced by double immunofluorescence with FITC-dUTP (TUNEL) and Texas red-labeled H11/HspB8 antibody (35–41% and 67–78% cells on days 2 and 3 after Dox, respectively), and it was blocked by the pan-caspase inhibitor z-VAD-fmk (100µM) (Fig. 3C). The data indicate that apoptosis is through activation of the intrinsic caspase pathway.
Fig. 3. Dox-induced H11/HspB8 overload triggers caspase activation.
A. H11/HspB8 transfected A2058 cells treated or not with Dox were immunoblotted with antibodies to caspase-9, caspase-3 or actin. B. NM1 cells, treated or not with Dox, were immunoblotted with antibodies to caspase-3 and actin. C. H11/HspB8 transfected SKMEL2 cells treated with Dox with or without z-VAD-fmk (100µM; 2 days) were stained with FITC-dUTP (TUNEL) and Texas red-H11-181 antibody (H11) (x200). Insert is magnification (x400)
TAK1 is bound and activated by H11/HspB8, but not its apoptosis-negative mutant W51C
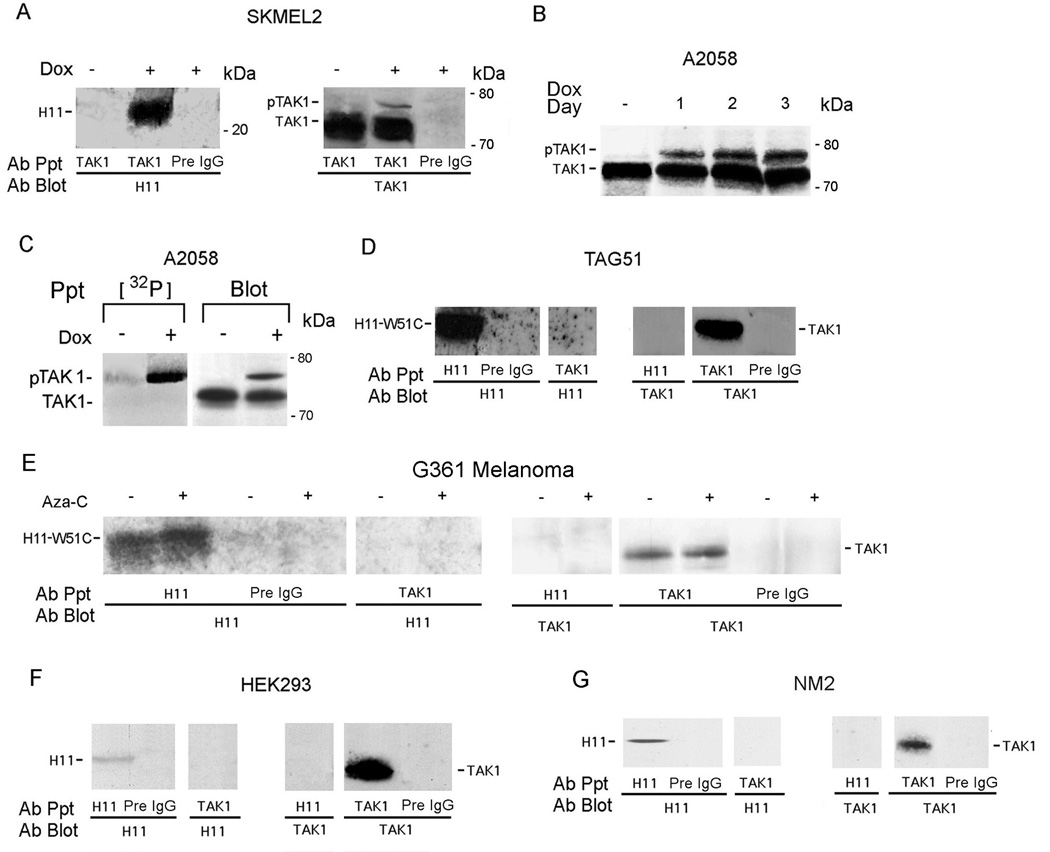
To examine whether apoptosis is through TAK1 activation, we used reciprocal pull-down assays with H11 and TAK1 antibodies. H11/HspB8 co-precipitated with TAK1 from Dox-treated (1–3 days), but not untreated, melanoma cells and the precipitates contained a slowly migrating band, consistent with activated TAK1 (pTAK1) (Kishimoto et al., 2000) (Fig. 4A,B). The slower band is, indeed, pTAK1, because it was also seen in the anti-TAK1 precipitates from Dox-treated [32P]-orthophosphate labeled cells (Fig. 4C). The TAK1-associated protein TAB1 was also in the H11/HspB8 precipitates (Supplement Fig. 2). TAK1 did not co-precipitate with the dominant anti-apoptotic H11/HspB8 mutant W51C, neither from G361 melanoma cells, which naturally express W51C (Gober et al., 2003), nor from TAG51 cells, which are stably transfected with W51C (Gober et al., 2003) and the precipitates did not contain pTAK1 (Fig. 4D,E). Co-precipitation and TAK1 activation were also not seen in HEK293 cells (Fig. 4F), or melanocytes (Fig. 4G), which were negative for apoptosis.
Fig. 4. TAK1 binds H11/HspB8, but not W51C.
A. Reciprocal immunoprecipitation/immunoblotting (pull-down) of H11/HspB8 transfected Dox-treated (2 days) SKMEL2 cells using antibodies H11-181, TAK1 or preimmune (Pre) IgG. B. Pull-down of H11/HspB8-transfected A2058 cells treated with Dox (1–3 days) using TAK1 antibody. C. Pull-down of H11/HspB8-transfected [32P]-orthophosphate labeled A2058 cells with H11-181 and TAK1 antibodies and Pre IgG. D–G. Reciprocal pull-down of TAG51 (D) G361 melanoma cells (E), HEK293 (F) and NM2 (G) cells with H11-181 or TAK1 antibodies. Representative molecular weights are on the right.
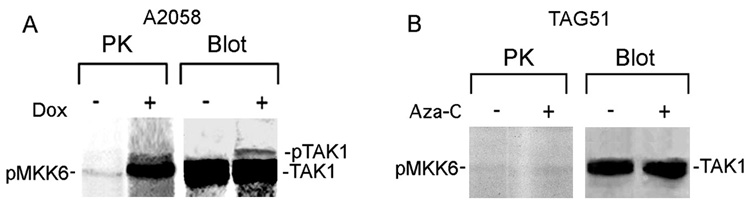
Immunocomplex PK assays with TAK1 antibody and the TAK1 substrate MKK6 (Singhirunnusorn et al., 2005), indicated that MKK6 was phosphorylated in Dox-treated, but not untreated melanoma cells and the precipitates were positive for pTAK1 (Fig. 5A). MKK6 was not phosphorylated in immunocomplex PK assays of G361 (data not shown) or TAG51 (Fig. 5B) cells. Collectively the data indicate that apoptosis caused by H11/HspB8 overload is associated with TAK1 activation.
Fig. 5. TAK1 is activated in Dox-treated melanoma cells.
Immunocomplex PK assay of H11/HspB8 transfected and Dox-treated A2058 (A) or Aza-C treated TAG51 (B) cells using TAK1 antibody and MKK6 substrate (PK). Kinase precipitates were blotted with TAK1 antibody (blot).
Dox treatment blocks β-catenin nuclear accumulation
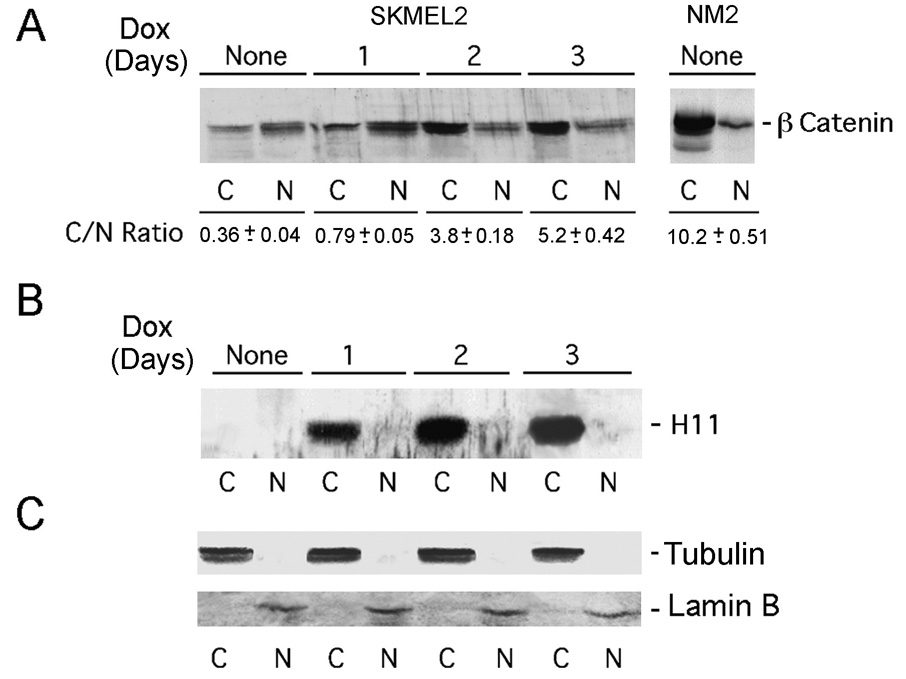
To examine the impact of TAK1 activation on β-catenin (Ishitani et al., 1999), the nuclear accumulation of which was associated with melanoma cell growth (Widlund et al., 2002; Saito et al., 2003), H11/HspB8-transfected melanoma cells were treated, or not, with Dox (1–3 days) and cytoplasmic (C) and nuclear (N) fractions were immunoblotted with β-catenin antibody. Normal melanocytes were studied in parallel. C/N ratios (determined by densitometric scanning) were obtained for 3 independent experiments and results are expressed as averages ± SEM. In melanocytes, β-catenin was primarily cytoplasmic (C/N = 10.2±0.51) while nuclear accumulation was evident in untreated melanoma cells (C/N = 0.36±0.04). Dox caused a time-dependent increase in the C/N ratio (5.2±0.42 on day 3 post treatment) (Fig. 6A). Re-probing of the stripped blots with H11/HspB8 antibody confirmed that it is strictly cytoplasmic (Fig. 6B), and fraction cross-contamination was excluded by further re-probing of the blots with antibodies to tubulin (cytoplasmic marker) and lamin B (nuclear marker) (Fig. 6C). The data indicate that H11/HspB8 overload interferes with β-catenin nuclear accumulation.
Fig. 6. Dox blocks β-catenin nuclear accumulation.
A. Cytoplasmic (C) and nuclear (N) fractions from H11/HspB8 transfected Dox-treated SKMEL2 and NM2 cells immunoblotted with β-catenin antibody. C/N are mean ± SEM for 3 independent experiments. Blots were re-probed with H11-181 (B), tubulin (cytoplasmic marker) and lamin B (nuclear marker) (C) antibodies.
β-catenin is phosphorylated by activated TAK1
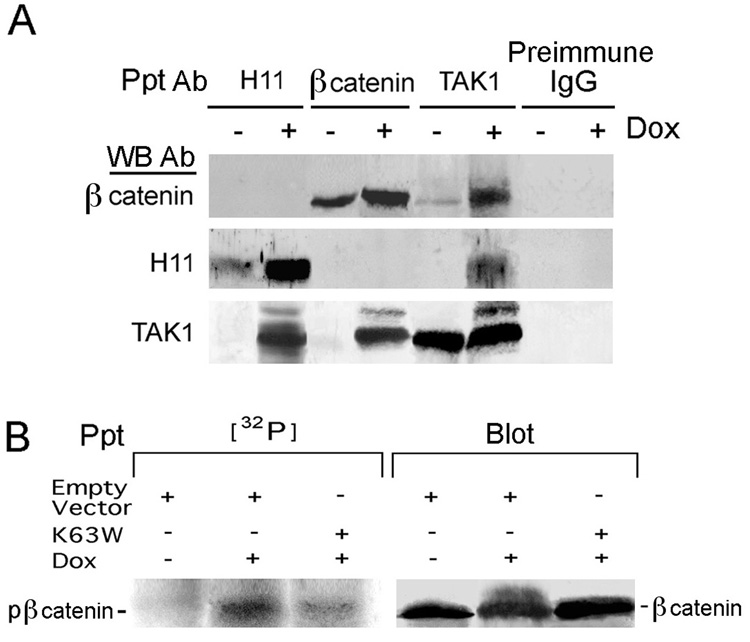
Two series of experiments were done to examine whether reduced nuclear accumulation of β-catenin in Dox-treated melanoma cells, is related to TAK1 activation. Reciprocal pull-down assays indicated that the H11/HspB8, TAK1 and β-catenin antibodies pulled down their respective cognate proteins from untreated cells and preimmune IgG was negative. By contrast, in Dox-treated cells, both the H11/HspB8 and β-catenin antibodies pulled down TAK1, and all 3 proteins were pulled down by TAK1 antibody. H11/HspB8 and β-catenin did not co-immunoprecipitate unless pTAK1 was present, suggesting that β-catenin complexed to TAK1 activated by binding H11/HspB8. Indeed, immunoblotting of the anti-TAK1 precipitates with TAK1 antibody revealed the presence of pTAK1 in the Dox-treated, but not untreated cells (Fig. 7A).
Fig. 7. TAK1 activation is involved in β-catenin phosphorylation.
A. Reciprocal pull-down of H11/HspB8 transfected Dox-treated (2 days) A2058 cells with H11-181, β-catenin or TAK1 antibodies. B. Cells as in A were transfected (24hrs) with FLAG-tagged K63W or empty vector, Dox treated labeled with [32P]-orthophosphate (4hrs; 500µCi/ml) and precipitated (32P) /blotted (blot) with β-catenin antibody.
In a second series of experiments, the cells were transfected with the dominant negative TAK1 mutant K63W (Diao et al., 2005) (or the empty vector) and, 24 hrs later, they were treated (or not) with Dox (2 days), labeled with [32P]-orthophosphate (4 hrs) and the extracts were immunoprecipitated with β-catenin antibody. A phosphorylated band, recognized by the β-catenin antibody, was seen in the precipitates from the [32P]-labeled Dox-treated cells transfected with the empty vector, but not in those transfected with K63W, and the untreated cells were negative (Fig. 7B). These findings are not an artifact due to improper protein levels, because the levels of β-catenin were virtually identical in all the precipitates (Fig. 7B). The data indicate that TAK1 activation is involved in β-catenin phosphorylation.
Dox inhibits MITF and CDK2 expression and causes growth arrest
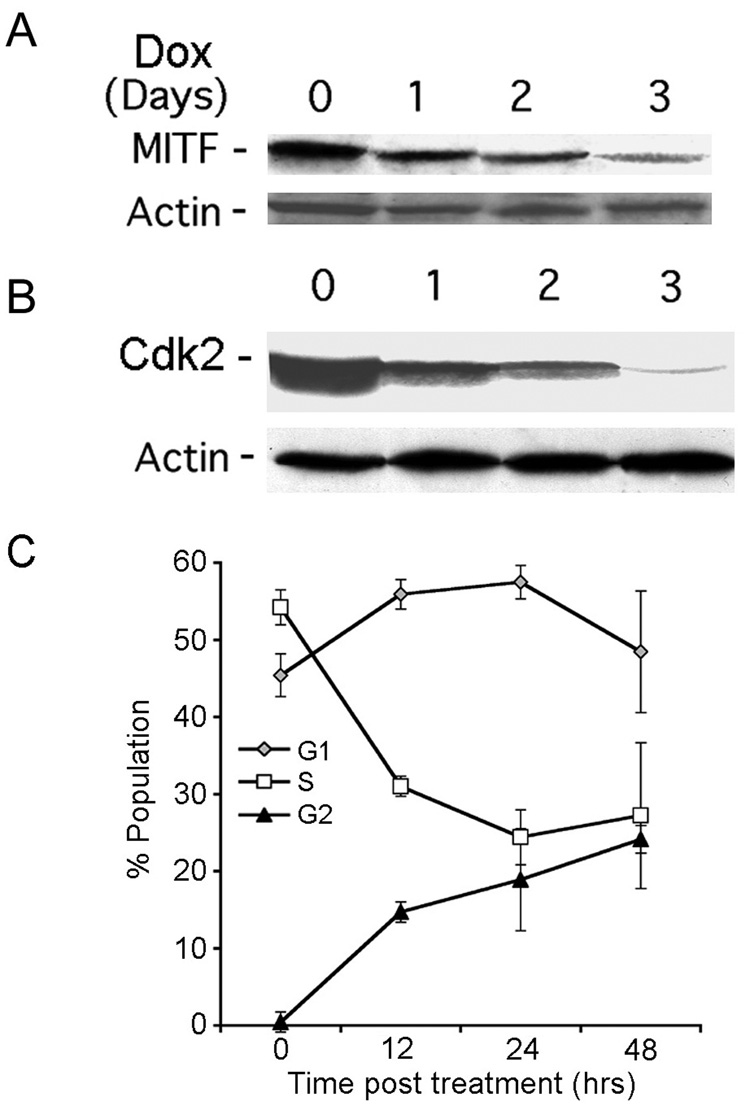
Having seen that Dox interferes with the nuclear accumulation of β-catenin, we wanted to know whether this causes growth arrest through downregulation of the β-catenin transcriptional target MITF and the MITF target CDK2, both of which are required for melanoma cell proliferation (Widlund et al., 2002; Du et al., 2004). Dox caused a time-dependent decrease in the levels of MITF (Fig. 8A) and CDK2 (Fig. 8B). It was accompanied by an increased proportion of cells in G1, and particularly in G2, and a decrease in the S-phase population, when compared to non-treated cells (Fig. 8C). The data suggest that H11 overload causes cell cycle arrest at G1 and G2, likely related to the function of CDK2 in both the G1/S and G2/M transitions of the cell cycle (Chae et al., 2004).
Fig. 8. Dox-induced MITF and CDK2 downregulation and growth arrest.
H11/HspB8 transfected Dox-treated A2058 cells were immunoblotted with antibodies to MITF (A), CdK2 (B) or actin. C. G1, S and G2/M populations in H11/HspB8 transfected Dox-treated A2058 cells. Results are mean ± SEM for 3 independent experiments.
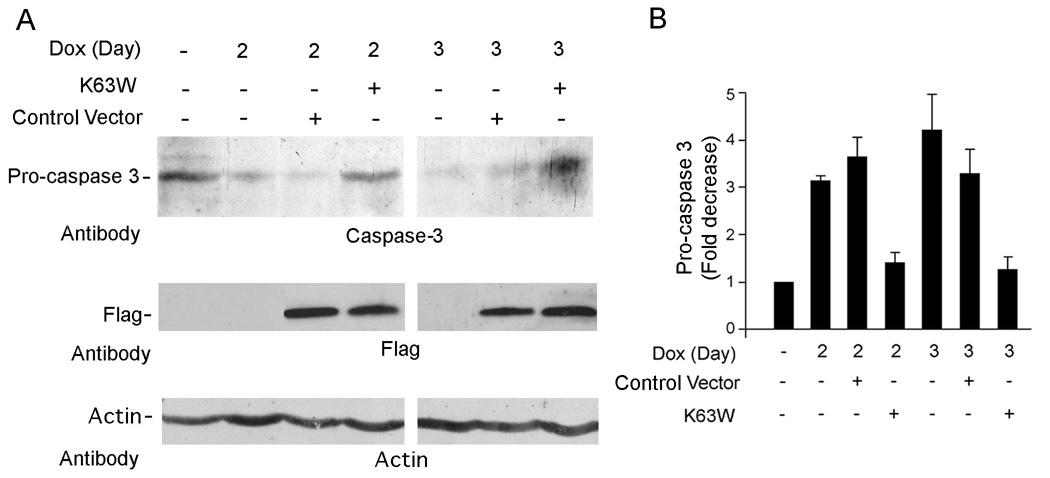
TAK1 activation is required for H11/HspB8-mediated apoptosis
To examine whether TAK1 activation is required for apoptosis, the H11/HspB8 positive melanoma cells were transfected with Flag-tagged K63W or empty vector, treated with Dox and immunoblotted with caspase-3 antibody. Procaspase-3 was decreased by Dox (indicative of activation) and restored to basal levels by transfection with K63W, but not the empty vector, although Flag was expressed equally well for both constructs (Fig. 9A). The results of 3 independent experiments (expressed as fold decrease ± SEM) indicate that Dox caused a 3-4-fold decrease in procaspase-3 levels, which was not seen in K63W transfected cells (Fig. 9B). The data indicate that TAK1 activation is required for caspase activation (apoptosis).
Fig. 9. Dox-induced caspase activation is TAK1-dependent.
A. H11/HspB8 transfected A2058 cells were transfected with FLAG-K63W or empty vector, treated (or not) with Dox and immunoblotted with antibodies to caspase-3, FLAG or actin. B. Procaspase-3 levels standardized to actin are expressed as fold-decrease relative to untreated cells.
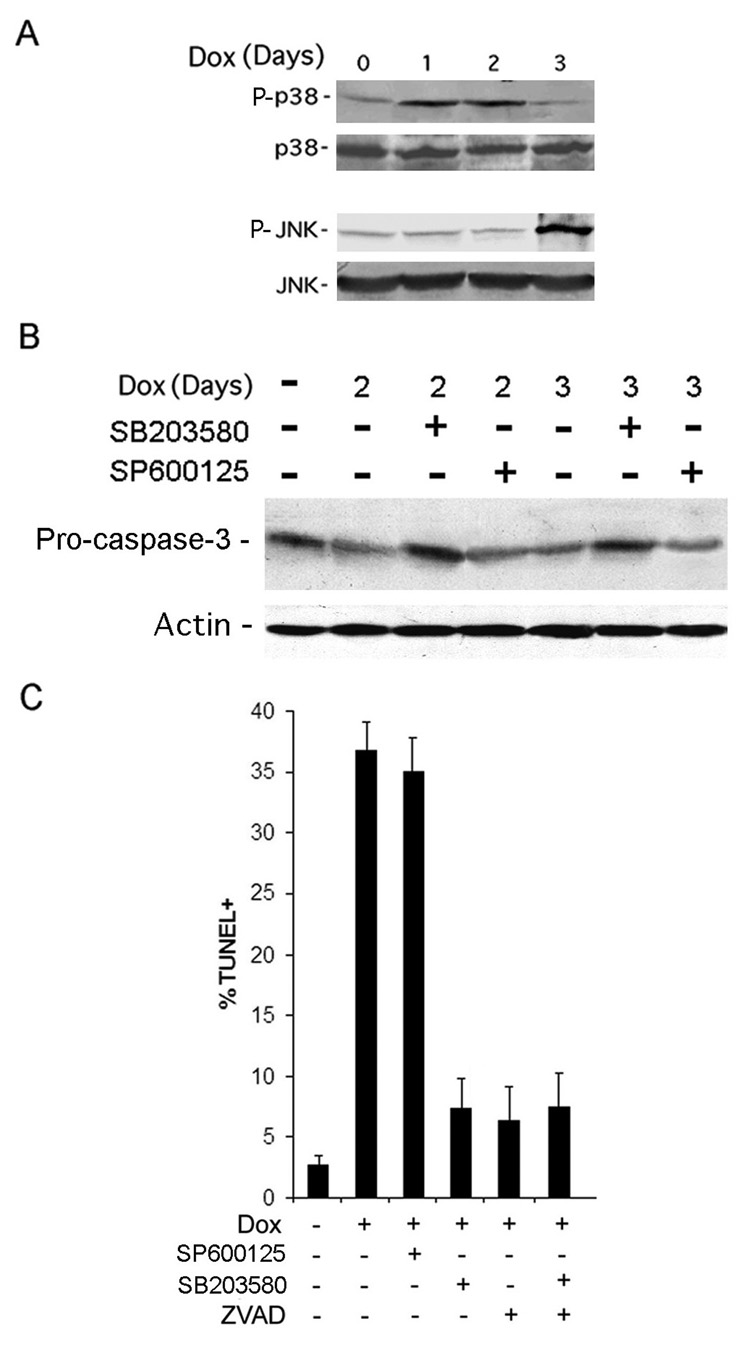
H11/HspB8 induced apoptosis is through TAK1-activated p38MAPK
To identify the TAK1 regulated pathways associated with Dox-induced apoptosis, the H11/HspB8-transfected melanoma cells were treated with Dox and immunoblotted with antibodies to phosphorylated (activated) p38MAPK (P-p38MAPK) or JNK (P-JNK) using antibodies to total p38MAPK and JNK as control. P-p38MAPK levels were increased on days 1 and 2 post treatment, concomitant with caspase activation. The levels of P-JNK were not increased until day 3 post-treatment, when apoptosis was already maximal (Fig 10A). We conclude that apoptosis is p38MAPK-dependent, because the p38MAPK inhibitor SB20350 blocked caspase activation (Fig. 10B) and restored TUNEL to its basal levels (Fig. 10C), while these were not altered by the JNK-specific inhibitor SP600125. The % TUNEL+ cells in SB203580-treated cells, was similar to that seen in cells treated with the pan-caspase inhibitor Z-VAD-fmk, and was not further reduced by treatment with SB203580 + Z-VAD-fmk (Fig. 10C).
Fig. 10. Dox-induced apoptosis is p38MAPK-dependent.
A. H11/HspB8 transfected Dox-treated SKMEL2 cells were immunoblotted with antibodies to activated p38MAPK (P-p38MAPK) or JNK (P-JNK) followed by antibodies to total p38MAPK or JNK. B. H11/HspB8 transfected A2058 cells treated with Dox and SB203580 or SP600125 (10µM) were immunoblotted with antibodies to caspase-3 and actin. C. H11/HspB8 transfected SKMEL2 cells treated with Dox with or without SB203580, SP600125, Z-VAD (100µM) or SB203580 + Z-VAD and assayed by TUNEL.
DISCUSSION
Melanoma is an aggressive cancer with increasing incidence and a growing lifetime risk (Margolin, 2004) that is intrinsically resistant to chemotherapy and apoptosis (Fink et al., 2001; Tsao and Sober, 2005). Apoptosis-based molecular therapies are desirable, but the identification of relevant gene target(s) is a major clinical challenge. The salient feature of our data presented is the finding that H11/HspB8 overload triggers apoptosis in melanoma, but not normal melanocytes, through TAK1 activation.
Recent interest has focused on de-methylation as a platform for cancer therapy. A remarkably limited number of genes are upregulated by de-methylation [0.67% of 25,940 screened by microarray (Karpf et al., 2004)]. CDKN2A, APAF-1, TIMP3, GAGED2, MAGE-1 and RARB are methylated in melanoma, but their relationship to apoptosis is unclear (Soengas et al., 2001; Zendman et al., 2002; van der Velden et al., 2003; Furuta et al., 2004). Aza-C sensitized a melanoma line to interferon-induced apoptosis (Reu et al., 2006) and DAC upregulated TSPY and suppressed the growth of two derivatives from another line (Gallagher et al., 2005). The role of TSPY in independently established melanoma lines, the contribution of the other 65 upregulated genes, and the mechanism of growth inhibition are still unresolved.
We found that Aza-C induced H11/HspB8 overload and caused cell death in 5/9 (55%) examined melanoma cultures, including 2 freshly isolated ones. Death was due to apoptosis caused by H11/HspB8 overload as evidenced by TUNEL inhibition with H11/HspB8 aODN, but not sODN. Apoptosis was also induced by Dox in melanoma cells stably transfected with Tet-regulated H11/HspB8, supporting the conclusion that it was mediated by H11/HspB8 overload. H11/HspB8 did not have apoptotic activity in melanocytes. Preliminary data suggest that in these cells, as in cardiac myocytes (Hase et al., 2005), H11/HspB8 protects from stress-induced apoptosis (unpublished). While genes involved in cell fate determination [viz. Ras and TGF-β (Cox and Der, 2003; Wakefield and Roberts, 2002)] have cell type and stimulus-specific pro- and anti-apoptotic activities through distinct protein-protein interactions, this is a novel paradigm for an Hsp.
The Tet-inducible system was used to examine the mechanism of H11/HspB8 mediated apoptosis. We used A2058 and SKMEL2 cells, to examine whether H11/HspB8 can override the effects of the respective activating mutations in B-Raf or Ras. In both lines, Dox-induced H11/HspB8 overload caused growth arrest followed by apoptosis, as determined by activation of caspases - 9 and 3 (intrinsic pathway) and DNA fragmentation (TUNEL+ cells). Apoptosis was not caused by deleterious effects of Dox on Hsp70 and Hsp27, which are anti-apoptotic (Cioca and Calderwood, 2005), because these are expressed equally well in untreated and Dox treated cells. Also, interaction of H11/HspB8 with Hsp27 (Sun et al., 2004) was not seen in pull-down assays of melanoma cells (Smith et al., 2000) and the bulk of the two proteins did not co-localize in sucrose gradient fractionation of extracts from Dox-treated melanoma cultures (Supplement Fig. 3). Apoptosis is through TAK1 activation, because: (i) TAK1 bound H11/HspB8 and was phosphorylated in Dox-treated, but not untreated cells, (ii) the TAK1 kinase substrate MKK6 was phosphorylated in Dox-treated, but not untreated, cells, (iii) TAK1 did not bind the H11/HspB8 mutant W51C, which is dominant negative for apoptosis (Gober et al., 2003), and (iv) caspase activation was inhibited by the dominant negative TAK1 mutant K63W.
In Dox-treated, but not untreated, melanoma cells, TAK1 bound β-catenin and was involved in its phosphorylation as evidenced by phosphorylation inhibition with the dominant negative TAK1 mutant K63W and TAK1-specific aODN (data not shown). To the extent of our knowledge, this is the first report that TAK1 is involved in β-catenin phosphorylation. β-catenin is phosphorylated by GSK-3β (on Tyrosine residues), or by cyclin-dependent CDK2 (on Ser33, Ser37, Thr41 and Ser45) promoting its proteosomal degradation (Park et al., 2004). However, GSK-3β was not activated in Dox-treated melanoma cells (unpublished) and Dox did not reduce the levels of phosphorylated β-catenin, suggesting that TAK1-mediated phosphorylation does not cause protein degradation and is likely to involve distinct serine-threonine residues. Its outcome appears to be reduced nuclear accumulation and inhibition of the β-catenin transcriptional activity, as evidenced by decreased levels of MITF in Dox-treated, as compared to untreated cells. While final conclusions relating to the effect of H11/HspB8 overload on β-catenin transcriptional activity must await the results of ongoing studies of TCF/LEF1-luciferase reporter gene plasmids, our data indicate that the MITF target CDK2 was also inhibited in Dox-treated cells. Because CDK2 functions in the G1/S and G2/M transitions (Chae et al., 2004), its inhibition is likely to be responsible for the growth arrest observed in the Dox-treated cells in G1, and particularly G2.
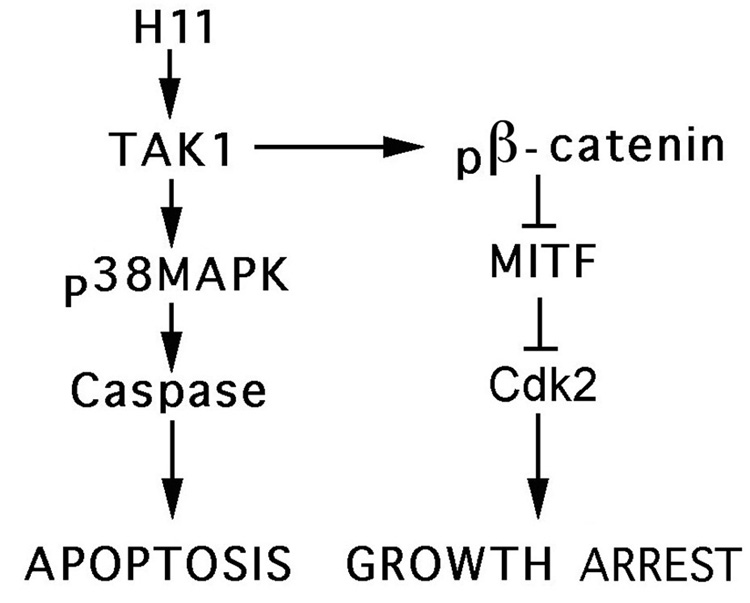
Apoptotic cells are predominantly derived from G2 arrested populations (Perez-Stable, 2006). Our data do not address this question, but suggest that β-catenin is not directly involved in Dox-induced apoptosis, because both caspase activation and TUNEL were completely inhibited by the p38MAPK inhibitor, SB23058. Indeed, p38MAPK was activated in Dox-treated, but not untreated, cells, and SB203580 was as effective as the caspase inhibitor Z-VAD-fmk, in inhibiting Dox-induced activation of caspase-3. Although JNK was also activated in Dox-treated cells, caspase activation and TUNEL were not inhibited by the JNK inhibitor SP600125. This is consistent with previous reports that TAK1 activated JNK and p38MAPK can play different roles in the regulation of signal-mediated gene expression/function (Yu et al., 2000) and activated p38MAPK suppresses the pro-survival NF-kB, which can also be activated by TAK1 (Ivanov et al., 2001). The mechanism of growth arrest/apoptosis induced by H11/HspB8 overload in melanoma cells is schematically represented in Fig. 11.
Fig. 11.
Schematic of H11/HspB8-induced growth arrest and apoptosis.
Melanoma-specific apoptosis by de-methylation induced overload of the endogenous H11/HspB8 is a promising therapeutic platform, with TAK1, p38MAPK and β-catenin as potential targets for future H11/HspB8-based therapies. Ongoing studies are using a PK negative H11/HspB8 mutant to examine whether TAK1 is directly or indirectly phosphorylated by H11/HspB8 kinase (Smith, et al., 2000) and considering the alternative possibility that H11/HspB8 facilitates TAK1 autophosphorylation which is required for kinase activation by an intramolecular mechanism, potentially involving TAB proteins (Kishimoto et al., 2000).
MATERIALS AND METHODS
Cells
SK-MEL-2, SK-MEL-28, SKMEL-31 (human melanoma), and HEK293 were cultured in EMEM with 10% fetal bovine serum (FBS), 1mM sodium pyruvate, 0.1mM non-essential amino acids. For SKMEL28, 4.5g/L glucose, 1500mg/ml sodium bicarbonate, and 4mM glutamine were added. Similarly supplemented DMEM was used for melanoma cells A375 and A2058, and McCoy’s 5A for melanoma cells G361 and HT144. HEK293 stably transfected with the H11 mutant W51C (TAG51) were described (Gober et al., 2003). LM and MR were the gift of Dr. Joseph Sinkovics (University of South Florida). They were freshly established from metastatic melanomas and cultured in RPMI 1640 with 10% FBS. Melanocyte cultures NM1 and NM2 (Cascade Biologicals) were passaged twice in medium 154 with HMGS, as per manufacturer instructions.
Antibodies and chemical reagents
H11/HspB8 antibody H11-181 was described (Smith et al., 2000). Antibodies M-579 (TAK-1), H-102 (β-catenin), C-20 (p38MAPK), C-11 (actin), H-50 (MITF), H277 (caspase-3), H-170 (caspase-9), FL (JNK1, p46, JNK2 p54, and JNK3), N18 (lamin A/C), H235 (β-tubulin), M2 (CDK2) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to P-p38MAPK and P-JNK1/2 were from Cell Signaling (Beverly, MA) and Promega (Madison WI), respectively. 5-aza 2’-deoxycytidine (Aza-C), Dox, and SP600125 were from Sigma Chemicals (St. Louis, MO), SB203580 and benzyloxcarbonyl-Val-Ala-Asp-fluormethyl ketone (z-VAD-fmk) from Calbiochem (La Jolla, CA) and G418 and hygromycin from Invitrogen (Carlsbad, CA).
Antisense oligonucleotides
The sequence and specificity of the phosphorothioate ODNs complementary to the H11/HspB8 translation initiation site [antisense ODN (aODN)] or sense ODN (sODN) and their use (30µM; 4 days; 37 °C) were described (Smith et al., 2000; Gober et al., 2003).
Plasmids and Transfection
The W51C expression vector was described (Gober et al., 2003). The FLAG-tagged TAK1 dominant negative mutant K63W is the gift of Dr. Chen Wang (University of Texas Southwestern Medical Center). K63W has a point mutation in the ATP binding site and is kinase negative (Diao et al., 2005). The empty expression vector p3xFLAG-CMV-10 (pFLAG) was from Sigma. Transient transfection was with 5µg of DNA and Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as per manufacturer’s instructions.
Tet–inducible H11/HspB8 retrovirus vector and cell infection
H11 DNA (0.6kb) was obtained by EcoR1/XbaI digestion of p3XFlag-CMV-H11/HspB8 (Smith et al., 2000). The Xba site was blunt ended and the fragment ligated into the EcoRI/SmaI site of pIRES2-EGFP vector (Clontech). A fragment containing H11/HspB8-IRES-EGFP was isolated by EcoR1/NotI digestion, blunt ended and ligated into the HpaI site of the pRev-TRE retroviral vector, in which the MMTV promoter drives a hygromycin resistance gene and the Tet-sensitive promoter (Tet response element upstream of minimal CMV IE promoter) drives H11/HspB8. To generate retroviruses, the PT57 packaging cell line was transfected with pRevTet-On (contains G418 resistance cassette) or pRevTRE-H11/HspB8 and Lipofectamine 2000. Virus titers were assayed as per manufacturer’s instructions. Melanoma cells were infected with the Tet-On retrovirus at a multiplicity of infection (moi) of 50. Clones were selected with G418 (1mg/ml), infected with the TRE-H11/HspB8 retrovirus (moi = 50) and selected with 800µg/ml hygromycin. H11/HspB8 overload was induced with Doxycycline (Dox; 2µg/ml).
Cellular fractionation
Cells were resuspended in 50mM Tris-HCl buffer (pH 8.0) with 50mM NaCl, 1% NP-40, 1mM dithiothreitol (DTT) and protease inhibitors (Sigma), incubated on ice 5 min and centrifuged (7,000 × g; 1 min). The supernatant is the cytoplasmic fraction. The nuclear pellet was resuspended in the same buffer but with 450mM NaCl (10 min; 4°C) and sonicated until in solution. The fractions were centrifuged (16,000 × g; 30 min) before use.
FACS and Cell Cycle Distribution
Low density cultures (2–5×105 cells/T75 flask) were trypsinized, the cells were fixed (70% ethanol ; 4°C; 12hrs), resuspended in PBS with 1 mg/ml RNase (RT, 30 min) and stained with propidium iodide (Sigma, 20µg/ml). Cell suspensions were filtered through a 40µm filter and analysed for the cell cycle profile with the FACSCalibur flow cytometry system (BD, Biosciences, Franklin Lakes NJ) (Wang et al., 2006).
Radiolabeling, Immunoprecipitation and Immunoblotting
Cells incubated (2hrs; 37°C) in phosphate-free EMEM with 10% dialyzed FBS were labeled (4 hrs) with [32P]-orthophosphate (250–500µC/ml; Perkin Elmer Life and Analytical Sciences, Wellesley MA) and processed for immunoprecipitation and immunoblotting, as described (Smith et al., 2000; Gober et al., 2003).
Immunocomplex Protein Kinase (PK) Assays
Immunocomplex PK assays were as described (Smith et al., 2000; Kishimoto et al., 2000) using MKK6 (1µg; Upstate Biotechnology, Lake Placid NY) as the TAK1 kinase substrate and 10µCi [32P]-ATP (3000C/mM; Perkin Elmer).
Terminal Deoxynucleotidyltransferase-mediated dUTP Nick End Labeling (TUNEL) and Immunofluorescence
TUNEL was assayed with the in situ cell death detection kit (Roche Applied Science, Indianapolis, IN) according to manufacturer’s instructions. Cells were counted in 5 randomly chosen microscopic fields (at least 250 cells) and results expressed as % TUNEL+ cells ± SEM. For double immunofluorescence, cells were fixed with 4% paraformaldehyde (RT; 1 hr), permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate (4°C; 2 min) and incubated with terminal deoxynucleotidyltranferase and FITC-conjugated dUTPs (RT; 1 hr). They were washed in PBS, blocked with 5% BSA and 5% normal goat serum (RT; 1 hr), and incubated with H11/HspB8 antibody (4°C; 12 hrs) and Texas Red conjugated secondary antibody (RT; 1 hr). Slides were mounted in VectaShield with DAPI (Vector) and visualized with a Nikon E4100 fluorescent microscope utilizing FITC (330–380nM), Texas Red (540–580nM) and UV (for DAPI) (465–495nM) cubes.
Supplementary Material
Dox has no effect on Hsp 27 and Hsp70 expression in melanoma cells. Extracts of H11/HspB8-transfected A2058 cells treated or not with Dox (2µg/ml) were immunoblottted with antibodies to Hsp70, Hsp27 or actin.
The H11/HspB8/TAK1 complex contains the TAK1-associated protein TAB1. Extracts of H11/HspB8 transfected A2058 cells treated or not with Dox (2µg/ml) were immunoprecipitated with TAK1 antibody and the precipitates were immunoblotted with TAB1 followed by H11-181 antibodies. The presence of TAB2 and/or TAB3 proteins in the precipitates is still unknown (Ishitani et al., EMBO J. 22:6277, 2003) is still unknown.
H11/HspB8 and Hsp27 do not co-localize in fractions from sucrose density gradients of Dox-treated melanoma cells. Extracts of H11/HspB8-transfected SKMEL2 cells treated or not with Dox (2µg/ml; 2d) were centrifuged a 10–35% w/v sucrose gradients (100, 000 × g; 16 hrs) and 0.2 ml fractions were collected. Aliquots from each fraction were immunoblotted with H11-181 antibody and the stripped blots were re-probed with antibody to Hsp27. Molecular weight standards are indicated on the top. H11/HspB8 was virtually undetectable in the fractions from the untreated cells. In Dox-treated cells it sedimented primarily at positions corresponding to 300–500kDa. Hsp27 was expressed equally well and primarily sedimented at positions corresponding to 60–160kDa in both untreated and Dox-treated melanoma cells. These sedimentation patterns are distinct from those previously reported in untreated Rhesus monkey heart cells, in which H11/HspB8 and Hsp27 co-localized in a high molecular weight complex (Sun et al J Biol Chem 279: 2394–2402, 2004), but they are consistent with the previous resolution of Hsp27 into distinct molecular weight complexes in different cell types (Kato et al; J Biol Chem 269:11274-78, 1994). The failure of the bulk of H11/HspB8 to associate with Hsp27 in Dox-treated melanoma cells may be related to its apoptotic function in these cells.
Ponceau staining of TAK1 and H11 immunoprecipitates. Extracts of H11/HspB8 transfected A2058 cells treated or not with Dox (2µg/ml; 2 days) were immunoprecipitated with TAK1 or H11-181 antibodies. Exogenous MKK6 (1µg) was added to one of the TAK1 precipitates and precipitates were transferred to nitrocellulose paper and stained with 0.1% Ponceau stain. Only the IgG and the MKK6 bands were detected in the precipitates.
ACKNOWLEDGEMENTS
Supported in part by grant AR42647 from NIAMD, NIH. BL, JML and MDG were supported by grant ES07263 from NIEHS, NIH.
REFERENCES
- Aurelian L. Curr Top Microbiol Immunol. 2005;289:79–11. doi: 10.1007/3-540-27320-4_4. [DOI] [PubMed] [Google Scholar]
- Chabaud S, Lambert H, Sasseville AM, Lavoie H, Guilbault C, Massie B, Landry J, Langelier Y. FEBS Lett. 2003;545:213–218. doi: 10.1016/s0014-5793(03)00547-7. [DOI] [PubMed] [Google Scholar]
- Chae HD, Yun J, Bang YJ, Shin DY. Oncogene. 2004;23:4084–4088. doi: 10.1038/sj.onc.1207482. [DOI] [PubMed] [Google Scholar]
- Chowdary TK, Raman B, Ramakrishna T, Rao CM. Biochem J. 2004;381:379–387. doi: 10.1042/BJ20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Der CJ. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Diao L, Zhang B, Xuan C, Sun S, Yang K, Tang Y, Qiao W, Chen Q, Geng Y, Wang C. Exp Cell Res. 2005;308:196–210. doi: 10.1016/j.yexcr.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE, Nishimura EK, Golub TR, Fisher DE. Cancer Cell. 2004;6:565–576. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Edlund S, Bu S, Schuster N, Aspenstrom P, Heuchel R, Heldin NE, ten Dijke P, Heldin CH, Landstrom M. Mol Biol Cell. 2003;14:529–544. doi: 10.1091/mbc.02-03-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink D, Schlagbauer-Wadl H, Selzer E, Lucas T, Wolff K, Pehamberger H, Eichler H, Jansen B. Melanoma Res. 2001;11:385–393. doi: 10.1097/00008390-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Furuta J, Umebayashi Y, Miyamoto K, Kikuchi K, Otsuka F, Sugimura T, Ushijima T. Cancer Sci. 2004;95:962–968. doi: 10.1111/j.1349-7006.2004.tb03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher WM, Bergin OE, Rafferty M, Kelly ZD, Nolan IM, Fox EJ, Culhane AC, McArdle L, Fraga MF, Hughes L, Currid CA, O'Mahony F, Byrne A, Murphy AA, Moss C, McDonnell S, Stallings RL, Plumb JA, Esteller M, Brown R, Dervan PA, Easty DJ. Carcinogenesis. 2005;26:1856–1867. doi: 10.1093/carcin/bgi152. [DOI] [PubMed] [Google Scholar]
- Gober MD, Smith CC, Ueda K, Toretsky JA, Aurelian L. J Biol Chem. 2003;278:37600–37609. doi: 10.1074/jbc.M303834200. [DOI] [PubMed] [Google Scholar]
- Gober MD, Wales SQ, Aurelian L. Frontiers in Bioscience. 2005;10:2788–2803. doi: 10.2741/1736. [DOI] [PubMed] [Google Scholar]
- Hase M, Depre C, Vatner SF, Sadoshima J. Biochem J. 2005;388:475–483. doi: 10.1042/BJ20041314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Fodstad O, Ronai Z. Expression of ring finger-deleted TRAF2 sensitizes metastatic melanoma cells to apoptosis via up-regulation of p38, TNFalpha and suppression of NF-kappaB activities. Oncogene. 2001;20:2243–2253. doi: 10.1038/sj.onc.1204314. [DOI] [PubMed] [Google Scholar]
- Karpf AR, Lasek AW, Ririe TO, Hanks AN, Grossman D, Jones DA. Mol Pharmacol. 2004;65:18–27. doi: 10.1124/mol.65.1.18. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. J Biol Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- Margolin KA. Cancer. 2004;101:435–438. doi: 10.1002/cncr.20402. [DOI] [PubMed] [Google Scholar]
- Park CS, Kim SI, Lee MS, Youn CY, Kim DJ, Jho EH, Song WK. J Biol Chem. 2004;279:19592–19599. doi: 10.1074/jbc.M314208200. [DOI] [PubMed] [Google Scholar]
- Perez-Stable C. Cancer Lett. 2006;231:49–64. doi: 10.1016/j.canlet.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Reu FJ, Leaman DW, Maitra RR, Bae SI, Cherkassky L, Fox MW, Rempinski DR, Beaulieu N, MacLeod AR, Borden EC. Cancer Res. 2006;66:2785–2793. doi: 10.1158/0008-5472.CAN-05-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Yasumoto K, Takeda K, Takahashi K, Yamamoto H, Shibahara S. Pigment Cell Res. 2003;16:261–265. doi: 10.1034/j.1600-0749.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- Sharma BK, Smith CC, Laing JM, Rucker DA, Byrnett JW, Aurelian L. Dermatology. doi: 10.1159/000095035. in press. [DOI] [PubMed] [Google Scholar]
- Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. J Biol Chem. 2005;280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- Smith CC, Yu YX, Kulka M, Aurelian L. J. Biol. Chem. 2000;275:25690–25699. doi: 10.1074/jbc.M002140200. [DOI] [PubMed] [Google Scholar]
- Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- Sun X, Fontaine JM, Rest JS, Shelden EA, Welsh MJ, Benndorf R. Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J Biol Chem. 2004;279:2394–2402. doi: 10.1074/jbc.M311324200. [DOI] [PubMed] [Google Scholar]
- Tsao H, Sober AJ. Melanoma Treatment Update. Dermatol Clin. 2005;23:323–333. doi: 10.1016/j.det.2004.09.005. [DOI] [PubMed] [Google Scholar]
- van der Velden PA, Zuidervaart W, Hurks MH, Pavey S, Ksander BR, Krijgsman E, Frants RR, Tensen CP, Willemze R, Jager MJ, Gruis NA. Int J Cancer. 2003;106:472–479. doi: 10.1002/ijc.11262. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Roberts AB. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Wang TH, Chan YH, Chen CW, Kung WH, Lee YS, Wang ST, Chang TC, Wang HS. Oncogene. 2006 doi: 10.1038/sj.onc.1209498. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Widlund HR, Horstmann MA, Price ER, Cui J, Lessnick SL, Wu M, He X, Fisher DE. J Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Chen C, Mo YY, Hebbar V, Owuor ED, Tan TH, Kong AN. J Biol Chem. 2000;275:39907–39913. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- Zendman AJ, van Kraats AA, den Hollander AI, Weidle UH, Ruiter DJ, van Muijen GN. Int J Cancer. 2002;97:195–204. doi: 10.1002/ijc.1584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dox has no effect on Hsp 27 and Hsp70 expression in melanoma cells. Extracts of H11/HspB8-transfected A2058 cells treated or not with Dox (2µg/ml) were immunoblottted with antibodies to Hsp70, Hsp27 or actin.
The H11/HspB8/TAK1 complex contains the TAK1-associated protein TAB1. Extracts of H11/HspB8 transfected A2058 cells treated or not with Dox (2µg/ml) were immunoprecipitated with TAK1 antibody and the precipitates were immunoblotted with TAB1 followed by H11-181 antibodies. The presence of TAB2 and/or TAB3 proteins in the precipitates is still unknown (Ishitani et al., EMBO J. 22:6277, 2003) is still unknown.
H11/HspB8 and Hsp27 do not co-localize in fractions from sucrose density gradients of Dox-treated melanoma cells. Extracts of H11/HspB8-transfected SKMEL2 cells treated or not with Dox (2µg/ml; 2d) were centrifuged a 10–35% w/v sucrose gradients (100, 000 × g; 16 hrs) and 0.2 ml fractions were collected. Aliquots from each fraction were immunoblotted with H11-181 antibody and the stripped blots were re-probed with antibody to Hsp27. Molecular weight standards are indicated on the top. H11/HspB8 was virtually undetectable in the fractions from the untreated cells. In Dox-treated cells it sedimented primarily at positions corresponding to 300–500kDa. Hsp27 was expressed equally well and primarily sedimented at positions corresponding to 60–160kDa in both untreated and Dox-treated melanoma cells. These sedimentation patterns are distinct from those previously reported in untreated Rhesus monkey heart cells, in which H11/HspB8 and Hsp27 co-localized in a high molecular weight complex (Sun et al J Biol Chem 279: 2394–2402, 2004), but they are consistent with the previous resolution of Hsp27 into distinct molecular weight complexes in different cell types (Kato et al; J Biol Chem 269:11274-78, 1994). The failure of the bulk of H11/HspB8 to associate with Hsp27 in Dox-treated melanoma cells may be related to its apoptotic function in these cells.
Ponceau staining of TAK1 and H11 immunoprecipitates. Extracts of H11/HspB8 transfected A2058 cells treated or not with Dox (2µg/ml; 2 days) were immunoprecipitated with TAK1 or H11-181 antibodies. Exogenous MKK6 (1µg) was added to one of the TAK1 precipitates and precipitates were transferred to nitrocellulose paper and stained with 0.1% Ponceau stain. Only the IgG and the MKK6 bands were detected in the precipitates.