Abstract
Accommodation and vergence help maintain single and focused visual experience while an object moves in depth. The relative importance of retinal blur and disparity, the primary sensory cues to accommodation and vergence, is largely unknown during development; a period when growth of the eye and head necessitate continual recalibration of egocentric space. Here we measured the developmental importance of retinal disparity in 192 typically developing subjects (1.9 months to 46 years). Subjects viewed high-contrast cartoon targets with naturalistic spatial frequency spectra while their accommodation and vergence responses were measured from both eyes using a PowerRefractor. Accommodative gain was reduced during monocular viewing relative to full binocular viewing, even though the fixating eye generated comparable tracking eye movements in the two conditions. This result was consistent across three forms of monocular occlusion. The accommodative gain was lowest in infants and only reached adult levels by 7 to 10 years of age. As expected, the gain of vergence was also reduced in monocular conditions. When 4- to 6-year-old children read 20/40-sized letters, their monocular accommodative gain reached adult-like levels. In summary, binocular viewing appears necessary under naturalistic viewing conditions to generate full accommodation and vergence responses in typically developing humans.
Keywords: accommodation, blur, binocular, disparity, human infant, monocular, occlusion, vergence, visual development
Introduction
Studies of animal models and human patients have demonstrated the importance of postnatal visual experience in the development of the neural visual system (e.g., Banks, Aslin, & Letson, 1975; Kiorpes, Kiper, O'Keefe, Cavanaugh, & Movshon, 1998; Mitchell & Timney, 1984). While these studies demonstrate that both focused and corresponding retinal images are essential for normal development, the fact that the immature neural visual system controls its own experience has received relatively little attention in this context.
Experiencing clear and single vision in a dynamic environment requires coordinated refocusing (through accommodation) and realignment (through vergence). The accommodative system responds directly to retinal blur and the vergence system responds directly to retinal disparity (Heath, 1956; Judge & Cumming, 1986; Maddox, 1886; Mays & Gamlin, 1995; Morgan, 1968). In addition, neural coupling between these systems (accommodative–vergence and vergence–accommodation) provides a mechanism by which the visual system can coordinate and facilitate oculomotor responses in a dynamic environment (Alpern & Ellen, 1956a, 1956b; Eadie & Carlin, 1995; Judge & Cumming, 1986; Mays & Gamlin, 1995; Morgan, 1968; Schor, 1992).
In adults, accommodative and vergence gains in binocular, naturalistic conditions are typically well matched to the stimulus (Ciuffreda & Kenyon, 1983; Morgan, 1968). Interestingly, an adult-like relationship between accommodation and vergence would be inappropriate during development (Aslin & Jackson, 1979), as infants typically have an increased accommodative demand resulting from their hyperopia (Cook & Glasscock, 1951; Mayer, Hansen, Moore, Kim, & Fulton, 2001), and a reduced vergence demand resulting from their smaller interpupillary distance (IPD) (MacLachlan & Howland, 2002; Pryor, 1969). How does the developing visual system coordinate its direct and coupled oculomotor responses to retinal blur and disparity to achieve appropriate experience during the critical period of neural development?
The basic neural components required for achieving clear and single vision are present in early postnatal development. The gains of accommodation and vergence in binocular naturalistic conditions can be almost adult-like at three months of age (Aslin, 1993; Banks, 1980; Brookman, 1983; Hainline, Riddell, Grose-Fifer, & Abramov, 1992; Haynes, White, & Held, 1965) and the coupled responses have been demonstrated to be present (Aslin & Jackson, 1979; Bobier, Guinta, Kurtz, & Howland, 2000; Turner, Horwood, Houston, & Riddell, 2002). The majority of studies involving measurement of accommodation and vergence during infancy have been performed in full cue, binocular viewing conditions (Aslin, 1993; Banks, 1980; Brookman, 1983; Hainline et al., 1992; Haynes et al., 1965; Horwood & Riddell, 2008). They, therefore, did not systematically assess the relative importance of retinal blur and disparity cues in generating accommodation and vergence responses during development. The two studies that have measured accommodative performance under monocular viewing conditions, in the absence of appropriate retinal disparity cues, have observed different results (Currie & Manny, 1997; Turner et al., 2002). Currie and Manny failed to observe any systematic difference between the size of monocular and binocular accommodative responses in their 1.5- and 3-month-old infants, while Turner and colleagues observed that the accommodative responses of 2−12 month olds and 3 year olds showed larger errors under monocular conditions than under binocular conditions.
Observations that young infants do not cooperate with tasks when one eye is occluded [visual acuity (Atkinson, Braddick, & Pimm-Smith, 1982); accommodation and vergence (Currie & Manny, 1997; Turner et al., 2002)] suggest, at least qualitatively, that retinal disparity may be central to coordinating the near response during early postnatal visual development. To test this hypothesis, we systematically assessed the developmental importance of retinal disparity as a cue for accommodation and vergence in a large group of subjects (n = 192) ranging from 1.9 months to 46 years of age. To our knowledge, this is also the first developmental study to use an additional independent measure to control for the cooperation of the subject and therefore define the importance of cues in conditions where the subject is demonstrated to be tracking the stimulus.
Methods
Subjects
A total of 192 individuals (age range: 1.83 months–46 years), with no significant ocular or medical conditions (by report), were recruited (Table 1). The infants and children were recruited from local birth records and were born within 3 weeks of their due date. Pre-presbyopic near-emmetropic (low amounts of hyperopia or up to 1.0 D of myopia) adults were recruited from the local academic department. They reported no accommodative abnormalities or ocular symptoms. The infants and children were expected to be typically hyperopic (Mayer et al., 2001) and did not wear any refractive correction during the experiment, to mimic their daily natural viewing conditions. Adults wore any habitual correction during the experiment (soft contact lenses only). The parents and adult subjects provided informed consent after the study had been approved by Indiana University's local Institutional Review Board.
Table 1.
The number of subjects recruited, the number of subjects from whom usable data were collected, and the percentage of responses that met the inclusion criteria in usable data sets, for the different age groups.
| Binocular viewing | Monocular viewing | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group (years) | <0.75 | 0.75−1.50 | 1.5−3.0 | 3.0−6.0 | 6.0−12.0 | 12.0−24.0 | 24.0−48.0 | Total | <0.75 | 0.75−1.50 | 1.5−3.0 | 3.0−6.0 | 6.0−12.0 | 12.0−24.0 | 24.0−48.0 | Total |
| Number recruited | 46 | 20 | 23 | 40 | 26 | 15 | 22 | 192 | 35 | 15 | 21 | 42 | 26 | 15 | 22 | 176 |
| Data | 43 | 17 | 23 | 39 | 26 | 14 | 22 | 184 | 31 | 10 | 19 | 41 | 26 | 14 | 22 | 163 |
| collection successful | 93% | 85% | 100% | 98% | 100% | 93% | 100% | 96% | 89% | 67% | 91% | 98% | 100% | 93% | 100% | 93% |
| Responses that met criteria | 37% | 33% | 38% | 47% | 49% | 60% | 77% | 16% | 27% | 38% | 40% | 42% | 64% | 72% | ||
Procedure
The accommodative response and gaze position of each eye, plus vergence, were measured simultaneously using a video-based dynamic (25 Hz) eccentric photorefractor (PowerRefractor (PR), Multi Channel Systems; Choi et al., 2000; Schaeffel, Wilhelm, & Zrenner, 1993). These measurements have been described in detail elsewhere and only a brief description will be provided here (Candy & Bharadwaj, 2007; Roorda, Campbell, & Bobier, 1997; Schaeffel et al., 1993). When using the PR the subject is aligned at 1.0 m from a set of LED light sources immediately beneath a camera aperture. Light from the LEDs passes into the eye and is reflected back from the retina through the pupil. The dioptric focus of the eye in one meridian is derived from the slope of a linear regression fit to the distribution of reflected light across the pupil (Figure 1B). The horizontal and vertical gaze positions are determined using the relative displacement of the first Purkinje image from the center of the image of the pupil (Brodie, 1987; Riddell, Hainline, & Abramov, 1994; Figure 1B), and vergence is calculated as the difference in horizontal gaze position between the two pupillary axes. Changes in vergence and gaze position are recorded by the PR in angular units of prism diopters (pd) and degrees, respectively. These units were converted and scaled into meter angles (MA, m−1), to compensate for changes in interpupillary distance (IPD) with age, using age appropriate IPD estimates derived by MacLachlan and Howland (2002) for infants and children. An IPD of 61 mm was used for all adult subjects.
Figure 1.
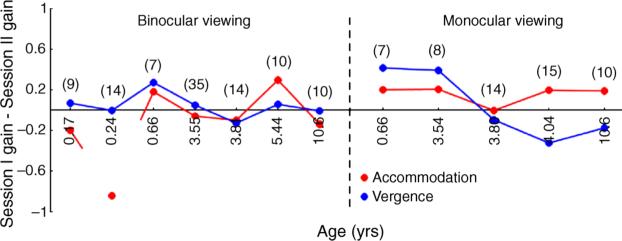
(A) A colored high-contrast cartoon target. (B) A photorefractor image from a 2.2-year-old subject taking part in the binocular condition (top panel) and in the monocular condition (bottom panel). The right eye was occluded in the monocular condition using an infrared Wratten #87 filter mounted on an adult-sized spectacle frame. The white arrowheads in the bottom panel of (B) indicate the medial edges of the IR filter. (C) The experimental equipment. The core components are labeled.
Infants (<12 months of age) viewed a colored high-contrast cartoon target (Figure 1A) while older children viewed a commercially available animated movie played at 30 frames per second. Adults were randomly assigned to view either the cartoon target or the animated movie. Both targets (subtending: 2.3° by 2.3° at 50 cm) contained broadband spatial frequency content and were displayed on an LCD screen (Figures 1A and 1C). The image from the LCD screen was reflected from a beamsplitter to reach the subject. Both the screen and beamsplitter were mounted on a motorized track that moved the screen between 33 and 80 cm from the subject in real space (Figure 1C). The subject was carefully aligned so that the target movement was centered on the midline between their eyes and therefore the unoccluded eye in the monocular viewing condition would be expected to generate an eye movement to track the moving target along the same trajectory as seen in binocular conditions. This eye movement response (represented in the gaze position data in Figure 2) was used to exclude trials in which the subject was apparently not attending to the target.
Figure 2.
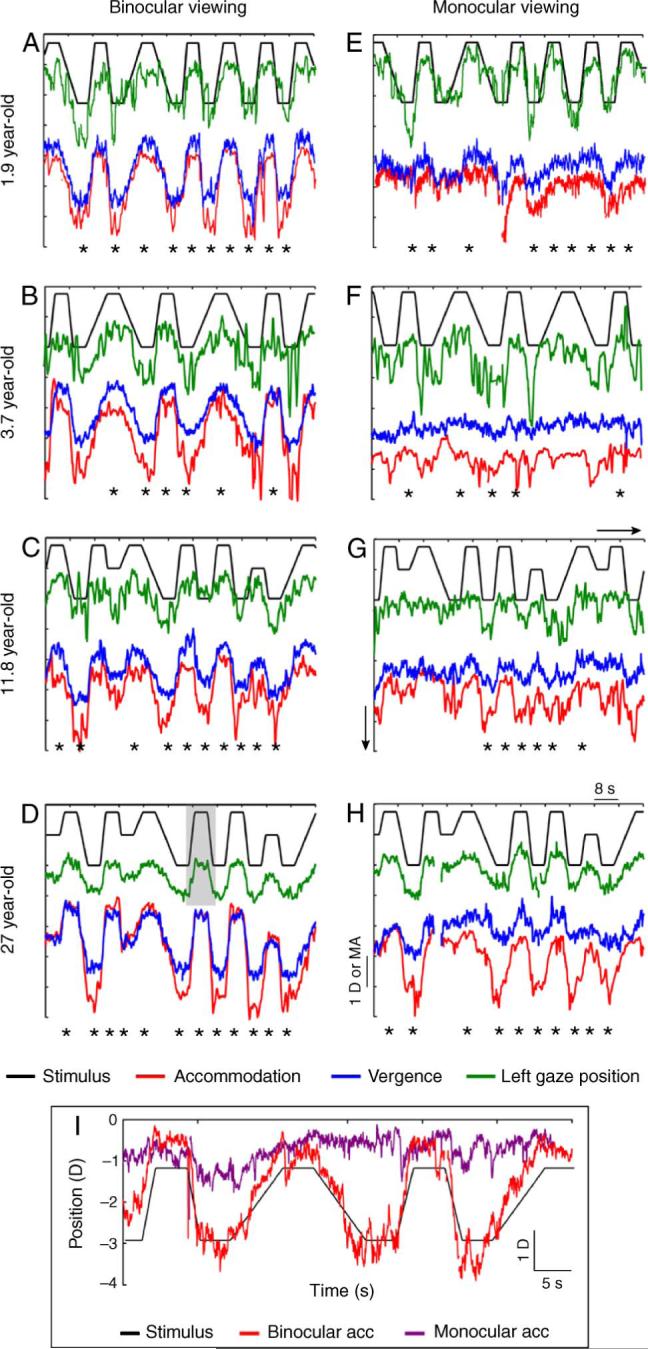
Accommodative, vergence, and left gaze position responses, displaced vertically for clarity, from four representative individuals plotted as a function of time. Stimulus position is plotted at the top in Figures 2A to 2H, followed by the left gaze position, vergence, and finally, accommodation responses. The gray bar (Figure 2D) shows a stimulus epoch used for the gaze position correlation criterion. Asterisks indicate responses that met the inclusion criteria. Accommodation, vergence, and gaze position responses were also measured at 50 cm from some subjects (e.g., Figure 2C), but only responses from 80 and 33 cm were considered for analysis. (I) Comparison of binocular and monocular accommodation on an absolute scale, with no vertical shifting, from a representative subject (3 years old). Negative values in the ordinate indicate an increase in stimulus and response.
To estimate the gain of each subject's accommodation, vergence, and gaze position, the target was moved eight times between 80 and 33 cm at one of three different speeds (0.25 D/s, 0.50 D/s, and 0.75 D/s), with a stable period of 4 s at each viewing distance before the next movement (Figure 2). The change in target position from 80 to 33 cm corresponded to a change in accommodative and vergence demand of 1.75 D or MA. In adults, 1.75 D of defocus or 1.75 MA of disparity can drive accommodation and vergence responses without requiring significant extra-retinal cues (e.g., a sense of target proximity; Fincham, 1951; Schor, Alexander, Cormack, & Stevenson, 1992; Schor, Wood, & Ogawa, 1984; Toates, 1972). Multiple target speeds were used to minimize the potential for predictive responses (Stark, 1968; van der Wildt, Bouman, & van de Kraats, 1974). No systematic difference in performance was noted across different target speeds and therefore the responses from all three target speeds were pooled during analyses.
Subjects viewed the stimulus either binocularly or monocularly while their responses were measured from both eyes. In the monocular viewing condition, the right eye was occluded with an infrared (IR) Kodak Wratten #87 filter (peak spectral transmission of 790 nm). This filter blocks most of the visible wavelengths while allowing the PR to collect data. Thus, all cues to accommodation and vergence (e.g., retinal blur, retinal disparity, proximity) were consistent with each other in the binocular viewing condition while retinal disparity was eliminated in monocular viewing. The IR filter was attached to a handheld adult-sized spectacle frame (75 mm × 75 mm) that entirely covered the subject's right eye and the surrounding region (Figure 1B, bottom panel). This minimized the possibility of the subject peeking around the edges of the IR filter. One hundred and ninety-two subjects took part in the binocular viewing condition and 176 subjects took part in the monocular viewing condition. An experimenter gently supported each infant or young child's chin during all of the conditions, to keep them aligned and minimize head movements. Older children and adults were instructed to hold their heads as stable as possible. No specific instructions were given to the adults regarding the task; they were merely asked to watch the target (Horwood, Turner, Houston, & Riddell, 2001; Stark & Atchison, 1994).
The photorefractor uses a population-average defocus calibration based on data collected from adults (Choi et al., 2000; Schaeffel et al., 1993). Similarly, a population-average Hirschberg ratio is used to calculate gaze position (Schaeffel et al., 1993). Individual defocus and eye position calibrations were not performed on every subject in the current study because the main analyses were conducted on within-subject data. Individual gain calibrations were performed on 23 subjects (age range: 5.2 months to 11.9 years), however, to confirm the absolute gains of accommodation and vergence in a subset of individuals. The defocus calibration procedure has been described in detail by Choi et al. (2000). Briefly, the subject fixated a target placed at 50 cm while the right eye was occluded using the IR filter. An estimate of the slope of the defocus calibration function was determined by placing trial lenses (+1 D to +4 D in steps of 1 D) in front of the occluded eye to induce anisometropia. Similarly, an estimate of the slope of the gaze position function was determined by optically shifting the Purkinje image of the occluded eye using base-out prisms placed in front of the filter (4 PD to 16 PD in steps of 4 PD). The measured changes in focus and gaze position were plotted as a function of lens power and prism power, respectively. The slopes of the functions were determined using linear regression and the individuals’ raw data from the experimental conditions were corrected for their calibration slope factor. Cycloplegic retinoscopy was also performed on a subset of 34 subjects (age range: 6.2 months to 16.4 years) to determine whether responses were dependent on their spherical equivalent refraction and therefore total accommodative demand. Cycloplegia was achieved 20−30 min after instilling 1 drop of 1% Cyclopentolate.
Control experiments
Control experiment I: ‘Monocular patch viewing’
The LED light source on the PR camera located at 1.0 m was somewhat visible when the right eye was occluded using the IR filter. To assess whether the image of the LEDs competed or combined with the image in the left eye to influence monocular accommodative performance (Flitcroft, Judge, & Morley, 1992; Flitcroft & Morley, 1997; Koh & Charman, 1998), the right eye was fully occluded with an opaque occluder while accommodation and gaze position were measured only from the left eye. Vergence responses could not be calculated in the absence of data from the right eye. Thirteen subjects (age range: 1.9 years to 10.9 years) who took part in the main binocular and monocular conditions also took part in this condition.
Control experiment II: ‘Monocular +20 D viewing’
The IR filter and the opaque patch both reduced the overall luminance of the retinal image in the right eye, creating a poor match to the left eye. The right eye of our subjects was occluded with a +20 D lens in control experiment II to determine whether the luminance reduction had affected behavior in the other conditions. The +20 D lens blurred the target dramatically, effectively creating a uniform luminance stimulus in the right eye. Inter-ocular differences in image contrast and image size of much smaller magnitudes have been shown to disrupt binocular vision in adults (Erickson & Schor, 1990; Ogle, Mussey, & Prangen, 1949; Schor & Heckmann, 1989). Accommodation and gaze position were measured from the left eye in this condition while data could not be collected from the right eye due to the presence of the high-powered lens. Thirteen subjects (age range: 3.4 months to 11.9 years) who took part in the main binocular and monocular conditions also took part in this condition.
Control experiment III: ‘Plano lens viewing’
It is possible that the presence of an object in front of one eye might have disturbed naturalistic behavior in the different monocular viewing conditions, by distracting the subject or inducing a proximal response. To test this hypothesis, a lens with no optical power was held in front of the subject's right eye in control experiment III. Any difference between the results of the main binocular experiment and this control experiment would be attributed to the presence of an object in front of one eye. Accommodation and left and right eye gaze positions could be measured from both eyes in this condition. Eighteen subjects (age range: 2.9 months to 11.9 years) who took part in the main binocular and monocular conditions also took part in this condition.
Control experiment IV: ‘Text reading’
In the main binocular and monocular conditions, our subjects did not engage in a task that required an explicit use of the high spatial frequency information present in the broadband cartoon target. To determine whether accommodative and vergence gains change for a task that is biased to higher spatial frequencies (Chung, Legge, & Tjan, 2002; Majaj, Pelli, Kurshan, & Palomares, 2002), we asked subjects to read aloud 20/40-sized letters and numbers displayed on an LCD screen that moved between 80 and 33 cm. Subjects performed the reading task under both binocular and monocular viewing conditions while their accommodation and gaze positions were measured from both eyes. Under monocular conditions, the right eye of the subject was occluded with the IR filter. The letters and numbers were changed during each target movement, to the correct angular subtense for the subsequent viewing distance, to avoid learning effects. Thirteen subjects (age range: 4.3 years to 6.5 years) who took part in the main binocular and monocular conditions also took part in this condition.
Data analysis
Data analyses were performed using Matlab®, Microsoft Excel®, and Igor Pro®. The raw stimulus position and response data (accommodation, vergence, and gaze position) were first smoothed using a 200-ms averaging window. The stimulus and response data were smoothed using the same window to maintain the temporal relationship between them. The stimulus profile was then divided into epochs, each containing a 4-s stable stimulus period plus the change in stimulus before and after this period (vertical gray rectangle in Figure 2D). The responses in each of the epochs were then examined and those that met the following criteria were included in the analysis.
The accommodation data were within the linear operating range of the instrument (+4.0 to −6.0 D) and the pupil diameters were between 3 and 8 mm (required for the instrument to collect data; Choi et al., 2000; Schaeffel et al., 1993).
The data were collected from a gaze eccentricity of less than 15° from the pupillary axis. The optical quality of the adult eye changes with gaze eccentricity (Jennings & Charman, 1981; Navarro, Artal, & Williams, 1993) and therefore the effects of peripheral refraction during intermittent peripheral fixations would not be distinguished from changes in accommodation. The threshold criterion was based on adult data as there are currently no comparable data from infants. The infants and adults typically maintained stable gaze on the target, and so very little data were excluded as a result of this criterion. As will be seen below, eye movement responses were similar in binocular and monocular conditions (Figure 3C) and so any effect of peripheral refraction would be similar across conditions.
The subject generated an eye movement to track the stimulus, and therefore, the accommodation and vergence responses represented a valid attempt to follow the target along the midline between the eyes. This criterion was applied to the data from the left eye in the binocular and monocular conditions (all monocular measurements were done with the left eye viewing) irrespective of what the right eye was doing. The criterion is reasonable because both adults (Lisberger, Morris, & Tychsen, 1987) and infants (Shea & Aslin, 1990) are capable of systematically pursuing the movement of a target. All response epochs with a correlation of 0.7 or greater between left eye position and stimulus position were automatically included in the analyses (responses indicated with an asterisk in Figure 2). Response epochs with correlations between 0.6 and 0.7 were visually inspected and included in the analyses only if the left gaze position appeared to systematically track the movement of the stimulus. The approach based on correlation was developed using a 2-alternative forced choice (‘accept or reject’) visual inspection assessment of 310 epochs, made by three independent observers (the two authors and one inexperienced observer). The correlation coefficient could remain above the 0.7 threshold value for latencies of up to 2 s between the stimulus and eye movement.
Figure 3.
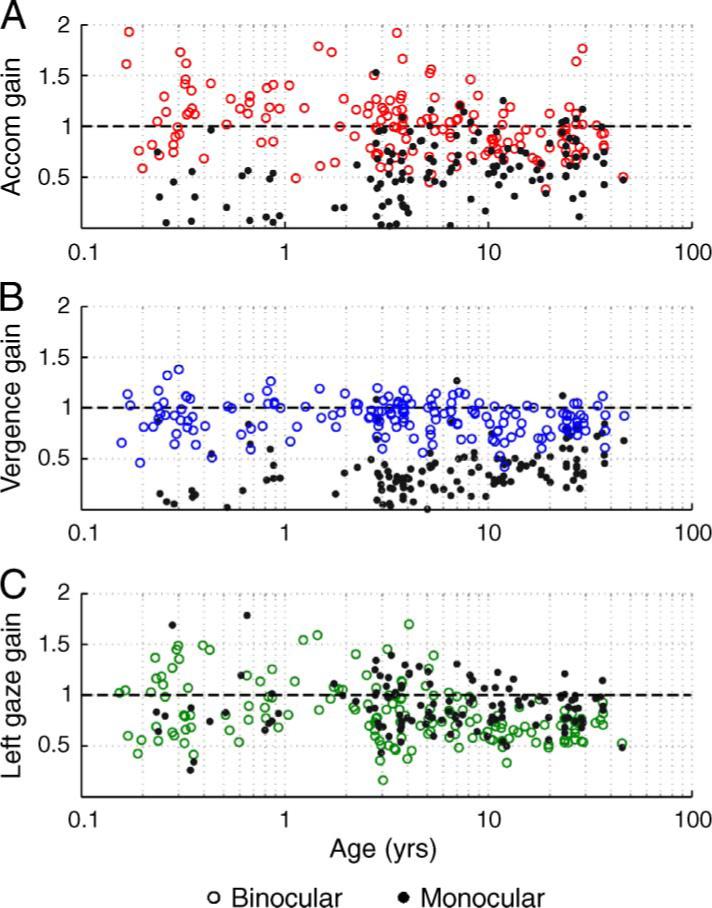
The gains of binocular (open circles) and monocular (filled circles) viewing accommodation (Figure 3A), vergence (Figure 3B), and left gaze position (Figure 3C) plotted as a function of age. The black dashed line in each panel denotes unity gain.
The final accommodation, vergence, and left eye gaze position responses were obtained by averaging 2.0 s (50 data points) of the stable portion of each usable epoch. Gains were then calculated as the ratio of the difference in response to the difference in stimulus between the two viewing distances. A gain of unity indicates a perfect response to the change in stimulus while a gain of zero indicates no response to the stimulus. If a subject provided multiple responses in each condition, the responses were averaged to obtain the overall mean accommodation, vergence, and left eye gaze position gains. The left and right eyes’ accommodative gains were well correlated with each other under both binocular (r = 0.86, p < 0.001) and monocular conditions (r = 0.89, p < 0.001). Hence, only the left eye's accommodative gains were used for the analyses. The subjects were divided into seven different age groups to determine the trend in data collection success rate (Table 1). However, as these age bins were somewhat arbitrary, age was treated as a continuous variable for the rest of the analyses.
Results
Data were successfully collected from 184 subjects in the binocular condition and from 163 subjects in the monocular condition (Table 1). Of the three inclusion criteria used, responses were typically rejected because they failed to meet the third inclusion criterion (correlation between gaze and stimulus position).
Both the binocular and monocular success rates increased systematically with age until almost complete cooperation was reached by 6 years (Table 1). Between 2 months and 6 years, the number of subjects who met the inclusion criteria and the number of usable responses obtained from each subject were higher under binocular conditions than under monocular conditions (Movie 1). For older children and adults, no notable difference was observed between the two viewing conditions. These trends, summarized in a 2-factor ANOVA (age × viewing condition), revealed a statistically significant effect of age (F (300, 6) = 40.7, p < 0.001) and viewing condition (F(300, 1) = 8.3, p < 0.004) on the percentage of responses that met the inclusion criteria. A post-hoc Games-Howell test (with no assumption about equal variance in each age group) showed that the number of usable responses obtained from age groups 1 to 3 were statistically significantly different from those of age groups 4 to 7 and the number of usable responses obtained from age groups 4 and 5 were statistically significantly different from those of age groups 6 and 7. The interaction between the two factors was also marginally statistically significant (F(300, 6) = 2.3, p < 0.03). Overall, these results suggest that the number of responses that met the inclusion criteria increased with age and that the increasing trend was somewhat different in the two viewing conditions.
Movie 1.
Example of a 5-month-old infant taking part in our experiment under binocular (first 1/3rd of the movie) and monocular (second 1/3rd of the movie) viewing conditions. The infant appears calm and cooperative under the binocular condition but becomes fussy and uncooperative when the IR filter is placed over the right eye (monocular condition). The last 1/3rd of the movie shows the infant taking part in the experiment with a ‘plano lens’ in front of the right eye (control experiment III). The infant remains calm and cooperative indicating that the presence of an object in front of the eye did not make the infants uncooperative.
The correlation between the stimulus position and the left eye gaze position for the usable responses ranged from 0.6 to 0.85. There was no statistically significant difference in the correlation coefficients obtained in the two viewing conditions (p = 0.2, t = 1.29, df = 115). This result indicates that although more usable responses were generated in the binocular conditions, the eye movement tracking during usable responses did not differ significantly between the binocular and monocular data.
Visual inspection of raw accommodation, vergence, and left gaze position data revealed two additional characteristics (Figure 2). First, unlike the gain of left gaze position, the gains of accommodation and vergence were smaller under monocular conditions than under binocular conditions. The vergence gains were attenuated more than the accommodative gains. Second, binocular and monocular accommodative responses tended to be similar at the farthest viewing distance (80 cm; 1.25 D) and differed at the near viewing distance (33 cm; 3.0 D; Figure 2I). Across subjects who provided usable data in both binocular and monocular viewing conditions (n = 117), the difference in accommodative states between the two viewing conditions were statistically insignificant at the 1.25 D viewing distance (t = 0.86, df = 115, p = 0.39) but statistically significant at the 3.0 D viewing distance (t = 4.05, df = 115, p < 0.001). This result implies that larger accommodative gain under binocular conditions was achieved by generating larger increases in accommodation from the farther viewing distance.
The mean gains of accommodation and vergence were close to unity in binocular viewing while they were less than unity under monocular viewing (Figures 3A and 3B). The binocular accommodative and vergence gains did not show any systematic age-related trend, but the monocular accommodation and vergence gains showed a gradual increase with age (Figures 3A and 3B). These trends occurred despite the fact that the left gaze position gain was equivalent in the two viewing conditions and did not show any systematic age-related trend (Figure 3C). This suggests that the subjects tracked the position of the target equally under the two viewing conditions, and that on average their accommodation and vergence gains were well matched to the stimulus demand in binocular conditions.
Five different summary gain ratios were computed for subjects who provided usable monocular and binocular measurements (n = 117; Figures 4A–4E). These were the ratios of monocular to binocular accommodative gain (Figure 4A), vergence gain (Figure 4B), and left gaze position gain (Figure 4C), plus the binocular vergence to binocular accommodation (Figure 4D) and monocular vergence to monocular accommodation gains (Figure 4E). As predicted from Figure 3, the ratios of monocular to binocular accommodation and vergence both increased with age (Figures 4A and 4B). They increased monotonically with age until reaching a plateau at an adult-like value. The maturation was described using a bilinear fit to estimate the age at which responses became adult-like (Equation 1):
| (1) |
where G = ratio of monocular to binocular gain, S = rate of change in gain ratio with age during the developmental phase, A = adult gain ratio, I = age at which the adult ratio was reached.
Figure 4.
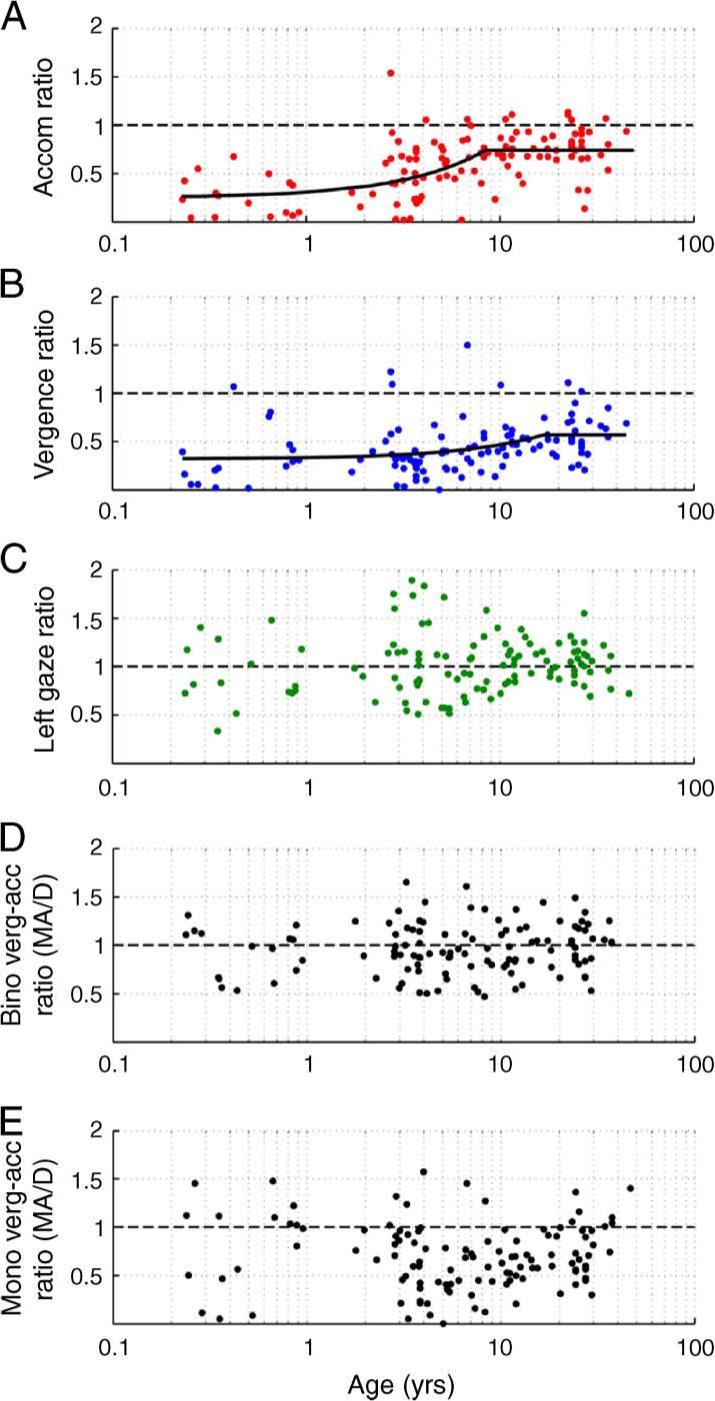
The gain ratios of monocular to binocular viewing accommodation (Figure 4A), vergence (Figure 4B), and left gaze position (Figure 4C), plus binocular vergence to binocular accommodation (Figure 4D) and monocular vergence to monocular accommodation (Figure 4E) as a function of age. The functions were fit to the data using Equation 1, as described in the text. Only functions that showed a significant age-related trend are shown in the figure (Figures 4A and 4B).
The early change in gain ratio with age was described using the slope of a linear regression, S, the stable adult-like ratio was described using a constant, A, and the age at which the gain ratio started to decrease from the adult-like level was estimated as the intersection, I. The three free parameters were estimated using a least-square algorithm and the standard deviation of each estimate was calculated using Igor Pro®. The standard deviations describe the range over which the coefficients can vary when the data are fit multiple times with different levels of artificially introduced Gaussian noise.
The gain ratio for accommodation reached an adult-like level of 0.75 at 8.2 years of age with a standard deviation of ±1.41 years and the gain ratio for vergence reached an adult-like level of 0.57 at 17.5 years with a standard deviation of ±6.41 years (Figures 4A and 4B, Table 2). Equation 1 was also used to describe the age-related trend in the remaining three gain ratios although none showed any systematic change with age. The gain ratios for left gaze position and binocular accommodation to vergence remained close to unity with age (Figure 4C, Table 2). The average ratio of vergence to accommodation in monocular viewing remained at a value of approximately 0.7−0.8 MA/D with age (Figure 4E, Table 2). This indicates that the change in vergence generated in response to blur (and proximity) in monocular conditions was less than the corresponding change in accommodation. Fitting the data with a different equation (e.g., higher order polynomial or sigmoid functions) did not yield significantly higher R2 values.
Table 2.
The coefficients (S, I, and A), R2, and p-values of the functions fit to the gain ratio data using Equation 1 (Figure 4). The standard deviation of each coefficient for functions that reached statistical significance is also given.
| S | I | A | R2 | p-value | |
|---|---|---|---|---|---|
| Monocular to binocular accommodation ratio | 0.058 ± 0.01 | 8.23 ± 1.41 | 0.75 ± 0.04 | 0.31 | <0.001 |
| Monocular to binocular vergence ratio | 0.014 ± 0.006 | 17.53 ± 6.41 | 0.57 ± 0.05 | 0.12 | <0.01 |
| Monocular to binocular gaze position ratio | 0.047 | 2.80 | 1.09 | 0.006 | 0.20 |
| Binocular convergence-accommodation ratio | 0.008 | 19.13 | 1.06 | 0.016 | 0.08 |
| Monocular convergence-accommodation ratio | 0.004 | 55.84 | 0.89 | 0.017 | 0.08 |
Individually calibrated responses
Individual calibrations of defocus and eye position were collected from 23 subjects (age range: 5.2 months to 11.9 years) who completed both the binocular and monocular viewing conditions. Across this age range, the slopes of the defocus and gaze position calibration functions were poorly correlated with age (defocus calibration: r = −0.15, p = 0.49; gaze position calibration: r = −0.05, p = 0.82; Figure 5A). For a 1.75 D accommodative stimulus, the mean binocular accommodative response after correction for the individual calibration was 1.80 ± 0.08 D (Figure 5B) and the mean monocular accommodative response was 0.96 ± 0.13 D (Figure 5C). Similarly, for a 1.75 MA vergence stimulus, the mean binocular viewing vergence response was 1.71 ± 0.13 MA (Figure 5B) and the mean monocular viewing vergence response was 0.65 ± 0.07 MA (Figure 5C). The difference between the mean binocular and monocular viewing accommodation and vergence responses was statistically significant (t = 4.8 for accommodation, t = 8.4 for vergence, df = 22, p < 0.001). These results illustrate that the gains of accommodation and vergence under binocular conditions are more accurate than in the presence of occlusion.
Figure 5.
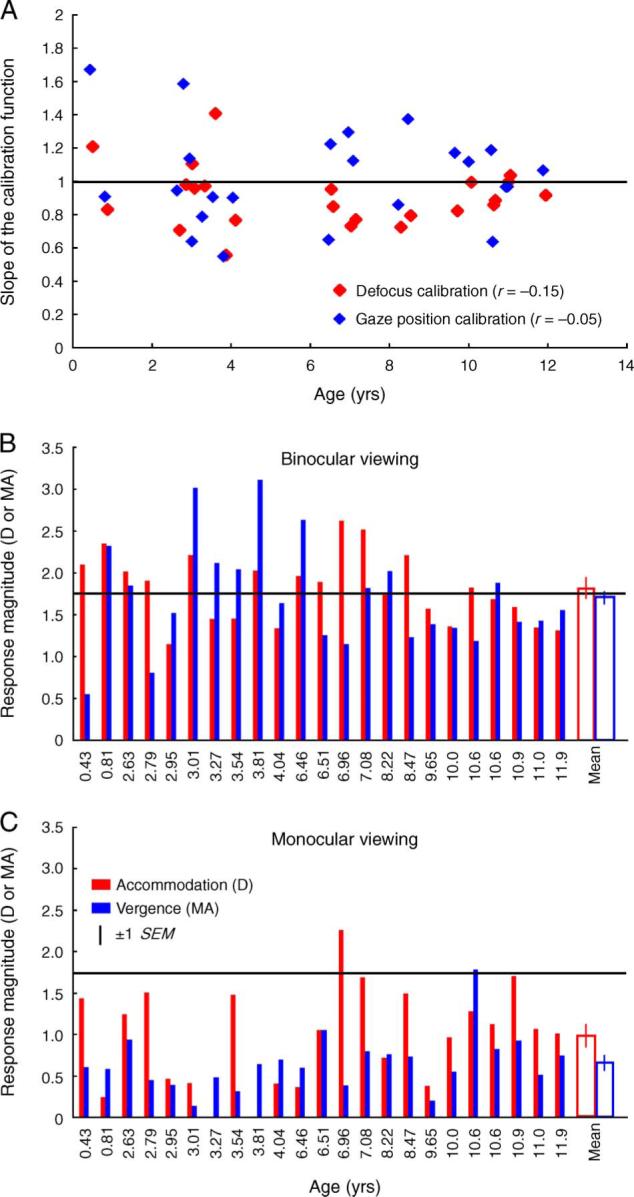
(Figure 5A) Slope of the defocus and gaze position calibration functions plotted as a function of age of the subjects. (Figures 5B and 5C) Individually calibrated accommodation and vergence responses to the 1.75 D and 1.75 MA stimulus (black horizontal line) under binocular (Figure 5B) and monocular (Figure 5C) viewing conditions. Each pair of bars represents data from one subject. Individual subjects are arranged by their age in an ascending order.
Cycloplegic refractive error
Infants are typically born hyperopic, with an average spherical equivalent refractive error (SER) of approximately 2.0 D and a standard deviation of ±2.0 D (Mayer et al., 2001). The accommodative demand of 1.75 D presented here therefore needs to be considered in relation to the refractive error of the individual (for instance, the stimulus would move between demands of 1.25 and 3 D in an individual with no refractive error and between 5.25 and 7 D in another with 4 D of hyperopia). The gains of accommodation and vergence were compared with the cycloplegic spherical equivalent refractive error of the left eye of 34 subjects (age range: 6.2 months to 16.4 years). Only individuals from whom both binocular and monocular data were available were included in the analysis (Figure 6). As expected, the cycloplegic SER was hyperopic in infants and then gradually reduced until near-emmetropia in the pre-school years (Figure 6A). The SER was poorly correlated with both binocular (r = 0.17, p = 0.34) and monocular accommodative gain (r = −0.20, p = 0.26) and with binocular (r = −0.20, p = 0.26) and monocular vergence gain (r = −0.10, p = 0.57; Figure 6B and 6C). These results indicate that binocular and monocular accommodative and vergence performance was not influenced dramatically by the subject's cycloplegic refractive error within the range studied.
Figure 6.
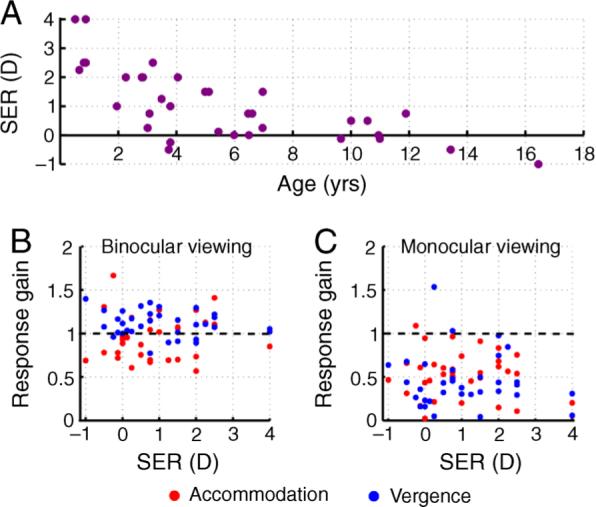
(Figure 6A) Cycloplegic spherical equivalent refraction (SER) plotted as a function of age. (Figures 6B and 6C) The gain of accommodation and vergence plotted as a function of the SER under binocular and monocular viewing conditions.
Repeatability
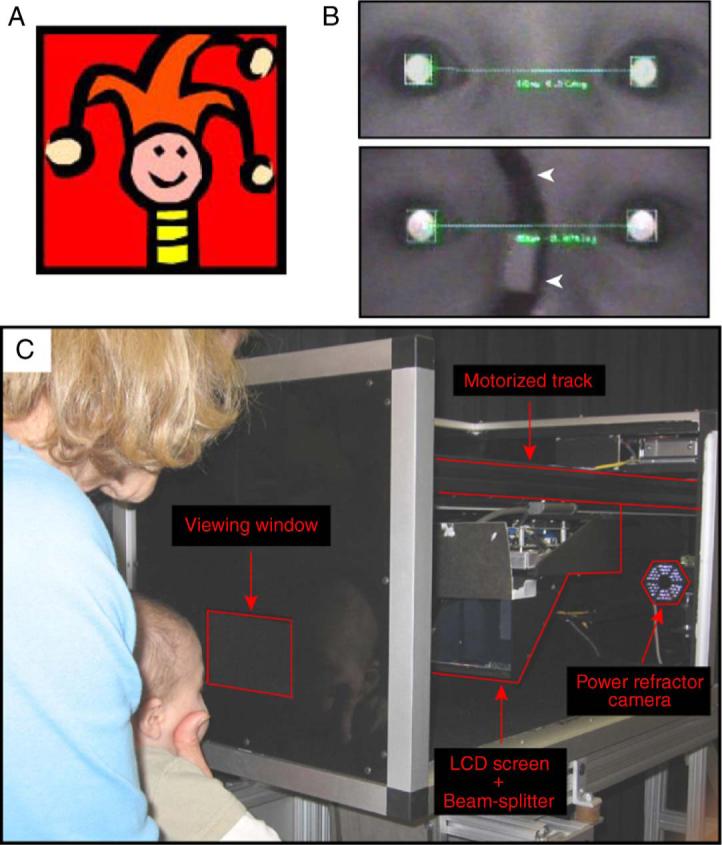
The repeatability of accommodative and vergence gains across different sessions was assessed under binocular (n = 7; age range: 2.1 months to 10.6 years) and monocular conditions (n = 5; age range: 7.9 months to 10.6 years; Figure 7). Both binocular and monocular repeatability data were available for two subjects (age: 7.9 months and 10.6 years). Qualitatively, three important trends were noted. First, binocular accommodative and vergence performance appeared relatively repeatable across the two sessions, with the difference in gains being less than 0.3 (except the 0.24-year-old subject's accommodation). Second, the inter-session variability of accommodative gain was similar under binocular and monocular conditions, but the variability of vergence gain increased under monocular conditions. This is presumably due to the absence of the retinal disparity cue and feedback in monocular viewing conditions. Third, the inter-session variability of accommodation and vergence gains did not appear to change systematically with the age.
Figure 7.
Inter-session differences in the gain of accommodation and vergence for ten subjects. Each pair of red and blue circles represents data from one subject. The number of days between the two sessions is noted in parentheses for each subject.
Control experiments
The monocular viewing gains of accommodation obtained in control experiment I (opaque patch occlusion) did not differ significantly from the monocular viewing gains of accommodation obtained with the IR filter (t = 0.26, df = 12, p = 0.72) (Figure 8). However, these gains differed significantly from the within-subject binocular viewing gains of accommodation (t = 3.1, df = 12, p < 0.01; Figure 8). The left eye gaze position gain did not differ significantly between binocular, monocular IR filter, and monocular patch viewing conditions. These results indicated that any effect of the LEDs could not account for the reduced accommodative gains seen under monocular viewing conditions with the IR filter.
Figure 8.
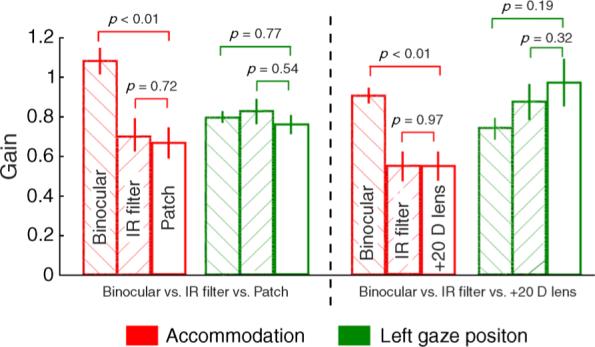
The mean gains of accommodation and left gaze position obtained under binocular, IR filter, and patch viewing conditions (control experiment I) and under binocular, IR filter, and +20 D lens viewing conditions (control experiment II). The error bars indicate ±1-SEM.
The results of control experiment II replicated those obtained in control experiment I. The monocular viewing gains of accommodation obtained with the +20 D lens did not differ significantly from the monocular viewing gains of accommodation obtained with the IR filter (t = 0.18, df = 12, p = 0.97; Figure 8). However, these gains differed significantly from the within-subject binocular viewing gains of accommodation (t = 3.1, df = 12, p < 0.01; Figure 8). The left eye gaze position gain did not differ significantly between the binocular, monocular IR filter, and monocular +20 D viewing conditions. Because different subjects took part in the first and second control experiments, the data from those two conditions could not be directly compared with each other. Qualitatively, however, the accommodation and gaze position gains were similar in the different occlusion conditions, indicating that the different forms of monocular occlusion (IR filter, opaque patch, and +20 D lens) had the same effect on the accommodative performance of the infants and children.
The presence of the plano lens in front of the subject's right eye (control experiment III) had minimal influence on the gain of accommodation, vergence, or left eye gaze position (Figure 9). There was no significant difference in accommodative gain (t = 0.98, df = 17, p = 0.36), vergence gain (t = 1.16, df = 17, p = 0.27), or left gaze position gain (t = 0.26, df = 17, p = 0.89) between the binocular and plano lens conditions. The gains of accommodation and vergence were however significantly different when the plano lens condition was compared with the monocular IR condition (t = 2.22 for accommodation, t = 4.38 for vergence, df = 17, p < 0.01). These results indicate that the presence of an object in front of the eye did not reduce the accommodative and vergence gain of infants and children. Instead, the reduction in response gain in the different monocular conditions appears attributable to the dissociation of the eyes.
Figure 9.
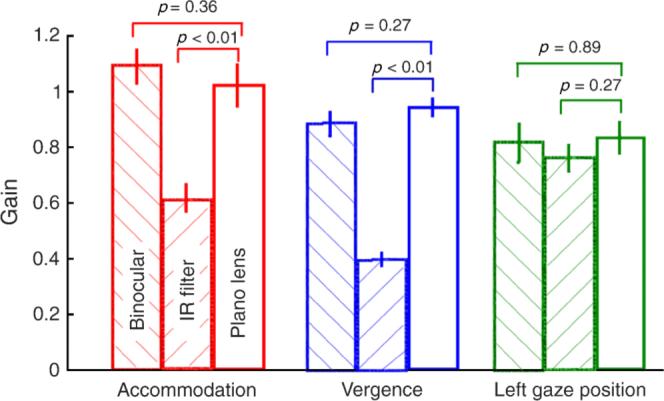
The mean accommodative, vergence, and left gaze position gains obtained under binocular, IR filter, and plano lens viewing conditions (control experiment III). The error bars indicate ±1-SEM.
In control experiment IV, all subjects correctly identified the 20/40 letter targets under both binocular and monocular viewing conditions. The binocular gains of accommodation and vergence when reading the letters and when watching a movie were close to unity and were insignificantly different from each other (t = 0.69, df = 13, p = 0.25 for accommodation and t = 0.75, df = 13, p = 0.56 for vergence; Figure 10). However, the monocular gains of accommodation and vergence were significantly higher for the letter-reading task (almost adult-like) when compared with watching the movie (t = 2.65, df = 13, p < 0.01 for accommodation and t = 2.16, df = 13, p = 0.03 for vergence; Figure 10). The increase in gain of monocular viewing vergence when reading letters is expected with the increase in the gain of monocular accommodation. The monocular viewing convergence to accommodation ratios obtained when watching the movie and when reading the text were statistically insignificantly different from each other (t = 1.08, df = 13, p = 0.30). The increased gain of monocular accommodation during the letter identification task suggests that children are capable of generating larger accommodative responses under visually demanding situations but they do not appear to do so under conditions requiring more naturalistic viewing.
Figure 10.
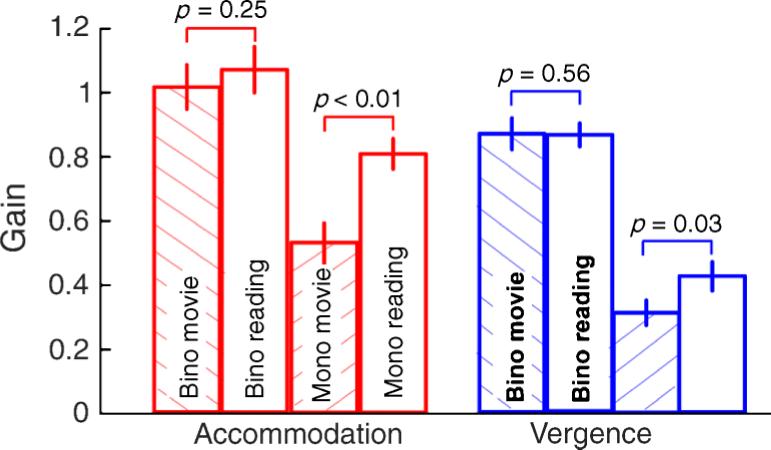
The mean gains of accommodation and vergence obtained when subjects watched the movie and read the letter targets under binocular and monocular viewing conditions (control experiment IV). The error bars indicate ±1-SEM.
Discussion
For all ages tested (2 months to 46 years), accommodative and vergence responses had larger gains and were more accurate in binocular conditions than in monocular conditions (Figures 2-4, and 6). The ratios of monocular to binocular gains of accommodation and vergence were lowest in infants and they increased with age until adult-like ratios were achieved at around 8 years for accommodation and 17 years for vergence (Figures 4A and 4B, Table 2). The reduced monocular viewing vergence gain is consistent with previous reports of accommodative-vergence coupling performance in adults (Fry, 1983; Morgan, 1968) and infants (Turner et al., 2002). The gradual increase in monocular vergence gain with age is unlikely to result from age-related changes in angle lambda (the angle formed between the pupillary axis and the line of sight). Angle lambda is larger in neonates (8.5°) and it quickly decreases to reach adult-like levels (4.7°) by 1 year of age (Riddell et al., 1994; Slater & Findlay, 1972). This angle would introduce a constant offset in gaze position that would not affect the change in position between two target distances, and its effect would also be equivalent in binocular and monocular viewing.
The reduced monocular viewing accommodative gain during infancy is in accordance with the results of Turner et al. (2002) but not with the results of Currie and Manny (1997). Turner et al. also observed that monocular viewing accommodative responses of infants less than 1 year of age had smaller gains than in binocular viewing. The difference between binocular and monocular responses was exaggerated at closer viewing distances (see Figure 2I above and their Figure 3). Currie and Manny, in contrast, failed to observe a systematic difference between monocular and binocular viewing accommodative response amplitudes for their three 1.5 month olds and seven 3 month olds. In addition to supporting the results of Turner et al., the current study systematically evaluated the developmental importance of sensory cues to accommodation and vergence in a wide range of subjects (1.9 months to 46 years) and determined that the reduced responses were consistent even when the subjects were demonstrated to be tracking the target. Overall, the consistently reduced monocular accommodative gain during infancy and early childhood suggests that the typically developing visual system is less efficient at generating an accommodative response in the absence of retinal disparity cues. Before considering this interpretation further, we considered three other explanations why the gain might have been reduced under monocular conditions.
1. Subject cooperation and data quality: Our youngest subjects regularly became uncooperative under monocular occlusion (Table 1, Figure 2, Movie 1) and similar behavior has been noted in previous studies, where the occluder was either placed directly in front of one eye (Atkinson et al., 1982; Currie & Manny, 1997) or in a remote location (Turner et al., 2002). Although the number of usable responses decreased under monocular conditions here (Table 1), the left eye gaze position data were comparable with those recorded in binocular conditions and therefore the data included in the analysis were equivalent in apparent cooperation. The data were also consistent across different forms of monocular occlusion (Figure 8), and the use of a plano lens did not reduce the accommodative gain from the value in binocular conditions (Figure 9).
Earlier studies of the development of accommodation were based on approximately 5 data samples from each infant (Currie & Manny, 1997; Turner et al., 2002). The current measurements were based on averaging a number of responses, each lasting 2 s (50 samples). Thus, the current mean accommodative gain estimates, gathered in a within-subjects design, are unlikely to be influenced by variability in steady-state accommodation (Candy & Bharadwaj, 2007). Taken together, these results suggest that the reduced monocular accommodative gains were not the result of poor cooperation or significant noise in the data.
2. Spatial and temporal characteristics of the accommodative target: The slope of the radially averaged spatial amplitude spectrum of the cartoon image used in this experiment was −1.32. Similarly, the mean and standard deviation of the slopes of the amplitude spectra of one hundred randomly sampled movie frames were −1.47 ± 0.03. These slopes are within the range described for ‘natural images’ (−0.8 to −1.5) (Tolhurst, Tadmor, & Chao, 1992) that support accurate accommodative responses in adults (Ciuffreda, 1991; Ciuffreda, Rosenfield, Rosen, Azimi, & Ong, 1990). Although the cartoon and movie have naturalistic amplitude spectra, the watching task does not require explicit use of high spatial frequency information. When children took part in a 20/40-letter identification task (control experiment IV), their monocular viewing accommodative gains improved significantly (Figure 10). This indicates that even though the developing visual system does not appear to use monocular cues effectively to accommodate under naturalistic viewing conditions, it is capable of generating accommodative responses that support detailed near vision in demanding circumstances, at least by 4 years of age.
The slopes of the spatial amplitude spectra also changed dynamically across movie frames (30 fps). Chauhan and Charman (1996) and Chauhan et al. (1992), however, observed that monocular accommodative responses to a static target were not significantly affected by temporal modulations in contrast. Taken together, the spatial and temporal characteristics of the cartoon and movie were unlikely to have an atypical influence on the gain of monocular accommodation.
3. Depth-of-focus (DOF): A closed-loop blur-driven accommodative response uses feedback to minimize retinal image blur until further reduction in blur cannot be detected (Carroll, 1982; Eadie & Carlin, 1995; Schor, 1992), at a point defined by the depth of focus of the accommodative system. Under this scheme, an increase in the DOF could result in a reduction in the accommodative response amplitude and hence a reduction in the gain. The developing visual system experiences both optical aberrations (Wang & Candy, 2005) and neural immaturities (Banks & Crowell, 1993) and so its DOF is predicted to be larger than that of adults (Green, Powers, & Banks, 1980). An adult-like spatial resolution of approximately 30 c/d is reached behaviorally by 36 months of age (Teller, McDonald, Preston, Sebris, & Dobson, 1986; Teller & Movshon, 1986) and an adult-like contrast sensitivity function is achieved by approximately 4.5 years of age (Banks & Salapatek, 1978; Bradley & Freeman, 1982; Gwiazda, Bauer, Thorn, & Held, 1997). These growth functions imply that, for comparable pupil sizes, a DOF based on detection of blur through contrast reduction should reach adult levels at least by 3 to 4.5 years (Green et al., 1980). Yet, the gain of the monocular accommodation remained attenuated in at least some subjects until the early teenage years (Figures 3 and 4). This suggests that an immature DOF is unlikely to be the limiting factor in the monocular accommodative performance observed here.
In summary, none of these alternative hypotheses appear to explain the immature monocular accommodative gains obtained under naturalistic viewing conditions, and therefore, it appears that binocular vision might be required by typically developing infants and children to generate both appropriate accommodation and vergence responses. In principle, the reduction in gain of accommodation under monocular conditions could result from the loss of any facet of binocular vision (retinal disparity, binocular summation, etc.). However, retinal disparity has been shown to drive accommodation (Cumming & Judge, 1986; Judge & Cumming, 1986; Mays & Gamlin, 1995) and there is little support for significant roles of other binocular functions. Indeed, Cumming and Judge (1986) have observed in adult monkeys that when accommodation is stimulated simultaneously in both eyes in the absence of retinal disparity alone, the accommodative gains are comparable to those observed under monocular viewing conditions. In fact, in the current study, a change in target size occurred with the change in retinal blur in all of the conditions and so the monocular gains reported here represent performance in the presence of this additional cue. It is feasible that the response to blur alone might be even smaller than reported here (Currie & Manny, 1997; Kruger & Pola, 1985, 1987; McLin, Schor, & Kruger, 1988).
The adult visual system can, in some senses, be considered to be biased toward retinal disparity cues over blur. For example, the thresholds for perceiving diplopia [0.08 MA (Schor et al., 1984)] and for eliciting a vergence response [0.01 MA (Duwaer & van den Brink, 1981)] are much smaller than the thresholds for perceiving blurred vision [0.18−0.25 D (Charman, 1983; Winn, Charman, Pugh, Heron, & Eadie, 1989)] and for eliciting an accommodative response [0.15 D (Kotulak & Schor, 1986; Ludlam, Wittenberg, Giglio, & Rosenberg, 1968)]. Second, in the presence of a cue conflict between retinal blur and disparity in adult humans and monkeys, the blur cue is more easily over-ridden by the disparity cue (Adamson & Fincham, 1939; Cumming & Judge, 1986; Fincham & Walton, 1957; Fry, 1983). Third, in the absence of a direct cue, retinal disparity is more efficient at driving accommodation [CA/C ratio: 0.9 D/MA (Fincham & Walton, 1957; Kersten & Legge, 1983)] than is retinal blur at driving vergence [AC/A ratio of 0.6 to 0.7 MA/D (Fincham & Walton, 1957; Morgan, 1968)]. However, unlike the developing visual system, adults appear more efficient at using monocular retinal cues to accommodate well in the absence of retinal disparity (Figures 3A and 4A; Lovasik, Kergoat, & Kothe, 1987; Ramsdale, 1979; Rosenfield, Portello, Blustein, & Jang, 1996; Seidemann & Schaeffel, 2003).
Why might low gains of accommodation and vergence to blur cues be an advantage during development? As described by Aslin and Jackson (1979), the accommodative demand for a typical infant is larger than for an adult as a result of their hyperopic refractive error (Mayer et al., 2001), while their vergence demand is smaller because of their narrow IPD (MacLachlan & Howland, 2002; Pryor, 1969). Adult-like neural coupling between accommodation and vergence might prevent the infant visual system from independently modulating blur-driven accommodation and disparity-driven vergence to overcome these immature and potentially conflicting demands (Aslin & Jackson, 1979; Bobier et al., 2000; Turner et al., 2002). If the gain of the accommodative-vergence coupling is adult-like from birth, a hyperopic infant is at risk of generating an over-convergence to their reduced vergence demand after they have accommodated to compensate for their hyperopia (this could theoretically precipitate a strabismus during the critical period of cortical development). It would therefore be advantageous to have a reduced near response gain to the blur cue if other cues could drive accurate performance in habitual conditions. Bobier et al. (2000) found that the convergence accommodation coupling was higher in 3- to 6- month-old infants than in adults, suggesting that the developing visual system may indeed be weighted toward driving larger accommodation responses through the retinal disparity and vergence pathways (see also the CA/C prism control data of Tondel and Candy (2008), which are in good agreement).
At least theoretically, the relationship between accommodation and vergence requires constant recalibration as the head grows and hyperopia decreases. Indeed, the response AC/A and CA/C ratios of adults change when their interpupillary distance (IPD) is artificially increased or decreased by wearing periscopic spectacles for 30 minutes (Bobier & McRae, 1996; Jiang & Ramamirtham, 2005; Judge & Miles, 1985; Miles, Judge, & Optican, 1987). We found that the ratio of vergence to accommodation under monocular conditions (response AC/A ratio) was approximately 0.7 to 0.8 MA/D and it did not change significantly with increase in interpupillary distance and age (Figure 4E). Similarly, Turner et al. (2002) observed that the response AC/A ratio of infants less than 1 year of age was not significantly different from that of adults. The constant AC/A ratio in meter angles per diopter implies that the angular rotation in degrees per diopter of accommodation changes with age. For instance, for an AC/A ratio of 0.75 MA/D, an infant with a 44-mm IPD would generate 1.9° of angular rotation per diopter of accommodation while an adult with a 61-mm IPD would generate 2.6° of angular rotation per diopter of accommodation (37% more than that of an infant). Perhaps the mean population AC/A ratio of 0.7 to 0.8 MA/D observed here and by others (Fry, 1983; Morgan, 1968; Turner et al., 2002) is being actively maintained through a neural recalibration process and is, therefore, in some sense optimal in habitual binocular conditions.
Acknowledgments
We would like to thank Bill Monette for developing the experimental equipment, Diane Goss for help with subject recruitment, and Drs. Jingyun Wang, Danielle Teel, and Kate Gray, and Kyle Gilbert, Sylvia Mishoulam, and Ashley Anderson for help with data collection. We would also like to thank the subjects and their parents for their participation.
This work was supported by R01 EY014460 awarded to TRC, K12 EY01550 support for D. Teel, and T35 EY013937 support for K. Gilbert and S. Mishoulam.
Footnotes
Commercial relationships: none.
Contributor Information
Shrikant R. Bharadwaj, Indiana University School of Optometry, Bloomington, IN, USA
T. Rowan Candy, Indiana University School of Optometry, Bloomington, IN, USA.
References
- Adamson J, Fincham EF. Effect of lenses and convergence on the state of accommodation of the eye. Transactions of the Ophthalmological Society. 1939;59:163. [Google Scholar]
- Alpern M, Ellen P. A quantitative analysis of the horizontal movements of the eyes in the experiment of Johannes Mueller. I. Method and results. American Journal of Ophthalmology. 1956a;42:289–296. doi: 10.1016/0002-9394(56)90380-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- Alpern M, Ellen P. A quantitative analysis of the horizontal movements of the eyes in the experiment of Johannes Mueller. II. Effect of variation in target separation. American Journal of Ophthalmology. 1956b;42:296–303. doi: 10.1016/0002-9394(56)90381-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- Aslin RN. Infant accommodation and convergence. In: Simons K, editor. Early visual development: Normal and abnormal. Oxford University Press; New York: 1993. pp. 30–37. [Google Scholar]
- Aslin RN, Jackson RW. Accommodative-convergence in young infants: Development of a synergistic sensory-motor system. Canadian Journal of Psychology. 1979;33:222–231. doi: 10.1037/h0081722. [PubMed] [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Pimm-Smith E. ‘Preferential looking’ for monocular and binocular acuity testing of infants. British Journal of Ophthalmology. 1982;66:264–268. doi: 10.1136/bjo.66.4.264. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MS. Infant refraction and accommodation. International Ophthalmology Clinics. 1980;20:205–232. doi: 10.1097/00004397-198002010-00010. [PubMed] [DOI] [PubMed] [Google Scholar]
- Banks MS, Aslin RN, Letson RD. Sensitive period for the development of human binocular vision. Science. 1975;190:675–677. doi: 10.1126/science.1188363. [PubMed] [DOI] [PubMed] [Google Scholar]
- Banks MS, Crowell JA. Front-end limitations to infant spatial vision: Examination of two analyses. In: Simons K, editor. Early visual development: Normal and abnormal. Oxford University Press; New York: 1993. pp. 91–115. [Google Scholar]
- Banks MS, Salapatek P. Acuity and contrast sensitivity in 1-, 2-, and 3-month-old human infants. Investigative Ophthalmology & Visual Science. 1978;17:361–365. [PubMed] [Article] [PubMed] [Google Scholar]
- Bobier WR, Guinta A, Kurtz S, Howland HC. Prism induced accommodation in infants 3 to 6 months of age. Vision Research. 2000;40:529–537. doi: 10.1016/s0042-6989(99)00196-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- Bobier WR, McRae M. Gain changes in the accommodative convergence cross-link. Ophthalmic & Physiological Optics. 1996;16:318–325. [PubMed] [PubMed] [Google Scholar]
- Bradley A, Freeman RD. Contrast sensitivity in children. Vision Research. 1982;22:953–959. doi: 10.1016/0042-6989(82)90031-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- Brodie SE. Photographic calibration of the Hirschberg test. Investigative Ophthalmology & Visual Science. 1987;28:736–742. [PubMed] [Article] [PubMed] [Google Scholar]
- Brookman KE. Ocular accommodation in human infants. American Journal of Optometry and Physiological Optics. 1983;60:91–99. doi: 10.1097/00006324-198302000-00001. [PubMed] [DOI] [PubMed] [Google Scholar]
- Candy TR, Bharadwaj SR. The stability of steady state accommodation in human infants. Journal of Vision. 2007;7(11):4, 1–16. doi: 10.1167/7.11.4. http://journalofvision. org/7/11/4/, doi:10.1167/7.11.4. [PubMed] [Article] [DOI] [PMC free article] [PubMed]
- Carroll JP. Control theory approach to accommodation and vergence. American Journal of Optometry and Physiological Optics. 1982;59:658–669. doi: 10.1097/00006324-198208000-00007. [PubMed] [DOI] [PubMed] [Google Scholar]
- Charman WN. The retinal image in the human eye. Progress in Retinal and Eye Research. 1983;2:1–50. [Google Scholar]
- Chauhan K, Charman WN. Accommodation responses to flickering stimuli. Ophthalmic & Physiological Optics. 1996;16:391–408. [PubMed] [PubMed] [Google Scholar]
- Chauhan K, Charman WN, Halnan AM, Kelly CM, Loughlin A, Neilson KJ, et al. Time-averaged accommodation response to flickering stimuli. Ophthalmic & Physiological Optics. 1992;12:327–334. [PubMed] [PubMed] [Google Scholar]
- Choi M, Weiss S, Schaeffel F, Seidemann A, Howland HC, Wilhelm B, et al. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor). Optometry and Vision Science. 2000;77:537–548. doi: 10.1097/00006324-200010000-00008. [PubMed] [DOI] [PubMed] [Google Scholar]
- Chung ST, Legge GE, Tjan BS. Spatial-frequency characteristics of letter identification in central and peripheral vision. Vision Research. 2002;42:2137–2152. doi: 10.1016/s0042-6989(02)00092-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ. The Glenn A. Fry invited lecture. Accommodation to gratings and more naturalistic stimuli. Optometry and Vision Science. 1991;68:243–260. doi: 10.1097/00006324-199104000-00001. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ, Kenyon RV. Accommodative vergence and accommodation in normals, amblyopes, and strabismics. In: Schor CM, Ciuffreda KJ, editors. Vergence eye movements: Basic & clinical aspects. Butterworths; Boston: 1983. pp. 101–163. [Google Scholar]
- Ciuffreda KJ, Rosenfield M, Rosen J, Azimi A, Ong E. Accommodative responses to naturalistic stimuli. Ophthalmic & Physiological Optics. 1990;10:168–174. doi: 10.1111/j.1475-1313.1990.tb00971.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. American Journal of Ophthalmology. 1951;34:1407–1413. doi: 10.1016/0002-9394(51)90481-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Cumming BG, Judge SJ. Disparity-induced and blur-induced convergence eye movement and accommodation in the monkey. Journal of Neurophysiology. 1986;55:896–914. doi: 10.1152/jn.1986.55.5.896. [PubMed] [DOI] [PubMed] [Google Scholar]
- Currie DC, Manny RE. The development of accommodation. Vision Research. 1997;37:1525–1533. doi: 10.1016/s0042-6989(97)85022-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Duwaer AL, van den Brink G. Diplopia thresholds and the initiation of vergence eye movements. Vision Research. 1981;21:1727–1737. doi: 10.1016/0042-6989(81)90205-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- Eadie AS, Carlin PJ. Evolution of control system models of ocular accommodation, vergence and their interaction. Medical & Biological Engineering & Computing. 1995;33:517–524. doi: 10.1007/BF02522508. [PubMed] [DOI] [PubMed] [Google Scholar]
- Erickson P, Schor C. Visual function with presbyopic contact lens correction. Optometry and Vision Science. 1990;67:22–28. doi: 10.1097/00006324-199001000-00006. [PubMed] [DOI] [PubMed] [Google Scholar]
- Fincham EF. The accommodation reflex and its stimulus. British Journal of Ophthalmology. 1951;35:381–393. doi: 10.1136/bjo.35.7.381. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham EF, Walton J. The reciprocal actions of accommodation and convergence. The Journal of Physiology. 1957;137:488–508. doi: 10.1113/jphysiol.1957.sp005829. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitcroft DI, Judge SJ, Morley JW. Binocular interactions in accommodation control: Effects of anisometropic stimuli. Journal of Neuroscience. 1992;12:188–203. doi: 10.1523/JNEUROSCI.12-01-00188.1992. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitcroft DI, Morley JW. Accommodation in binocular contour rivalry. Vision Research. 1997;37:121–125. doi: 10.1016/s0042-6989(96)00146-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- Fry GA. Basic concepts underlying graphical analysis. In: Schor CM, Ciuffreda K, editors. Vergence eye movements: Basic and clinical aspects. Butterworths; Boston: 1983. pp. 605–646. [Google Scholar]
- Green DG, Powers MK, Banks MS. Depth of focus, eye size and visual acuity. Vision Research. 1980;20:827–835. doi: 10.1016/0042-6989(80)90063-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Bauer J, Thorn F, Held R. Development of spatial contrast sensitivity from infancy to adulthood: Psychophysical data. Optometry and Vision Science. 1997;74:785–789. doi: 10.1097/00006324-199710000-00017. [PubMed] [DOI] [PubMed] [Google Scholar]
- Hainline L, Riddell P, Grose-Fifer J, Abramov I. Development of accommodation and convergence in infancy. Behavioral Brain Research. 1992;49:33–50. doi: 10.1016/s0166-4328(05)80192-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Haynes H, White BL, Held R. Visual accommodation in human infants. Science. 1965;148:528–530. doi: 10.1126/science.148.3669.528. [PubMed] [DOI] [PubMed] [Google Scholar]
- Heath GG. Components of accommodation. American Journal of Optometry and Archives of American Academy of Optometry. 1956;33:569–579. doi: 10.1097/00006324-195611000-00001. [PubMed] [DOI] [PubMed] [Google Scholar]
- Horwood AM, Riddell PM. Gender differences in early accommodation and vergence development. Ophthalmic & Physiological Optics. 2008;28:115–126. doi: 10.1111/j.1475-1313.2008.00547.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Horwood AM, Turner JE, Houston SM, Riddell PM. Variations in accommodation and convergence responses in a minimally controlled photorefractive setting. Optometry and Vision Science. 2001;78:791–804. doi: 10.1097/00006324-200111000-00009. [PubMed] [DOI] [PubMed] [Google Scholar]
- Jennings JA, Charman WN. Off-axis image quality in the human eye. Vision Research. 1981;21:445–455. doi: 10.1016/0042-6989(81)90091-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- Jiang BC, Ramamirtham R. The adaptive effect of narrowing the interocular separation on the AC/A ratio. Vision Research. 2005;45:2704–2709. doi: 10.1016/j.visres.2005.03.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. Journal of Neurophysiology. 1986;55:915–930. doi: 10.1152/jn.1986.55.5.915. [PubMed] [DOI] [PubMed] [Google Scholar]
- Judge SJ, Miles FA. Changes in the coupling between accommodation and vergence eye movements induced in human subjects by altering the effective interocular separation. Perception. 1985;14:617–629. doi: 10.1068/p140617. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kersten D, Legge GE. Convergence accommodation. Journal of the Optical Society of America. 1983;73:332–338. doi: 10.1364/josa.73.000332. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. Journal of Neuroscience. 1998;18:6411–6424. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh LH, Charman WN. Accommodative responses to anisoaccommodative targets. Ophthalmic & Physiological Optics. 1998;18:254–262. [PubMed] [PubMed] [Google Scholar]
- Kotulak JC, Schor CM. The accommodative response to subthreshold blur and to perceptual fading during the Troxler phenomenon. Perception. 1986;15:7–15. doi: 10.1068/p150007. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kruger PB, Pola J. Changing target size is a stimulus for accommodation. Journal of the Optical Society of America A, Optics and Image Science. 1985;2:1832–1835. doi: 10.1364/josaa.2.001832. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kruger PB, Pola J. Dioptric and non-dioptric stimuli for accommodation: Target size alone and with blur and chromatic aberration. Vision Research. 1987;27:555–567. doi: 10.1016/0042-6989(87)90042-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annual Review of Neuroscience. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H, Kothe AC. The influence of letter size on the focusing response of the eye. Journal of the American Optometric Association. 1987;58:631–639. [PubMed] [PubMed] [Google Scholar]
- Ludlam WM, Wittenberg S, Giglio EJ, Rosenberg R. Accommodative responses to small changes in dioptric stimulus. American Journal of Optometry. 1968;45:483–506. doi: 10.1097/00006324-196808000-00001. [DOI] [PubMed] [Google Scholar]
- MacLachlan C, Howland HC. Normal values and standard deviations for pupil diameter and interpupillary distance in subjects aged 1 month to 19 years. Ophthalmic & Physiological Optics. 2002;22:175–182. doi: 10.1046/j.1475-1313.2002.00023.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Maddox EE. Investigations in the relation between convergence and accommodation of the eyes. Journal of Anatomy and Physiology. 1886;21:21–42. [PubMed] [Article] [PMC free article] [PubMed] [Google Scholar]
- Majaj NJ, Pelli DG, Kurshan P, Palomares M. The role of spatial frequency channels in letter identification. Vision Research. 2002;42:1165–1184. doi: 10.1016/s0042-6989(02)00045-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Archives of Ophthalmology. 2001;119:1625–1628. doi: 10.1001/archopht.119.11.1625. [PubMed] [DOI] [PubMed] [Google Scholar]
- Mays LE, Gamlin PD. Neuronal circuitry controlling the near response. Current Opinion in Neurobiology. 1995;5:763–768. doi: 10.1016/0959-4388(95)80104-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- McLin LN, Jr., Schor CM, Kruger PB. Changing size (looming) as a stimulus to accommodation and vergence. Vision Research. 1988;28:883–898. doi: 10.1016/0042-6989(88)90098-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Miles FA, Judge SJ, Optican LM. Optically induced changes in the couplings between vergence and accommodation. Journal of Neuroscience. 1987;7:2576–2589. [PubMed] [Article] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE, Timney BN. Postnatal development of function in the mammalian visual system. In: Kandel ER, editor. Handbook of physiology: The nervous system. Vol. 3. American Physiological Society; Washington D.C.: 1984. pp. 507–555. [Google Scholar]
- Morgan MW. Accommodation and vergence. American Journal of Optometry and Archives of American Academy of Optometry. 1968;45:417–454. doi: 10.1097/00006324-196807000-00002. [PubMed] [DOI] [PubMed] [Google Scholar]
- Navarro R, Artal P, Williams DR. Modulation transfer of the human eye as a function of retinal eccentricity. Journal of the Optical Society of America A, Optics and Image Science. 1993;10:201–212. doi: 10.1364/josaa.10.000201. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ogle KN, Mussey F, Prangen AD. Fixation disparity and the fusional processes in binocular single vision. American Journal of Ophthalmology. 1949;32:1069–1087. doi: 10.1016/0002-9394(49)90649-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pryor HB. Objective measurement of interpupillary distance. Pediatrics. 1969;44:973–977. [PubMed] [PubMed] [Google Scholar]
- Ramsdale C. Monocular and binocular accommodation. Ophthalmic Optometry. 1979;19:606–622. [Google Scholar]
- Riddell PM, Hainline L, Abramov I. Calibration of the Hirschberg test in human infants. Investigative Ophthalmology & Visual Science. 1994;35:538–543. [PubMed] [Article] [PubMed] [Google Scholar]
- Roorda A, Campbell MC, Bobier WR. Slope-based eccentric photorefraction: Theoretical analysis of different light source configurations and effects of ocular aberrations. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1997;14:2547–2556. doi: 10.1364/josaa.14.002547. [PubMed] [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Portello JK, Blustein GH, Jang C. Comparison of clinical techniques to assess the near accommodative response. Optometry and Vision Science. 1996;73:382–388. doi: 10.1097/00006324-199606000-00005. [PubMed] [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: Relation to age and refractive errors. The Journal of Physiology. 1993;461:301–320. doi: 10.1113/jphysiol.1993.sp019515. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor C, Heckmann T. Interocular differences in contrast and spatial frequency: Effects on stereopsis and fusion. Vision Research. 1989;29:837–847. doi: 10.1016/0042-6989(89)90095-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Schor C, Wood I, Ogawa J. Binocular sensory fusion is limited by spatial resolution. Vision Research. 1984;24:661–665. doi: 10.1016/0042-6989(84)90207-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- Schor CM. A dynamic model of cross-coupling between accommodation and convergence: Simulations of step and frequency responses. Optometry and Vision Science. 1992;69:258–269. doi: 10.1097/00006324-199204000-00002. [PubMed] [DOI] [PubMed] [Google Scholar]
- Schor CM, Alexander J, Cormack L, Stevenson S. Negative feedback control model of proximal convergence and accommodation. Ophthalmic & Physiological Optics. 1992;12:307–318. [PubMed] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F. An evaluation of the lag of accommodation using photorefraction. Vision Research. 2003;43:419–430. doi: 10.1016/s0042-6989(02)00571-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- Shea SL, Aslin RN. Oculomotor responses to step-ramp targets by young human infants. Vision Research. 1990;30:1077–1092. doi: 10.1016/0042-6989(90)90116-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Slater AM, Findlay JM. The measurement of fixation position in the newborn baby. Journal of Experimental Child Psychology. 1972;14:349–364. doi: 10.1016/0022-0965(72)90056-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- Stark L. Neurological control systems: Studies in Bioengineering. Plenum; New York: 1968. [Google Scholar]
- Stark LR, Atchison DA. Subject instructions and methods of target presentation in accommodation research. Investigative Ophthalmology & Visual Science. 1994;35:528–537. [PubMed] [Article] [PubMed] [Google Scholar]
- Teller DY, McDonald MA, Preston K, Sebris SL, Dobson V. Assessment of visual acuity in infants and children: The acuity card procedure. Developmental Medicine and Child Neurology. 1986;28:779–789. doi: 10.1111/j.1469-8749.1986.tb03932.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Teller DY, Movshon JA. Visual development. Vision Research. 1986;26:1483–1506. doi: 10.1016/0042-6989(86)90169-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- Toates FM. Accommodation function of the human eye. Physiological Reviews. 1972;52:828–863. doi: 10.1152/physrev.1972.52.4.828. [PubMed] [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Tadmor Y, Chao T. Amplitude spectra of natural images. Ophthalmic & Physiological Optics. 1992;12:229–232. doi: 10.1111/j.1475-1313.1992.tb00296.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Tondel GM, Candy TR. Accommodation and vergence latencies in human infants. Vision Research. 2008;48:564–576. doi: 10.1016/j.visres.2007.11.016. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Horwood AM, Houston SM, Riddell PM. Development of the response AC/A ratio over the first year of life. Vision Research. 2002;42:2521–2532. doi: 10.1016/s0042-6989(02)00268-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- van der Wildt GJ, Bouman MA, van de Kraats J. The effect of anticipation on the transfer function of the human lens system. Optica Acta. 1974;21:843–860. [Google Scholar]
- Wang J, Candy TR. Higher order monochromatic aberrations of the human infant eye. Journal of Vision. 2005;5(6):6, 543–555. doi: 10.1167/5.6.6. http://journalofvision.org/5/6/6/, doi:10.1167/5.6.6. [PubMed] [Article] [DOI] [PMC free article] [PubMed]
- Winn B, Charman WN, Pugh JR, Heron G, Eadie AS. Perceptual detectability of ocular accommodation microfluctuations. Journal of the Optical Society of America A, Optics and Image Science. 1989;6:459–462. doi: 10.1364/josaa.6.000459. [PubMed] [DOI] [PubMed] [Google Scholar]