Abstract
Children with unilateral pre- or perinatal brain injury (BI) show remarkable plasticity for language learning. Previous work highlights the important role that lesion characteristics play in explaining individual variation in plasticity in the language development of children with BI. We ask here whether the linguistic input that children with BI receive from their caregivers also contributes to this early plasticity, and whether linguistic input plays a similar role in children with BI as it does in typically developing (TD) children. We model growth in vocabulary and syntactic production for 80 children (53 TD, 27 BI) between 14 and 46 months. We find that caregiver input is an equally potent predictor of vocabulary growth in children with BI and in TD children. In contrast, we find that input is a more potent predictor of syntactic growth for children with BI than for TD children. Controlling for input, lesion characteristics (lesion size, type, seizure history) also affect the language trajectories of children with BI. Thus, our findings illustrate how both variability in the environment (linguistic input) and variability in the organism (lesion characteristics) work together to contribute to plasticity in language learning.
Typically developing children vary widely in the rate at which they acquire various aspects of language. A growing body of research finds that variations in linguistic input can account for many of these early differences in child language growth. Children with early unilateral brain injury also vary in their rate and breadth of language learning. However, and in contrast to research on typically developing children, previous research has not looked to linguistic input to explain variation in children with brain injury. Rather, most research has focused almost exclusively on variations in lesion characteristics as a way to explain language-learning differences in children with brain injury.
But early functional plasticity does not arise in a vacuum. Linguistic input seems almost certain to have some impact on the rate at which children with early brain injury acquire language, much as it does in children with an intact brain. The question we ask here is whether this impact is conditioned by the learner’s atypical brain. We address this question by exploring whether linguistic input plays the same role for children with brain injury as it does for children without early focal brain injury.
One possibility is that linguistic input plays the same role in children with and without early brain injury. Such a finding would indicate that language acquisition is buffered early in life—so buffered that it proceeds in more or less the same manner even when the learner has suffered a significant organic insult. A finding of this sort would provide evidence that the process, as well as the product, of language development is spared in the face of early brain injury. Alternatively, linguistic input could play a less important role in the language development of children with brain injury, suggesting that early lesions limit the impact that linguistic input can have on language development, and thus alter how language is learned. Finally, linguistic input could play a more important role for children with focal brain injury than it does for typically developing children (cf., Wilcox & Shannon, 1996). Such a finding would indicate that augmentation from the environment might be needed to offset early organic injury. In this event, plasticity following early lesions should be thought of as the joint product of organic injury and input from the environment.
Background
Children with pre- or perinatal unilateral brain injury, even those whose lesions involve classical language areas in the left hemisphere, show remarkable plasticity for language learning. They typically do not exhibit aphasic symptoms that are common when such lesions are incurred during adulthood (e.g., Bates & Roe, 2001; Bates, Reilly, Wulfeck et al., 2001) and, in some cases, intact brain regions, either ipsi-lesional and/or contra-lesional, become involved in language functions that would normally be carried out by the damaged regions (e.g., Booth, MacWhinney, Thulborn, Sacco, Voyvodic, & Feldman, 2000; Fair, Brown, Peterson & Schlaggar, 2006; Raja, Josse, Suriyakham, et. al., 2006; Staudt, Lidzba, Grodd, Wildgruber, Erg, Krageloh-Mann, 2002; Stiles, Reilly, Paul & Moses, 2005). Positive language outcomes in children with early lesions are typically attributed to the greater plasticity of the young nervous system (e.g., Kolb, 1995; Lansdell, 1969; Huttenlocher, 2002; Rasmussen & Milner, 1977; Strass, Satz & Wada, 1990).
Despite this developmental plasticity, early lesions of either hemisphere can result in delayed language milestones for children with BI, compared to typically developing children. But there is considerable variability among children with regard to the extent, and even the presence, of a delay (e.g, Bates, Vicari, & Trauner, 1999; Feldman, 2005). To date, research has focused on characterizing this variability in terms of the location and size of lesions, and/or the presence or absence of seizure disorders (e.g., Staudt et al., 2002; Bates et al., 1999; Vargha-Khadem, Isaacs, van der Werf, Robb, & Wilson, 1992; Levine, Kraus, Alexander, Suriyakham, & Huttenlocher, 2005). We review this literature briefly below and suggest an additional factor worthy of consideration—variation in linguistic input.
Lesion location
There are two current views of how lesion laterality relates to language difficulties in children with early unilateral brain injury. According to one view, early left hemisphere lesions result in more marked language deficits than early right hemisphere lesions (e.g., Annett, 1973; Aram, Ekelman, Rose, & Whitaker, 1985; Aram & Ekelman, 1986; Baranes et al., 1993; Dennis & Whitaker, 1976; Levine et al., 1987; Rankin et al., 1981; Riva & Cazzaniga, 1986; Woods & Teuber, 1978). For example, Chilosi and colleagues (2001) found that children with left hemisphere injury generally scored lower than those with right hemisphere injury on measures of expressive vocabulary and syntax. The second view, also supported by research (e.g., Bates et al., 2001, 1998; Dall’Oglio, Bates, Volterra, Di Capua, & Pezzini, 1994; Feldman et al., 1992; Marchman, Miller & Bates, 1991; Reilly et al., 1998; Vargha-Khadem, Isaacs, Muter, 1994), is that language deficits are just as likely following early injury to the left and right hemispheres. Thus, it is not yet clear what role lesion location plays in the early language development of children with BI.
Lesion size and type
Larger lesions are generally associated with greater deficits in language, reading, and other cognitive functions (Booth et al., 2000; Levine et al., 2005). Our own research shows a strong link between lesion size and early language growth trajectories for receptive and productive vocabulary, as well as for syntax comprehension and the syntactic complexity of utterances (Brasky, Nikolas, Meanwell, Levine, & Goldin-Meadow, 2005; Levine, Brasky, & Nikolas, 1995; Levine, Kraus, Alexander, Suriyakham, & Huttenlocher, 2005). Bates and colleagues (1999) found a curvilinear relation between lesion size and outcome, with smaller and larger lesions associated with better outcomes than medium lesions; however, this pattern was not replicated in a larger sample (Bates, Vicari, & Trauner, 1999).
One fundamental concern in comparing results across lesion studies is that the type of injury is often confounded with the size and site of lesion as well as with the timing of the lesion. In particular, periventricular lesions tend to occur in the early to mid third trimester and predominantly affect white matter tracts, whereas cerebrovascular infarcts tend to occur in the late third trimester of preganancy or perinatally and predominantly affect gray matter structures in the middle cerebral artery (MCA) territory (e.g., Krageloh-Mann, 2004; Staudt et al., 2004). Further, cerebrovascular infarcts tend to be larger in size than periventricular lesions. While we are not aware of previous research looking at lesion type and language development, a study by Staudt and colleagues (2004), found that lesion type was related to hand motor dysfunction; patients with infarcts to the middle cerebral artery showed more dysfunction than those with periventricular white matter lesions. Staudt and colleagues (2004) suggest that the greater plasticity shown by patients with periventricular lesions stems from the earlier timing of these lesions relative to cerebral infarcts during the gestational period.
Presence or absence of seizures
Current research is inconclusive with respect to whether seizures are associated with more pronounced deficits in cognitive/language skills in children with early lesions. Bates et al. (1999) stress that children with early unilateral brain injury who have never experienced seizures may still have deficits. Indeed, some studies find no significant effects of seizures on language functioning or IQ in this population (Bates et al., 1997; Levine et al., 2005). Other studies, however, find that seizures negatively affect functioning in children with early brain injury. Notably, Vargha-Khadem and colleagues (1992) report that children with early unilateral lesions who had experienced seizures had lower Verbal and Performance IQ than those who had not had seizures. A large study of the relation between seizures and IQ suggests that it may be recurrent seizures that are responsible for intellectual deficits (Huttenlocher & Hapke, 1990). Note, however, that it is difficult to tease apart whether deficits are attributable to the seizure disorder itself, the underlying pathology that led to both seizures and lower functioning, and/or the effects of anticonvulsant medications (e.g., Vargha-Khadem et al., 1992).
Linguistic input
Studies of language development in typically developing children find significant differences in the quantity and quality of caregiver speech children receive (e.g., Bee, Van Egeren, Streissguth, Nyman & Leckie, 1969; Farian & Haskins, 1980; Hart & Risley, 1995; Hoff, 2003; Huttenlocher, Vasilyeva, Waterfall, Vevea, & Hedges, 2007; Rowe, Pan & Ayoub, 2005), which are often correlated with background characteristics, such as socioeconomic status (SES). These input differences appear to be related to vocabulary growth (Hart & Risley, 1995; Huttenlocher, Haight, Bryk, Seltzer, & Lyons, 1991; Pan, Rowe, Singer & Snow, 2005) and growth in sentence length (Barnes, Gutfreund, Satterly, & Wells, 1983) and complexity (Huttenlocher et al., in prep). For example, more educated and advantaged parents talk more and use more varied and complex language with their children than parents with less education and fewer economic advantages, and children from advantaged households show faster language growth and greater language skills compared to peers from less advantaged backgrounds (Hart & Risley, 1992; Hoff, 2003). Further, variability in preschool teacher speech is related to growth of syntactic comprehension and production skills over the course of the school year and, importantly, is not related to children’s language levels at the start of the school year (Huttenlcoher, Vasilyeva, Cymerman, & Levine, 2002). Thus, the nature of the linguistic input children receive appears to play a role in shaping the course of language development in typically developing children, even when speech is produced by adults who are not biologically related to the child.
The present study
In the study that follows we investigate the interaction between early brain injury and linguistic input in language learning by addressing the following research questions: (1) What does development in vocabulary and syntax production look like for typically developing children and children with brain injury between 14 and 46 months of age? (2) Does linguistic input play a similar or different role in the language development of typically developing children and children with brain injury, controlling for SES? (3) Do particular lesion characteristics affect the language development trajectories of children with brain injury, even when SES and linguistic input are controlled? We take a developmental approach in that we use longitudinal methods to model growth in children’s observed vocabulary and syntax production over the first few years of language learning. Specifically, we use Hierarchical Linear Modeling (HLM) procedures (Raudenbush, Bryk, Cheong, & Congdon, 2000) with a 2-level, longitudinal model. This approach allows us to address all three of our research questions by fitting one multilevel model for change for each language measure. Specifically, the level-1 portion of the model focuses on within-person change and tells us about individual change over time in vocabulary and syntactic production. The level-2 portion of the model focuses on how these individual changes vary across typically developing or brain injured groups, across families with different levels of input and SES, and across children with various lesion characteristics (Raudenbush et al., 2000; Singer & Willett, 2003).
Methods
Participants
The participants were 53 typically-developing (TD) children and their primary caregivers and 27 children who experienced pre- or peri-natal unilateral brain injury (BI) and their primary caregivers, observed between the ages of 14 and 46 months. These 80 families were drawn from a larger sample of 104 families (64 TD, 40 BI) participating in a longitudinal study of language development in the greater Chicago area.1
The TD children were recruited via direct mailings to roughly 5,000 families living in targeted zip codes and an advertisement in a free, monthly parent magazine. Interested parents were interviewed about background characteristics and a final sample was selected to be representative of the greater Chicago area in terms of ethnicity and income. Annual income levels varied from less than $15,000 to over $100,000 (M = $62,889), and children came from more than five different ethnic groups. On average, parents had 16 years of education (the equivalent of a college degree) when they entered the study; however, the range was from 10 years (less than high school degree) to 18 years (Master’s degree or more). The TD sample included 27 boys and 26 girls. Primary caregivers consisted of 52 mothers and 1 father.
The children who had experienced pre- or peri-natal brain injury were sampled using a different approach as the incidence of such lesions is estimated to be approximately 1 in 4,000 births (Lynch & Nelson, 2001), making these children difficult to find. We recruited the BI sample by contacting pediatric neurologists in the greater Chicago area and neighboring states. In addition, we established relationships with parent support groups in the area (Childhood Stroke and Hemiplegia Connections of Illinois, CSHC; Pediatric Stroke Network, PSN; and Children’s Hemiplegia and Stroke Association, CHASA), which we visited to inform interested parents about our research project. We included every family that was interested as long as the child had experienced a pre- or peri-natal unilateral brain injury and was reported by the parent to be born at 36 weeks gestation or later. The sample included 18 girls and 9 boys. Twenty four of the children are Caucasian, and three are of mixed race. All primary caregivers were mothers. Maternal education ranged from 12 to 18 years (M = 16), and annual family income ranged from $30,000 to over $100,000 (M = $86,583). All families in both the TD and BI samples were raising their children as monolingual English speakers.
Procedure
Parent-child dyads were visited in the home every four months between child ages 14 and 46 months, resulting in nine visits covering a 32 month period. As in most longitudinal studies, families occasionally missed a data collection period. In the TD sample of 53 families, 45 families participated in all 9 visits, and 7 families participated in 8 of 9 visits. In one family both parents participated in the final 3 visits; thus only data from the first 6 visits are included here. The children with BI entered the study at a variety of different ages, as children are often not diagnosed until after 14 months of age and families became aware of our study when children were of various ages. In addition, we accepted children into the study over a period of several years so some children had not yet completed all nine visits at the time the current analyses were conducted. In all, two children completed 8 visits, two completed 7 visits, three completed 6 visits, three completed 5 visits, six completed 4 visits, three completed 3 visits, three completed 2 visits, and five completed 1 visit. One of the advantages of using growth modeling methods is that the multilevel model for change is designed to deal with longitudinal data sets in which there are varying numbers of waves per person and variable spacing of these waves (Raudenbush & Bryk, 2005; Singer & Willett, 2003). Thus, all 80 parent-child dyads could be and were included in the analysis.
At each home visit, parent-child dyads were videotaped for 90 minutes while engaging in their ordinary activities. The particular activities dyads engaged in varied, yet sessions typically included time playing with toys, book reading, and a meal or snack time. All of the speech by parents and children was transcribed. The unit of transcription was the utterance, defined as any sequence of words preceded and followed by a pause, a change in conversational turn, or a change in intonation pattern. All dictionary words, as well as onomatopoeic sounds (e.g., woof-woof) and evaluative sounds (e.g., uh-oh), were counted as words. Transcription reliability was established by having a second individual transcribe 20 percent of the videotapes; reliability was achieved when the second coder agreed with the first on 95 percent of the transcription decisions.
Measures
Child vocabulary
Automated analyses of the transcripts yielded data on number of different word types (i.e., number of different intelligible word roots) produced by each child at each session. Several decisions were made as to what constitutes a word type. Morphologically inflected variants of words (e.g., run, running) were considered a single type. Words produced in imitation of the mother were included in the corpus of child word types, as were words that the parent or child produced while reading. The number of word types produced by each child at each session serves as our measure of vocabulary growth2.
Child syntax
Automated analyses of the transcripts yielded data on the mean length of the children’s utterances (MLU), measured in number of words. For example, the utterance “My shoes” counts as 2 words (my+shoes). Note that our measure differs from many studies in which MLU is measured in morphemes, i.e., “my shoes” would count as 3 morphemes (my+shoe+s).
Parent input
The same analyses were conducted for parent speech. Parent word types and MLU produced at child age 30-months (the 5th visit) were used as input measures predicting child language growth. The 30-month visit was chosen because it is half-way through the period of data collection. Parent input values were imputed for dyads where 30-month data were not available (n = 13; 1 TD, 12 BI), either by averaging values from the 26- and 34-month visits, when available, or by using values from the closest available visit to 30-months. Parent word types served as our measure of vocabulary input and parent MLU as our measure of syntactic input3. One might question our use of MLU as a measure of syntactic complexity for adults, as studies with children have shown the measure to be less useful after children reach an MLU of 4.5 (Brown, 1973). To address this potential concern, we looked at the association between our MLU measure and the proportion of complex sentences in a subset of parents in the study on whom coded data were available (n = 44 TD parents). In this subsample, there was a strong linear relation between MLU and proportion of complex sentences, with a correlation of 0.794 (p<.0001) (J. Huttenlocher, personal communication). Thus, it appears that parent MLU in words is measuring a similar underlying construct as parent proportion of complex sentences and is a reasonable measure of the syntactic complexity of the input.
Parent education.4
The education level of the primary caregiver was measured categorically and each category was assigned a value equivalent to years of education (less than high school = 10 years, high school = 12 years, some college or associates degree = 14 years, college degree = 16 years, more than college = 18 years). There were no significant differences in education level of the primary caregiver in the TD and BI samples. The TD parents averaged 15.8 years of education (SD = 2.2), with a range from 10 to 18 years, and the BI parents averaged 15.7 years of education (SD = 1.7), with a slightly smaller range from 12 to 18 years.
Coding characteristics of brain lesions
Families provided copies of clinical MRI films (26 children), or detailed medical reports (1 child). In addition, six children were scanned using a 3Tesla GM Scanner at the University of Chicago when they were 5 years old or older (i.e., when scans could be obtained without sedation). Images were acquired in the axial plane and five sequences were included in the scan: (1) Localizer, (2) Mprage Volume sequence (high resolution 1.5 mm), (3) Flair (fluid attenuated inversion recovery), (4) Phase map (for DTI), and (5) DTI (Diffusion Tensor Imaging). These various sources provided lesion information on all 27 of the children with BI. Scans were evaluated by pediatric neurologists who coded lesions according to location, size, and type; discrepancies were few and were resolved through discussions. Only one child had a gestational age of 36 weeks; the rest of our sample was not born prematurely.
The specific biological measures considered in the current analysis include lesion laterality (left vs right), lesion size (small, medium, large), lesion type (periventricular (PV) versus cerebrovascular (CV)), and whether or not the child experienced recurrent seizures treated with medication (determined from medical reports). These data are presented for each child in Table 1. Lesions were classified on the basis of the following criteria. Small lesions affected only one lobe, or minimally affected subcortical regions. Medium lesions extended into more than one lobe or subcortical region. Large lesions affected three or four lobes and were typically cerebrovascular infarcts; these lesions affected multiple cortical areas and often involved the thalamus and subcortical regions. Regarding lesion type, cerebrovascular lesions (CV) are infarcts of the middle cerebral artery territory, and tend to affect the inferior frontal and/or superior temporal regions. Periventricular lesions (PV) are primarily subcortical and involve white matter tracts, the thalamus, basal ganglia, and/or the medial temporal lobe. While periventricular leukomalacia in very low birthweight, prematurely-born children has been the focus of much previous literature, periventricular lesions in full-term children has also been studied. For example, in a recent review, Krägeloh-Mann and Horber (2007) cite over 6 papers on full-term patients with periventricular lesions. In our study, one child was born at 36 weeks, but the rest are full-term as we did not include very pre-term babies in our study. Thus our sample of children with periventricular lesions differs from samples of very premature children with periventricular leukomalacia.
Table 1.
Lesion characteristics for brain-injured children (n = 27).
| ID# | Sex | Size | Type | Seizure | Areas Affected |
|---|---|---|---|---|---|
| Children with center-hemisphere lesions (n = 16) | |||||
| 30 | F | L | CV | N | F, T, P, O, subcortical |
| 32 | F | M | CV | - | F, T, P, subcortical |
| 35 | F | M | PV | N | subcortical |
| 95 | F | L | CV | - | F, T, P, subcortical |
| 116 | M | S | CV | - | thalamus |
| 129 | M | M | PV | N | subcortical |
| 132 | F | S | PV | N | T, subcortical |
| 133 | F | L | CV | N | F, T, P |
| 140 | F | L | CV | N | F, T, P, subcortical |
| 144 | M | M | CV | Y | F, T, P, subcortical |
| 147 | M | S | PV | Y | F, T, subcortical |
| 148 | F | L | CV | N | F, T, P, O, subcortical |
| 149 | F | L | CV | Y | F, T, P, subcortical |
| 151 | M | S | CV | Y | O, subcortical |
| 153 | M | L | CV | - | F, T, P, O, subcortical |
| 157 | F | S | PV | N | unknown |
| Children with right-hemisphere lesions (n = 11) | |||||
| 34 | F | L | PV | - | WM, insula, subcortical |
| 93 | M | S | PV | Y | subcortical |
| 94 | F | S | PV | N | T, P, subcortical |
| 117 | F | M | CV | Y | F, T, P, subcortical |
| 121 | M | M | PV | Y | T, subcortical |
| 135 | F | S | CV | Na | F, P |
| 139 | F | S | CV | N | F, T, P |
| 141 | F | M | PV | N | T, subcortical |
| 152 | F | L | CV | N | F, T, P, O, subcortical |
| 156 | F | L | CV | Y | F, T, P, subcortical |
| 159 | M | S | PV | N | T, subcortical |
Note: Codes are Sex (M = male, F = female), Size (S = small, M = medium, L = large), Type (PV = periventricular, CV = cerebrovascular), Seizure (Y = history of seizures, N = no history), Areas affected (F = frontal, T = temporal, P = parietal, O = occipital; Periventricular lesions involve the thalamus, basal ganglia, the medial temporal lobe and/or white matter tracts). Lesion information for #157 was determined based on medical notes.
Neonatal seizures resolved without medication.
Based on reviews of available MRI scans, Flair images, and medical notes by two pediatric neurologists, 16 children were classified as having left hemisphere lesions and 11 were classified as having right hemisphere lesions. Although PV lesions are likely to have some contralateral effects (Inder et al., 2005) the children in our study had predominantly unilateral lesions. Further, 10 children had lesions that were categorized as small, 7 as medium, and 10 as large. Finally, 16 of the children had lesions identified as having resulted from cerebrovascular infarcts (CV), whereas 11 had periventricular lesions (PV) involving the white matter tracts and enlarged ventricles. Of the 22 children for whom we had information about seizure history, 8 experienced recurrent seizures, and 14 did not (either no seizures, a single febrile seizure during the first year of life, or in the case of one child neonatal seizures that were resolved early without medication). Of the 8 children who experienced recurrent seizures, six were on medication for the seizures during some portion of the study, one child had seizures from 6–9 months only and was on medication at that time but not during the study, and one child had sporadic petit mal seizures and was never on medication.
Results and Discussion
We begin by providing a descriptive analysis of the growth in vocabulary and MLU between 14 and 46 months of age for both TD and BI groups. Next we present longitudinal analyses to determine (1) whether input plays a similar or different role in the language development of TD children and children with BI, controlling for SES (e.g., parent education), and (2) whether certain biological characteristics (lesion side, size, type, or seizure history) affect the language development trajectories of children with BI, controlling for education and linguistic input. Child gender was not a significant predictor of intercept or growth rates in any of our analyses for TD children, children with BI, or the combined groups; hence, this variable is not considered in the results reported below.
Describing and modeling growth in child language data
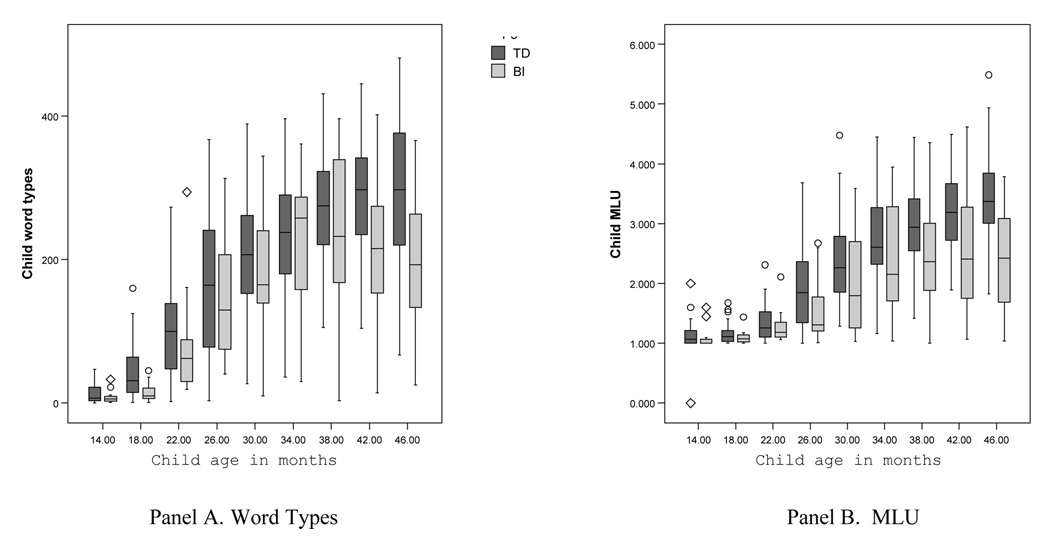
Panel A in Figure 1 presents the distribution of children’s word types at each session for the TD children and children with BI. At 14 months, children are at the very early stages of productive vocabulary use, producing an average of only 9 (BI) and 13 (TD) word types in a 90-minute session. During the 20-month period between 18 and 38 months, children increase dramatically in their production of word types; by 38 months, they are producing an average of 234 (SD = 107) (BI) and 276 (SD = 74) (TD) word types in a 90-minute session. Group comparisons of word type production at each time point showed significant differences favoring the TD children at 18 months, t(60) = 3.93, p < .001, and at 46 months, t(60) = 3.05, p < .01, but not at any other age. These group comparisons should be interpreted cautiously, however, as the particular children included in the BI group differ over time.
Figure 1.
Distribution of children’s word types (Panel A) and MLU (Panel B) at each age for TD (dark) and BI (light) children.
Note: The boxes in the graphs present the median values and inter-quartile range; the tails represent the 5th and 95th percentiles; outliers are noted by circles; extreme values are noted by diamonds.
After 38 months, growth in word type production during our 90-minute observation sessions starts to level off (not surprisingly as once children reach a certain level of vocabulary, our measure of their vocabulary improvement is limited by the length of time they are observed, which was fixed at 90 minutes). However, the BI group decreased slightly, on average, in word type production after 38 months, rather than leveling off as the TD children did. We can only speculate as to why this is the case. First, it may be that the leveling off in the TD group is a signal that they have reached a point in development where increases in vocabulary are more difficult to attain. Getting beyond this level of difficutly, or threshold, may be particularly hard for children with BI; as a result, these children may hover around the threshold level for an extended period of time, causing average scores to fluctuate between 38–46 months. Second, it is possible that the children with BI who entered the study at later timepoints had lower vocabularies than those who entered earlier, pulling the word types average down for the BI group. At the later ages, two children with low vocabularies did, in fact, begin the study. However, four other children with BI who had entered the study earlier produced fewer word types at 42 and 46 months than they did at 38 months. Thus, the average decrease in vocabulary production for the BI group may be due to a combination of sampling and the increasing difficulty of producing more diverse vocabulary in a fixed period of time at older ages.
Panel B in Figure 1 presents the distribution of children’s MLU at each session for the TD children and the children with BI. At 14 months most children are producing primarily one word utterances5. By 18 months all children were talking and MLU values averaged 1.16 (SD = 0.16)(TD) and 1.11 (SD = 0.14) (BI) words per utterance. MLU values increased steadily and by 30 months children were, on average, in the two-word stage of language production, (M(TD) = 2.41 (SD = 0.75); M(BI) = 1.99 (SD = 0.77). MLU values continued to increase over time, yet more steadily once the transition to two-word speech was accomplished. By 46 months, TD children were producing an average MLU of 3.40 (SD = 0.70), compared to an average MLU of 2.47 (SD = 0.86) for the children with BI. Group comparisons of MLU production at each time point showed differences favoring the TD children at our later observation time points; at 38 months t(69) = 2.37, p < .05; at 42 months, t(62) = 2.07, p = .06; and at 46 months, t(60) = 3.35, p < .01. Again, these group comparisons should be interpreted cautiously, as the particular children included in the BI group differ over time.
In sum, our initial descriptive results corroborate previous findings—in the face of early brain injury, language development is a relatively resilient process. Between 14 and 46 months, the children with BI in our sample lagged slightly behind their TD peers in vocabulary production, but there was wide and overlapping variation within and across the groups. Differences across groups in MLU were more pronounced, particularly after 3 years of age, adding support to previous findings that deficits often emerge only on more difficult linguistic tasks (e.g., producing complex syntactic forms; MacWhinney, Feldman, Sacco, & Vaaldes-Perez, 2000; Weckerly, Wulfeck, & Reilly, 2004). Thus, language development after pre- or perinatal brain injury may appear more resilient when earlier, less complex aspects of language development are considered.
To further examine the effects of brain injury and other environmental factors on language development, we next moved to longitudinal analyses using HLM procedures (Raudenbush, et al., 2000). We first developed an appropriate Level 1 model that describes the growth rates of the individual children. To do so, we examined the empirical growth trajectories for each child over time. Visual inspection of these trajectories indicated a fair amount of variation in the rate and shape of growth in word types and MLU for both TD children and children with BI. However, on average, for both language measures, children start out with low values, increase rapidly between 18 and 38 months, and then level off around 42 to 46 months. We anticipated that this type of growth would be best fit by a cubic model, or s-shaped curve. Indeed, after exploring a wide range of possibilities, we found that the best fitting Level 1 specification (the model with the lowest value on the -2 log-likelihood [−2LL] statistic using full maximum likelihood method) for both the TD and the BI data separately and combined included linear, quadratic, and cubic components for both word types and MLU. These models are represented by the following equation:
| (1) |
In Equation 1, Word Types/MLUit represents the word types or MLU production of child i at time t. By centering child age around 30-months, the individual growth parameters have the following interpretations: π0i represents child i’s intercept (level of word types or MLU at 30 months); π1i represents child i’s linear rate of growth (in word types or MLU); π2i represents child i’s quadratic growth over time (in word types or MLU); π3i represents child i’s cubic change over time (in word types or MLU). The residual in equation 1 (εit) represents that portion of child i’s word types or MLU production at age t that is not predicted by age. The Level 2 or between-person portion of the multilevel model for change uses the individual growth parameters from the Level 1 (within-person) model as outcome measures and enables us to determine whether children vary in their initial status, linear, quadratic, or cubic rates of change, and if so, what predicts that variation. For the following growth modeling analyses, child MLU values were multiplied by 100 to avoid potential rounding and nonconvergence problems (Singer & Willett, 2003).
Table 2 presents the unconditional growth models for word types and MLU for the sample of TD children and children with BI combined. Although we expected the variance of observations to be larger for the children with brain injury than the typically developing children (which would have led to a violation of the homoscedasticity assumption), we did not find this to be case. Thus, we include the BI and TD children together in one sample for subsequent analyses.
Table 2.
Estimates of fixed and random effects from individual growth models using child age to predict average child word types and MLU production at 30-months (intercept) and linear, quadratic, and cubic rate of change (simultaneously) in child word type and MLU production between 14 and 46 months in the combined sample of TD children and children with BI (n = 80).
| Parameter Estimate (standard error) | ||
|---|---|---|
| Word Types | MLU | |
| Fixed Effects | ||
| Intercept | 186.51*** | 213.22*** |
| (8.56) | (6.74) | |
| Age | 12.64*** | 10.37*** |
| (0.42) | (0.44) | |
| Age2 | −0.17*** | −0.01 |
| (0.03) | (0.02) | |
| Age3 | −0.02*** | −0.01*** |
| (0.002) | (0.00) | |
| Variance Components | ||
| Level 2: Intercept | 5125.57*** | 3145.60*** |
| (71.59) | (56.09) | |
| Level 2: Slope (linear) | 4.93*** | 5.43*** |
| (2.22) | (2.33) | |
| Level 2: Slope (quadratic) | 0.04** | 0.02*** |
| (0.20) | (0.15) | |
| Goodness of fit | ||
| Deviance (−2LL) | 5850.03 | 5820.95 |
p<.05
p<.01
p<.001
Notes: The child MLU outcome measure was multiplied by 100. Age is measured in months and centered at 30 months
For child word types, the fixed effects for intercept, linear, quadratic, and cubic terms are all statistically significant (see Table 2). The effects tell us that the intercept is significantly different from zero, and that children’s linear, quadratic and cubic growth in vocabulary changes significantly over time. We estimate that the average child spoke approximately 187 word types at 30 months, and that the child’s linear rate of growth in word types at this age was approximately 13 words per month. The significant Level 2 random effects6 tell us that there is sufficient variation in the intercept and growth rates to be explained by predictor variables.
For child MLU, the fixed effects for the intercept, linear, and cubic terms are all statistically significant. These effects tell us that the intercept is significantly different from zero, and that children’s linear and cubic growth in MLU changes at a significant rate over time. The quadratic growth, or rate of acceleration, was not statistically different from zero. We estimate that the average child had an MLU of approximately 2.13 at 30 months, and that the child’s linear rate of growth in MLU at this age was approximately 0.10 words per month. As for vocabulary, the significant Level 2 random effects tell us that there is sufficient variation in the intercept and growth rates to be explained by predictor variables.
Explaining variation in child language growth
The role of input in the language development of TD children and children with BI
Before examining the role of input as a predictor of children’s language development it is important to examine whether the caregiver input differs for children with BI and TD children. Our analyses did not show any differences in input measures for parents of the TD children, and parents of the children with BI. Specifically, parents of TD children produced an average of 426 (SD = 126) word types and an MLU of 4.07 (SD = 0.61) at 30-months, compared to 430 (SD = 137) word types and an MLU of 4.03 (SD = 0.57) for parents of children with BI. We were surprised by this finding, as some previous research has found that parents of children with Specific Language Impairment or language delay use more simplified speech with their children than parents of typically-developing children (see Conti-Ramsden, 1994 for a review). We examined the input further by conducting individual t-tests on parent word types and MLU at the later ages (38–46 months), as we were curious about whether parents of children with BI produced less diverse vocabulary and/or more simplified input later when their children, on average, lagged further behind the TD children. The only effect we found was a marginal difference in parent MLU at 46 months (p <.10), suggesting that parents of TD children used slightly longer sentences at that time. Again, these comparisons need to be interpreted cautiously because the families in the BI group changed over time. Thus, the parents of TD children and the parents of children with BI in our sample used similar language when addressing their children.
To determine whether language input has an effect on intercept or growth rates of child vocabulary or utterance length, we include parent input as a level 2 predictor in our growth models, along with parent education as a socioeconomic control. We also include a dummy variable for brain injury status (BI vs. TD) and test whether the effects of input differ for BI versus TD children by including interaction effects in our models. The final models predicting intercept and growth in child word types and MLU using parent education, parent input, brain injury status, and the interaction between input and brain injury status are presented in Table 3 and displayed in Figure 2.
Table 3.
Estimates of fixed effects from individual growth models in which SES (parent education), language input (word types or MLU), brain injury status (BI versus TD), and the interaction between input and brain injury status predict average child word types and MLU production at 30-months (intercept) and linear, quadratic, and cubic rate of change (simultaneously) in child word type and MLU production between 14 and 46 months in the combined sample of TD children and children with BI (n = 80).
| Parameter Estimate (standard error) | |||
|---|---|---|---|
| Fixed Effects Time/Age | Word Types | MLU | |
| Intercept | 183.55*** | 210.12*** | |
| (7.52) | (5.75) | ||
| Age | 12.62*** | 9.94*** | |
| (0.48) | (0.47) | ||
| Age2 | −0.17*** | −0.01 | |
| (0.03) | (0.02) | ||
| Age3 | −0.02*** | −0.01*** | |
| (0.002) | (0.00) | ||
| Education | |||
| Intercept | 4.77 | 3.18 | |
| (3.71) | (2.94) | ||
| Age | 0.40* | 0.27 | |
| (0.20) | (0.21) | ||
| Age2 | −0.003 | −0.02~ | |
| (0.02) | (0.01) | ||
| Age3 | −0.00 | −0.00 | |
| (0.00) | (0.00) | ||
| Input | |||
| Intercept | 0.25*** | 39.91*** | |
| (0.06) | (10.04) | ||
| Age | 0.005 | 3.62*** | |
| (0.003) | (0.80) | ||
| Age2 | −0.001** | −0.08~ | |
| (0.00) | (0.04) | ||
| Age3 | −0.00 | −0.01* | |
| (0.00) | (0.00) | ||
| Brain Injury | |||
| Intercept | −35.83* | −45.57** | |
| (16.41) | (12.69) | ||
| Age | −0.40 | −2.25~ | |
| (1.18) | (1.14) | ||
| Age2 | 0.05 | 0.07 | |
| (0.08) | (0.06) | ||
| Age3 | −0.003 | −0.00 | |
| (0.01) | (0.01) | ||
| Input*Brain Injury | |||
| Intercept | ns | 15.30 | |
| (22.40) | |||
| Age | ns | 4.62* | |
| (2.00) | |||
| Age2 | ns | 0.02 | |
| (0.10) | |||
| Age3 | ns | −0.01~ | |
| (0.01) | |||
| Goodness of fit | |||
| −2LL | 5818.88 | 5775.45 | |
p<.10
p<.05
p<.01
p<.001
Note: The child MLU outcome measure was multiplied by 100; Age is centered at 30-months; Education, Input and Brain Injury are centered at their mean values. Input is parent word types for the models predicting child word types, and parent MLU for the models predicting child MLU.
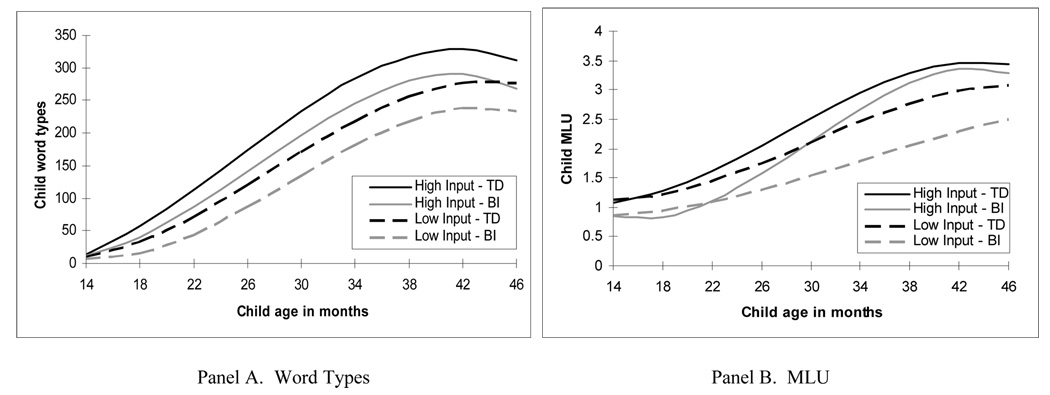
Figure 2.
Effect of parent input and brain injury status (TD vs. BI) on intercept and change over time in child word types (Panel A) and MLU (Panel B), controlling for parent education (n = 80).
In the model predicting word types (first column, Table 3), there was an effect of parent education on linear growth rate (p < .05), an effect of parent input (word types) on intercept (p < .001) and quadratic growth (p < .01), and an effect of brain injury status on intercept (p < .05). There was no interaction between brain injury status and parent input. Panel A of Figure 2 shows a plot of this model, presenting the effects of input and brain injury status on vocabulary growth, controlling for parent education. In this figure, the black lines represent the estimated vocabulary growth for TD children who received high (1 SD above mean, solid black line) and low (1 SD below mean, dashed black line) input. The gray lines represent the estimated vocabulary growth for children with BI who received high (1 SD above mean, solid gray line) and low (1 SD below mean, dashed gray line) input. The effect of brain injury status on the intercept is reflected in the fact that the estimated vocabulary growth for children with BI (the gray lines) is lower, yet parallel, to the corresponding estimated trajectories for TD children (the black lines). The significant effects of parent input on intercept and quadratic growth are reflected in the fact that (1) for all children, those with less input (the dashed lines) have lower estimated vocabularies at 30-months than those with more input (the solid lines), and (2) the acceleration in growth is more rapid for children with high input than for those with low input (the solid lines show steeper increase than the dashed lines). In sum, controlling for input and parent education, children with BI, on average, produce approximately 36 fewer word types than TD children at 30 months, yet they grow in vocabulary at the same rate as the TD children. Importantly, caregiver input is a significant predictor of vocabulary level and acceleration for all children, and the effect of input does not differ based on brain injury status.
In terms of vocabulary growth, controlling for socioeconomic status (i.e., parent education), children whose parents produce a large number of word types during interaction at 30 months have greater estimated vocabularies themselves at 30 months, and accelerate at a faster rate over time, than children of parents who produce fewer word types. This result is consistent with previous findings showing positive effects of input on the vocabulary growth of TD children during this same developmental period (Hart & Risley, 1995; Hoff, 2003; Huttenlocher et. al., 1991; Pan et. al., 2005). The fact that input plays the same role in the vocabulary growth of TD children and children with early brain injury indicates that brain injury does not limit a child’s ability to make effective use of the linguistic input in his or her home environment to build a vocabulary. Thus, the process of vocabulary development appears to be the same for children with BI as it is for TD children with respect to the importance of parental linguistic input.
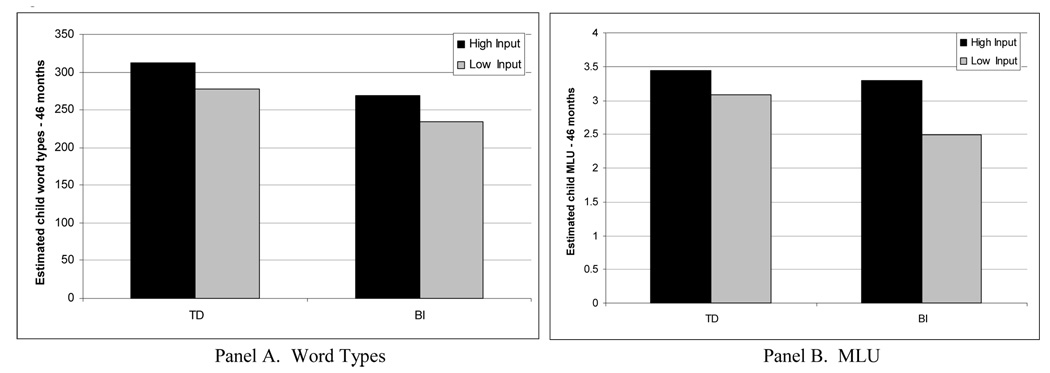
In the model predicting MLU (second column, Table 3), there was no effect of parent education on intercept or growth (the effect on acceleration was marginal), yet we keep education in the model to ensure an SES control. There was an effect of parent input (MLU) on intercept (p < .001), linear (p < .001), quadratic (p = .06), and cubic (p < .05) growth. There also was an effect of brain injury status on intercept (p < .001) and on linear growth (p = .05). More importantly, unlike the patterns for vocabulary growth, there was a significant interaction effect between input and brain injury status on linear growth (p < .05), and a marginal interaction effect on cubic growth (p < .10) for MLU. Figure 2, Panel B, shows a plot of this model, presenting the effects of input and brain injury status on growth in MLU, controlling for parent education. In this figure, the black lines represent the estimated growth in MLU for TD children who received high (1 SD above mean, solid black line) and low (1 SD below mean, dashed black line) input. The gray lines represent the estimated growth in MLU for children with BI who received high (1 SD above mean, solid gray line) and low (1 SD below mean, dashed gray line) input. The significant interaction effect between brain injury status and input on linear growth is represented by the fact that there is a greater difference between the estimated rates of growth in MLU for the high and low input children with BI (the difference between the solid and dashed gray lines) than for the high and low input TD children (the difference between the solid and dashed black lines). The estimated effects of input for the TD and BI groups at 46 months based on the models presented in Table 2 are displayed in Figure 3. For example, at child age 46 months, the difference in the estimated MLU of TD children who experience high and low caregiver MLU is 0.36 words per utterance, compared to 0.80 words per utterance for children with BI who experience high and low caregiver MLU input. By 46 months, the estimated MLUs of TD children who receive high or low input and of BI children who receive high input are all at least 3.0 words per utterance, whereas the estimated MLU of children with BI who receive low input is only 2.5.
Figure 3.
Estimated child word types (Panel A) and MLU (Panel B) at 46 months for TD children and children with BI whose parents produce high (1 SD above mean) and low (1 SD below mean) input based on final growth models presented in Table 3 (n = 80).
In sum, linguistic input (parent MLU) is a more potent predictor of growth in MLU for children with BI than for TD children. Controlling for socioeconomic status (i.e., parent education), the difference in rate of growth in MLU between children whose parents produce high and low input is greater for children who have experienced brain injury than it is for typically developing children. In fact, the children with BI who receive high MLU input look almost identical in terms of MLU growth between 30 and 46 months to the TD children who receive high MLU input. In contrast, the children with BI who receive low MLU input show very slow growth in MLU across the entire developmental period, and are even slower than TD children who receive low input.
What might it mean that linguistic input has a greater effect on the syntactic growth of children with BI than TD children? One possibility is that brain injury makes it difficult for children to compensate for impoverished linguistic input on relatively difficult aspects of linguistic processing such as syntax learning (as opposed to easier tasks such as vocabulary learning). TD children who experience below-average MLU input may be able to capitalize on the small number of long utterances that they do hear, using them in a way that children with BI cannot, to ultimately produce more complex utterances of their own. Previous research has, in fact, shown that children who do not have brain injury are able to make generalizations from relatively sparse and inconsistent data. For example, deaf children learning American Sign Language (ASL) from deaf parents who were late-language-learners and thus inconsistent users of ASL were able to generalize from their spotty input, producing highly consistent sentences themselves (Singleton & Newport, 2004). Children with BI might need more consistent, high quality input as the basis for generalization. Indeed, our findings suggest that if they receive high quality input, children with BI can achieve syntactic growth comparable to growth in TD children; but if they do not, their syntactic growth may suffer more than it does in TD children.
Although input appears to be having a different effect on syntax development than it has on vocabulary development in children with and without brain injury, the crucial difference may not be type of linguistic skill but rather difficulty level. In other words, if we looked at measures of more complex vocabulary, we might find the same interaction effect between children with and without brain injury for vocabulary growth as we have found for sentence growth. If so, our findings may generalize to harder vs. easier tasks in other domains. That is, input may be differentially important for children with BI on harder tasks, regardless of domain (Levine et al., 2005).
The role of lesion characteristics in language growth of children with BI
We have found that linguistic input plays an important role in accounting for vocabulary and MLU growth in children with BI. What about lesion characteristics? In the next analyses, we control for parent education and linguistic input and ask whether lesion laterality (right vs. left), lesion size (S, M, L), lesion type (CV vs. PV), and seizure history (Y vs. N) predict vocabulary or MLU growth in the children with BI. We include these factors in our growth models in place of the brain injury status dummy variable used in our previous HLM analysis. Our initial analyses indicated that lesion laterality (right vs. left hemisphere) was not a significant predictor of intercept or growth in child word types or MLU. We therefore do not consider lesion laterality in further analyses.
In predicting vocabulary growth, controlling for parent education and input, we found that when each characteristic is considered on its own, lesion size and type both predict vocabulary intercept. Specifically, children with large lesions produce an estimated 52 word types fewer at 30 months than TD children (p < .05); children with medium and small lesions do not differ significantly from TD children. Similarly, children with CV lesions produce an estimated 58 word types fewer at 30 months than TD children (p < .01); children with PV lesions do not differ from TD children. These significant effects of lesion size and type support previous findings that larger lesions result in greater language and cognitive deficits than smaller lesions (Banich et al., 1997; Booth et al., 2000; Levine et al., 2005) and that type of lesion may be an important factor in predicting developmental outcomes, possibly due to the timing of these lesions during development (Staudt et al., 2004). Lesion size also has an effect on linear growth—children with medium lesions increase their word type production at an estimated rate of 5.9 words-per-month slower than TD children (p < .01); children with large or small lesions do not differ from TD children in growth rates. This finding supports Bates’ hypothesis (1999) that there is a curvilinear relation between lesion size and language outcomes (this result should be interpreted cautiously, however, as we had only 7 children with medium-sized lesions in our sample). There were no differences between TD children and BI children who did or did not experience seizures.
In predicting MLU growth, controlling for parent education and input, we found that when each lesion characteristic is considered on its own, lesion size, type, and seizure history all predict MLU intercept, and lesion size and type also predict linear growth of MLU. Specifically, Children with BI who have large lesions have an estimated MLU at 30 months that is 0.53 words smaller than TD children (p < .01); children with small and medium lesions showed no significant differences. The linear rate of change in MLU for children with large lesions is 0.04 words per utterance per month slower than TD children (p = .06); children with small and medium lesions showed no significant differences in rate of change relative to TD children. Similarly, children with CV lesions have an estimated MLU at 30 months that is 0.60 words smaller than TD children (p < .001); children with PV lesions showed no significant differences. The linear rate of change in MLU for children with CV lesions is 0.04 words per utterance per month slower than TD children (p < .05); children with PV lesions showed no significant differences in rate of change relative to TD children. Here again, the results support previous findings that lesion size and type are important factors in predicting developmental outcomes (Banich et al., 1997; Booth et al., 2000; Levine et al., 2005; Staudt et al., 2004). Finally, children who experienced seizures have an estimated MLU at 30 months that is 0.39 words smaller than TD children (p = .06); children who did not experience seizures do not differ from TD children in estimated MLU at 30 months. In the above models of word type and MLU growth, parent input measures always remained significant when considering the effects of lesion characteristics. Further, we found no significant interactions between input and specific lesion characteristics.
Our analysis of biological characteristics of lesions shows that the overall differences between BI and TD children in estimated word types at 30 months (Table 3 and Figure 2A) and in MLU intercept and growth (Table 3, Figure 2B) are driven by the children with BI who have large lesions, have CV lesions, and, to a lesser extent, experienced seizures (for MLU only). We are not able to disentangle the effects of lesion size, type, or seizure history because of sample size limitations and the collinearity of predictors (of the 10 children with large lesions, 9 have CV lesions).
In sum, taken together, our results indicate that, controlling for environmental factors (linguistic input, SES), lesion characteristics remain important predictors of language development in children with early unilateral brain injury. Conversely, when controlling for lesion characteristics, environmental factors continue to play an important role in predicting language development in children with early unilateral brain injury (i.e., the effects of linguistic input and parent education remain significant). Our findings thus point to the importance of lesion characteristics and linguistic input in the language learning of children with BI. It is important to note that our findings do not show causal relations, nor do they determine who is influencing whom (parent or child). With that caveat in mind, the results suggest that linguistic input plays the same role in vocabulary development in children with early brain injury as it does in typically developing children. However, linguistic input appears to play a more critical role in the development of early sentences in children with early unilateral brain injury than in children without brain injury.
Conclusion
Previous research highlights the impact of the environment and experience on the growing brain. Variations in early life stress and cognitive stimulation lead to measurable differences in the brains of animals (Greenough, Black & Wallace, 1987), and differences in social class affect neurocognitive systems (including language systems) in human children (Noble, Norman & Farah, 2005). The effects of social class on language systems are presumed to be due, in part, to the linguistic input that caregivers provide their children during the early stages of language learning (Hart & Risley, 1995; Huttenlocher et al, 1991).
Although we know of no prior empirical research investigating the effects of linguistic input in children with brain injury, researchers have hypothesized that input may be more important in the language development of children with “atypical biological potentials” than in children who are developing typically (Wilcox & Shannon, 1996). We put this hypothesis to the test in our study and found that it holds for the more complex aspects of language (early sentence generation), but does not hold for simpler aspects (early vocabulary acquisition). Thus, it is essential to pay attention to linguistic input, as well as the characteristics of brain lesions, in explaining the functional plasticity that underlies language learning. We have found that the same variation in linguistic input can lead to wider variations in linguistic development in children with brain injury than in uninjured children, suggesting that attention to environmental input may be that much more important in children who come to language-learning with early focal brain injuries.
Our findings are consistent with recent pediatric research stressing that early environmental interventions may prevent adverse neurodevelopmental outcomes in extremely low-birth-weight infants, particularly those born into poverty (Msall, 2004a; 2004b; Msall, Nier, LaGasse, Tremont & Lester, 1998). Our findings suggest that enhancing the early childhood environments of children with early pre- or peri-natal brain injury may lessen risks of language and cognitive delays in this population as well.
Studying the effects of early brain injury is, of course, important for understanding language development in children with brain lesions and holds the promise of laying the basis for future interventions. But brain injury also creates a “natural experiment,” allowing us to ask questions about the role that the environment (in particular, linguistic input) plays in cases where development is at risk. The study of children with brain injury thus provides evidence that there are cumulative effects of lesion characteristics and environmental variation on the communicative abilities of young children. Since we cannot alter lesion characteristics, our best bet is to focus our interventions on environmental factors. Determining how much and in what ways the environment needs to be manipulated to offset organic injury is our next challenge.
Acknowledgements
We thank Peter Huttenlocher and Martin Staudt for help in coding brain lesions, Stephen Raudenbush for analytical advice, Adele Diamond and two anonymous reviewers for helpful comments on the manuscript, Kristi Schonwald and Jason Voigt for administrative and technical support, and Karyn Brasky, Laura Chang, Elaine Croft, Kristin Duboc, Jennifer Griffin, Sarah Gripshover, Kelsey Harden, Lauren King, Carrie Meanwell, Erica Mellum, Molly Nikolas, Jana Oberholtzer, Lilia Rissman, Becky Seibel, Meredith Simone, Calla Trofatter, Kevin Uttich, and Julie Wallman for help in data collection and transcription. We are grateful to the participating children and families. The research was supported by grants from the National Institute of Child Health and Human Development: P01HD40605 to Susan Goldin-Meadow and F32HD045099 to Meredith L. Rowe, and by a grant from the Brain Research Foundation to Susan C. Levine.
Footnotes
The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/dev/
Eleven TD children from the larger sample were excluded from this analysis because the children were either diagnosed with a disorder that affected their language development (n =1), had primary caregivers who changed over the course of the study (n = 2), or had two primary caregivers present during data collection and thus participated in triadic rather than dyadic interaction (n = 8). Thirteen of the Children with BI from the larger sample were excluded from this analysis because they either entered the study after 46 months of age (n = 4), had two primary caregivers present during data collection (n = 3), or do not yet have the detailed information on their brain lesions necessary for the current analysis (n = 6).
We opted to use number of word types produced at each session rather than calculating cumulative vocabulary size over time for several reasons. First, calculating cumulative vocabularies would be problematic in the BI sample of children since many missed several visits. Furthermore, previous research examining growth in non-cumulative vocabulary word use (Pan et al., 2005) shows similar effects of input as those examining cumulative vocabulary growth (Huttenlocher et al., 1991) during this developmental period.
We also investigated using parent word types as a predictor of child growth in MLU, yet parent MLU was a stronger predictor of child MLU than was parent word types.
Parent education serves as a socioeconomic control in this study. Education and income were positively related (r = .44), and previous work with this sample shows that parent education is more strongly related to parent input and child language outcomes (than family income) (Huttenlocher et al., 2007).
There were a few TD children who did not talk at all at 14 months, and thus had an MLU of zero. All children with BI produced at least one word.
The random cubic term was “fixed” in models for word types and MLU, as there was little variance to explain and the model with the cubic term fixed fit better than the models where the term was allowed to vary. In addition, models predicting child vocabulary (word types) were fit with the level-1 variance allowed to increase over time in order to account for the fact that, as children got older and increased in language abilities, variation increased.
References
- Annet M. Handedness in families. Annals of Human Genetics. 1973;37:93–105. doi: 10.1111/j.1469-1809.1973.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Aram DM, Ekelman BL. Cognitive profiles of children with early onset of unilateral lesions. Developmental Neuropsychology. 1986;2(3):115–172. [Google Scholar]
- Aram DM, Ekelman BL, Rose DF, Whitaker HA. Verbal and cognitive sequel following unilateral lesions acquired in early childhood. Journal of Clinical & Experimental Neuropsychology. 1985;7(1):55–78. doi: 10.1080/01688638508401242. [DOI] [PubMed] [Google Scholar]
- Baranes R, Albro E, Levine SC. Narrative production after early unilateral lesions. Biannual Meeting of the Society for Research on Child Development; New Orleans. 1993. [Google Scholar]
- Barnes S, Gutfreund M, Satterly D, Wells G. Characteristics of adult speech which predicts children’s language development. Journal of Child Language. 1983;10(1):65–84. doi: 10.1017/s0305000900005146. [DOI] [PubMed] [Google Scholar]
- Bates E, Roe K. Language development in children with unilateral brain injury. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001. pp. 281–307. [Google Scholar]
- Bates E, Reilly J, Wulfeck B, Druonkers N, Opie M, Fenson J, Kriz S, Jeffries R, Miller L, Herbst K. Differential effects of unilateral lesions on language production in children and adults. Brain and Language. 2001;79(2):223–265. doi: 10.1006/brln.2001.2482. [DOI] [PubMed] [Google Scholar]
- Bates E, Thal D, Trauner D, Fenson J, Aram D, Eisele J, Nass R. From first words to grammar in children with focal brain injury. Developmental Neuropsychology. 1997;13:447–476. [Google Scholar]
- Bates E, Vicari S, Trauner D. Neural mediation of language development: Perspectives from lesion studies of infants and children. In: Tager-Flusberg H, editor. Neurodevelopmental Disorders. Cambridge, MA: MIT Press; 1999. pp. 533–581. [Google Scholar]
- Bee HL, Van Egeren LF, Streissguth AP, Nyman BA, Leckie MA. Social class differences in maternal teaching strategies and speech patterns. Developmental Psychology. 1969;1:726–734. [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic JT, Feldman HM. Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Developmental Neuropsychology. 2000;18:139–169. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- Brasky K, Nikolas M, Meanwell C, Levine S, Goldin-Meadow Language development in children with unilateral brain injury: Effects of lesion size. Poster presented at Symposium on Research in Child Language Disorders; Madison, Wisconsin. 2005. [Google Scholar]
- Brown R. A first language: The early stages. Cambridge MA: Harvard University Press; 1973. [Google Scholar]
- Chilosi AM, Cipriani P, Bertuccelli B, Pfanner L, Cioni G. Early cognitive and communication development in children with focal brain lesions. Journal of Child Neurology. 2001;16:309–319. doi: 10.1177/088307380101600502. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G. Language interaction with atypical language learners. In: Gallaway C, Richards B, editors. Input and interaction in language acquisition. Cambridge, UK: Cambridge University Press; 1994. pp. 183–196. [Google Scholar]
- Dall’Oglio AM, Bates E, Volterra V, Di Capua M, Pezzini G. Early cognition, communication and language in children with focal brain injury. Developmental Medicine and Child Neurology. 1994;36:1076–1098. doi: 10.1111/j.1469-8749.1994.tb11810.x. [DOI] [PubMed] [Google Scholar]
- Dennis M, Whitaker HA. Language acquisition following hemidecortication: Linguistic superiority of the left over the right hemisphere. Brain and Language. 1976;3:404–433. doi: 10.1016/0093-934x(76)90036-5. [DOI] [PubMed] [Google Scholar]
- Fair DA, Brown TT, Peterson SE, Schlaggar BL. fMRI reveals novel functional neuroanatomy in a child with perinatal stroke. Neurology. 2006;67:2246–2249. doi: 10.1212/01.wnl.0000249348.84045.0e. [DOI] [PubMed] [Google Scholar]
- Farian DC, Haskins R. Reciprocal influence in the social interaction of mothers and three-year-old children from different socioeconmic backgrounds. Child Development. 1980;51:780–791. [Google Scholar]
- Feldman HM. Language learning with an injured brain. Language Learning and Development. 2005;3 & 4:265–288. [Google Scholar]
- Feldman HM, Holland AL, Kemp SS, Janowsky JE. Language development after unilateral brain injury. Brain and Language. 1992;42(1):89–102. doi: 10.1016/0093-934x(92)90058-m. [DOI] [PubMed] [Google Scholar]
- Hart B, Risley T. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995. [Google Scholar]
- Hoff E. The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Development. 2003;74:1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, Lyons T. Early vocabulary growth: Relation to language input and gender. Developmental Psychology. 1991;27(2):236–248. [Google Scholar]
- Huttenlocher PR. Neural plasticity: the effects of environment on the development of the cerebral cortex. Cambridge, MA: Harvard University Press; 2002. [Google Scholar]
- Huttenlocher PR, Hapke RJ. A follow-up study of intractable seizures in childhood. Annals of Neurology. 1990;30(1):115. doi: 10.1002/ana.410280516. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Vasilyeva M, Cymerman E, Levine SC. Language input at home and at school: Relation to syntax. Cognitive Psychology. 2002;45:337–374. doi: 10.1016/s0010-0285(02)00500-5. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Vasilyeva M, Waterfall H, Vevea J, Hedges L. The varieties of speech to young children. Developmental Psychology. 2007;43:1062–1083. doi: 10.1037/0012-1649.43.5.1062. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure in present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: A systematic review. Developmental Medicine & Child Neurology. 2007;49:144–151. doi: 10.1111/j.1469-8749.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- Kolb B. Brain plasticity and behavior. Mahwah, NJ: Erlbaum; 1995. [Google Scholar]
- Lansdell H. Verbal and Nonverbal Factors in Right-Hemisphere Speech: Relation to Early Neurological History. J. Comp. Physiol. Psychol. 1969;LXIX:734–738. doi: 10.1037/h0028306. [DOI] [PubMed] [Google Scholar]
- Levine SC, Brasky K, Nikolas M. The role of gesture in language development in brain injured children. Paper presented at Interanational Association for the Study of Child Language; Berlin, Germany. 2005. [Google Scholar]
- Levine SC, Huttenlocher P, Banich M, Duda E. Factors affecting the cognitive functioning of hemiplegic children. Developmental Medicine and Child Neurology. 1987;29:27–35. doi: 10.1111/j.1469-8749.1987.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Levine SC, Kraus R, Alexander E, Suriyakham LW, Huttenlocher P. IQ decline following early unilateral brain injury: A longitudinal study. Brain and Cognition. 2005;59(2):114–123. doi: 10.1016/j.bandc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Current Opinions in Pediatrics. 2001;13:499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- MacWhinney B, Feldman H, Sacco K, Vaaldes-Perez Online measures of basic language skills in children with early focal brain lesions. Brain and Language. 2000;71:400–431. doi: 10.1006/brln.1999.2273. [DOI] [PubMed] [Google Scholar]
- Marchman V, Miller R, Bates E. Babble and first words in children with focal brain injury. Applied Psycholinguistics. 1991;12:1–22. [Google Scholar]
- Msall ME. Supporting vulnerable preschool children: Connecting the dots before kindergarten. Pediatrics. 2004a;114(4):1086. doi: 10.1542/peds.2004-1655. [DOI] [PubMed] [Google Scholar]
- Msall ME. Developmental vulnerability and resilience in extremely preterm infants. JAMA. 2004b;292(19):2399–2401. doi: 10.1001/jama.292.19.2399. [DOI] [PubMed] [Google Scholar]
- Msall ME, Bier J, LaGasse L, Tremont M, Lester B. The vulnerable preschool child: The impact of biomedical and social risks on neurodevelopmental function. Seminars in Pediatric Neurology. 1998;5(1):52–61. doi: 10.1016/s1071-9091(98)80019-3. [DOI] [PubMed] [Google Scholar]
- Pan BA, Rowe ML, Singer JD, Snow CE. Maternal correlates of growth in toddler vocabulary production in low-income families. Child Development. 2005;76:763–782. doi: 10.1111/j.1467-8624.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Price P. A study of mother-child interaction strategies with mothers of young developmentally delayed children. In: Berg J, editor. Perspectives and progress in mental retardation (Sixth Congress of the International Association for the Scientific Study of Mental Deficiency) Baltimore, MD: University Park Press; 1984. [Google Scholar]
- Rankin JM, Aram DM, Horowitz SJ. Language ability in right and left hemiplegic children. Discussions in Neuroscience. 1981;10(2):130–136. doi: 10.1016/0093-934x(81)90081-x. [DOI] [PubMed] [Google Scholar]
- Raja AC, Josse G, Suriyakham LW, Fisher JA, Huttenlocher PR, Levine SC, Small SL. Regional brain activation after early left and right hemisphere stroke: Relation to cognitive functioning. 12th Annual Meeting of the Organization for Human Brain Mapping; July 2006; Florence, Italy. 2006. [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining the lateralization of cerebral speech functions. Annals of the New York Academy of Sciences. 1977;229:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Reilly JS, Bates EA, Marchman VA. Narrative discourse in children with early focal brain injury. Brain and Language. 1998;61:333–375. doi: 10.1006/brln.1997.1882. [DOI] [PubMed] [Google Scholar]
- Riva D, Cazzaniga L. Late effects of brain lesions sustained before and after age one. Neuropsychologia. 1986;24(3):423–428. doi: 10.1016/0028-3932(86)90029-1. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd edition. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Raudenbush S, Bryk A, Cheong YF, Congdon R. HLM5: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International; 2000. [Google Scholar]
- Rowe ML, Pan BA, Ayoub C. Predictors of variation in maternal talk to children: A longitudinal study of low-income families. Parenting: Science and Practice. 2005;5:285–310. [Google Scholar]
- Singleton JL, Newport EL. When learners surpass their models: The acquisition of American Sign Language from inconsistent input. Cognitive Psychology. 2004;49:370–407. doi: 10.1016/j.cogpsych.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Staudt M, Gerloff C, Grodd W, Hodhausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Annals of Neurology. 2004;56:854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lidzba K, Grodd W, Wildgruber D, Erb M, Krageloh-Mann I. Right-hemispheric organization of language following early left-sided brain lesions: Functional MRI topography. Neuroimage. 2002;16:954–967. doi: 10.1006/nimg.2002.1108. [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends in Cognitive Sciences. 2005;9:136–143. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Strauss E, Satz P, Wada J. An examination of the crowding hypothesis in epileptic patients who have undergone the carotid amytal test. Neuropsychologia. 1990;28:1221–1227. doi: 10.1016/0028-3932(90)90057-u. [DOI] [PubMed] [Google Scholar]
- Thal D, Marchman V, Stiles J, Aram D, Trauner D, Nass R, Bates E. Early lexical development in children with focal brain injury. Brain and Language. 1991;40:491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Muter V. A review of cognitive outcome after unilateral lesions sustained during childhood. Journal of Child Neurology. 1994;9:67–73. [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Van Der Werf S, Robb S, Wilson J. Development of intelligence and memory in children with hemiplegic cerebral palsy. Brain. 1992;115:315–329. doi: 10.1093/brain/115.1.315. [DOI] [PubMed] [Google Scholar]
- Weckerly J, Wulfeck B, Reilly J. The development of morphosyntactic ability in atypical populations: The acquisition of tag questions in children with early focal lesions and children with specific-language impairment. Brain and Language. 2004;88(2):190–201. doi: 10.1016/S0093-934X(03)00098-1. [DOI] [PubMed] [Google Scholar]
- Wilcox MJ, Shannon M. Integrated early intervention practices in speech-language pathology: Issues, strategies, and further directions. In: McWilliam R, editor. Proceedings of the 19th Annual Boston University Conference on Language Development. Vol 2 1996. [Google Scholar]
- Woods BT, Teuber H. Mirror movements after child hemiparesis. Neurology. 1978;28(11):1152–1157. doi: 10.1212/wnl.28.11.1152. [DOI] [PubMed] [Google Scholar]