Abstract
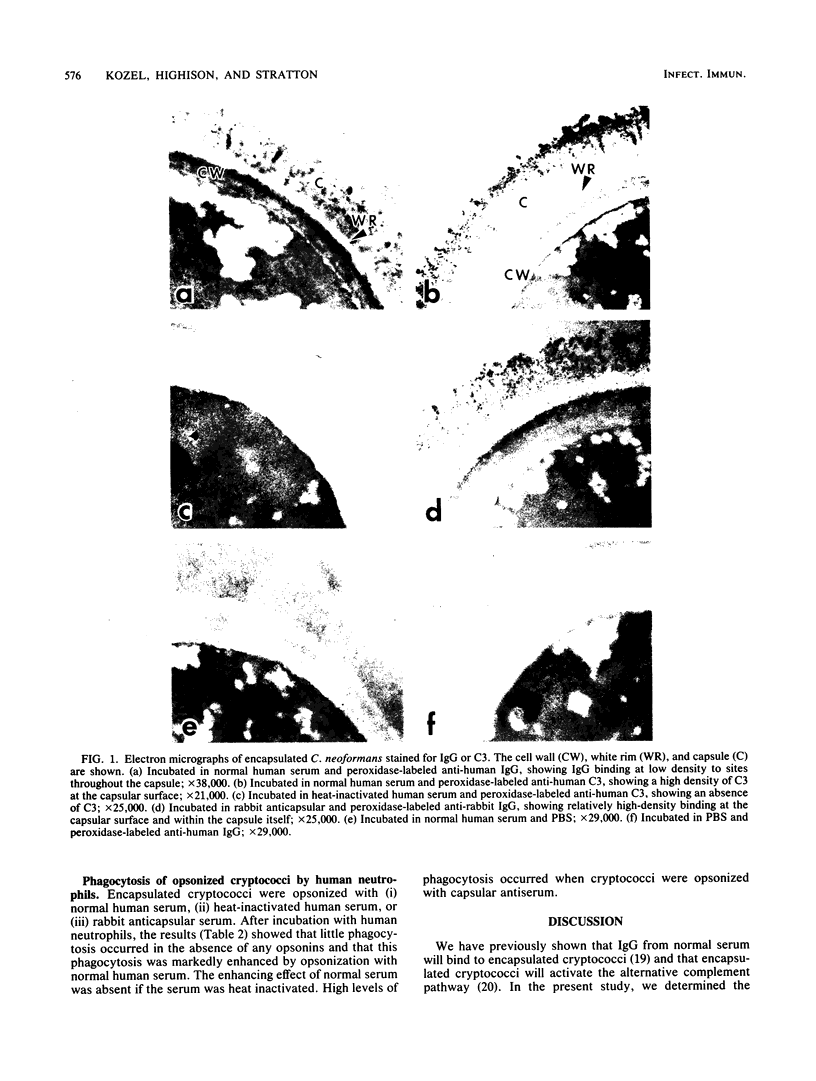
Previous studies have shown that the cryptococcal capsule inhibits phagocytosis of Cryptococcus neoformans by macrophages and neutrophils. In this study, the binding sites of potential serum opsonins in immune and nonimmune sera were determined by immunoelectron microscopy, and the results were compared with the results of phagocytosis of the yeasts by mouse peritoneal macrophages and human neutrophils. Immunoglobulin G (IgG) from normal human serum showed low-density binding at the capsular surface and at sites throughout the capsule. Complement component C3 from normal serum bound heavily at the capsular surface. IgG from rabbit capsular antiserum showed relatively dense deposition at the capsular surface and at sites throughout the capsule. Cells opsonized with heat-inactivated human serum were engulfed poorly by both macrophages and neutrophils, indicating that the low-density deposition of IgG produced by normal serum was not adequate for opsonization. Yeasts opsonized with normal human serum were engulfed in large numbers by neutrophils and to a lesser extent by macrophages, indicating that neutrophils in particular were able to effectively utilize the opsonically active C3 which normal human serum deposited at the capsular surface. Yeasts opsonized with rabbit anticapsular serum were engulfed by both macrophages and neutrophils, indicating that the high density of surface IgG produced by capsular antiserum is an effective opsonin for both cells. These results suggest that the complement-neutrophil system is a possible defense mechanism in the nonimmune host.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. 3. Inhibition of phagocytosis. J Bacteriol. 1968 Jan;95(1):5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M., Frank M. M., Bennett J. E. The role of late complement components and the alternate complement pathway in experimental cryptococcosis. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):312–315. doi: 10.3181/00379727-144-37580. [DOI] [PubMed] [Google Scholar]
- Edwards M. R., Gordon M. A., Lapa E. W., Ghiorse W. C. Micromorphology of Cryptococcus neoformans. J Bacteriol. 1967 Sep;94(3):766–777. doi: 10.1128/jb.94.3.766-777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Warren J. Immunofluorescence studies of reactions at the Cryptococcal capsule. J Infect Dis. 1968 Apr;118(2):215–229. doi: 10.1093/infdis/118.2.215. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr Roles of macrophage Fc and C3b receptors in phagocytosis of immunologically coated Cryptococcus neoformans. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3853–3857. doi: 10.1073/pnas.78.6.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. F., Wilkinson B. J. Binding of human immunoglobulin G to protein A in encapsulated Staphylococcus aureus. Infect Immun. 1981 Sep;33(3):666–672. doi: 10.1128/iai.33.3.666-672.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Follette J. L. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect Immun. 1981 Mar;31(3):978–984. doi: 10.1128/iai.31.3.978-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., McGaw T. G. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979 Jul;25(1):255–261. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977 Apr;16(1):99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxalt K. A., Kozel T. R. Chemotaxigenesis and activation of the alternative complement pathway by encapsulated and non-encapsulated Cryptococcus neoformans. Infect Immun. 1979 Nov;26(2):435–440. doi: 10.1128/iai.26.2.435-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaw T. G., Kozel T. R. Opsonization of Cryptococcus neoformans by human immunoglobulin G: masking of immunoglobulin G by cryptococcal polysaccharide. Infect Immun. 1979 Jul;25(1):262–267. doi: 10.1128/iai.25.1.262-267.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. G., Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972 Apr;5(4):491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Pease D. C., Peterson R. G. Polymerizable glutaraldehyde-urea mixtures as polar, water-containing embedding media. J Ultrastruct Res. 1972 Oct;41(1):133–159. doi: 10.1016/s0022-5320(72)90043-3. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Wilkinson B. J., Schmeling D., Michael A. F., Quie P. G. Dichotomy between opsonization and serum complement activation by encapsulated staphylococci. Infect Immun. 1978 Jun;20(3):770–775. doi: 10.1128/iai.20.3.770-775.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- Swanson J., Gotschlich E. C. Electron microscopic studies on streptococci. II. Group A carbohydrate. J Exp Med. 1973 Jul 1;138(1):245–258. doi: 10.1084/jem.138.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Nguyen B. Y., Sisson S. P., Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982 Oct;129(4):1681–1687. [PubMed] [Google Scholar]
- Wilkinson B. J., Peterson P. K., Quie P. G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsule: model for the antiphagocytic effect of bacterial cell surface polymers. Infect Immun. 1979 Feb;23(2):502–508. doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K. Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun. 1979 Dec;26(3):1159–1163. doi: 10.1128/iai.26.3.1159-1163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




