Abstract
Previous studies have shown that the right hemisphere processes the visual details of objects and the emotionality of information. These two roles of the right hemisphere have not been examined concurrently, and in the present study, we examined whether right hemisphere processing would lead to particularly good memory for the visual details of emotional stimuli. Participants viewed positive, negative, and neutral objects, displayed to the left or right of a fixation cross. Later, participants performed a recognition task in which they evaluated whether items were “same,” (same visual details) “similar,” (same verbal label, different visual details) or “new” (unrelated) in comparison to the studied objects. Participants remembered the visual details of negative items well, and this advantage in memory specificity was particularly pronounced when the items had been presented directly to the right hemisphere (i.e., to the left of the fixation cross). These results suggest that there is an episodic memory benefit conveyed when negative items are presented directly to the right hemisphere, likely because of the specialization of the right hemisphere for processing both visual detail and also negatively valenced emotional information.
We sometimes remember information with tremendous visual specificity. Yet at other times, our memories can lack visual details. We may be able to describe in perfect detail the dress that our favorite movie star wore to the Oscars, but we may have no idea what our professor was wearing at this morning's meeting.
When it comes to remembering the precise visual details of an object, it is believed that the right hemisphere (RH) may play a particularly important role. Kosslyn and colleagues have proposed that coordinate-based visuospatial processing, including the spatial relationships between objects, or among an object's features, may preferentially occur in the RH, whereas categorical-based processing may arise through left hemisphere (LH) involvement (Kosslyn, 1987; Kosslyn et al., 1989). Marsolek and colleagues have similarly proposed that processing of specific exemplars may operate more efficiently in the RH whereas processing of abstract categories may occur more effectively in the LH (e.g., Burgund & Marsolek, 2000; Marsolek, 1995, 1999).
In support of a distinction between the processing that occurs in the RH and LH, a number of studies have revealed that memory for item-specific information can be improved when stimuli are presented to the left visual field (LVF) and thus processed by the RH. By contrast, reliance on abstract or heuristic information can be enhanced when information is presented in the right visual field (RVF) and thus processed directly by the LH (Marsolek, 1995, 1999; Marsolek, Schacter, & Nichols, 1996; Marsolek, Squire, Kosslyn, & Lulenski, 1994; Marsolek & Burgund, in press). Within the realm of object processing, neuroimaging studies have suggested that the fusiform gyrus, in particular, may process information differently depending upon the hemisphere. The right fusiform gyrus responds strongly to item-specific attributes, such as an object's size, color, shape, or orientation, while the left fusiform gyrus responds more generally to global item features. Thus, the right fusiform gyrus shows robust habituation if the same exemplar of an item is shown repeatedly (e.g., if the same kitten is shown multiple times), but it shows little habituation if different exemplars of the same type of item are shown repeatedly (e.g., if different kittens are shown). By contrast, the left fusiform gyrus shows significant habituation even when it is different exemplars that are repeated (Koutstaal et al., 2001; Simons et al., 2003). Activity in the right fusiform gyrus also shows a strong correspondence to a person's ability to remember the precise visual details of an object, whereas activity in the left fusiform gyrus relates to later memory for the general object type but not for the object's visual details (Garoff et al., 2005; Kensinger, Garoff-Eaton, & Schacter, 2007c).
A separate literature has suggested that the RH may be specialized not only for processing specific object representations but also for processing emotional - and perhaps specifically negative - information (reviewed by Borod, 1992; Davidson, 1992; Kucharska-Pietura, 2006). For example, patients with brain damage in the LH (but a preserved RH) are more likely to dwell on negative information and to suffer from depressive symptoms, while patients with brain damage in the RH are more likely to display a shift away from the negative, sometimes displaying mania (e.g., Lee et al., 1990; Morris et al., 1996; Paradiso et al., 1999; Sackeim et al., 1982; Starkstein et al., 1989). Distinctions between LH and RH processing of emotional information also are readily apparent in healthy individuals. If a chimeric face is presented to a participant, with the left half of the face (presented to the RH) displaying one expression, and the right half of the face (presented to the LH) displaying another expression, participants will be more likely to label the expression presented to the RH (see recent review by Bourne, 2008). Affective priming also is more likely to occur when stimuli are presented to the LVF/RH than when they are presented to the RVF/LH (Sato & Aoki, 2006; see also Collins & Cooke, 2005 for evidence that priming for emotional information can be greater following right hemisphere processing). Though many theories regarding the hemispheric processing of emotion processing have focused on laterality differences within prefrontal cortex (Davidson, 1995; Davidson & Irwin, 1999), it also has been proposed that the right visual cortex is more sensitive to emotional stimuli than the left visual cortex, perhaps because of strong connections between the right amygdala and the right visual cortex (Landis, 2006).
Though a few studies have indicated that implicit memory for emotional items can vary as a function of hemispheric presentation, the present study asked whether this overlap in RH specialization for processing both the visual details of objects and also the emotional content of information would lead to a benefit in episodic memory. In particular, we examined whether memory for the visual details of emotional items would be enhanced when those items were presented to the LVF/RH. It has been shown previously that negative items are more likely than neutral ones to be remembered with specific visual details, but that such benefits occur only with sufficiently long presentation durations (Kensinger, Garoff-Eaton, & Schacter, 2006). The present experiment examined whether benefits in remembering the specific visual details of negative items would occur even when items were presented briefly, if those items were presented directly to the RH (via LVF presentation).
Method
Participants
Participants were 26 Boston College undergraduate or graduate students (18 males and 8 females; mean years of education = 13.5 years) between the ages of 18 and 26 (mean age = 19.5 years). Participants were native English speakers with normal or corrected-to-normal vision. No participant reported taking any medication that affected the central nervous system. No participant reported a history of depression or other psychiatric illness nor any neurological disorder. All participants reported that they were right handed, and handedness was assessed via the Edinburgh Handedness Inventory (Oldfield, 1971). This inventory asks whether participants have a strong or weak handedness preference when performing a series of activities (writing, drawing, throwing, striking match, opening box lid, holding scissors, toothbrush, knife, spoon, or broom handle). Scores for each activity range from 1 (always use left hand) to 5 (always use right hand). This inventory confirmed that all of our participants were strongly right handed (M = 4.5, SE = .06). Informed consent was obtained from all participants in a manner approved by the Boston College Institutional Review Board.
Materials
Materials comprised 180 pairs of photo objects (from Kensinger, Garoff-Eaton, & Schacter, 2007c). Pairs shared the same verbal label (e.g., were both canoes) but differed in other perceptual features (e.g., color, shape, size, orientation). The pairs were selected from a larger set that had been rated for valence and arousal by a separate group of adults. One-third of the selected objects were negative and arousing (i.e., valence less than 3.5 on a 9-point likert scale, with low numbers signifying negative valence; arousal ratings greater than 5 on a 9-point likert scale), one-third were positive and arousing (i.e., valence ratings greater than 5.5 and arousal ratings greater than 5), and one-third were neutral (i.e., valence ratings between 3.5 and 5.5 and arousal ratings less than 5). The positive and negative objects did not differ from one another in arousal nor in absolute valence (i.e., distance from a neutral valence rating of 5), both p>.15, and positive and negative objects were significantly more arousing than neutral ones, p<.001.
Pairs also had been rated for the overall similarity of the two items, the dimensions (color, size, shape, orientation) that differed between the two items, and the familiarity of the items (see Kensinger, Garoff-Eaton, & Schacter, 2006 for details of matching procedures). Negative, positive, and neutral pairs were carefully matched on each of these dimensions (all p>.15). In particular, negative, positive, and neutral objects were selected to be similar in visual complexity and to be from the same types of categories (e.g., included approximately the same number of animals, manmade objects, foods). We also examined the word frequencies for the object names (as reported by Colthart, 1981) and selected items so that there were no differences in word frequency across the three categories of emotional items.
Procedure
During the study phase, participants were asked to view a series of 144 objects (one-third of each emotional valence) while their eyes remained focused on a dark fixation cross in the center of the screen. Each object was presented for 250 milliseconds, and there was a 3750 millisecond interstimulus interval. Half of the objects from each emotional valence category were presented to the left of the fixation cross and half were presented to the right of the cross. So that objects were presented directly to only one hemisphere, the center of each object was presented approximately 7° to the left or right of the fixation cross. The order of the presented items was randomized for each odd-numbered participant; the even-numbered participants saw the same items, in the same order as the previous participant, but each item was presented in the opposite hemifield (e.g., if participant one saw a canoe presented in the LVF followed by a grenade presented in the RVF, then participant two saw a canoe presented in the RVF followed by a grenade presented in the LVF). To avoid fatigue, participants were given short breaks every approximately two and a half minutes. A “Get Ready” prompt appeared and participants made a mouse click when they were ready to continue the task.
Immediately following completion of the study phase, participants were asked to perform a surprise recognition memory task. Participants had believed that the purpose of the study phase was to assess how hemispheric presentation influenced the speed with which decisions could be made about objects, and so participants were not expecting that their memory for these objects would be tested. Debriefing forms confirmed that the incidental encoding manipulation was successful; no participant reported that he or she had thought that the experiment would assess memory for the objects.
On the recognition memory task, participants were once again asked to view a series of objects presented on a computer screen. This time, each of 180 objects were presented in the center of the screen. 72 objects (24 negative, 24 positive, 24 neutral) were the identical object that had been studied (e.g., the same canoe). 72 objects shared the same verbal label as a studied object but differed in visual details (e.g., a similar snake), and 36 objects (12 of each emotional valence) did not share a verbal label with a presented object (a new hammer; see Kensinger, Garoff-Eaton, & Schacter, 2006 for more information on these methods). Participants were instructed to indicate, by button press, whether the picture was “same” (identical), “similar,” or “new,” in relation to the previous study list.
Only one object within the object pair was tested across all participants (i.e., participants never saw both a same and a similar snake). The same recognition task was given to all participants. The studied items were counterbalanced between subjects to manipulate the condition of each object shown at recognition (i.e., whether it was same, similar, or new) and the side of the screen on which the item had been studied.
A pilot sample of participants was tested while eye tracking patterns were recorded during the study phase, to assure that participants could maintain fixation on the central point. The behavioral data from these participants, during both the study and test phases, paralleled the data from those participants whose eye gaze was not objectively measured. These pilot participants made only a small number of fixation errors (fewer than 3% of all trials) and errors did not differ as a function of hemispheric presentation or emotional valence category. Thus, we have no reason to believe that the participants in the present study were unable to fixate on the cross during the study phase1.
Data Analysis
We focused our analyses on three types of memory that have been defined previously using this paradigm (Kensinger, Garoff-Eaton, & Schacter, 2007; Payne et al., in press): general recognition memory, or memory for at least the gist of the items, with or without memory for visual detail; memory for specific visual detail; and gist-only memory, lacking in visual detail. For general recognition memory, we computed d' sensitivity measures (Snodgrass & Corwin, 1988), considering “same” or “similar” responses to same or similar items to reflect general recognition hits and considering “same” or “similar” responses to new items to reflect general false alarm responses. That is, for same and similar items given either a “same” or a “similar” response, participants had to remember at least that a particular type of object had been studied (e.g., that they had seen a canoe) because otherwise they would have instead indicated that the item was “new.” Thus, these general recognition scores reflect a participant's tendency to remember at least the gist of the items (with or without visual detail). By contrast, when participants gave a “same” or “similar” response to a new item, these responses reflected false alarms, revealing a participant's bias to indicate that a general item type had been studied. Because by definition the new items were not studied, there was only one false alarm rate (it did not vary based upon hemispheric presentation). d' scores were calculated as recommended by Snodgrass and Corwin (1988) using these hit and false alarm rates.
For visually specific recognition memory, we examined what proportion of the same items for which general recognition occurred (i.e., for which “same” or “similar” responses were given) also were associated with specific recognition (i.e., a “same” response to a same item2). These proportional scores, reflecting specific recognition hits, were calculated as: “same” responses to same items/ (“same” + “similar” responses to same items). Specific recognition false alarms, reflecting participants' tendencies to say incorrectly that a familiar item was “same,” were considered to be the comparable proportion for similar items: “same” response to similar items/(“same” + “similar” responses to similar items). Though similar items had not been studied, we considered a similar item to be associated with RVF/LH presentation if its corresponding object had been studied in that condition (e.g., if penguin A was studied in the RVF/LH condition, then penguin B was considered to be a similar object associated with RVF/LH presentation). d' measures of sensitivity (Snodgrass & Corwin, 1988) were computed using these hit and false alarm rates.
For gist-only memory, we considered “similar” responses to same items to reflect gist-only hits and “similar” responses to new items to reflect gist-only false alarms. Using these computations, d' sensitivity values were calculated. Because by definition the new items were not studied, there was only one false alarm rate (it did not vary based upon hemispheric presentation).
We also computed response bias measurements (C) for each of the memory measures described above. Because these measurements never were influenced by hemispheric presentation (i.e., ANOVAs revealed no main effects of hemisphere nor any interactions between emotional valence and hemisphere), we do not report these analyses.
Results
General Recognition
An ANOVA conducted on the general recognition d' values with emotional valence (positive, negative, neutral) and hemispheric presentation (left, right) as within-subject factors indicated only a main effect of emotion, F(2,50) = 3.47, p<.05, partial eta-squared = .12, with negative and positive items having higher general recognition than neutral items. The hemisphere of presentation did not influence general recognition ability (p>.25), nor did the interaction between the two factors.
Memory for Specific Visual Detail
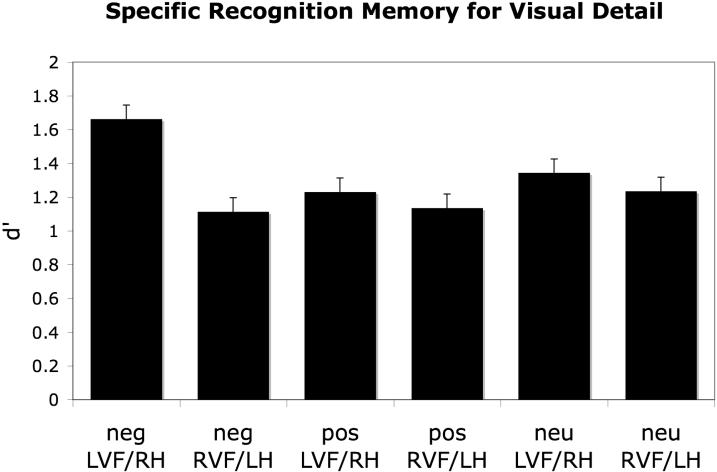
An ANOVA conducted on the specific recognition d' scores revealed a main effect of hemispheric presentation, F(1,25) = 4.29, p<.05, partial eta-squared = .18, with items presented directly to the LVF/RH remembered with better specificity than items presented to the RVF/LH. The ANOVA also revealed an interaction between hemispheric presentation and emotional valence, F(2,50) = 3.30, p<.05, partial eta-squared = .12. As can be seen in Figure 1, this interaction reflected the fact that specific recognition was highest when items were both negative and also were presented to the LVF/RH. In fact, the benefit of negative emotion on memory specificity existed only when negative items were presented to the LVF/RH (p<.001) and not when they were presented to the RVF/LH (p>.15).
Figure 1.
When items were presented directly to the LVF/RH, participants were more likely to remember the visual details of negative items than they were to remember the visual details of positive or neutral items. No such advantage existed when items were presented directly to the RVF/LH.
Gist-Only Memory
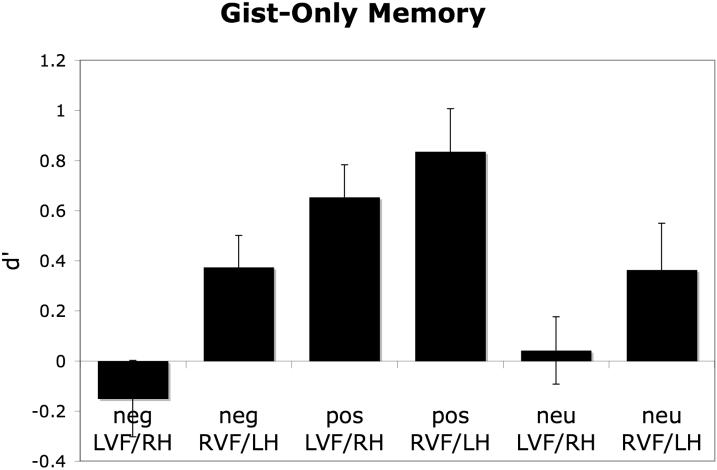
An ANOVA conducted on the gist-only d' scores revealed a main effect of emotional valence, F(2,50)=13.39, p<.001, partial eta-squared = .35, with positive items more likely to be remembered with only gist-only information than negative or neutral items, and a main effect of hemispheric presentation, F(1,25)=17.28, p<.001, partial eta-squared = .41, with items presented in the right hemifield (i.e., directly to the left hemisphere) more likely to be remembered with only gist information (see Figure 2). There was no interaction between the two factors (p>.25).
Figure 2.
Memory for gist-only information was more likely to be remembered when items had been presented directly to the RVF/LH than when they had been presented directly to the LVF/RH. Regardless of whether items were presented directly to the LVF/RH or RVF/LH, the low d' values for negative and neutral items suggest that participants rarely remembered the gist of those items without their specific visual details. By contrast, this type of gist-only recognition occurred more reliably for the positive items.
Discussion
The present results provide strong evidence for a unique memory benefit when items are both negative and also are presented directly to the RH (via LVF presentation). In this instance, even with brief presentation, episodic memory for the visual details of negative items is enhanced. By contrast, when the items are presented briefly and directly to the LH (via RVF presentation), or are presented briefly in the center of the screen (Kensinger et al., 2006), no such memory benefits exist.
Given the proposed role of the RH in processing the visual specifics or relational details of objects, and also in processing emotional, and perhaps particularly negative, information, it makes sense that LVF/RH processing would lead to enhanced memory for the visual details of negative items. Nevertheless, this is one of the few studies to show such an effect of hemispheric processing on episodic encoding for objects (but see Berrini et al., 1982; Evans & Federmeier, 2007; Federmeier & Benjamin, 2005; Hannay & Malone, 1976 for evidence that hemifield presentation can influence subsequent episodic memory for words), and the first to show that the magnitude of the emotion-related enhancement in episodic memory can be modulated by hemifield presentation. Thus, these results are intriguing in suggesting that the magnitude of memory benefit conveyed by emotional information may vary depending upon which hemisphere first processes information. The enhancement in memory specificity for negative information is greatest when the right hemisphere receives high fidelity information rapidly. This finding is broadly consistent with recent neuroimaging evidence suggesting that when the right fusiform and right amygdala are engaged during encoding, negative items are particularly likely to be remembered with specific visual detail (Kensinger, Garoff-Eaton, & Schacter, 2007c). The present results suggest that this right-lateralized processing may be facilitated by giving the RH a “head start” in processing the information.
More broadly, the results of the present study further support a distinction between the types of processing mediated by the LH and RH. In the present study, when information was presented directly to the LVF/RH, memory for specific visual detail was enhanced, whereas when information was presented directly to the RVF/LH, information was more likely to be remembered with only gist information. This pattern of results is consistent with the proposal that the RH is specialized for processing the visual specifics or relational details of objects while the LH is recruited for the processing of more general or conceptual features of objects (e.g., Burgund & Marsolek, 2000; Kosslyn, 1987; Kosslyn et al., 1989; Marsolek, 1995, 1999). This finding also is consistent with recent event-related potential evidence that the neural processes involved in determining whether a test item is a perceptual match to a studied item are more likely to be involved if the item was studied in the LVF/RH than if it was studied in the RVF/LH (Evans & Federmeier, 2007). Taken together with the results of the present study, these results provide convincing evidence that RH processing can lead to better retention of the details of studied information, making it easier to determine whether another item is a perceptual match to that studied item.
The fact that the effects of RH presentation on memory for visual specificity interacted with emotion, but the effects of LH presentation on memory for gist-only information did not, may reflect the lack of specialized emotional processing in the LH. As outlined in the introduction, many lines of evidence suggest that the RH may play a particularly important role in processing information with emotional meaning (e.g., Bourne, 2008). However, it also has been proposed that there are valence-specific effects, with the RH specialized for processing negative emotion, and the LH optimized for processing positive emotion (e.g., Davidson, 1995; Natale et al., 1983; Sackeim et al., 1982; Schaffer et al., 1983). If the latter hypothesis were true, then it might be expected that there should be an interaction between emotion and subsequent memory in the LH as well, with the “gist” of positive items being more likely to be remembered when they are presented directly to the LH. At the present time, it is unclear whether our measure of gist-only recognition is insufficiently sensitive to detect interactions with emotion or whether no such interactions exist. It will be important for future studies to examine whether there can be interactions between emotional valence and subsequent episodic memory performance when information is presented directly to the LH.
There are a few limitations of the present study which should be noted. First, these findings are based upon a small sample drawn from a college campus, preventing us from examining whether the effects vary with age, gender, or other individual differences. Second, we saw only modest effects of direct hemispheric presentation on memory for neutral items (see Figure 1), though such an effect would be hypothesized to occur due to the RH role in processing exemplar-specific details. It is possible that the parameters of this study (e.g., stimulus presentation duration, encoding task, study-test delay) were not optimal for detecting the effects of direct hemispheric presentation on memory for neutral items. Alternately, it is possible that intermixing negative, positive, and neutral items changes the way in which neutral information is attended and processed (and see Gruhn et al., 2007; Strange et al., 2000 for evidence), reducing the link between direct RH presentation and encoding of specific visual details. Future studies comparing mixed lists to blocked lists could address this possibility. More generally, future research will be needed to examine whether the effects of direct hemispheric presentation always are greater for negative information than for positive or neutral information, or whether the benefit for negative information can be modulated based on stimulus characteristics (e.g., the arousal level of the stimulus, whether the stimulus is a word or an object) or task demands (e.g., how attention is directed to the information).
In summary, the results of the present study indicate that when information initially is processed directly in the LVF/RH, there is a lasting benefit in memory for the visual details of negative objects. Thus, the RH specialization for processing both negative affect (e.g., Natale et al., 1983) and exemplar-specific details (e.g., Marsolek, 1999) has critical implications for the type of information that is encoded into episodic long-term memory. By contrast, when information is presented directly to the RVF/LH, there is an increased tendency to remember only the gist of the information, and this tendency is not influenced by the emotional valence of the information. These results emphasize the complex interplay between emotional valence, hemispheric processing, and memory specificity, and highlight the importance of considering differences in how the two hemispheres process information when examining the effects of emotional valence on memory.
Table 1.
Proportion of “same,” “similar,” and “new” responses given to objects as a function of object type (same, similar, new), emotion type (negative, positive, neutral) and hemispheric presentation (LVF/RH, RVF/LH). Because new items were only presented at test, they are not associated with a hemispheric presentation at study.
| Same item | ||||||
| Negative | Positive | Neutral | ||||
| LVF/RH | RVF/LH | LVF/RH | RVF/LH | LVF/RH | RVF/LH | |
| “same” | .63 (.04) | .46 (.04) | .44 (.04) | .41 (.05) | .42 (.04) | .39 (.03) |
| “similar” | .15 (.02) | .27 (.02) | .27 (.03) | .32 (.02) | .23 (.03) | .26 (.02) |
| “new” | .22 (.03) | .27 (.03) | .29 (.04) | .27 (.04) | .34 (.05) | .34 (.04) |
| Similar item | ||||||
| Negative | Positive | Neutral | ||||
| LVF/RH | RVF/LH | LVF/RH | RVF/LH | LVF/RH | RVF/LH | |
| “same” | .14 (.02) | .15 (.02) | .12 (.02) | .10 (.03) | .13 (.02) | .12 (.04) |
| “similar” | .52 (.03) | .50 (.03) | .46 (.05) | .40 (.05) | .42 (.04) | .44 (.04) |
| “new” | .33 (.04) | .35 (.03) | .43 (.05) | .50 (.05) | .45 (.05) | .44 (.05) |
| New item | ||||||
| Negative | Positive | Neutral | ||||
| “same” | .03 (.01) | .01 (.01) | .02 (.01) | |||
| “similar” | .18 (.03) | .10 (.01) | .16 (.02) | |||
| “new” | .78 (.03) | .89 (.01) | .82 (.02) | |||
Acknowledgments
This research was supported by NSF grant BCS 0542694 and NIH grant MH080833 to E.A.K. This project was conducted as the independent research project of the second author, under the supervision of the first author. We thank Ruben Gur and Chad Marsolek for helpful comments.
Footnotes
We did not perform eyetracking with the participants in this experiment because we were concerned that the novel and somewhat stressful eyetracking environment might induce mood effects which could influence our results.
Note that responses to similar items are harder to interpret with regard to memory specificity. A “similar” response to a similar item could reflect memory for specific details (e.g., maybe participants give a “similar” response to a similar snake because they remember the exact snake they studied and know that this is not that same snake). However, a “similar” response to a similar item also might reflect memory for only the gist of an item (e.g., maybe a person does not remember what the snake looked like, and so claims this is a “similar” one).
References
- Berrini R, Della Sala S, Spinnler H, Sterzi R, Vallar G. In eliciting hemisphere asymmetries which is more important: The stimulus input side or the recognition side? A tachistoscopic study on normals. Neuropsychologia. 1982;20:91–94. doi: 10.1016/0028-3932(82)90091-4. [DOI] [PubMed] [Google Scholar]
- Borod JC. Interhemispheric and intrahemispheric control of emotion: a focus on unilateral brain damage. Journal of Consulting and Clinical Psychology. 1992;60:339–348. doi: 10.1037//0022-006x.60.3.339. [DOI] [PubMed] [Google Scholar]
- Bourne VJ. Chimeric faces, visual field bias, and reaction time bias: Have we been missing a trick? Laterality. 2008;13:92–103. doi: 10.1080/13576500701754315. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Marsolek CJ. Viewpoint-invariant and viewpoint-dependent object recognition in dissociable neural subsystems. Psychonomic Bulletin and Review. 2000;7:480–489. doi: 10.3758/bf03214360. [DOI] [PubMed] [Google Scholar]
- Collins MA, Cooke A. A transfer appropriate processing approach to investigating implicit memory for emotional words in the cerebral hemispheres. Neuropsychologia. 2005;43:1529–1545. doi: 10.1016/j.neuropsychologia.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Cerebral asymmetry, emotion, and affective style. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. MIT Press; Cambridge, MA: 1995. pp. 361–387. [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–20. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Evans KM, Federmeier KD. The memory that's right and the memory that's left: event-related potentials reveal hemispheric asymmetries in the encoding and retention of verbal information. Neuropsychologia. 2007;45:1777–1790. doi: 10.1016/j.neuropsychologia.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, Benjamin AS. Hemispheric asymmetries in the time course of recognition memory. Psychonomic Bulletin and Review. 2005;12:993–998. doi: 10.3758/bf03206434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: the role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Grühn D, Scheibe S, Baltes PB. Reduced negativity effect in older adults' memory for emotional pictures: the heterogeneity-homogeneity list paradigm. Psychology and Aging. 2007;22:644–649. doi: 10.1037/0882-7974.22.3.644. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Malone DR. Visual field effects and short-term memory for verbal material. Neuropsychologia. 1976;14:203–9. doi: 10.1016/0028-3932(76)90049-x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54:99–112. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. Journal of Gerontology. 2007a;62:P208–215. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of Memory and Language. 2007b;56:575–591. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. How negative emotion enhances the visual specificity of a memory. Journal of Cognitive Neuroscience. 2007c;19:1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Koenig O, Barrett A, Cave CB, Tang J, Gabrieli J. Evidence for two types of spatial representations: Hemispheric specialization for categorical and coordinate relations. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:723–735. doi: 10.1037//0096-1523.15.4.723. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Seeing and imagining in the cerebral hemispheres: a computational approach. Psychological Review. 1987;94:148–175. [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pietura K. Disordered emotional processing in schizophrenia and onesided brain damage. Progress in Brain Research. 2006;156:467–479. doi: 10.1016/S0079-6123(06)56026-1. [DOI] [PubMed] [Google Scholar]
- Landis T. Emotional words: what's so different from just words? Cortex. 2006;42:823–830. doi: 10.1016/s0010-9452(08)70424-6. [DOI] [PubMed] [Google Scholar]
- Lee GP, Loring DW, Meader KJ, Brooks BB. Hemispheric specialization for emotional expression: a reexamination of results from intracarotid administration of sodium amobarbital. Brain and Cognition. 1990;12:267–280. doi: 10.1016/0278-2626(90)90019-k. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ. Abstract visual-form representations in the left cerebral hemisphere. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:375–386. doi: 10.1037//0096-1523.21.2.375. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ. Dissociable neural subsystems underlie abstract and specific object recognition. Psychological Science. 1999;10:111–118. [Google Scholar]
- Marsolek CJ, Schacter DL, Nichols CD. Form-specific visual priming for new associations in the right cerebral hemisphere. Memory & Cognition. 1996;24:539–556. doi: 10.3758/bf03201082. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ, Squire LR, Kosslyn SM, Lulenski ME. Form-specific explicit and implicit memory in the right cerebral hemisphere. Neuropsychology. 1994;8:588–597. [Google Scholar]
- Marsolek CJ, Burgund ED. Dissociable neural subsystems underlie visual working memory for abstract categories and specific exemplars. Cognitive, Affective, and Behavioral Neuroscience. doi: 10.3758/cabn.8.1.17. in press. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Natale M, Gur RE, Gur RC. Hemispheric asymmetries in processing emotional expressions. Neuropsychologia. 1983;21:555–565. doi: 10.1016/0028-3932(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, Hichwa RD. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. American Journal of Psychiatry. 1999;156:1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychological Science. doi: 10.1111/j.1467-9280.2008.02157.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions: Neurological Evidence. Archives of Neurology. 1982;39:210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- Sato W, Aoki S. Right hemispheric dominance in processing of unconscious negative emotion. Brain and Cognition. 2006;62:261–266. doi: 10.1016/j.bandc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schaffer CE, Davidson RJ, Saron C. Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biological Psychiatry. 1983;18:753–762. [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG, Honig MA, Parikh RM, Joselyn J, Price TR. Mood changes after right-hemisphere lesions. British Journal of Psychiatry. 1989;155:79–85. doi: 10.1192/bjp.155.1.79. [DOI] [PubMed] [Google Scholar]
- Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12:425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]