Abstract
Human malignant neuroblastoma is characterized by poor differentiation and uncontrolled proliferation of immature neuroblasts. Retinoids such as all-trans-retinoic acid (ATRA), 13-cis-retinoic acid (13-CRA) and N-(4-hydroxyphenyl) retinamide (4-HPR) at low doses are capable of inducing differentiation, while flavonoids such as with (−)-epigallocatechin-3-gallate (EGCG) and genistein (GST) at relatively high dose can induce apoptosis. We used combination of retinoid and flavonoid for controlling growth of malignant neuroblastoma cells. SH-SY5Y cells were treated with a retinoid (1 μM ATRA, 1 μM 13-CRA, or 0.5 μM 4-HPR) for 7 days and then with a flavonoid (25 μM EGCG or 25 μM GST) for 24 h. Treatment of cells with a low dose of a retinoid for 7 days induced neuronal differentiation with downregulation of telomerase activity and N-Myc but over-expression of neurofilament protein (NFP) and subsequent treatment with a relatively high dose of a flavonoid for 24 h increased apoptosis in the differentiated cells. Besides, retinoids reduced the levels of inflammatory and angiogenic factors. Apoptosis was associated with increases in intra-cellular-free [Ca2+], Bax expression, cytochrome c release from mitochondria and activities of calpain and caspases. Decreases in expression of calpastatin (endogenous calpain inhibitor) and baculovirus inhibitor-of-apoptosis repeat containing (BIRC) proteins (endogenous caspase inhibitors) favored apoptosis. Treatment of SH-SY5Y cells with EGCG activated caspase-8, indicating induction of the receptor-mediated pathway of apoptosis. Based on our observation, we conclude that combination of a retinoid and a flavonoid worked synergistically for controlling the malignant growth of human neuroblastoma cells.
Keywords: apoptosis, caspases, flavonoids, neuroblastoma, retinoids
Introduction
The clinical hallmark of neuroblastoma includes heterogeneity and the likelihood of tumor progression varies widely according to age at diagnosis and extent of the disease (1). A number of prognostic parameters for this disease have been identified, including age, loss of heterozygosity at chromosome 1p36, DNA ploidy, histopathological stage and amplification of the N-Myc proto-oncogene. The latter condition is strongly correlated with advanced disease, insensitivity to chemotherapy and poor outcome (2). The long-term survival rate of such high-risk patients has remained very low over the last decades. This situation has called for development of innovative therapeutic approaches. In recent years, the concept of ‘combination therapy’ has gained considerable interest. We applied this strategy to a human malignant neuroblastoma cell line representing immature multipotent cells lacking differentiation to induce terminal differentiation and permanent cell-cycle arrest by a retinoid that sensitized the cells to a flavonoid for increasing apoptosis.
Retinoid can also induce differentiation of neuroblastoma cell lines in vitro and are being employed in the therapy of neuroblastoma patients who have shown increase in survival rates (3). All-trans retinoic acid (ATRA) and 13-cis retinoic acid (13-CRA) induce differentiation in neuroblastoma cells and have been used in clinical trials in children with advanced neuroblastoma (4). While natural retinoid as a differentiation-promoting agent has entered clinical trials, it is only recently that the synthetic retinoid N-(4-hydroxyphenyl)retinamide (4-HPR) has been found to be of potential clinical value in cancer chemoprevention and treatment. Thus far, no extensive amounts of differentiation data exist on the effects of 4-HPR on neural crest-derived tumor cells (5). Besides, very little is known about the effects of retinoids on the expression of angiogenic factors such as vascular endothelial growth factor (VEGF) that may fuel the malignant growth of neuroblastoma cells. If retinoids at a low dose can induce differentiation in neuroblastoma cells and act synergistically with apoptosis-inducing agents, this combination strategy can provide opportunities for controlling the malignant growth of neuroblastoma.
Flavonoids are polyphenolic compounds that are ubiquitous in plants. The role of dietary flavonoids in cancer prevention is widely recognized (6). Epigallocatechin-3-gallate (EGCG), a major polyphenol found in green tea, is a widely studied chemopreventive agent with potential anti-cancer activity (6). The anti-tumor mechanism of EGCG in culture is due to modulation of the expression of key molecules in cell-cycle progression, inhibition of the inflammatory molecule nuclear factor-κB (NF-κB), binding to Fas and activation of mitogen-activated protein kinase cascade (7). Genistein (GST) is a natural isoflavonoid found in Leguminosae. This isoflavonoid has been shown to have a strong inhibitory effect on protein tyrosine kinase and it can produce cell-cycle arrest and apoptosis in leukemic cells (8). GST substantially inhibited the growth of five tumor cell lines (N2A, JC, SKNSH, MSN and Lan5) through induction of apoptosis and modulation of protein tyrosine kinase activity and N-Myc expression (9).
This investigation was designed to examine a dual approach for controlling the malignant growth of neuroblastoma by promoting differentiation with a retinoid (ATRA, 13-CRA or 4-HPR) and increasing apoptosis with a flavonoid (EGCG or GST).
Materials and methods
Cell culture and treatments
Human malignant (N-type) neuroblastoma SH-SY5Y cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown in 75-cm2 flasks containing 10 ml of RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin in a fully-humidified incubator containing 5% CO2 at 37°C. Prior to drug treatments, the cells were starved in RPMI-1640 supplemented with 1% FBS for 24 h. ATRA, 13-CRA and 4-HPR (Sigma Chemical, St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO) and stored as aliquots of 1000x stocks at −70°C. Because of light sensitivity of ATRA, 13-CRA and 4-HPR, all incubations involving retinoids were performed under subdued lighting. The concentration of DMSO in each experiment was always ≤0.01%, which was not toxic and did not induce differentiation. Dose-response studies were conducted to determine the suitable doses of the drugs used for induction of apoptosis in the experiments. Finally, cells were treated with 25 μM EGCG and 25 μM GST for 24 h for induction of apoptotic death. Following treatments, apoptosis was determined morphologically and biochemically. The cells were also examined for alterations in expression and activity of proteins related to apoptosis.
Methylene blue staining for detection of morphological features of differentiation
SH-SY5Y cells were grown in monolayer in 9-cm diameter plates in absence and presence of 1 μM ATRA, 1 μM 13-CRA or 0.5 μM 4-HPR for 7 days. Culture medium was aspirated and washed twice with ice-cold PBS, pH 7.4. Each plate was placed on ice and 5 ml of ice-cold 50% (v/v) ethanol was added to fix the cells. Ethanol was aspirated followed by the addition of 5 ml of ice-cold 0.2% (w/v) methylene blue solution (made up in 50% ethanol). Cells were stained for 30 sec, washed twice with ice-cold water and the plates were dried in the air. Cells were examined under the light microscope at x400 magnification.
Wright staining and ApopTag peroxidase assay for morphological and biochemical features of apoptosis
SH-SY5Y cells from each treatment were sedimented onto the microscopic slide and fixed in methanol before examination of apoptosis by Wright staining and ApopTag assay (10,11). Wright staining detected characteristic apoptotic features such as chromatin condensation, cell-volume shrinkage and membrane-bound apoptotic bodies. ApopTag assay kit (Intergen, Purchase, NY, USA) was used for biochemical detection of DNA fragmentation in apoptotic cells. The nuclei containing DNA fragments were stained dark brown with ApopTag assay and were not counterstained with methyl green that, however, stained normal nuclei pale to medium green. After ApopTag assay, cells were counted to determine percentage of apoptosis.
Telomerase activity assay
Telomerase activity was examined using the telomerase repeat amplification protocol (TRAP) (10). Briefly, cells were washed once with ice-cold PBS and again later with ice-cold wash buffer (10 mM HEPES-KOH, pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT). Cells were lysed in ice-cold lysis buffer (10 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 1 mM EGTA, 0.1 mM PMSF, 0.5% CHAPS, 10% glycerol) for 30 min on ice and centrifuged in a microfuge at 4°C. The cells are homogenized and stored on ice for 30 min. The supernatant was removed and quick-frozen on dry ice. The protein concentration was measured by Coomassie Brilliant Blue assay. The TRAP reaction products were electrophoresed on 12% polyacrylamide gels. After ethidium bromide staining, the gels were examined under UV (303 nm) light and photographed.
Determination of intracellular free [Ca2+] using Fura-2 assay
The level of intracellular free [Ca2+] was measured in human neuroblastoma SH-SY5Y cells using the fluorescence Ca2+ indicator Fura-2/AM, as described previously (6). The value of Kd, a cell-specific constant, was determined experimentally to be 0.451 μM for the SH-SY5Y cells using standards of the Calcium Calibration Buffer kit with magnesium (Molecular Probes, Eugene, OR, USA).
Analysis of mRNA expression
Extraction of total RNA, reverse transcription-polymerase chain reaction (RT-PCR) and agarose gel electrophoresis were performed, as we described previously (11). All human primers for RT-PCR experiments were designed using human cDNA sequences of specific genes (Table I) and Oligo software (National Biosciences, Plymouth, MN, USA). The level of ß-actin gene expression served as an internal control.
Table I.
Human primers used to determine levels of mRNA expression of specific genes.
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| ß-actin | Sense: 5′-TAT CCC TGT ACG CCT CT-3′ | 460 |
| Antisense: 5′-AGG TCT TTG CGG ATG T-3′ | ||
| Baxα | Sense: 5′-AAG AAG CTG AGC GAG TGT-3′ | 265 |
| Antisense: 5′-GGA GGA AGT CCA ATG TC-3′ | ||
| Bcl-2α | Sense: 5′-CTT CTC CCG CCG CTA C-3′ | 306 |
| Antisense: 5′-CTG GGG CCG TAC AGT TC-3′ | ||
| BIRC-2 | Sense: 5′-CAG AAA GGA GTC TTG CTC GTG-3′ | 536 |
| Antisense: 5′-CCG GTG TTC TGA CAT AGC ATC-3′ | ||
| BIRC-3 | Sense: 5′-GGG AAC CGA AGG ATA ATG CT-3′ | 368 |
| Antisense: 5′-ACT GGC TTG AAC TTG ACG GAT-3′ | ||
| BIRC-4 | Sense: 5′-AAT GCT GCT TTG GAT GAC CTG-3′ | 470 |
| Antisense: 5′-ACC TGT ACT CAG CAG GTA CTG-3′ | ||
| BIRC-5 | Sense: 5′-GCC CCA CTG AGA ACG-3′ | 302 |
| Antisense: 5′-CCA GAG GCC TCA ATC C-3′ | ||
| BIRC-6 | Sense: 5′-AGC CGA AGG ATA GCG A-3′ | 385 |
| Antisense: 5′-GCC ATC CGC CTT AGA A-3′ | ||
| BIRC-7 | Sense: 5′-GCC TCC TTC TAT GAC T-3′ | 283 |
| Antisense: 5′-CGT CTT CCG GTT CT-3′ | ||
| BIRC-8 | Sense: 5′-GTG AGC GCT CAG AAA GAC ACT AC-3′ | 209 |
| Antisense: 5′-CAC ATG GGA CAT CTG TCA ACT G-3′ | ||
| M-calpain | Sense: 5′-CCC TCC CAA CCT GTT CAA G-3′ | 440 |
| Antisense: 5′-GCC TCC AGT TCC CAT CCA-3′ | ||
| Calpastatin | Sense: 5′-TCA CCT GTG GGT CGC CTA C-3′ | 351 |
| Antisense: 5′-GCT CTG GCA ATA GTG GTT TTC C-3′ |
Antibodies
Monoclonal antibody against α-spectrin (Affiniti, Exeter, UK) was used to measure the activities of calpain and caspase-3. Monoclonal antibody against ß-actin (clone AC-15, Sigma Chemical) was used to standardize protein loadings on the gels. The secondary antibody used was goat anti-mouse IgG conjugated with alkaline horseradish peroxidase (HRP) (ICN Biomedicals, Aurora, OH, USA) except in the case of calpain and α-spectrin where it was goat anti-rabbit IgG conjugated with alkaline HRP (ICN Biomedicals).
Western blot analysis of specific proteins
Western blot analysis was performed, as we described previously (10,11). The autoradiograms were scanned using Photoshop software (Adobe Systems, Seattle, WA, USA) and the optical density (OD) of each band was determined using Quantity One software (Bio-Rad, Hercules, CA, USA).
Colorimetric assay for the measurement of caspase-8, -9 and -3 activities
Measurements of caspase activities in cells were performed with the commercially available caspase-8, -9 and -3 assay kits (Sigma). The colorimetric assays were based on the hydrolysis of the Ac-IETD-pNA by caspase-8, Ac-LEHD-pNA by caspase-9 and Ac-DEVD-pNA by caspase-3, resulting in the release of the p-nitroaniline (pNA) moiety using the commercially available colorimetric assay kits (Sigma Chemical). Concentration of the p-NA released from the substrate was calculated from the absorbance at 405 nm.
Statistical analysis
All results obtained from different treatments of SH-SY5Y cells were analyzed using StatView (Abacus Concepts, Berkeley, CA, USA). Data were expressed as mean ± standard error of mean (SEM) of separate experiments (n≥3) and compared by one-way analysis of variance (ANOVA) followed by Fisher's post hoc test. Significant difference between control (CTL) and EGCG, and CTL and GST was analyzed. Difference between two treatments was considered significant at P≤0.01 or P≤0.05.
Results
Retinoids induced differentiation with downregulation of telomerase and N-Myc but overexpression of neurofilament protein (NFP)
We examined the morphological and biochemical features of neuronal differentiation in SH-SY5Y cells following treatment with retinoids (Fig. 1). ATRA, 13-CRA, or 4-HPR induced morphological features of neuronal differentiation in SH-SY5Y cells (Fig. 1a). Telomerase activity was evaluated by the TRAP assay (Fig. 1b). Our results showed inhibition of telomerase activity in SH-SY5Y cells following treatment with retinoids. We also used Western blotting to examine the level of expression of hTERT (the catalytic subunit of telomerase), which was downregulated following treatment with retinoids (Fig. 1c). Inhibition of N-Myc expression either results in suppression of cell proliferation or induction of differentiation. Our results showed that the expression of N-Myc was decreased due to treatment with reinoids, compared with parental SH-SY5Y cells (Fig. 1c). Expression of 68 kDa NFP occurs in neuroblasts with signs of differentiation, but not in immature small, round cells. Also, 68 kDa NFP is present in cell bodies as well as in cytoplasmic processes of differentiated neuroblasts. The presence of 68 kDa NFP is related to the degree of differentiation of tumor cells. Our Western blotting indicated increase in expression of 68 kDa NFP in differentiated SH-SY5Y cells (Fig. 1c). The uniform level of expression of ß-actin was used as a loading control. Thus, our findings suggested that ATRA, 13-CRA, or 4-HPR at a low dose induced differentiation with downregulation of telomerase and N-Myc and overexpression of 68 kDa NFP in SH-SY5Y cells.
Figure 1.
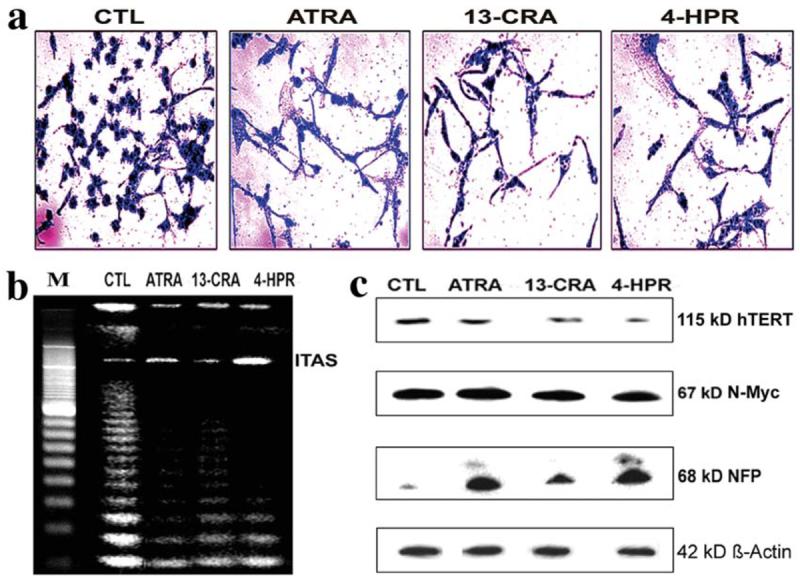
Morphological and biochemical features of neuronal differentiation in SH-SY5Y cells following treatment with retinoids. Treatment with retinoid (7 days): control (CTL), 1 μM ATRA, 1 μM 13-CRA and 0.5 μM 4-HPR. (a) Methylene blue staining to identify morphological features of neuronal differentiation. (b) Application of TRAP assay to determine any decrease in the telomerase activity. (c) Western blotting to examine levels of hTERT, N-Myc, NFP and ß-actin.
Detection of morphological and biochemical features of apoptosis following single and double treatments
The amount of apoptotic death was determined using Wright staining and ApopTag assay (Fig. 2). Wright staining and ApopTag assay were used to demonstrate apoptotic features morphologically and biochemically, respectively. Treatment of SH-SY5Y cells with EGCG or GST for 24 h induced apoptotic death with manifestation of morphological features as evident from Wright staining (Fig. 2a) and DNA fragmentation as evident from ApopTag assay (Fig. 2b). Following ApopTag assay, control cells showed little or no brown color confirming almost absence of apoptosis. But cells treated with EGCG or GST for 24 h demonstrated prominent brown color apoptotic cells. Treatment of cells with a low dose of ATRA, 13-CRA, or 4-HPR for 7 days did not induce apoptosis but increased sensitivity of differentiated cells to EGCG or GST for increasing the amounts of apoptosis, as determined on the basis of ApopTag assay (Fig. 2c). The results showed that the combination of a retinoid and a flavonoid worked synergistically for increasing apoptosis in malignant neuroblastoma cells.
Figure 2.
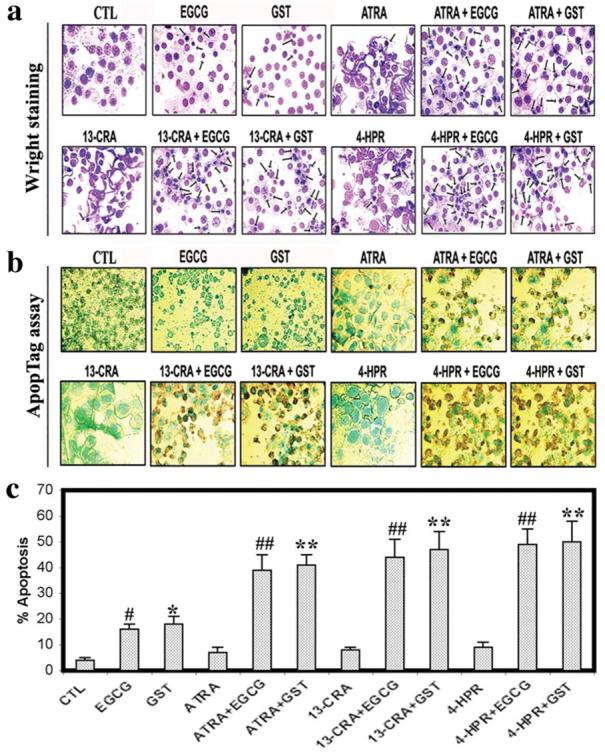
Morphological and biochemical features of apoptosis in SH-SY5Y cells. Treatment with retinoid (7 days) and flavonoid (24 h): CTL, 25 μM EGCG, 25 μM GST, 1 μM ATRA, 1 μM ATRA + 25 μM EGCG, 1 μM ATRA + 25 μM GST, 1 μM 13-CRA, 1 μM 13-CRA + 25 μM EGCG, 1 μM 13-CRA + 25 μM GST, 0.5 μM 4-HPR, 0.5 μM 4-HPR + 25 μM EGCG and 0.5 μM 4-HPR + 25 μM GST. (a) Wright staining for morphological features of apoptosis. (b) ApopTag assay for labeling of DNA fragmentation in apoptotic cells. (c) Bar diagram to show the percentage of apoptosis. Significant difference between control (CTL) and EGCG is indicated by #. Significant difference between CTL and GST is indicated by *.
Retinoids reduced the levels of inflammatory and angiogenic factors
Increased expression of the inflammatory mediator NF-κB may be correlated to malignant growth of neuroblastoma. Although the anti-inflammatory effect of retinoids has been investigated for several decades, the underlying mechanisms responsible for this effect are largely unknown. In this study, we demonstrated that ATRA, 13-CRA, or 4-HPR inhibited inflammatory response in SH-SY5Y cells by decreasing the expression of NF-κB (Fig. 3). Moreover, our results suggested that the anti-inflammatory effect of ATRA, 13-CRA, or 4-HPR could be potentiated by EGCG or GST in SH-SY5Y cells. Thus, targeting the inhibition of NF-κB activation would lead to the development of new strategy for treatment of neuroblastoma. Downregulation of the angiogenic factor VEGF in cancer cells by retinoids may contribute to the therapeutic effects (12). Our data demonstrated that retinoids could be potent inhibitors of VEGF production in human neuroblastoma cells (Fig. 3). Thus, one of the molecular mechanisms by which retinoids inhibited VEGF expression appeared to correlate with the inhibition of NF-κB activation. Our studies demonstrated that the combination of a retinoid (ATRA, 13-CRA or 4-HPR) and a flavonoid (EGCG or GST) could reduce the levels of NF-κB and VEGF so as to favor induction of apoptosis in SH-SY5Y cells.
Figure 3.
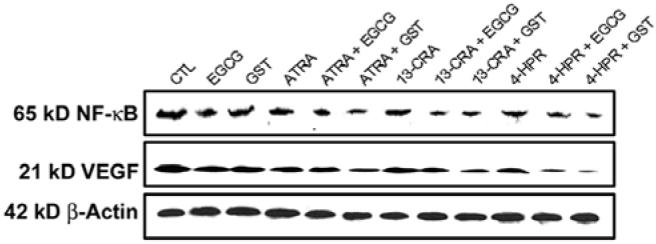
Western blotting to examine the levels of inflammatory and angiogenic factors in SH-SY5Y cells. Treatment with retinoid (7 days) and flavonoid (24 h): CTL, 25 μM EGCG, 25 μM GST, 1 μM ATRA, 1 μM ATRA + 25 μM EGCG, 1 μM ATRA + 25 μM GST, 1 μM 13-CRA, 1 μM 13-CRA + 25 μM EGCG, 1 μM 13-CRA + 25 μM GST, 0.5 μM 4-HPR, 0.5 μM 4-HPR + 25 μM EGCG and 0.5 μM 4-HPR + 25 μM GST. Representative Western blots to show levels of NF-κB, VEGF and ß-actin.
Treatment of neuroblastoma cells with EGCG induced caspase-8 activation and activity
We examined the induction of death receptor pathway of apoptosis in the caspase-8 activation and activity following the treatments of SH-SY5Y cells (Fig. 4). Caspase-8 is the apical caspase in death receptor pathway of apoptosis. Caspase-8 is activated upstream of caspase-3 and has been implicated to play a prominent role in potentiating the mitochondria-mediated pathway of apoptosis. Our Western blotting showed that treatment of SH-SY5Y cells with EGCG induced generation of 18 kDa caspase-8 fragment indicating the caspase-8 activation (Fig. 4a). Pretreatment of the cells with a retinoid dramatically increased the action of EGCG for caspase-8 activation. ß-Actin expression was monitored to ensure that equal amounts of protein were loaded in all lanes (Fig. 4a). Further, our colorimetric assay showed that caspase-8 activity was increased in the cells after treatment with EGCG (Fig. 4b). Combination of a retinoid (ATRA, 13-CRA or 4-HPR) and EGCG highly induced caspase-8 activation and activity (Fig. 4). Notably, GST alone or in combination with a retinoid did not induce death receptor-mediated pathway of apoptosis.
Figure 4.
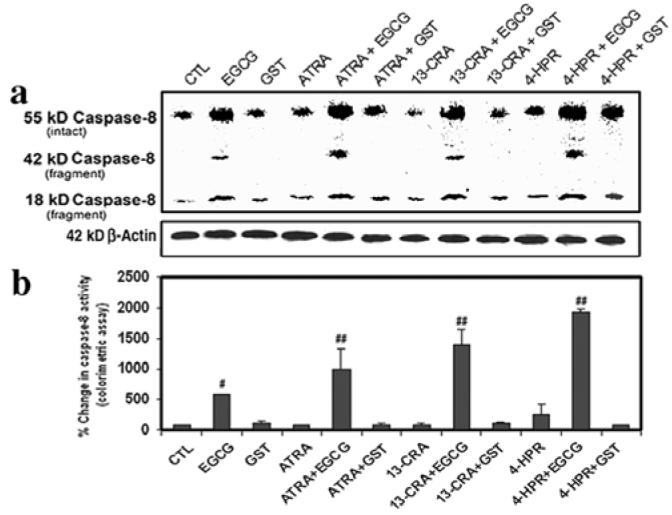
Determination of caspase-8 activation and activity in SH-SY5Y cells. Treatment with retinoid (7 days) and flavonoid (24 h): CTL, 25 μM EGCG, 25 μM GST, 1 μM ATRA, 1 μM ATRA + 25 μM EGCG, 1 μM ATRA + 25 μM GST, 1 μM 13-CRA, 1 μM 13-CRA + 25 μM EGCG, 1 μM 13-CRA + 25 μM GST, 0.5 μM 4-HPR, 0.5 μM 4-HPR + 25 μM EGCG and 0.5 μM 4-HPR + 25 μM GST. (a) Representative Western blots to show levels of caspase-8 and ß-actin. (b) Determination of caspase-8 activity by colorimetric assay.
Induction of apoptosis with activation of mitochondria-dependent caspase cascade
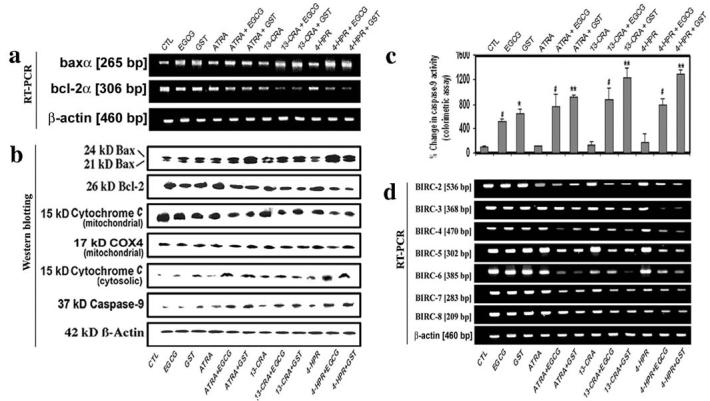
Members of the Bcl-2 family, with proteins such as Bax functioning as inducer of apoptosis and proteins such as Bcl-2 as suppressor of cell death, play major roles in governing the mitochondria-dependent caspase cascade. We examined the level of expression or location of the molecules that activate the mitochondria-dependent pathway of apoptosis (Fig. 5). Our results showed that cells treated with a flavonoid (EGCG or GST) increased the Bax (pro-apoptotic protein) expression at mRNA (Fig. 5a) and protein (Fig. 5b) levels. Importantly, treatment of cells with the combination of a retinoid (ATRA, 13-CRA, or 4-HPR) and a flavonoid (EGCG or GST) most effectively increased Bax expression and decreased Bcl-2 expression to trigger mitochondria-dependent caspase cascade. Our results showed that treatment of cells with the combination of retinoid and flavonoid clearly prompted the disappearance of 15 kDa cytochrome c from the mitochondrial fraction (Fig. 5b), indicating mitochondrial release of cytochrome c. Due to the release from mitochondria, 15 kDa cytochrome c appeared in the cytosolic fraction (Fig. 5b). We monitored the level of expression of COX4 as an internal control in mitochondrial fraction (13). ß-Actin expression was examined to ensure that equal amounts of cytosolic protein were loaded in all lanes. The release of cytochrome c from mitochondria to cytosol could cause activation of mitochondria-dependent caspase cascade. Indeed, we observed that treatments of SH-SY5Y cells with a flavonoid, especially with the combination of retinoid and flavonoid, increased caspase-9 activation with generation of active 37 kDa caspase-9 fragment as revealed by Western blotting (Fig. 5b) and also increased the total caspase-9 activity as confirmed by a colorimetric assay (Fig. 5c).
Figure 5.
Examination of mitochondrial pathway of apoptosis in SH-SY5Y cells. Treatment with retinoid (7 days) and flavonoid (24 h): CTL, 25 μM EGCG, 25 μM GST, 1 μM ATRA, 1 μM ATRA + 25 μM EGCG, 1 μM ATRA + 25 μM GST, 1 μM 13-CRA, 1 μM 13-CRA + 25 μM EGCG, 1 μM 13-CRA + 25 μM GST, 0.5 μM 4-HPR, 0.5 μM 4-HPR + 25 μM EGCG and 0.5 μM 4-HPR + 25 μM GST. (a) The representative gel pictures with RT-PCR products to show the mRNA levels of baxα, bcl-2α and ß-actin. (b) Representative Western blots to show levels of Bax, Bcl-2, cytochrome c, COX4, caspase-9 and ß-actin. (c) Determination of caspase-9 activity using a colorimetric assay. (d) Representative gel pictures with RT-PCR products to show the mRNA levels of BIRC-2 to BIRC-8 and ß-actin.
As any decrease in BIRC contents could favor sustained activation of caspase cascade, we used RT-PCR experiments to assess levels of expression of BIRC-2 to BIRC-8 in SH-SY5Y cells after the treatments (Fig. 5d). Our results showed that treatment with the combination of retinoid and flavonoid decreased the levels of expression of BIRC-2 to BIRC-6 in SH-SY5Y cells. However, the levels of BIRC-7 and BIRC-8 did not go down dramatically after the treatments. The results indicated that EGCG or GST induced apoptosis in differentiated SH-SY5Y cells with dramatic decrease in levels of expression of BIRC-2 to BIRC-6.
Treatments increased intracellular-free [Ca2+] and activities of calpain and caspase-3
We examined the consequences of treatments, especially combination of flavonoid and retinoid, in alterations of the intracellular-free [Ca2+] and activities of calapin and caspase-3 (Fig. 6). We used Fura-2 assay to determine the intracellular-free [Ca2+] in SH-SY5Y cells (Fig. 6a). Treatment with EGCG or GST for 48 h increased intracellular-free [Ca2+] significantly in undifferentiated cells and very significantly in differentiated cells, compared with control cells (Fig. 6a). Our results showed that treatments increased m-calpain at mRNA and protein levels (Fig. 6b). The endogenous calpain inhibitor calpastatin is fragmented by calpain and caspase(s) to various extent during apoptosis (14). Our result showed that the levels of mRNA (Fig. 6b) and protein (Fig. 6c) of calpastain were decreased after the treatments. The degradation of 270 kDa (α-spectrin to 145 kDa spectrin breakdown product (SBDP) and 120 kDa SBDP has been attributed to activation of calpain and caspase-3, respectively (6). So, we performed Western blotting to determine activities of calpain and caspase-3 in the generation of calpain-specific 145 kDa SBDP and caspase-3-specific 120 kDa SBDP, respectively (Fig. 6c). Treatment of the cells with a flavonoid (EGCG or GST), especially combination of flavonoid and retinoid, caused an increase in 145 kDa SBDP and 120 kDa SBDP suggesting increased activity of calapin and caspase-3, respectively. Moreover, we used a colorimetric assay to confirm that combination of retinoid and flavonoid worked synergistically to increase caspase-3 activity in SH-SY5Y cells (Fig. 6d). Importantly, our results showed that combination of 4-HPR and GST was the most suitable treatment for increasing both proteolytic activity and apoptosis in human malignant neuroblastoma cells.
Figure 6.
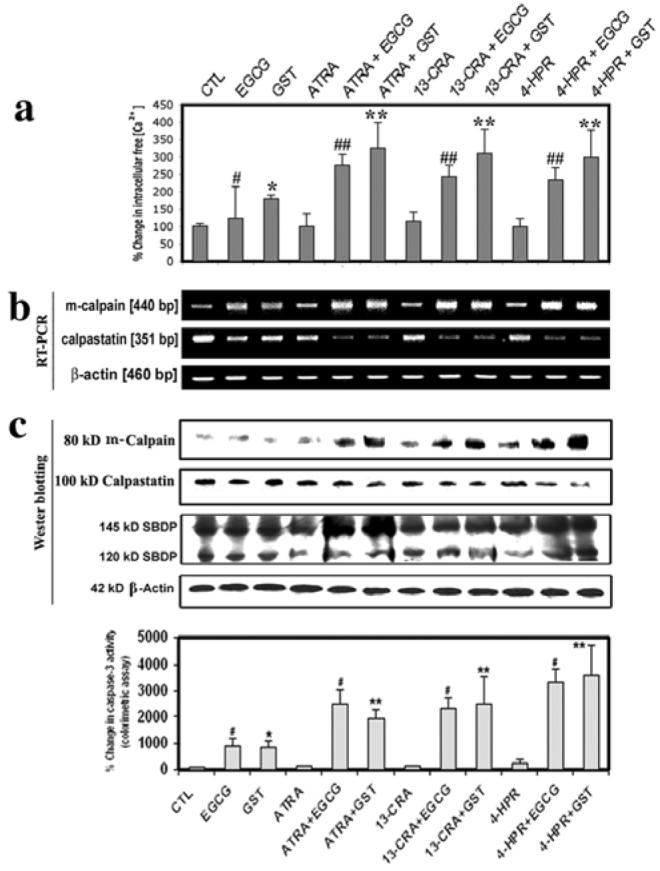
Determination of intracellular free [Ca2+] and activities of calpain and caspase-3 in SH-SY5Y cells. Treatment with retinoid (7 days) and flavonoid (24 h): CTL, 25 μM EGCG, 25 μM GST, 1 μM ATRA, 1 μM ATRA + 25 μM EGCG, 1 μM ATRA + 25 μM GST, 1 μM 13-CRA, 1 μM 13-CRA + 25 μM EGCG, 1 μM 13-CRA + 25 μM GST, 0.5 μM 4-HPR, 0.5 μM 4-HPR + 25 μM EGCG and 0.5 μM 4-HPR + 25 μM GST. (a) Determination of percentage of increase in intracellular-free [Ca2+]. (b) Representative gel pictures with RT-PCR products to show the mRNA levels of m-calpain, calpastain and ß-actin. (c) Representative Western blots to show the levels of m-calpain, calpastatin, SBDP and ß-actin. (d) Determination of caspase-3 activity using a colorimetric assay.
Discussion
Chemotherapy with a single agent may be limited by both toxicity and lack of potency. Therefore, combination chemotherapeutic strategies are currently being explored to reduce toxicity and enhance therapeutic efficacy. For many years, it has been known that exposure of neuroblastoma cells to a retinoid causes decrease in tumor cell proliferation and induces differentiation (15). There are no clear roadmaps yet for prioritizing which retinoid or combination strategy should be put forward for clinical evaluation. Flavonoids have been reported to exert multiple effects on signal transduction pathways, cell-cycle regulatory molecules and cell growth in diverse cell lines. Upregulation of N-Myc plays an important role in promoting proliferation of neuroblastoma cells (16). Here, we observed that a low dose of a retinoid (ATRA, 13-CRA or 4-HPR) induced neuronal differentiation with downregulation of N-Myc expression and telomerase activity but overexpression of 68 kDa NFP in SH-SY5Y cells (Fig. 1). Treatment with a retinoid inhibited not only N-Myc expression and telomerase activity in neuroblastoma cells (Fig. 1) and but also increased sensitivity to a flavonoid (EGCG or GST) for increasing the amount of apoptosis (Fig. 2).
Angiogenesis is necessary for cancer growth and dissemination. In addition to angiogenesis, it has become increasingly clear that inflammation is a key component in cancer insurgence that can promote angiogenesis. It is now known that NF-κB belongs to a family of transcription factors (17), which play central roles in the expression of genes involved in cell mobilization, proliferation and inflammation. In particular, NF-κB activation appears to drive a number of inflammatory diseases including cancers. Thus, targeting NF-κB activation would lead to the development of new therapeutic strategy that would provide novel treatment for these diseases. Our results showed a decrease in cytotolic level of NF-κB in SH-SY5Y cells after the treatments (Fig. 3). Thus, the repression of the NF-κB pathway suggests an anti-inflammatory effect of these compounds. Our results also showed for the first time that combination of a retinoid and a flavonoid very effectively downregulated the angiogenic factor VEGF in SH-SY5Y cells (Fig. 3).
Caspase-8 activation acts as a key determinant for induction of apoptosis by some cytotoxic drugs (18). The resistance of neuroblastoma cells to anti-cancer therapy has been related to absent or reduced caspase-8 expression. We reported herein that SH-SY5Y cells constitutively express normal caspase-8 and that activation of caspase-8 is a key determinant in induction of apoptotic death by EGCG in undifferentiated and especially in differentiated SH-SY5Y cells (Fig. 4). Previous studies showed that green tea polyphenols induced apoptosis in human prostate carcinoma cells and monocytic leukemia U937 cells due to caspase-8 activation (19). Generally, green tea polyphenol induces death receptor-mediated caspase-8 activation followed by caspase-3 activation. We found that EGCG activated caspase-8 in SH-SY5Y cells (Fig. 4), indicating involvement of death receptor pathway of apoptosis.
Pro-apoptotic and anti-apoptotic members of the Bcl-2 family regulate the release of cytochrome c from the mitochondrial intermembrane space into the cytosol. Cytochrome c then interacts with the pro-caspase-9 and Apaf-1 to activate caspase-9 to switch on caspase-3 activity leading to apoptosis. So, we examined changes in expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 at mRNA and protein levels (Fig. 5). Treatment of cells with a flavonoid (EGCG or GST), especially combination of a retinoid and a flavonoid, increased total Bax expression and decreased Bcl-2 expression (Fig. 5) resulting in increase in Bax:Bcl-2 ratio (data not shown) to promote mitochondrial release of pro-apoptotic factors. The mitochondrial involvement in apoptosis was manifested in the disappearance of 15 kDa cytochrome c from the mitochondria and its concurrent appearance in the cytosol (Fig. 5). As an internal control, we monitored expression of COX4 in mitochondrial fraction whereas ß-actin expression in cytosolic fraction. The mitochondrial release of cytochrome c into the cytosol could cause activation of caspase-9. Treatments with EGCG or GST and also combination of a retinoid and EGCG or GST increased active 37 kDa caspase-9 fragment as detected by Western blotting (Fig. 5b) and also increased caspase-9 activity as determined by colorimetric assay (Fig. 5c). The inhibitory effects of various BIRC proteins on caspases and apoptosis have been examined in different cell culture models (20). We examined changes in the levels of BIRC expression and found down-regulation of BIRC-2 to BIRC-6 (Fig. 5) to promote apoptosis.
Previously, it has been reported that a novel Ca2+-mediated calpain-dependent apoptotic pathway exists in breast cancer cells and also regulation of intracellular free [Ca2+] in normal and cancer mammary epithelial cells is different (21). Our findings provide evidence for a sustained increase in intracellular-free [Ca2+] for activation of the Ca2+-dependent calpain for induction of apoptotic mechanism in SH-SY5Y cells following treatment with EGCG or GST alone and in combination with a retinoid (Fig. 6). Calpain can play a dual role, mediation of Ca2+ influx and proteolysis subsequent to Ca2+ influx, during cell death (22). Our findings support a direct relationship between an increase in intracellular-free [Ca2+] (Fig. 6a) and cell death with elevation of calpain expression and activity (Fig. 6b) following exposure to EGCG or GST. We also observed the decrease in calpastatin at mRNA and protein levels (Fig. 6). The increases in calpain and caspase-3 activities were confirmed in the cleavage of α-spectrin at specific sites for generating calpain-specific 145 kDa SBDP and caspase-3-specific 120 kDa SBDP. Overall, the results from this investigation showed that pretreatment of human malignant neuroblastoma cells with a retinoid induced differentiation and increased sensitivity to a flavonoid for increasing proteolytic activities for apoptotic death.
In conclusion, our present findings showed that the combination of a retinoid (especially 4-HPR) with a flavonoid (especially GST) could provide a novel therapeutic strategy for controlling the growth of human maliganant neuroblastoma cells.
Acknowledgements
This study was supported in part by the R01 grants (CA-91460 and NS-57811) from the National Institutes of Health (Bethesda, MD, USA) to S.K.R.
References
- 1.Brodeur GM. Meeting summary for advances in neuroblastoma research-2000. Med Pediatr Oncol. 2000;35:727–728. doi: 10.1002/1096-911x(20001201)35:6<727::aid-mpo54>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Guzhova I, Hultquist A, Cetinkaya C, Nilsson K, Påhlman S, Larsson LG. Interferon-gamma cooperates with retinoic acid and phorbol ester to induce differentiation and growth inhibition of human neuroblastoma cells. Int J Cancer. 2001;94:97–108. doi: 10.1002/ijc.1443. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds CP, Kane DJ, Einhorn PA, Matthay KK, Crouse VL, Wilbur JR, Shurin SB, Seeger RC. Response of neuroblastoma to retinoic acid in vitro and in vivo. Prog Clin Biol Res. 1991;366:203–211. [PubMed] [Google Scholar]
- 4.Veal GJ, Errington J, Redfern CP, Pearson AD, Boddy AV. Influence of isomerisation on the growth inhibitory effects and cellular activity of 13-cis and all-trans retinoic acid in neuroblastoma cells. Biochem Pharmacol. 2002;63:207–215. doi: 10.1016/s0006-2952(01)00844-9. [DOI] [PubMed] [Google Scholar]
- 5.Lovat PE, Corazzari M, Goranov B, Piacentini M, Redfern CP. Molecular mechanisms of fenretinide-induced apoptosis of neuroblastoma cells. Ann NY Acad Sci. 2004;28:81–89. doi: 10.1196/annals.1322.009. [DOI] [PubMed] [Google Scholar]
- 6.Das A, Banik NL, Ray SK. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int J Cancer. 2006;119:2575–2585. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–1378. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- 8.Uchiyama S, Yamaguchi M. Genistein and zinc synergistically stimulate apoptotic cell death and suppress RANKL signaling-related gene expression in osteoclastic cells. J Cell Biochem. 2007;101:529–542. doi: 10.1002/jcb.21208. [DOI] [PubMed] [Google Scholar]
- 9.Brown A, Jolly P, Wei H. Genistein modulates neuroblastoma cell proliferation and differentiation through induction of apoptosis and regulation of tyrosine kinase activity and N-myc expression. Carcinogenesis. 1998;19:991–997. doi: 10.1093/carcin/19.6.991. [DOI] [PubMed] [Google Scholar]
- 10.Das A, Banik NL, Ray SK. Differentiation decreased telomerase activity in rat glioblastoma C6 cells and increased sensitivity to IFN-γ and taxol for apoptosis. Neurochem Res. 2007;32:2167–2183. doi: 10.1007/s11064-007-9413-y. [DOI] [PubMed] [Google Scholar]
- 11.Das A, Banik NL, Ray SK. Retinoids induced astrocytic differentiation with down regulation of telomerase activity and enhanced sensitivity to taxol for apoptosis in human glioblastoma T98G and U87MG cells. J Neurooncol. 2008;87:9–22. doi: 10.1007/s11060-007-9485-1. [DOI] [PubMed] [Google Scholar]
- 12.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1α, 25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Mott JL, Chang SW, Stevens M, Mikolajczak P, Zassenhaus HP. Mitochondrial DNA mutations activate programmed cell survival in the mouse heart. Am J Physiol Heart Circ Physiol. 2005;288:H2476–H2483. doi: 10.1152/ajpheart.00670.2004. [DOI] [PubMed] [Google Scholar]
- 14.Wang KK, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon RA, Talanian RV, Keegan M, Herzog L, Allen H. Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch Biochem Biophys. 1998;356:187–196. doi: 10.1006/abbi.1998.0748. [DOI] [PubMed] [Google Scholar]
- 15.Smith MA, Anderson B. Where to next with retinoids for cancer therapy? Clin Cancer Res. 2001;7:2955–2957. [PubMed] [Google Scholar]
- 16.Raschellà G, Negroni A, Giubilei C, Romeo A, Ferrari S, Castello MA, Dominici C. Transcription of N-myc and proliferation-related genes is linked in human neuroblastoma. Cancer Lett. 1991;56:45–51. doi: 10.1016/0304-3835(91)90192-k. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-κB signaling module. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 18.Bozzo C, Sabbatini M, Tiberio R, Piffanelli V, Santoro C, Cannas M. Activation of caspase-8 triggers anoikis in human neuroblastoma cells. Neurosci Res. 2006;56:145–153. doi: 10.1016/j.neures.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Roy M, Chakrabarty S, Sinha D, Bhattacharya RK, Siddiqi M. Anti-clastogenic, anti-genotoxic and apoptotic activity of epigallocatechin gallate: a green tea polyphenol. Mutat Res. 2003;523/524:33–41. doi: 10.1016/s0027-5107(02)00319-6. [DOI] [PubMed] [Google Scholar]
- 20.Silke J, Vaux DL. Two kinds of BIR-containing protein - inhibitors of apoptosis, or required for mitosis. J Cell Sci. 2001;114:1821–1827. doi: 10.1242/jcs.114.10.1821. [DOI] [PubMed] [Google Scholar]
- 21.Sergeev IN, Norman AW. Calcium as a mediator of apoptosis in bovine oocytes and preimplantation embryos. Endocrine. 2003;22:169–175. doi: 10.1385/ENDO:22:2:169. [DOI] [PubMed] [Google Scholar]
- 22.Ray SK, Fidan M, Nowak MW, Wilford GG, Hogan EL, Banik NL. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000;852:326–334. doi: 10.1016/s0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]