Abstract
Objective
To evaluate cognitive behavioral therapy to enhance medication adherence and reduce depression (CBT-AD) in individuals with HIV.
Design
A two arm, randomized, controlled, cross-over trial comparing CBT-AD, to enhanced treatment as usual only (ETAU). ETAU, which both groups received, included a single-session intervention for adherence and a letter to the patient’s provider documenting her or his continued depression. The intervention group also received 10 to 12 sessions of CBT-AD.
Main Outcome Measures
Adherence to antiretroviral therapy as assessed by Medication Event Monitoring Systems (MEMs) and depression as assessed by blinded structured evaluation.
Results
At the acute outcome assessment (3-months), those who received CBT-AD evidenced significantly greater improvements in medication adherence and depression relative to the comparison group. Those who were originally assigned to the comparison group who chose to cross over to CBT-AD showed similar improvements in both depression and adherence outcomes. Treatment gains for those in the intervention group were generally maintained at 6 and 12-month follow-up assessments. By the end of the follow-up period, those originally assigned CBT-AD demonstrated improvements in plasma HIV RNA concentrations, though these differences did not emerge before the cross-over, and hence there were not between-group differences.
Conclusions
CBT-AD is a potentially efficacious approach for individuals with HIV struggling with depression and adherence. Replication and extension in larger efficacy trials are needed.
Keywords: HIV, adherence, Cognitive behavioral therapy
INTRODUCTION
Depressed mood is a distressing, interfering, and clinically significant problem frequent among individuals with medical illnesses (e.g., Frasure-Smith, Lesperance & Talajic, 1995; Januzzi, Stern, Pasternak & DeSantis, 2000; Pirl & Roth, 1999; Silverstone, 1990 Spiegel & Giese-Davis, 2003) and particularly for HIV-infected patients (e.g., Atkinson & Grant, 1994; Dew et al., 1997; Lyketsos, Hanson, Fishman, McHugh, & Treisman, 1994; Maldonado, Santiago, & Pacheco, 1998; Perkins, Stern & Golden, 1994; Rabkin, 1996; Thompson, Silverman, Dzeng, & Treisman, 2006). The prevalence of depressive mood disorders in people with HIV has been documented as high as 37% in some cohorts (Asch et al 2003; Atkinson & Grant, 1994; Bing et al., 2007; Dew et al., 1997). Depressed mood places people living with HIV at serious disadvantage through its associations with poorer disease course and health risk behaviors. Depressed symptoms in patients living with HIV have been related to more rapid decline in CD4 cells and faster HIV viral load increase (Ironson et al, 2005; Burack et al 1993), progression to AIDS (Page-Shafer, Delorenze, Satariano & Winkelstein, 1996; Leserman et al 1999, 2002,) and mortality (Mayne, Vittinghoff, Chesney, Barrett & Coates, 1996, Patterson et al, 1996, Ickovics et al 2001). Similarly, depressed mood has been related to higher levels of substance use (Dixit & Crum, 2000; Kalichman, Kelly & Rompa, 1997), more risky sexual behavior (Lehman et al., 1998; Kennedy et al., 1993), and poorer adherence to anti-HIV medication.
The relationship between depressed mood and poor adherence is well documented across various medical conditions (see DiMatteo, Lepper & Groghan, 2000; Dunbar-Jacob, Burke & Puczynski, 1995) including HIV (Catz, Kelly, Bogart, Benotsch & McAuliffe, 2000; Gordillo, del Amo, Soriano, Gonzalez-Lahoz, 1999; Holzemer et al., 1999; Paterson et al., 2000; Safren et al., 2001; Singh et al., 1996). In one of our previous intervention trials (Safren et al., 2001) that used a single session adherence counseling intervention (Safren et al., 1999), we found that baseline symptoms of depression were negatively associated with baseline adherence over and above additional psychosocial predictors - social support, adherence self-efficacy, and punishment beliefs about HIV.
In HIV, high levels of adherence to antiretroviral therapy for HIV are necessary to maximize treatment outcomes. Although the relationship between adherence and medication resistance in HIV is complex (see Bansgberg et al., 2000, Bangsberg, Weiser, Guzman & Riley, 2005), less than optimal adherence can contribute to the development of medication resistance, and several studies have established through self-report, pill count, or electronic pill-cap assessment that poor adherence is associated with worse medical outcomes as measured by viral load or CD4 cell count (e.g., Bangsberg et al., 2000; Catz et al., 2000; Gifford et al., 2000; Montaner et al., 1998; Hecht, Colfax, Swanson & Chesney, 1998; Paterson et al., 2000; Stansell et al., 2001). Although attaining a 95% medication adherence may be optimal, particularly with some regimens (Paterson et al., 2000), in patients with less than perfect adherence, improvements of even 10% in adherence may lead to enhanced biological outcomes (Bangsberg et al., 2001; Liu et al., 2006).
Given the association of depression to poor self-care across medical illnesses including HIV, the purpose of the present program of research has been to develop and test cognitive behavioral therapy for adherence and depression (CBT-AD) as applied to HIV medication adherence. CBT-AD integrates our previously tested approach to increasing adherence (Safren et al., 1999) with cognitive behavioral interventions for depression relevant to individuals with a medical condition. Although one research program that we know of has examined adding adherence counseling into an intervention for stress management (e.g. Antoni et al., 2006; Carrico et al., 2006) we are unaware of any study of a psychosocial intervention that has sought to specifically integrate the treatment of diagnosed clinical depression with adherence skills enhancement in any medical illness (see Olatunji et al., 2006 for a review of treatment studies of depression in HIV).
Prior to the present study, we completed a small, successful open-phase pilot trial of the approach with 5 HIV-infected gay men (Safren et al., 2004). However, as with any open trial, confounds due to time, maturation, and other variables limit the results. Hence, the current study is an initial randomized controlled trial of CBT-AD in individuals with HIV and depression. Following a staged approach to treatment development and testing (Rounsaville, Carroll, & Onken, 2001), the primary emphasis was on the acute (post-treatment) outcome comparison between the intervention and comparison condition. Other goals of the study included further feasibility and acceptability testing, an examination of the degree to which participants who crossed-over from the comparison group to CBT-AD made similar improvements, and an examination of whether improvements in those who completed CBT-AD were maintained. Accordingly, we hypothesized that the CBT-AD condition would have better adherence and improved depression compared to the ETAU condition at post-treatment, that those who cross over from ETAU to CBT-AD would make similar gains, and that gains would be maintained at follow-ups.
METHODS
Study Design
Forty-five men and women with HIV and depression were randomly assigned, in blocks of two by the study coordinator, stratified by sex, depression severity, and viral load (suppressed versus not) to one of two conditions. Enrollment occurred between June 2002 and November 2004. The last follow up occurred in February of 2006. The comparison condition was an enhanced treatment as usual condition, and included a single session intervention for HIV medication adherence, called “Life-Steps” (Safren et al., 1999). It also included monitoring of adherence, and a letter to one’s provider documenting his or her depression or other psychiatric disorders. The experimental condition included all of the components of the comparison condition plus 10 to 12 sessions of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV, following a modular approach to treatment. Those who were assigned to the comparison condition were invited to “cross over” into the CBT-AD condition at the post-treatment assessment if they still met study entry criteria.
After an initial evaluation to determine study eligibility, and two-week monitoring period to collect pre-intervention adherence data, there were four major study assessment visits: baseline (T1, time 1), acute outcome (T2, time 2), 3-month post-intervention follow-up (T3, time 3; approximately 6 months after baseline), and 9-month follow up (T4, time 4; approximately 12 months after baseline). The major assessments consisted of an assessment of HIV medication adherence as assessed with an electronic pill cap (MEMS), depression as assessed by an independent assessor who was blind to study condition and by self-report, as well as the determination of HIV plasma RNA concentration as drawn for the study or as abstracted from participants’ medical records if one was done in the past month.
The following inclusion/exclusion criteria were employed. Included were those who were diagnosed with a depressive mood disorder. This included 37 individuals with major depressive disorder, 1 with dysthymia, and 7 with stable bipolar disorder with the most recent episode being depressed (4 Bipolar II and 3 Bipolar II). Having residual symptoms of depression was defined as having a Clinical Global Impression Score (CGI; NIMH 1985) of 2 (“Minimally ill” = depressive symptoms, but able to function in all areas). Individuals also needed to be diagnosed with HIV, and on antiretroviral therapy for 4 months. Excluded were those with another psychiatric disorder (i.e. psychotic) that would interfere with study participation or consent, active substance abuse or dependence (those who had been in recovery for at least three months were not excluded), other inability to consent, or current or history of structured CBT for depression. Randomization happened after the independent assessment and confirmation of meeting all eligibility criteria.
Setting and recruitment
The study took place at Fenway Community Health, the largest provider of outpatient HIV care in New England. Recruitment occurred at both Fenway Community Health and selected Harvard Medical School hospitals through the affiliation of the Harvard Center for AIDS Research (CFAR). At Fenway Community Health and at Massachusetts General Hospital, a research assistant or research nurse met with potentially interested individuals when they came in for routine HIV care visits. Recruitment also involved community outreach and advertising, including outreach to local HIV service agencies and community health centers.
Description of CBT-AD and Enhanced Treatment as Usual
The treatment approach, cognitive behavioral therapy for HIV medication adherence and depression (CBT-AD), was based on traditional CBT approaches to the treatment of depression combined with intervention techniques most applicable to persons with chronic illness in general (see Thomason, Bachanas, & Campos, 1996; Nezu, Nezu, Friedman, Faddis, & Houts, 1998; Nezu, Nezu, Friedman, Houts, & Faddis, 1997), and prior clinical experience caring for HIV-infected individuals. A more detailed description of the intervention as employed in this current study can be found elsewhere (Safren, Gonzalez & Soroudi, 2007a; 2007b). Sessions were approximately 50 minutes long. In the present study, therapists were 2 clinical psychologists, a psychology intern, a post-doctoral fellow in psychology, and pre-doctoral graduate student in psychology. All had some prior experience with CBT, and all except the first author were under the supervision of the first author through weekly group meetings. This supervision involved review and feedback from audio-taped sessions as well as verbal review between therapists.
CBT-AD begins with a single-session intervention on HIV medication adherence (Safren et al., 1999), which involves eleven informational, problem-solving, and cognitive behavioral steps (e.g., education about adherence, scheduling, cue control strategies including the use of a watch alarm, adaptive thoughts about adherence, cues, provider communication). In each step, participants and the clinician define the problem, generate alternative solutions, make decisions about the solutions, and develop a plan for implementing them. Participants also receive adherence tools such as assistance with a schedule and a cue-dosing watch that can sound two alarms per day.
The remaining 11 sessions continued to address strategies for and barriers to medication adherence, with a review of electronic pill-cap (MEMS) data on adherence at the beginning of each session, and discussion of progress or difficulties with adherence. At each session, patients also completed the Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and proceeded with the completion of specific modules of treatment. Although the treatment manual provided guidance for the number of sessions for each module, flexibility in the number of sessions devoted to any module was allowed to address the complexity and variability of issues facing patients with HIV and depression. Module 1 first provided psychoeducation about HIV and depression, and then transitioned to motivational interviewing exercises (e.g. pros and cons of changing to improve one’s depression and adherence) designed to set the stage for behavioral change interventions to follow. Module 2 provided behavioral activation interventions across one session, and was designed to increase regularly occurring activities that involve pleasure and mastery. Module 3 provided cognitive restructuring interventions across 3 sessions following procedures outlined by Beck (1987), with attention specifically to negative automatic thoughts that relate to HIV medication adherence. Module 4 provided problem solving interventions across 3 sessions, and followed guidelines by D’Zurilla (1986) and Nezu et al. (1997) to help patients engage in a process that results in the selection of an action plan, and break this plan into manageable steps. Module 5 provided progressive muscle relaxation training/and diaphragmatic breathing skills across 1 session. A description of our updated approach is available (Safren, Gonzalez & Soroudi, 2007a; 2007b.).
Overview of assessment measures and assessment procedures
Four strategies were followed for the assessment of primary outcomes: 1) assessment of HIV medication adherence using MEMs (Medication Event Monitoring, AARDEX Inc) cap technology, 2) evaluation of depression using an independent assessor blind to treatment assignment, 3) self-report on a battery of measures, and 4) an HIV plasma RNA test. As was done in past studies, we included a two-week monitoring period of adherence, using MEMs cap data, as part of the baseline evaluation (Safren, Hendriksen, DeSousa, Boswell & Mayer, 2003.) in order to have a comparison point for pre-post analyses.
Clinician administered assessments
Enrollment visit
The initial evaluation for study entry criteria included a diagnostic evaluation of DSM-IV diagnoses using the Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, Gibbon, & Williams, 1995). The SCID-IV is the most widely used diagnostic instrument to reliably determine Axis I disorders in clinical populations (First et al. 1995). This evaluation was completed by one of the study therapists (before randomization), and all new cases were reviewed for review and diagnostic consensus by the study team in a weekly group supervision meeting. Before conducting the SCID-IV on one’s own, the study therapists had standard training in the approach, involving matching on audio-recorded interviews, and observing interviews by an already “certified” interviewer, and conducting interviews under observation.
Independent Assessments
An independent assessor, who remained blind to study condition and was not the therapist, conducted the clinician-administered outcome assessments. The independent assessment visits included administration of: 1) the Structured Version (Williams, 1988) of the Hamilton Depression Scale (HAM-D; Hamilton, 1959), 2) a rating of global distress and impairment for depression using the Clinical Global Impression (CGI; National Institute of Mental Health [NIMH], 1985) for severity (1 = not ill, to 7 = extremely ill), and 3) The Global Assessment of Functioning (GAF; APA, 1994) which accounted for overall impairment accounting for all DSM-IV axes and disorders. The HAM-D and CGI scores were regularly reviewed through audiotape supervision with another assessor who was also blind to treatment assignment, and consensus scores were assigned. A CGI score of 3 or above indicated that the patient met full criteria for depression, and a score of 2 indicated subsyndromal symptoms. Training in the administration of the Hamilton and CGI occurred through didactics and matching to videotaped example interviews. The independent assessor also had ongoing supervision and review of audio-taped assessments with an expert in these measures who also remained blind to treatment assignment.
Participant measures
Adherence assessment
We used an electronic pill-cap (Medication Event Monitoring System; MEMS; AARDEX inc.), recording the date and time of each instance of bottle opening, to monitor the antiretroviral medication that was “most difficult to remember or most difficult to take.” If participants reported that his or her medications were equally difficult to remember or take, the participants used the cap for the pill that they took most frequently. To account for doses that participants may have taken without opening the pill cap (e.g., took out afternoon doses when they opened it in the morning), we counted a dose as taken if participants could recall specific instances when they took their medications but did not use the pill cap. This procedure is consistent with recent studies comparing multiple measures of adherence with HIV outcomes, and recommending use of composite adherence scores (e.g., Liu et al., 2001, 2006; Llabre et al., in press). Medication adherence scores were calculated by dividing the number of doses taken by the number of prescribed doses. A dose was considered missed if it was not taken or if it was taken more than two hours before or after the designated time. The designated time was established with the research assistant and the patient at the baseline visit, but could be modified as part of formal interventions. At each assessment point, two week monitoring periods were used for adherence outcomes. Hence, we asked participants to monitor their medications for two weeks between the initial diagnosis/enrollment and the independent assessment in order to establish a baseline for medication adherence. This also allowed us to retrieve viral load results if they were needed between enrollment and randomization. Those who had a suppressed viral load had higher two-week MEMs adherence than those with detectable viral loads at baseline, and this association approached significance (t(40) = 1.95, p = .06).
Self report assessment of depression
Participants completed the self-reported Depression Inventory (BDI; Beck et al., 1961) during the major study assessments, as well as during each of weekly therapy sessions. The BDI has a strong history of psychometric reliability and validity and is widely used in psychiatric research.
Plasma HIV RNA determinations
At the major study assessment visits, participants who did not have an HIV plasma RNA test conducted in the previous month were asked to provide blood for viral load testing. For those who had a recent viral load examination, clinic chart extraction was used. We chose viral load over CD4 for analysis because we believed it would be the most proximal biological endpoint. This is because there is a lag between improved adherence to improved viral load, and then another lag between improved adherence to improved CD4.
Statistical Analyses
Forty-five participants were randomized to two conditions (experimental and comparison) and were assessed at baseline (T1) and three follow-up assessments (3 months (T2), 6 months (T3) and 12 months (T4) post baseline). At T2 participants in the comparison condition who continued to meet inclusion criteria were invited to cross over to the experimental condition (n = 13). For the main analyses, acute treatment effects, we compared outcome scores at T2 across the two conditions (N=45 randomized/intent to treat; N = 42 completers) covarying for baseline (T1) scores using ANCOVA. To complement traditional significance testing, for the acute outcome analyses, we computed between-group effect sizes (d =Mean change CBT-AD group -Mean change comparison-group / SD pooled of the change scores) for the continuous outcome measures, and used the table provided by Glass, McGaw, and Smith (1981) for computation of categorical outcomes. We also examined whether those who elected to cross over to CBT-AD from the comparison condition made similar gains by conducting within subjects t-tests for T2 (before CBT-AD for this condition) to T3 (post-CBT-AD for this condition) scores.
To examine maintenance of gains for those who received CBT-AD initially, we used within-subjects repeated measures analyses of variance, with a priori comparisons examining differences across the four time points. The hypothesis was that baseline scores (T1) would differ from acute outcome (T2) and follow-up (T3 and T4) scores, but that acute outcome (T2) and follow-up scores (T3 and T4) would not differ. There was not a large enough sample to statistically examine follow up in those who crossed over.
For acute outcome analyses, both intent-to-treat (last observation carried forward) and completer analyses (using data only from those who arrived at the follow-up assessment) were conducted. For follow-up analyses, only completer analyses were conducted. This was considered a more conservative approach because intent-to-treat analyses could artificially bias the analyses toward maintenance of gains.
Log transformed viral load data were analyzed following a similar approach. Additionally, because of the potential lag between improved adherence and viral load changes, which did not correspond with the acute outcome assessment where individuals could cross-over, we conducted exploratory analyses of viral load data from baseline to the 6 and 12 month outcome assessments in patients who received CBT-AD.
RESULTS
Participant Characteristics
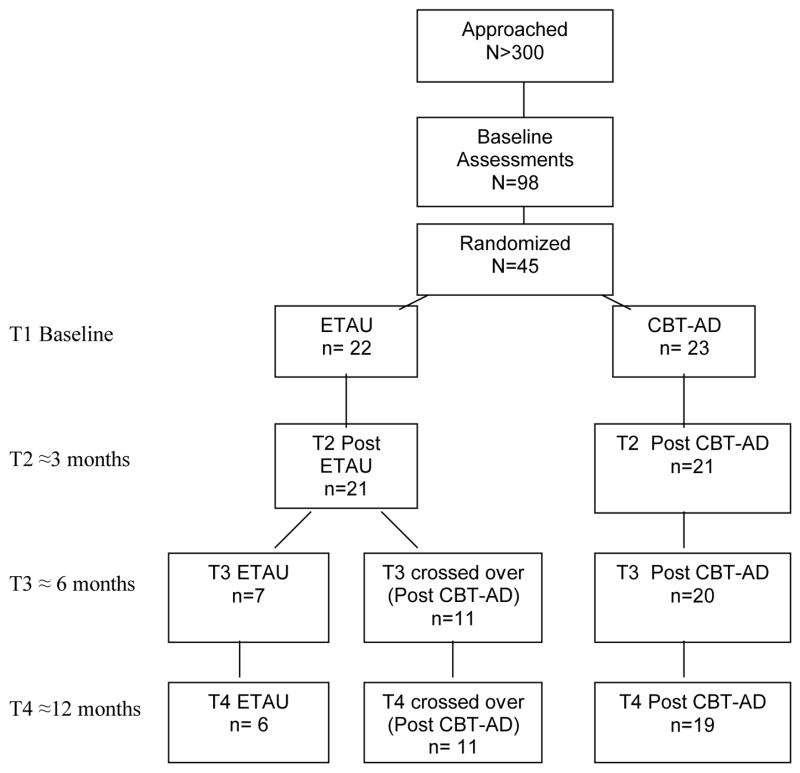
A diagram of participant flow is depicted in Figure 1. Over 300 in-person or telephone initial screens were conducted to attain 98 baseline, in-person, full screening evaluations. Of these, 45 participants were randomized. The following are the reasons for non-randomization of the remaining 53: 10 did not complete the full baseline evaluation (e.g., decided not to participate during the evaluation), 5 were lost to follow-up before randomization (randomization occurred after the second visit), and 38 did not meet inclusion criteria. The most frequent reasons for ineligibility were current active substance abuse or dependence (n=16, 42%), absence of a lack of a diagnosis of a depressive disorder (n=13, 34%), or a depressive disorder not being the primary psychiatric diagnosis (n = 6, 16%). For the acute outcome assessment, we had a 93% (42 of 45) retention rate, and for the 12 month follow up, we had an 80% (36 of 45) retention rate.
Figure 1.
Participant flow
Of the 45 participants randomized, 51% (n=23) were white and not Hispanic or Latino, 31% (n=14) were African-American, 18% Latino or Hispanic (n=8), 7% (n=3) Native American (percentages do not add to 100 because of overlap and some people reporting more than one race or ethnic group). Race and ethnicity did not differ by study arm. Regarding psychiatric comorbidity, sixty-four percent (n= 29) had at least one additional current DSM-IV diagnosis, and 18 (29%) had two additional current DSM-IV diagnoses. The most common were PTSD (n=14, 31%), panic disorder (n=12, 27%), and social anxiety disorder (n=10, 22%). The mean viral load log for the sample was 1.99 (sd = 1.98), and this did not differ across the two conditions at study entry. The number of participants with an undetectable viral load also did not vary between the groups at study entry. Of those randomized, 37 of the 38 of the male participants self-identified as being gay or bisexual, and of the women, 2 of the 7 self-identified as being gay or bisexual. At baseline, 20 (44%) participants had an undetectable viral load.
Acute outcome analyses – adherence and depression
For those randomized to CBT or who crossed over, the median and modal number of sessions was 12. The mean, however, was 10.42 (SD 2.81), which was influenced by the three participants who discontinued early did so after the first CBT session. The primary adherence outcome variable was percentage of doses taken within a two-hour window over the previous two weeks as indicated by MEMs (with corrections of pocketed doses as described above). The depression outcomes were overall clinical global impression (severity) scores as assessed by the CGI-S and rated by the independent assessor, as well as HAM-D and BDI scores.
Acute adherence outcome
Table 1 depicts means and standard deviations for the acute adherence outcomes. In the intent to treat analyses, those in the CBT-AD condition exhibited better adherence post-treatment than those in the comparison condition when controlling for baseline scores (F(1,42) = 21.94, p < .0001). The controlled effect size for the difference in the change scores between the two conditions was d = 1.0, which exceeded Cohen’s (1992) designation of large (.80). The completer analysis yielded a similar result (F(1,35) = 16.53, p<.0001), with the controlled, between group effect size for the difference in the change scores being d = 1.1. Four participants in the comparison condition did not have any valid pill cap data (i.e., did not use it or could no longer find it) at the acute outcome assessment and are not included in the completer analyses.
Table 1.
Acute Adherence and Depression Outcomes
| ITT | Completer | |||
|---|---|---|---|---|
| Baseline | T2 | Baseline | T2 | |
| MEMS | ||||
| ETAU | 54.41 (30.94)a | 54.00 (33.00)a | 61.59 (29.15)b | 61.06 (32.10)b |
| CBT-AD | 62.26 (26.82)c | 87.61 (14.27)c | 61.38 (27.76)d | 89.14 (13.58)d |
| CGI | ||||
| ETAU | 3.77 (0.97)a | 3.68 (1.04)a | 3.76 (.995)d | 3.67 (1.07)d |
| CBT-AD | 3.96 (1.02)c | 2.78 (1.17)c | 4.00 (1.05)d | 2.71 (1.19)d |
| HAM-D | ||||
| ETAU | 18.05 (6.45)a | 16.77 (5.77)a | 18.05 (6.61)d | 16.71 (5.91)d |
| CBT-AD | 20.39 (7.09)c | 13.30 (6.20)c | 20.71 (7.35)d | 12.95 (6.31)d |
| BDI | ||||
| ETAU | 23.86 (8.89)a | 19.54 (9.19)a | 24.14 (9.01)d | 19.61 (9.41)d |
| CBT-AD | 23.65 (11.99)c | 12.26 (10.55)c | 22.86 (11.76)d | 10.38 (8.23)d |
Note. n = 22.
n = 17.
n = 23.
n = 21.
ETAU=enhanced treatment as usual; CBT-AD= cognitive behavioral therapy. ITT= intent to treat; Completer= participant completed all sessions and assessments. MEMS= medication event monitoring systems; CGI= Clinical Global Impression Score; HAM-D= Hamilton Depression Scale; BDI= Beck Depression Inventory.
We examined two categorical outcomes: adherence at post-treatment greater than 90% and adherence at post-treatment greater than 95%. At the acute outcome in the intent-to-treat analysis, 57% of those assigned to CBT-AD evidence 90% adherence or greater compared to 18% of those in the comparison group (p = .013; d = .97). Using the 95% designation in the intent-to-treat analysis, 44% of those assigned CBT-AD evidenced 95% adherence or greater, compared to 14% of those in the comparison (p = .047, d = .91). At baseline, there were no significant differences between the groups on these adherence measures. At the acute outcome in the completer analysis, 61% of those assigned CBT-AD achieved 90% adherence or greater compared to 24% of those in the comparison group (p = .025, d = .67). Using the 95% designation in the completer analysis, 48% of those assigned CBT-AD achieved adherence or greater, compared to 14% of those in the comparison (p = .086, d = .91).
Acute Depression Outcomes
Table 1 also depicts means and standard deviations for acute depression outcomes. In the intent-to-treat analyses, across all three indicators of severity of depressed mood, the CBT-AD group exhibited decreased ratings compared to the comparison group1. This was for the independent-assessor rated Clinical Global Impression (F(1,42) = 9.68, p < .01), the independent-assessor rated Hamilton Depression Rating Scale (F(1,42) = 6.32, p < .02), and the self-reported Beck Depression Inventory (F(1,42) = 9.78, p < .01). For all three outcome variables, the controlled effect size calculations for between group differences in change scores were in the large (Cohen, 1992) range (ds = .91, .82, and .81 respectively).
The completer analyses generated a similar pattern of results for the independent-assessor rated Clinical Global Impression (F(1,39) = 10.19, p < .01), the independent-assessor rated Hamilton Depression Rating Scale (F(1,39) = 6.94, p < .02), and the self-reported Beck Depression Inventory (F(1,39) = 15.52, p < .001). Between group effect sizes for change scores for completer analyses for these variables also exceeded the large range (Cohen, 1992) (d = 1.0, .89, and .93 respectively).
Cross-over analyses
Thirteen individuals in the comparison condition still met initial inclusion criteria at the T2 assessment and chose to cross over to CBT-AD. Two individuals in this condition died before the T3 assessment. Table 2 depicts mean and standard deviation scores for the outcome variables for the cross over analyses. As compared to T2 levels, participants achieved significant additional adherence gains after crossing over to CBT-AD in both the intent-to-treat (t(12) = −4.186, p < .002) and completer (t(9) = −2.914, p< .02) analyses. Similar benefits were evident for the treatment of depression in both the intent-to-treat (CGI, t(12)=4.788, p < .0001; HAM-D, t(12) = 4.802, p < .0001; BDI, t(12) = 2.815, p < .02) and completer (CGI, t(10)=5.882, p < .0001; HAM-D, t(10) = 5.908, p < .0001; BDI, t(10) = 3.357, p < .01) analyses. The magnitude of gains was such that patients crossing over to CBT-AD achieved similar outcomes to those who originally received CBT-AD - all effect sizes exceeded the large range (>.8 except BDI completer was .75)
Table 2.
Adherence and Depression Cross-Over Outcomes
| ITT | Completer | |||
|---|---|---|---|---|
| T2 | T3 | T2 | T3 | |
| MEMS | 54.85 (36.12)a | 81.46 (16.32)a | 64.20 (36.14)b | 87.00 (13.14)b |
| CGI | 3.85 (0.90)a | 2.62 (1.12)a | 3.73 (0.91)c | 2.27 (0.79)c |
| HAM-D | 17.92 (5.33)a | 11.38 (6.49)a | 17.55 (5.47)c | 9.82 (5.46)c |
| BDI | 21.38 (8.65)a | 14.23 (9.51)a | 19.82 (7.90)c | 10.91 (5.07)c |
Note. n = 13.
n = 10.
n = 11.
ETAU=enhanced treatment as usual; CBT-AD= cognitive behavioral therapy. . ITT= intent to treat; Completer= participant completed all sessions and assessments. MEMS= medication event monitoring systems; CGI= Clinical Global Impression Score; HAM-D= Hamilton Depression Scale; BDI= Beck Depression Inventory.
Maintenance of change for individuals originally assigned to CBT-AD – adherence and depression
To examine maintenance of adherence gains, we examined the group that was originally assigned CBT-AD. Means and standard deviations for adherence and depression scores for those originally assigned CBT-AD are presented in Table 3.
Table 3.
Maintenance of change: CBT-AD Condition Only
| CBT-AD | ||||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| MEMS | 61.38 (27.76)a | 89.14 (13.58)a | 93.56 (8.88)c | 84.60 (17.08)b |
| CGI | 4.00 (1.05)a | 2.71 (1.19)a | 2.50 (1.28)f | 2.79 (1.40)e |
| HAM D | 20.71 (7.35)a | 12.95 (6.39)a | 11.85 (8.79)f | 13.53 (8.58)e |
| BDI | 22.86 (11.76)a | 10.38 (8.23)a | 10.21 (10.97)e | 11.22 (8.99)d |
Note. n = 21.
n = 15.
n = 16.
n = 18.
n = 19.
n = 20.
ETAU=enhanced treatment as usual; CBT-AD= cognitive behavioral therapy. MEMS= medication event monitoring systems; CGI= Clinical Global Impression Score; HAM-D= Hamilton Depression Scale; BDI= Beck Depression Inventory.
For adherence (MEMs scores), a repeated measures ANOVA across completers in the four time points (baseline, acute, 6 month, 12 month) yielded significance (F(3,33) = 8.467, p < .0001). Mean comparisons (using available data at the respective time points) were consistent with the hypothesized pattern of results: baseline adherence scores were significantly lower than acute outcome scores (t(20) = −5.010, p < .0001), time 3 scores (t(15) = −4.507, p < .0001), and time 4 scores (t(14) = −3.092, p < .01). As hypothesized, acute outcome scores did not differ from 6 or 12-month follow-up scores, and 6 month and 12 month follow-up scores did not differ from each other.
For depression, repeated measures ANOVAs across the four time points (baseline, acute, 6 month, 12 month) for completers yielded significant improvements over time for all indicators (CGI; F(3,51) = 8.115, p < .0001) (HAM-D; F(3,51) = 8.115, p < .0001) (BDI; F(3,45) = 17.053, p < .0001). Follow-up comparisons (using available data) were consistent with the hypothesized pattern of results. Baseline scores were significantly higher than outcome scores immediately after treatment (CGI; t(20) = 4.500, p < .0001) (HAMD; t(20) = 4.886, p < .0001) (BDI; t(20) = 5.978, p < .0001), at the time 3 assessment (CGI; t(19) = 4.04, p < .01) (HAMD; t(19) = 4.059, p < .01) (BDI; t(18) = 5.458, p < .0001), and at the time 4 assessment (CGl; t(18) = 3.13, p < .01) (HAMD; t(18) = 3.445, p < .01) (BDI; t(17) = 4.210, p < .005). Generally, outcome scores immediately after treatment did not significantly differ from follow-up scores at time 3 or time 4, and time 3 and time 4 scores did not differ from each other for any of the depression variables. The one exception is that for the BDI, time 4 scores were marginally higher than time 3 scores (t(15) = −2.051, p = .058).
Plasma HIV RNA
The analysis of treatment effects on viral load change was complicated by the study design. Specifically, there was not sufficient time for the intervention to impact viral load at the T2 post treatment assessment when comparing the two conditions, and these effects were not significant for both the completer analysis and intent to treat analysis (all ps >.1). In addition, because non-responders in the control condition were invited to cross over to the treatment condition at T2, our ability to detect a treatment effect on viral load at follow-up assessments T3–T5 was greatly reduced.
Paired t-tests revealed significant reductions in viral load log within the treatment group from baseline to T3 (t (20) = 2.22, p <.05) and to T4 (t (20) = 2.15, p<.05) follow-up. The mean reductions in log plasma HIV RNA were .85 and .86 respectively. These results were consistent using intent to treat for T3 (t (22) = 2.20, p <.05) and T4 (t(22) = 2.14, p <.05). Here, the mean reductions in log plasma HIV RNA were .78 for both. A similar pattern of results emerged combining the CBT group with those control participants who crossed over such that completer analysis identified a significant reduction in viral load log for the combined group from baseline to 6 months post-CBT (t(31) = 2.07, p <.05). These results were consistent using intent to treat analysis (t(33) = 2.06, p <.05). The mean reductions in log plasma HIV RNA were .60 and .56 respectively.
DISCUSSION
Despite the convergence of studies demonstrating a high association of depression with medical adherence (e.g., DiMatteo et al., 2000), to our knowledge this is the first randomized controlled trial to integrate evidence-based psychosocial treatment for depression (CBT) with interventions to increase HIV medication adherence in a medically ill population. We conducted this research in a population in particular need - those with HIV and depression. Our intervention was both feasible and, in this first trial, efficacious.
We found that this treatment led to significantly better outcomes in both adherence and depression than an enhanced-care comparison group. Furthermore, individuals crossing over to CBT-AD from the comparison group achieved similar gains. When starting ART, the expected decline of patient viral load is usually 1 log after the first month, and those who are adherent to their regimen typically achieve undetectable plasma HIV RNA levels following 6 months of therapy (Hammer et al, 2006). It is plausible that the absence of group differences in HIV viral load at the acute study outcome is due to the brief treatment format and insufficient elapsed time of the participants to reap the immunological benefit of treatment related gains in adherence. However, significant reductions in viral loads were evident for patients who were assigned the treatment by the six-month and 1-year follow-up assessments.
The average increase in adherence in the treatment group was 25.35 percentage points (going from 62.26 to 87.61), which is clinically significant considering there are two studies suggesting that even a 10% increase in adherence is associated with medically significant HIV outcomes (Bangsberg et al., 2001; Liu et al., 2006). These results encourage further study and application of CBT-AD, particularly given evidence that, controlling for antiretroviral medications, in some clinics viral loads tend to increase rather than decrease over time (.014 viral load log units per month; Ironson et al., 2005). Future studies of this approach should assess plasma HIV RNA, keep experimental groups distinct until sufficient time has elapsed to be maximally sensitive to viral load changes, and, potentially, improvements in CD4. Additionally, future studies with a larger sample sizes will be able to test a mediational model, whereby improved depression leads to better benefit from an adherence intervention, which leads to decreased viral load, and ultimately leads to increased CD4 counts.
Although the experimental group had significantly improved depression compared to the control condition, with a large effect size, symptoms were still slightly elevated in both groups at the endpoints. A strict definition of clinical significance would be a return to normal functioning (Jacobson, Berns, & McGlinchey, 1999; Kendall, Marrs-Garcia, Nath, & Sheldrick, 1999) which here, would be a CGI of, on average 1.0. This was likely not achieved because the patient population was generally quite complex – with multiple comorbidities and both medical and psychiatric symptoms. The average CGI at entry approached a 4, which indicates exceeding full criteria for clinical depression, and of moderate severity. Each average CGI at the follow-ups is under 3, indicating not meeting full criteria for depression, with an average improvement of 1.23 in the ITT analyses and 1.46 in the completer analyses. Hence, in terms of clinical significance to the patient, this reduction is meaningful.
Two important limitations of the present study are the sample size and the fact that it did not control for differences in therapist contact between the treatment and comparison conditions. The lack of an attention-matched control precludes the interpretation that the particular components of this psychosocial approach to treating depression are better than others. Markowitz et al (1998), for example, in depressed HIV-infected patients, found that CBT and supportive psychotherapy were similar in their efficacy in reducing depression. Subsequent studies may be conducted to account for the effects of nonspecific or supportive treatment elements in understanding the contribution of CBT-AD interventions to clinical outcomes. One goal of the present program of research, however, was to determine if adherence counseling alone is useful for those with individuals who are diagnosed both with depression and with HIV, or whether one would need to optimize the treatment of depression in order to maximize the gains that could be made from adherence counseling. Accordingly, our current study utilized one method for optimizing the treatment of depression, and found that this approach (CBT-AD) was associated with better outcomes than a single session of Life-Steps and a letter to one’s provider. The large effect sizes for reductions in depression and increases in medication adherence speak to the potential utility of this approach, and encourage efforts for replication and extension of these promising findings with a larger sample size.
There are several other limitations of the present study, which highlight the need for replication and extension with a larger sample. One issue inherent with conducting interventions that target adherence concerns the fact that those who are not adherent to their medical intervention may not be adherent to the requirements of a study. The current study had more people drop out or have unused MEMs caps in the comparison group than the intervention group (though this difference in missing data was not statistically significant). In some ways, this is a methodological problem inherent in adherence intervention research. If the goal is to demonstrate a better effect in the experimental intervention, the comparison group would do worse with respect to adherence. If those who are non-adherent to HIV medications are less likely to be adherent to study procedures, and the intervention helps with adherence, then it is likely that those in the control group will not improve adherence, and remain worse at both medication and study adherence. Future studies that examine alternative ways of following and assessing individuals assigned to comparison group may be required (e.g., unannounced pill counts – Bangsberg, Weiser, Guzman, & Riley, 2005). Although we allowed psychiatric comorbidity, we did exclude those with active substance abuse. Given the high prevalence of substance abuse in HIV (e.g. Bing et al., 2001; Dixit & Crum, 2000; Kalichman, Kelly & Rompa, 1997), the potential overlap between depression and substance abuse, and the fact that we screened out individuals with active substance abuse or dependence, further study of this intervention approach in those with comorobid substance abuse is warranted.
Nonetheless, the present study adds to the literature a successful intervention for HIV medication adherence, targeting a psychiatrically-comorbid sample: individuals with a clinical diagnosis of depression. Although other approaches have utilized cognitive behavioral techniques, particularly relaxation training, for stress management in HIV (CBSM; e.g. Antoni et al., 2006; Carrico et al., 2006), the present approach is unique because it integrates adherence training, a health promotion behavior, into each and every session of the intervention and also targets the treatment of a clinical diagnosis of depression. Although relaxation training was a component of the current approach, it typically was done in only one session, with the main emphasis on treating depression and increasing adherence using cognitive and behavioral approaches designed for medically-ill populations. Targeting both the treatment of a comorbid psychiatric condition and increasing self-care behaviors, may make it possible to help individuals with HIV and high levels of impairment maximize both medical and quality of life outcomes.
Acknowledgments
This research was supported by the National Institute of Mental Health, grant number R21MH06660 to Dr. Steven A. Safren.
The authors would like to thank Dr. Robert Knauz, Dr. Jonathan Lerner, Rodney Vanderwarker, Matthew Mimiaga, and Christopher Sterling for their assistance carrying out this project.
Footnotes
We thank the anonymous reviewers for their comments. One was to rerun the BDI analyses using just the cognitive-affective subscale. When we did this, the pattern of results were the same. We also did this for the Hamilton, and again, the pattern of results were the same.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/hea/
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Antoni MH, Carrico AW, Duran RE, Spitzer S, Penedo F, Ironson G, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosom Med. 2006;68:143–151. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- Asch SM, Kilbourne AM, Gifford AL, Burnam MA, Turner B, Shapiro MF, et al. Underdiagnosis of depression in HIV: Who are we missing? Journal of General Internal Medicine. 2003;18:450–460. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Grant I. Natural history of neuropsychiatric manifestations of HIV disease. The Psychiatric Clinics of North America. 1994;17:17–33. [PubMed] [Google Scholar]
- Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. Journal of the American Medical Association. 1993;270:2568–2573. [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Weiser SD, Guzman D, Riley ED. 95% adherence is not necessary for viral suppression to less than 400 copies/mL in the majority of individuals with NNRTI regimens. Presented at the Twelfth Conference on Retroviruses and Opportunistic Infections; Boston. 2005. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck A. Cognitive therapy of depression. New York: Guilford Press; 1987. [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Antoni MH, Duran RE, Ironson G, Penedo F, Fletcher MA, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with hiv-positive gay men treated with haart. Ann Behav Med. 2006;31:155–164. doi: 10.1207/s15324796abm3102_7. [DOI] [PubMed] [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19:124–133. [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;1:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Dew MA, Becker JT, Sanchez J, Caldararo R, Lopez OL, Wess J, et al. Prevalence and predictors of depressive, anxiety and substance use disorders in HIV-infected and uninfected men: a longitudinal evaluation. Psychological Medicine. 1997;27:395–409. doi: 10.1017/s0033291796004552. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Dixit AR, Crum RM. Prospective study of depression and the risk of heavy alcohol use in women. American Journal of Psychiatry. 2000;157:751–8. doi: 10.1176/appi.ajp.157.5.751. [DOI] [PubMed] [Google Scholar]
- Dunbar-Jacob J, Burke LE, Puczynski S. Clinical assessment and management of adherence to medical regimens. In: Nicassio PM, Smith TW, editors. Managing Chronic Illness. Washington DC: American Psychological Association; 1995. pp. 313–349. [Google Scholar]
- D’Zurilla TJ. Problem solving therapy: A social competence approach to clinical interventions. New York: Springer; 1986. [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders, patient edition. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Frasure-Smith N, Lesperance F, Talajic M. The impact of negative emotions on prognosis following myocardial infarction: Is it more than depression? Health Psychology. 1995;14:388–398. doi: 10.1037//0278-6133.14.5.388. [DOI] [PubMed] [Google Scholar]
- Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. Journal of Acquired Immune Deficiency Syndromes. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- Glass Gene V, McGaw B, Smith ML. Meta-analysis in social research. Beverly Hills: Sage Publications; 1981. [Google Scholar]
- Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1959;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SM, Saag MS, Schechter M, Montaner JSG, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 Recommendations of the International AIDS Society- USA panel. Journal of the American Medical Association. 2006;296:827–843. [Google Scholar]
- Hecht FM, Colfax G, Swanson M, Chesney MA. Adherence and effectiveness of protease inhibitors in clinical practice. 5th Conference on Retroviruses and Opportunistic Infections; Chicago, Ill. 1998. [Google Scholar]
- Holzemer WL, Corless IB, Nokes KM, Turner JG, Brown MA, Powell-Cope GM, et al. Predictors of self-reported adherence in persons living with HIV disease. AIDS Patient Care and STDS. 1999;13:185–197. doi: 10.1089/apc.1999.13.185. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. Journal of the American Medical Association. 2001;285:1460–1465. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Ironson G, O’Cleirigh C, Fletcher MA, Laurenceau JP, Balbin E, Klimas N, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosomatic Medicine. 2005;67:1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining clinical significance for treatment effects: Description, application, and alternatives. Journal of Consulting and Clinical Psychology. 1999;67:300–307. doi: 10.1037//0022-006x.67.3.300. [DOI] [PubMed] [Google Scholar]
- Januzzi JL, Stern TA, Pasternak RC, DeSantis RW. The influence of anxiety and depression on outcomes of patients with coronary artery disease. Archives of Internal Medicine. 2000;160:1913–1921. doi: 10.1001/archinte.160.13.1913. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Kelly JA, Rompa D. Continued high-risk sex among HIV seropositive gay and bisexual men seeking HIV prevention services. Health Psychology. 1997;16:369–375. doi: 10.1037//0278-6133.16.4.369. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Marrs-Garcia A, Nath SR, Sheldrick RC. Normative comparisons for the evaluation of clinical significance. Journal of Consulting and Clinical Psychology. 1999;67:285–299. doi: 10.1037//0022-006x.67.3.285. [DOI] [PubMed] [Google Scholar]
- Kennedy CA, Skurnick J, Wan JY, Quattrone G, Sheffet A, Quionenes M, et al. Psychological distress, drug and alcohol use as correlates of condom use in HIV-serodiscordant heterosexual couples. AIDS. 1993;7:1493–1499. doi: 10.1097/00002030-199311000-00014. [DOI] [PubMed] [Google Scholar]
- Lehman JS, Hecht FM, Fleming PL, Coleman S, Chesney M, Bindman A, et al. HIV testing behavior among at-risk populations: why do persons seek, defer, or avoid getting tested in the United States? International Conference on AIDS. 1998;12:867. abstract no. 136/43103. [Google Scholar]
- Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, et al. Progression to AIDS: The effects of stress, depressive symptoms, and social support. Psychosomatic Medicine. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychological Medicine. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- Liu H, Golin CE, Miller L, Hays RD, Beck K, Sanadaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;2001:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- Liu H, Miller LG, Golin CE, Wu T, Wenger NS, Kaplan AH. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. Journal of Acquired Immune Deficiency Syndrome. 2006;41:315–322. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Weaver KE, Durán RE, Antoni MH, McPherson-Baker S, Schneiderman N. A measurement model of medication adherence to highly active antiretroviral therapy and its relation to viral load in HIV+ adults. AIDS Patient Care and STDs. doi: 10.1089/apc.2006.20.701. (In Press) [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Hanson AL, Fishman M, McHugh PR, Treisman GJ. Screening for psychiatric morbidity in a medical outpatient clinic for HIV infection: The need for a psychiatric presence. International Journal of Psychiatry and Medicine. 1994;24:103–113. doi: 10.2190/URTC-AQVJ-N9KG-0RL4. [DOI] [PubMed] [Google Scholar]
- Maldonado C, Santiago L, Pacheco E. Depresión in women living with HIV: high rates and decreasing trend. Paper presented at 12th World AIDS Conference; Geneva, Switzerland. 1998. [Google Scholar]
- Markowitz JC, Kocsis JH, Fishman B, Spielman LA, Jacobsberg LB, Frances AJ, et al. Treatment of depressive symptoms in human immunodeficiency virus-positive patients. Arch Gen Psychiatry. 1998;55:452–457. doi: 10.1001/archpsyc.55.5.452. [DOI] [PubMed] [Google Scholar]
- Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Archives of Internal Medicine. 1996;156:2233–2238. [PubMed] [Google Scholar]
- Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, et al. A randomized, double-blind trial comparing combinations of nevarpine, didanosine, and zidovudine for HIV-infected patients. Journal of the American Medical Association. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. CGI: Clinical Global Impression Scale - NIMH. Psychopharmacology Bulletin. 1985;21:839–844. [Google Scholar]
- Nezu AM, Nezu CM, Friedman SH, Houts PS, Faddis S. Project genesis: Application of problem-solving therapy to individuals with cancer. The Behavior Therapist. 1997;9:155–158. [Google Scholar]
- Nezu AM, Nezu CM, Friedman SH, Faddis S, Houts PS. Helping cancer patients cope: A problem-solving approach. Washington, D.C.: American Psychological Association; 1998. [Google Scholar]
- Olatunji BO, Mimiaga MJ, O’Cleiright C, Safren SA. A review of treatment studies of depression in HIV. Topics in HIV Medicine. 2006;14:112–124. [PubMed] [Google Scholar]
- Page-Shafer K, Delorenze GN, Satariano W, Winkelstein W., Jr Comorbidity and survival in HIV infected men in the San Francisco Men's Health Survey. Annals of Epidemiology. 1996;6:420–430. doi: 10.1016/s1047-2797(96)00064-6. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Vergis EN, Squire C, Wagener MM, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Shaw WS, Semple SJ, Cherner M, McCutchan A, Atkinson JH, et al. Relationship of psychosocial factors to HIV disease progression. Annals of Behavior Medicine. 1996;18:30–39. doi: 10.1007/BF02903937. [DOI] [PubMed] [Google Scholar]
- Perkins D, Stern R, Golden R. Mood disorders in HIV infection: prevalence and risk factor in a non-epicenter of the AIDS epidemic. American Journal of Psychiatry. 1994;151:233–236. doi: 10.1176/ajp.151.2.233. [DOI] [PubMed] [Google Scholar]
- Pirl WF, Roth AJ. Diagnosis and treatment of depression in cancer patients. Oncology. 1999;13:1293–1301. [PubMed] [Google Scholar]
- Rabkin JG. Prevalence of psychiatric disorders in HIV illness. International Review of Psychiatry. 1996;8:157–166. [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clinical Psychology: Science and Practice. 2001;8:133–142. [Google Scholar]
- Safren SA, Gonzalez JS, Soroudi N. Coping with Chronic Illness: Cognitive behavioral therapy for adherence and depression in individuals with chronic illness, Client Workbook. Oxford University Press; 2007a. [Google Scholar]
- Safren SA, Gonzalez JS, Soroudi N. Coping with Chronic Illness: Cognitive behavioral therapy for adherence and depression in individuals with chronic illness, Therapist Guide. Oxford University Press; 2007b. [Google Scholar]
- Safren SA, Hendriksen ES, DeSousa N, Boswell SL, Mayer KH. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care. 2003;15:787–793. doi: 10.1080/09540120310001618630. [DOI] [PubMed] [Google Scholar]
- Safren SA, Hendriksen ES, Mayer KH, Mimiaga MJ, Pickard R, Otto MW. Cognitive-behavioral therapy for HIV medication adherence and depression. Cognitive and Behavioral Practice. 2004;11:415–423. [Google Scholar]
- Safren SA, Otto MW, Worth J. Life-Steps: Applying cognitive-behavioral therapy to patient adherence to HIV medication treatment. Cognitive and Behavioral Practice. 1999;6:332–341. [Google Scholar]
- Safren SA, Otto MW, Worth J, Salomon E, Johnson W, Mayer K, Boswell S. Two strategies to increase adherence to HIV antiretroviral medication: Life-Steps and medication monitoring. Behavioural Research and Therapy. 2001;39:1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Safren SA, Radomsky AS, Otto MW, Salomon E. Predictors of psychological well-being in a diverse sample of HIV-positive patients on highly active antiretroviral therapy. Psychosomatics. 2002;43:478–485. doi: 10.1176/appi.psy.43.6.478. [DOI] [PubMed] [Google Scholar]
- Silverstone PH. Changes in depression scores following life-threatening illness. Journal of Psychosomatic Research. 1990;34:659–663. doi: 10.1016/0022-3999(90)90110-p. [DOI] [PubMed] [Google Scholar]
- Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu VL. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: Prospective assessment with implications for enhancing compliance. AIDS Care. 1996;10:1033–1039. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Giese-Davis J. Depression and cancer: Mechanisms and disease progression. Biological Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- Stansell J, Holtzer C, Mayer S, DeGuzman D, Hamel E, Lapins D. Factors affecting treatment outcomes in a medication event monitoring system. 8th Conference on Retroviruses and Opportunistic Infections; Chicago, Ill. 2001. [Google Scholar]
- Thompson A, Silverman B, Dzeng L, Treisman G. Psychotropic medications and HIV. Clinical Infectious Diseases: An official publication of the Infectious Diseases Society of America. 2006;42:1305–1310. doi: 10.1086/501454. [DOI] [PubMed] [Google Scholar]
- Thomason B, Bachanas PJ, Campos PE. Cognitive behavioral interventions with persons affected by HIV/AIDS. Cognitive and Behavioral Practice. 1996;3:417–442. [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]