Abstract
It is proposed that the mind and brain often work at a gross level and only with fine tuning or inhibition act in a more differentiated manner, even when one might think the domains being issued the global command should be distinct. This applies to disparate findings in cognitive science and neuroscience in both children and adults. Thus, it is easier to switch everything, or nothing, than to switch one thing (the rule one is following or which button to press) but not the other. It is easier to issue the same command to both hands than to move only one hand. If one needs to respond to the opposite (or antonym) of a stimulus, one is faster if the correct response is to the side opposite the stimulus. People tend to think of the nervous system as sending out very precise commands only to the relevant recipient, but it appears that often the command goes out more globally and then parts of the system need to be inhibited from acting on the command.
Keywords: task switching, bimanual coordination, Simon effect, card sort test, inhibition
Developmental psychologists, neuroscientists, pediatricians, and teachers have long known that early in life the nervous system lacks precision in many ways, often functioning in a global, diffuse way. For example, when a young child intends to do something with only one hand, there is often motor overflow to the other hand. The nervous system command to do the action goes to both hands, lacking the intended precision that was for the command to go to one hand only. Bruner and I independently documented the frustration of infants and toddlers, who having forcefully pushed a lid up with both hands, intend to remove one hand to reach for a treat under the lid. That darn lid, though, keeps coming down because when the child lowers one hand, the other hand (the one that should be holding the lid up) comes down as well (Bruner, 1970; Diamond, 1990; see Figure 1). Here, the command “lower” has gone to both hands, though it was intended for only one hand. Such mirror movements of the limbs are not only seen in infants but are normal in children through at least 7 years of age (Abercrombie, Lindon, & Tyson, 1964; Lazarus & Todor, 1987; Mayston, Harrison, & Stephens, 1999).
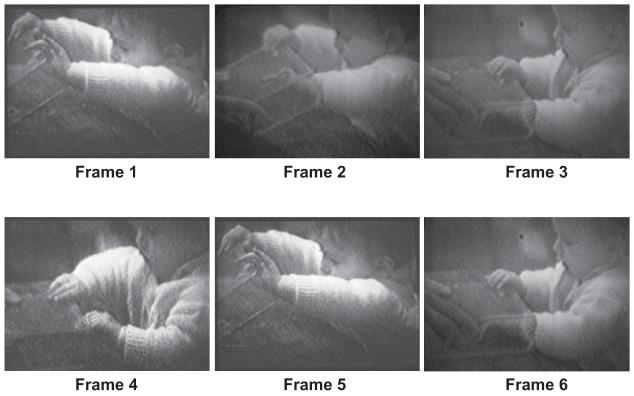
Figure 1.
A girl of 7.5 months performing Diamond’s (1990) object retrieval task where the front of the box is open and the toy is on the table near the rear of the box. The baby needs to be able to see her pacifier through the box opening to succeed in reaching in to retrieve the toy. Cleverly, the baby raises the box with both hands so that she can see in through the opening (Frame 1). Then she lowers one hand to start to reach in for the toy (Frame 2). The hand that was supposed to keep the box up fails her, however, and comes down too (Frame 3), creating a problem. She is no longer looking through the opening, so she withdraws the hand that had started to reach in (Frame 4). In Frame 5, she tries again, once again raising the box with both hands. Once again, when one hand comes down to reach, the hand that was supposed to keep the box raised also comes down, and once again the baby is not able to succeed in retrieving the toy.
Another example of early lack of precision in neural communication is the underspecification of neuronal projections, earning them the name exuberant projections. The sensory areas initially project somewhat globally to the thalamus, so that some nonvisual inputs go to the visual thalamus (the lateral geniculate) and some nonauditory inputs go to the auditory thalamus (the medial geniculate), and conversely some early projections from the visual thalamus go to nonvisual areas, and some early projections from the auditory thalamus go to nonauditory areas (Bhide & Frost, 1999; Cooper & Cowey, 1990; Frost, 1986; Ramoa & Yamasaki, 1996; D. K. Simon, & O’Leary, 1992; Sur, 1988; Sur, Garraghty, & Roe, 1988). The nervous system relies on projections that have gone to the wrong place being pruned away when they do not match up as well with the recipient site as projections intended for that site.
What is less widely recognized, and is explored in this article, is that such global modes appear to (a) characterize the mind and brain not just in children but also in adults and (b) characterize the mind and brain in far more respects than in just motor or sensory processing—not in all respects but in surprisingly many. While people tend to think of gross commands as only characterizing the immature brain, they are very much true of the mature adult brain as well. I offer the hypothesis that the mind and brain often tend to work at a relatively gross level, and only with effort (often in the form of inhibition) do they work in a more selective manner. The lack of specification that surprised neuroscientists when they first discovered exuberant projections decades ago seems to often be the rule across many domains of cognition, perception, and action. It is proposed that several seemingly unrelated findings in cognitive psychology and neuroscience provide support for this principle. People are more integrated than they often appreciate. To act in a less integrated fashion often requires inhibition of the global-command default mode.
Corollary 1
One corollary of the global default principle is as follows: It is easier to switch everything or nothing than to switch one thing (e.g., the rule or the response) but not another (Crone, Ridderinkhof, Worm, Somsen, & van der Molen, 2004; Davidson, Amso, Anderson, & Diamond, 2006; Hommel, Musseler, Aschersleben, & Prinz, 2001; Kleinsorge, 1999; Schuch & Koch, 2004). Similarly, it is easier to slow down across the board than to fine tune that more precisely.
Thus, if the rule changes, people are faster to respond if where they should respond also changes. This has been demonstrated in children (Crone et al., 2004), young adults (e.g., Kleinsorge, 1999), and older adults (Mayr, 2001). Only with effort can people switch one thing but not another; it is much easier to switch everything across the board. For example, Davidson et al. (2006) had participants randomly switch between the rule to respond on the same side as the stimulus and the rule to respond on the side opposite the stimulus. (If the stimulus was gray, participants were to press the button on the same side as the stimulus, and if the stimulus was striped, participants were to respond on the opposite side.) Participants were much faster on task-switch trials when the location of the correct response also changed than on trials where the rule switched but not the response (Davidson et al., 2006). They were faster on rule-repeat trials when the response site also remained the same than when the correct response changed. It is as if it is easier for a person’s mind or brain to issue a global command, “switch” or “repeat,” than to issue the more refined command, “Switch rules but do not switch response site,” though conceptually where one responds should be orthogonal to what rule one is following.
Consider the Simon effect, named after the person who first described it (J. R. Simon, Acosta, Mewaldt, & Speidel, 1976; J. R. Simon, Sly, & Vilapakkam, 1981; J. R. Simon & Small, 1969). On Simon tasks the rules are as follows: “For A, press right, and for B, press left.” The stimuli are always presented individually to the right or left. People are consistently faster to respond when a stimulus appears on the same side as its associated response than when it appears on the opposite side; that difference in response time is called the Simon effect (Hommel, Proctor, & Vu, 2004; Lu & Proctor, 1995). However, if one is supposed to press the color opposite to the color of a stimulus, people are faster to also press the button on the side opposite the stimulus (a reverse Simon effect; Hedge & Marsh, 1975; J. R. Simon et al., 1981). Again, it appears that issuing a global command (in this case, “opposite”) is preferred by one’s neural machinery over a more selective command to just the action system or to just one aspect of cognition.
Also consistent with Corollary 1, if the stimulus changes but the rule and response remain the same as on the previous trial, people are slower to respond than if the rule and response also change when the stimulus changes (Mayr & Bryck, 2005). That is, upon a change of stimulus, a person’s default mode is to change everything rather than, in a more refined way, to stay with the same rule and response despite the change of stimulus. People can do the latter, but it goes counter to their default and so takes longer.
When comparing performance on single-task blocks (where all trials present the same task) to task-switch blocks (where two tasks are intermixed), researchers consistently find that people slow down across the board when two tasks are intermixed (Davidson et al., 2006; Duncan, 1979; Allport & Wylie, 2000; Los, 1996; Rogers & Monsell, 1995). That is true even for easy trials—trials that require acting in accord with one’s dominant tendency (e.g., read the word and ignore the color of the ink) and trials that present the same task as on the preceding trial. It is as if the system has been given a gross-level command, “slow down,” even when slowing down might not really be needed on a subset of those trials.
Corollary 2
A second corollary of the principle of a default global-command mode is that it is easier to take into account multiple salient properties of a stimulus than only one of its properties. Indeed, it is often difficult to ignore irrelevant properties of an attended stimulus.
On Simon tasks (described above), all a participant needs to know to respond correctly is the identity of the stimulus; nothing else about a stimulus is ever relevant. People evidently cannot ignore the location of the stimulus, however, as their performance is affected by that irrelevant information; hence, the Simon effect (faster reaction times when a stimulus appears on the same side as its associated response than when it appears on the opposite side). This is true from the youngest age that children can perform the Simon task (3.5-4 years) and, if anything, is more exaggerated at the youngest ages than thereafter (Davidson et al., 2006).
Giving practice in overcoming the tendency to respond faster when stimulus and response are on the same side, such as by presenting a series of opposite-side (incongruent) trials can produce a reverse Simon effect when one is asked to switch back to responding on the same side as the stimulus (Logan & Zbrodoff, 1979). That too shows the influence of stimulus location, which is irrelevant to the task. The point is that when same-side and opposite-side trials are intermixed, the responses of children (Davidson et al., 2006), young adults (Hommel et al., 2004), and older adults (Van der Lubbe & Verleger, 2002) are affected by a stimulus dimension participants know is irrelevant but which their strong tendency is to process anyway. It appears that because the commands to the motor system are to respond on the right or left, right-left location becomes globally salient (even in the visual perception of the stimuli).
Similarly, since Garner (1974), psychophysicists have known that if a person is attending to length, although orientation and/or width might be wholly irrelevant, those properties are also processed (e.g., Dick & Hochstein, 1988; though this does not extend to all possible stimulus properties [e.g., Cant, Large, McCall, & Goodale, 2008]). The behavioral paradigm Garner pioneered was a speeded-classification task that assesses how efficiently people can process one dimension of an object while ignoring other dimensions. In the baseline condition, only the relevant dimension varies, while the value of an irrelevant dimension is kept constant. In the filtering condition, both the relevant and irrelevant dimensions vary. If participants could selectively process only the relevant dimension, their speed and accuracy would be identical in the two conditions. Since participants cannot help attending to the orientation of lines when asked to discriminate their length, responses to length are slower and less accurate when both dimensions vary than when the stimuli vary only in length.
It is many years since Duncan (Duncan, 1980; Duncan, Emslie, & Williams, 1996; Duncan, Humphreys, & Ward, 1997) theorized that directing attention to one feature of an object results in selection of other features of that object, both relevant and irrelevant ones. A particularly striking example of people’s tendency to be influenced by properties of a stimulus that they know are irrelevant is provided by Pratt and Hommel (2003, Experiment 4; see Figure 2). They demonstrated that not only is an irrelevant feature (color) of a stimulus processed by adults, but if that irrelevant feature (the same color) then appears as part of a wholly irrelevant stimulus, that wholly irrelevant stimulus then influences adults’ performance. This has yet to be tested in children, but I see no reason why children’s performance would not be similarly influenced.
Figure 2.
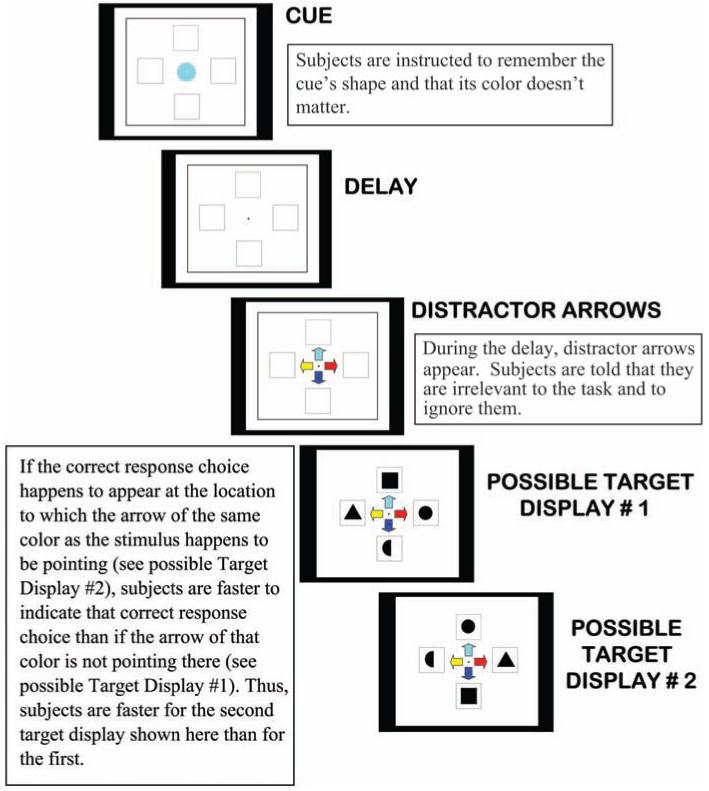
Illustration of a trial in Pratt and Hommel (2003, Experiment 4). Adapted from “Symbolic Control of Visual Attention: The Role of Working Memory and Attentional Control Settings,” by J. Pratt & B. Hommel, 2003, Journal of Experimental Psychology: Human Perception and Performance, 29, p. 837. Copyright 2003 by the American Psychological Association.
The difficulty that 3-year-olds display in changing dimensions on the dimensional change card sort task also demonstrates this in stark relief (Diamond, Carlson, & Beck, 2005; Diamond & Kirkham, 2005; Zelazo, Frye, & Rapus, 1996). Here, cards can be sorted by color or shape. Each stimulus matches the label for one sorting bin (the model card) on one dimension but not the other (e.g., a red truck stimulus matches a blue truck model card on shape but not on color). Hence the correct sorting response for one sorting rule is the wrong response for sorting by the other dimension. Unlike the procedure for the Wisconsin card sorting task, participants are told when the sorting criterion changes and what it is changing to, and on each trial they are either reminded of the currently relevant sorting rules or quizzed on them with feedback. Three-year-olds can sort flawlessly by either color or shape, but when they are supposed to switch to the other sorting dimension (e.g., switch from sorting by color to sorting by shape), they seem unable to ignore the previously relevant dimension. Thus, although a child may have just told the experimenter that trucks go with the blue truck model and stars go with the red star model, and although the experimenter presents the stimulus by labeling only the relevant dimension (“Here’s a truck”), a 3-year-old who previously sorted by color will take the red truck card, look at it, say “But it’s red,” and sort it with the red star model.
Importantly, if color and shape are always present on the stimulus cards but are not properties of the same object (e.g., instead of presenting a red truck on a white background, a black truck is presented on a red background), then many more 3-year-olds are able to succeed. Here they do not need to selectively attend to only one property of a thing; color is a property of the background, not of the shape (Diamond et al., 2005; Kloo & Perner, 2005). Adults, of course, can switch from sorting by color or shape to sorting by the other dimension if they are told when the switch is occurring and what to switch to. Even adults, however, show a cost in switching from always sorting by color or shape to always sorting by the other; adults are slower on the second block and remain slower on the second dimension throughout the testing session (Diamond & Kirkham, 2005). That is, both children and adults find it difficult to ignore the previously relevant property of a stimulus, especially if it was the first relevant property in the testing session, even though they know that property is now irrelevant. Children show this in a more extreme form, but this is still present in adults. It presumably reflects an inertial tendency; indeed, Kirkham, Cruess, and Diamond (2005) labeled it attentional inertia. Hommel and colleagues have elaborated on why this inertial effect is found (Hommel, 2004; Waszak, Hommel, & Allport, 2003).
Many people have written about the binding problem (e.g., Caiqi, Guifang, Zhicheng, & Jian, 2004; Treisman, 1996). For example, how do stimulus identity and stimulus location, presumably encoded by different neural systems, get bound together? Note that for all of the examples above, it is unbinding (rather than binding) that is the problem. O’Craven, Downing, and Kanwisher (1999) and Schoenfeld et al. (2003) provided evidence at the neural level for such rapid, automatic binding. Unbinding would require inhibiting or undoing that process. (O’Craven and colleagues, 1999, had participants view a face transparently superimposed on a house, one moving and the other stationary. Attending to one attribute of an object [e.g., the motion of a moving face] enhanced the neural representation not only of that attribute but also of other, task-irrelevant attributes of the same object [e.g., the face] but not of attributes of the other object [e.g., the house].)
Corollary 3
A third corollary of the global default principle is that it is easier to inhibit a dominant response all the time than only some of the time.
One of the most demanding cognitive requirements is to switch back and forth, to overcome inertial tendencies that favor continuing in whatever mindset one is in. Once in a groove, even if it was a difficult one to settle into, it is not that difficult to continue along that path. What is most demanding is switching back and forth, overcoming inertial tendencies (Diamond et al., 2005; Fagot, 1994; Los, 1996; Schuch & Koch, 2004; Waszak et al., 2003). Thus, it is easier to respond on the same side as a stimulus than on the opposite side (the Simon effect), but it is much easier (both for children and adults) to always respond on the side opposite the stimulus than to switch back and forth between sometimes responding on the same side and sometimes responding on the opposite side (Davidson et al., 2006; Lu & Proctor, 1995).
Proficient readers are used to attending to the meaning of words and to ignoring their surface features such as the color of the ink in which they are printed. It is difficult for readers to ignore a word’s meaning and only focus on the color of the ink. Hence, if a color word (say, green) is printed in the ink of another color (say, red), people are slower to name the ink color than to read the word (the Stroop effect; MacLeod, 1991; Stroop, 1935). However, people are far faster to always name the ink color of color words (inhibiting the dominant response on all trials) than they are to name the ink color on some trials and read the word on other trials (inhibiting the dominant response on only some trials, the inknaming ones, e.g., Allport & Wylie, 2000; Wylie & Allport, 2000). To try this yourself, see Figure 3. It is far easier to keep doing the same thing (to obey a global command relevant for an entire block of trials) even if that is a command to do something relatively difficult than it is to operate in a more fine-grained manner, sometimes following the difficult rule and sometimes the easy one. This is true even though it may seem counterintuitive that one would be faster if all trials require one to follow a difficult rule than if only some of the trials require that and other, intermixed trials allow one to follow an easy rule.
Figure 3.
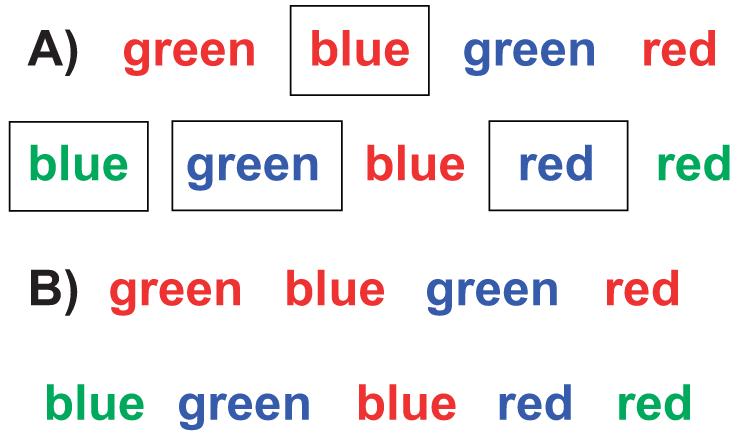
Here, color words are printed in the ink of another color. Compare for yourself the difficulty of saying the color of the ink only some of the time (in a mixed block) and saying the color of the ink all the time (in a single-task block). For Group A, some of the words are enclosed within a box. For those words, read the word instead of saying the color of the ink. For all others, say the color of the ink. For Group B, say the color of the ink for all words. You should notice quite a big difference in difficulty. When reading a study that uses the Stroop task, it is important to look at how the stimuli were administered (in single-task blocks or mixed blocks).
Similarly, in the standard flanker paradigm (press in the direction indicated by a central stimulus, ignore the flanking stimuli; Eriksen & Eriksen, 1974), participants can get in the groove of only attending to the center stimulus. Hence, one sees little developmental improvement after 7 years of age (Rueda et al., 2004), one sees only small (though well-replicated) reaction time differences between when the flanking stimuli are congruent with the central target and when the flankers are mapped to the opposite response (the Flanker effect), and the Flanker effect disappears if the stimuli are not very small or not very close together (e.g., Paquet, 2001). Our variation of the flanker task includes reverse flanker trials (attend to the flankers and ignore the central stimulus). When classical Flanker and reverse Flanker trials are intermixed, even ignoring all trials where the rule switches from the previous trial, because participants cannot settle into relying on one global command (e.g., focus only on the stimulus in the center), one finds a Flanker effect that is 6-12 times larger than in the standard task and is insensitive to stimulus characteristics such as whether the stimuli are large or small (Munro, Chau, Gazarian, & Diamond, 2006).
Corollary 4
Certainly, it has long been known in motor control that it is easier to do the same thing (or mirror image) with both hands than to do one type of movement with one hand and a different type of movement with the other hand, or even to move one hand and do nothing with the other. That is, the default seems to be a global command to both hands to do a certain action.
To keep one hand from doing the same action as the other requires inhibition of that default. Thus, when young children intend to make a movement with only one hand, they involuntarily activate homologous or mirror-image muscle groups of the contralateral hand (called mirror movements or motor overflow). This is normal in children until almost 8 years of age (Abercrombie et al., 1964; Lazarus & Todor, 1987; Mayston et al., 1999; Todor & Lazarus, 1986). Older children and adults manage not to make such associated movements of the contralateral hand by inhibiting that tendency. If adults are cognitively distracted or fatigued (Bodwell, Mahurin, Waddle, Price, & Cramer, 2003) or have to work against high resistance (Armatas, Summers, & Bradshaw, 1994), that inhibition fails and mirror movements are released.
Under normal circumstances, unilateral hand movements of adults are accompanied by (a) transient inhibition of corticospinal neurons innervating homologous muscles in the resting hand (Duque et al., 2005; Leocani, Cohen, Wassermann, Ikoma, & Hallett, 2000; Liepert, Dettmers, Terborg & Weiller, 2001; Sohn, Jung, Kaelin-Lang, & Hallett, 2003; Weiss, Weiller, & Liepert, 2003) and (b) transient transcallosal inhibition of neurons of the contralateral primary hand area of motor cortex (Heinen et al., 1998; Meyer, Roricht, Von Einsiedel, Kruggel, & Weindl, 1995; Schnitzler, Kessler, & Benecke, 1996).
Not only does a command to make a movement go to both limbs even when movement of only one limb is intended (requiring inhibition of that movement for the hand not meant to make the movement) but the command to not act (to stop a movement one is ready to make just before executing it) is also nonselective (Coxon, Stinear, & Byblow, 2006; Hoshiyama et al., 1996). “The inhibitory “No-go” process which is probably generated in the prefrontal cortex and/or SMA [supplementary motor area] is not specific to the target muscles, but causes general suppression over the pyramidal tract” (Hoshiyama et al., 1996, p. 429).
Complementary findings obtain for bimanual coordination (e.g., Fagard, Morioka, & Wolff, 1985; Swinnen, 2002; Wenderoth, Puttemans, Vangheluwe, & Swinnen, 2003). It is far easier to make bimanual mirror-image movements (e.g., mirror-image circles in the air) or the same movement simultaneously with both hands (e.g., alternately placing one’s hands down flat or in a fist) than to have one’s hands do different things simultaneously (e.g., executing the palm-fist alternation offset between the two hands so that one hand executes the palm-flat movement while the other makes a fist). Well before children start kindergarten they can do the former, whereas the latter is beyond the ability of many children as old as 8 years (Fagard, Hardy-Leger, Kervella, & Marks, 2001; Njiokiktjien, Driessen, & Habraken, 1986). Even many healthy, young adults cannot move one arm in a circle while moving the other in a square, or move one arm at one speed and the other arm at a different speed even if the movements of the two arms are the same or a mirror image.
Corollary 5
A fifth corollary of the global default principle is that if one is thinking about a sentence that implies movement in one direction, the prepotent tendency is to move one’s body in that direction; to move in the opposite direction requires inhibition of that tendency.
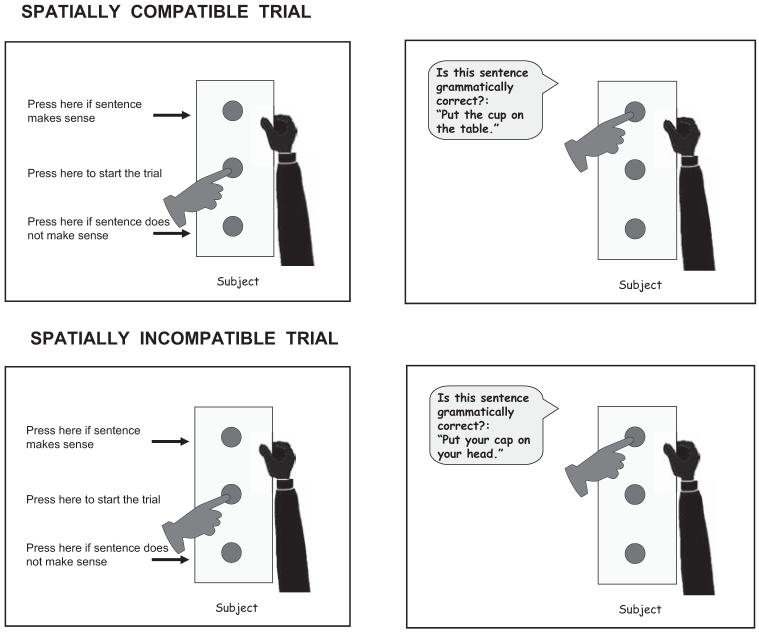
This is based on the work of Glenberg and Kaschak (2002), who in ingenious experiments have shown that if participants are asked to judge, say, whether a sentence is grammatical or not by reaching to a button away from their body if the sentence is grammatical and reaching to a button toward their body if the sentence is ungrammatical, people are faster to hit the grammatical button if the content of the sentence implies an action outward from oneself than if the content of the sentence whose grammaticality they are judging implies an action toward oneself (see Figure 4). Conceptually, it should be as easy to judge whether the sentences “Put the cup on the table” or “Put the cap on your head” are grammatical or not. Indeed people are just as fast to make that judgment verbally or by a left or right keypress. Conceptually, it should not matter whether the response required is to one’s left, right, toward the person, or away from the person. However, when the response is a keypress that requires reaching out, away from one’s body, people are faster to respond to the cup sentence than to the cap sentence.
Figure 4.
Two illustrative trials in Glenberg and Kaschak (2002). Adapted from “Grounding Language in Action,” by A. M. Glenberg & M. P. Kaschak, 2002, Psychonomic Bulletin & Review, 9, 358-365. Copyright 2002 by the Psychonomic Society.
Conclusions
Neuroscientists have known for sometime that neural connections are initially grossly specified and later fine tuned. However, people have not considered that gross, global commands might be the default at all stages of development (even in young adults) and across many contexts. Thus, global commands “Repeat,” “Change,” “Pick the opposite,” “Slow down,” “Make Movement X,” or “Encode the properties of this stimulus” seem often to be sent in a less finely tuned manner than one might have expected (e.g., to both one’s conceptual and one’s motor apparatus or to both the right and left hands). To execute more differentiated commands often seems to require inhibition of the global-command default.
Thus, it is proposed that the mind and brain often work at a relatively gross level and only with fine tuning act in a more differentiated manner, even in adults and even when one might think the domains being issued the same global command should be distinct. This simple principle applies to disparate work within cognitive science and neuroscience. It is consistent with findings that it is easier to switch everything, or nothing, than to switch one thing (e.g., the rule one is following or which button to press) but not the other. Though response site and rule should be orthogonal, evidently both seem to be affected by a global command to “change” or “repeat.” It is easier to take into account multiple salient properties of a stimulus than only some of its properties; indeed, it is often difficult to ignore irrelevant properties of an attended stimulus. If the manual responses differ by left-right location, it is difficult to ignore left-right location differences of the stimuli even if they are irrelevant to the task or even impede task performance. It is easier to inhibit a dominant response all of the time than only some of the time or to execute a relatively difficult response all of the time rather than only some of the time. It is easier to issue the same command to both hands than to do one movement with one hand and a different movement with the other, or even to move one hand and not the other. If one needs to make a conceptual judgment about a sentence (e.g., Is the sentence grammatical?) and the sentence implies movement in one direction, one is faster to respond if the movement required is in the same direction as that implied in the sentence. If one needs to respond to the opposite of a stimulus, one is faster if the correct response is also to the side opposite the stimulus (e.g., the reverse Simon effect). If one needs to slow down for some trials in a set, one tends to slow down for all trials in that set, even easy ones. People tend to think of cognition as separate from action; some even still think of action and perception as quite distinct from one another, and object location (the dorsal stream) as separate from object identity (the ventral stream), but it appears that we are more integrated than we often appreciate (e.g., Anderson, 2003; Bertenthal & Clifton, 1998; Fagioli, Ferlazzo, & Hommel, 2007; Goodale & Westwood, 2004; Thelen, Schöner, Scheier, & Smith, 2001). To act in a less integrated fashion appears to often require inhibition of the global-command default mode. People tend to think of the nervous system as sending out very precise commands only to the relevant recipient, but it appears that often the command goes out more globally and then parts of the system need to be inhibited from acting on the command.
Acknowledgments
I gratefully acknowledge support from National Institute on Drug Abuse Grant R01 DA19685 during the writing of this article.
References
- Abercrombie ML, Lindon RL, Tyson MC. Associated movements in normal and physically handicapped children. Developmental Medicine & Child Neurology. 1964;10:93–97. doi: 10.1111/j.1469-8749.1964.tb02795.x. [DOI] [PubMed] [Google Scholar]
- Allport A, Wylie G. Task switching, stimulus-response bindings, and negative priming. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVII. MIT Press; Cambridge, MA: 2000. pp. 35–70. [Google Scholar]
- Anderson ML. Embodied cognition: A field guide. Artificial Intelligence. 2003;149:91–130. [Google Scholar]
- Armatas CA, Summers JJ, Bradshaw JL. Mirror movements in normal adult subjects. Journal of Clinical and Experimental Neuropsychology. 1994;16:405–413. doi: 10.1080/01688639408402651. [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Clifton RK. Perception and action. In: Kune D, Siegler R, editors. Handbook of child psychology. Vol. 2. Wiley; New York: 1998. pp. 51–102. [Google Scholar]
- Bhide PG, Frost DO. Intrinsic determinants of retinal axon collateralization and arborization patterns. Journal of Comparative Neurology. 1999;411:119–129. [PubMed] [Google Scholar]
- Bodwell JA, Mahurin RK, Waddle S, Price R, Cramer SC. Age and features of movement influence motor overflow. Journal of the American Geriatrics Society. 2003;51:1735–1739. doi: 10.1046/j.1532-5415.2003.51557.x. [DOI] [PubMed] [Google Scholar]
- Bruner JS. The growth and structure of skill. In: Connolly KJ, editor. Mechanics of motor skill development. Academic Press; New York: 1970. pp. 63–94. [Google Scholar]
- Caiqi C, Guifang F, Zhicheng J, Jian L. The binding problem in cognitive processes—Studies of the cognitive neuroscience paradigm. Psychological Science (China) 2004;27:590–594. [Google Scholar]
- Cant JS, Large M-E, McCall L, Goodale MA. Independent processing of form, colour, and texture in object perception. Perception. 2008;37:57–78. doi: 10.1068/p5727. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Cowey A. Development and retraction of a crossed retinal projection to the inferior colliculus in neonatal pigmented rats. Neuroscience. 1990;35:335–344. doi: 10.1016/0306-4522(90)90087-k. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. Journal of Neurophysiology. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Crone EA, Ridderinkhof RK, Worm M, Somsen RJM, van der Molen MW. Switching between spatial stimulus-response mappings: A developmental study of cognitive flexibility. Developmental Science. 2004;7:443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases, of inhibitory control in reaching. Annals of the New York Academy of Sciences. 1990;608:637–676. doi: 10.1111/j.1749-6632.1990.tb48913.x. [DOI] [PubMed] [Google Scholar]
- Diamond A, Carlson SM, Beck DM. Preschool children’s performance in task switching on the dimensional change card sort task: Separating the dimensions aids the ability to switch. Developmental Neuropsychology. 2005;28:689–729. doi: 10.1207/s15326942dn2802_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Kirkham N. Not quite as grown-up as people like to think: Parallels between cognition in childhood and adulthood. Psychological Science. 2005;16:291–297. doi: 10.1111/j.0956-7976.2005.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick M, Hochstein S. Interactions in the dimensions and absolute judgments of orientation and length. Perception. 1988;17:177–189. doi: 10.1068/p170177. [DOI] [PubMed] [Google Scholar]
- Duncan J. Divided attention: The whole is more than the sum of its parts. Journal of Experimental Psychology: Human Perception and Performance. 1979;5:216–228. doi: 10.1037//0096-1523.5.2.216. [DOI] [PubMed] [Google Scholar]
- Duncan J. The locus of interference in the perception of simultaneous stimuli. Psychological Review. 1980;87:272–300. [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P. Intelligence and the prefrontal lobe: The organization on goal-directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Current Opinion in Neurobiology. 1997;7:255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cerebral Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Fagard J, Hardy-Leger I, Kervella C, Marks A. Changes in interhemispheric transfer rate and the development of bimanual coordination during childhood. Journal of Experimental Child Psychology. 2001;80:1–22. doi: 10.1006/jecp.2000.2623. [DOI] [PubMed] [Google Scholar]
- Fagard J, Morioka M, Wolff PH. Early stages in the acquisition of a bimanual motor skill. Neuropsychologia. 1985;23:535–543. doi: 10.1016/0028-3932(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Fagioli S, Ferlazzo F, Hommel B. Controlling attention through action: Observing actions primes action-related stimulus dimensions. Neuropsychologia. 2007;14:3351–3355. doi: 10.1016/j.neuropsychologia.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Fagot C. Unpublished doctoral dissertation. University of California; San Diego: 1994. Chronometric investigation of task switching. [Google Scholar]
- Frost DO. Development of anomalous retinal projections to nonvisual thalamic nuclei in Syrian hamsters: A quantitative study. Journal of Comparative Neurology. 1986;252:95–105. doi: 10.1002/cne.902520106. [DOI] [PubMed] [Google Scholar]
- Garner WR. The processing of information and structure. Erlbaum; Potomac, MD: 1974. [Google Scholar]
- Glenberg AM, Kaschak MP. Grounding language in action. Psychonomic Bulletin & Review. 2002;9:358–365. doi: 10.3758/bf03196313. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. An evolving view of duplex vision: Separate but interacting cortical pathways for perception and action. Current Opinion in Neurobiology. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Hedge A, Marsh NWA. The effect of irrelevant spatial correspondences on two-choice response-time. Acta Psychologica. 1975;39:427–439. doi: 10.1016/0001-6918(75)90041-4. [DOI] [PubMed] [Google Scholar]
- Heinen F, Glocker FX, Fietzek U, Meyer BU, Lucking CH, Korinthenberg R. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Annals of Neurology. 1998;43:608–612. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- Hommel B. Event files: Feature binding in and across perception and action. Trends in Cognitive Sciences. 2004;8:494–500. doi: 10.1016/j.tics.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Hommel B, Musseler J, Aschersleben G, Prinz W. The theory of event coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences. 2001;24:849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Hommel B, Proctor RW, Vu KP. A feature-integration account of sequential effects in the Simon task. Psychological Research. 2004;68:1–17. doi: 10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Koyama S, Kitamura Y, Shimojo M, Watanabe S, Kakigi R. Effects of judgement process on motor evoked potentials in go/no-go hand movement task. Neuroscience Research. 1996;24:427–430. doi: 10.1016/0168-0102(95)01013-0. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6:449–467. [Google Scholar]
- Kleinsorge T. Response repetition benefits and costs. Acta Pschologica. 1999;103:295–310. doi: 10.1016/s0001-6918(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Kloo D, Perner J. Disentangling dimensions in the dimensional change card sorting task. Developmental Science. 2005;8:44–56. doi: 10.1111/j.1467-7687.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- Lazarus JA, Todor JI. Age differences in the magnitude of associated movement. Developmental Medicine & Child Neurology. 1987;29:726–733. doi: 10.1111/j.1469-8749.1987.tb08817.x. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clinical Neurophysiology. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misled: Facilitative effects of increasing the frequency of conflicting stimuli in a Stroop-like task. Memory & Cognition. 1979;7:166–174. [Google Scholar]
- Los SA. On the origin of mixing costs: Exploring information processing in pure and mixed blocks of trials. Acta Psychologica. 1996;94:145–188. [Google Scholar]
- Lu CH, Proctor RW. The influence of irrelevant location information on performance: A review of the Simon and spatial Stroop effects. Psychonomic Bulletin and Review. 1995;2:174–207. doi: 10.3758/BF03210959. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Mayr U, Bryck RL. Sticky rules: Integration between abstract rules and specific actions. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:337–350. doi: 10.1037/0278-7393.31.2.337. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Annals of Neurology. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfer between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Munro S, Chau C, Gazarian K, Diamond A. Dramatically larger flanker effects (6-fold elevation); Paper presented at the Cognitive Neuroscience Society Annual Meeting; San Francisco. 2006, April. [Google Scholar]
- Njiokiktjien C, Driessen M, Habraken L. Development of supination-pronation movements in normal children. Human Neurobiology. 1986;5:199–203. [PubMed] [Google Scholar]
- O’Craven KM, Downing PE, Kanwisher N. fMRI evidence for objects as the units of attentional selection. Nature. 1999 October 7;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- Paquet L. Eliminating flanker effects and negative priming in the flankers task: Evidence for early selection. Psychonomic Bulletin & Review. 2001;8:301–306. doi: 10.3758/bf03196165. [DOI] [PubMed] [Google Scholar]
- Pratt J, Hommel B. Symbolic control of visual attention: The role of working memory and attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:835–845. doi: 10.1037/0096-1523.29.5.835. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Yamasaki EN. Transient retinal ganglion cells in the developing rat are characterized by specific morphological properties. Journal of Comparative Neurology. 1996;368:582–596. doi: 10.1002/(SICI)1096-9861(19960513)368:4<582::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Kessler KR, Benecke R. Transcallosally mediated inhibition of interneurons within human primary motor cortex. Experimental Brain Research. 1996;112:381–391. doi: 10.1007/BF00227944. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Tempelmann C, Martinez A, Hopf J-M, Sattler C, Heinze H-J, Hillyard SA. From the cover: Dynamics of feature binding during object-selective attention. Proceedings of the National Academy of Sciences, USA. 2003;100:11806–11811. doi: 10.1073/pnas.1932820100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch S, Koch I. The costs of changing the representation of action: Response repetition and response-response compatibility in dual tasks. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:566–582. doi: 10.1037/0096-1523.30.3.566. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DD. Development of topographic order in the mammalian retinocollicular projection. Journal of Neuroscience. 1992;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Acosta E, Mewaldt SP, Speidel CR. The effect of an irrelevant directional cue on choice reaction time: Duration of the phenomenon and its relation to stages of processing. Perception & Psychophysics. 1976;19:16–22. [Google Scholar]
- Simon JR, Sly PE, Vilapakkam S. Effect of compatibility of S-R mapping on reaction toward the stimulus source. Acta Psychologica. 1981;47:63–81. doi: 10.1016/0001-6918(81)90039-1. [DOI] [PubMed] [Google Scholar]
- Simon JR, Small AM. Processing auditory irrelevant information: Interference from an irrelevant cue. Journal of Applied Psychology. 1969;53:433–435. doi: 10.1037/h0028034. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Jung HY, Kaelin-Lang A, Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Experimental Brain Research. 2003;148:176–185. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sur M. Development and plasticity of retinal X and Y axon terminations in the cat’s lateral geniculate nucleus. Brain Behavior Evolution. 1988;31:243–251. doi: 10.1159/000116592. [DOI] [PubMed] [Google Scholar]
- Sur M, Garraghty PE, Roe AW. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988 December 9;242:1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: From behavioural principles to neural-network interactions. Nature Reviews Neuroscience. 2002;3:348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Thelen E, Schöner G, Scheier C, Smith L. The dynamics of embodiment: A field theory of infant perseverative reaching. Brain and Behavioral Sciences. 2001;24:1–33. doi: 10.1017/s0140525x01003910. [DOI] [PubMed] [Google Scholar]
- Todor JI, Lazarus JA. Exertion level and the intensity of associated movements. Developmental Medicine & Child Neurology. 1986;28:205–212. doi: 10.1111/j.1469-8749.1986.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Treisman A. The binding problem. Current Opinion in Neurobiology. 1996;6:171–178. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RHJ, Verleger R. Aging and the Simon task. Psychophysiology. 2002;39:100–110. doi: 10.1017/S0048577202001221. [DOI] [PubMed] [Google Scholar]
- Waszak F, Hommel B, Allport A. Task-switching and longterm priming: Role of episodic stimulus-task bindings in task-shift costs. Cognitive Psychology. 2003;46:361–413. doi: 10.1016/s0010-0285(02)00520-0. [DOI] [PubMed] [Google Scholar]
- Weiss AC, Weiller C, Liepert J. Pre-movement motor excitability is reduced ipsilateral to low force pinch grips. Journal of Neural Transmission. 2003;110:201–208. doi: 10.1007/s00702-002-0780-x. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Puttemans V, Vangheluwe S, Swinnen SP. Bimanual training reduces spatial interference. Journal of Motor Behavior. 2003;35:296–308. doi: 10.1080/00222890309602142. [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of “switch costs.”. Psychology Research. 2000;63:212–233. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11:37–63. [Google Scholar]